Adsorption of the rhNGF Protein on Polypropylene with Different Grades of Copolymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Atomic Force Microscopy (AFM) Analysis
2.3. Quartz Crystal Microbalance with Dissipation (QCM-D) Monitoring
2.4. Thermal Analysis
2.5. Static Contact Angle (CA) Analysis
2.6. X-ray Photoelectron Spectroscopy (XPS) Analysis
3. Results
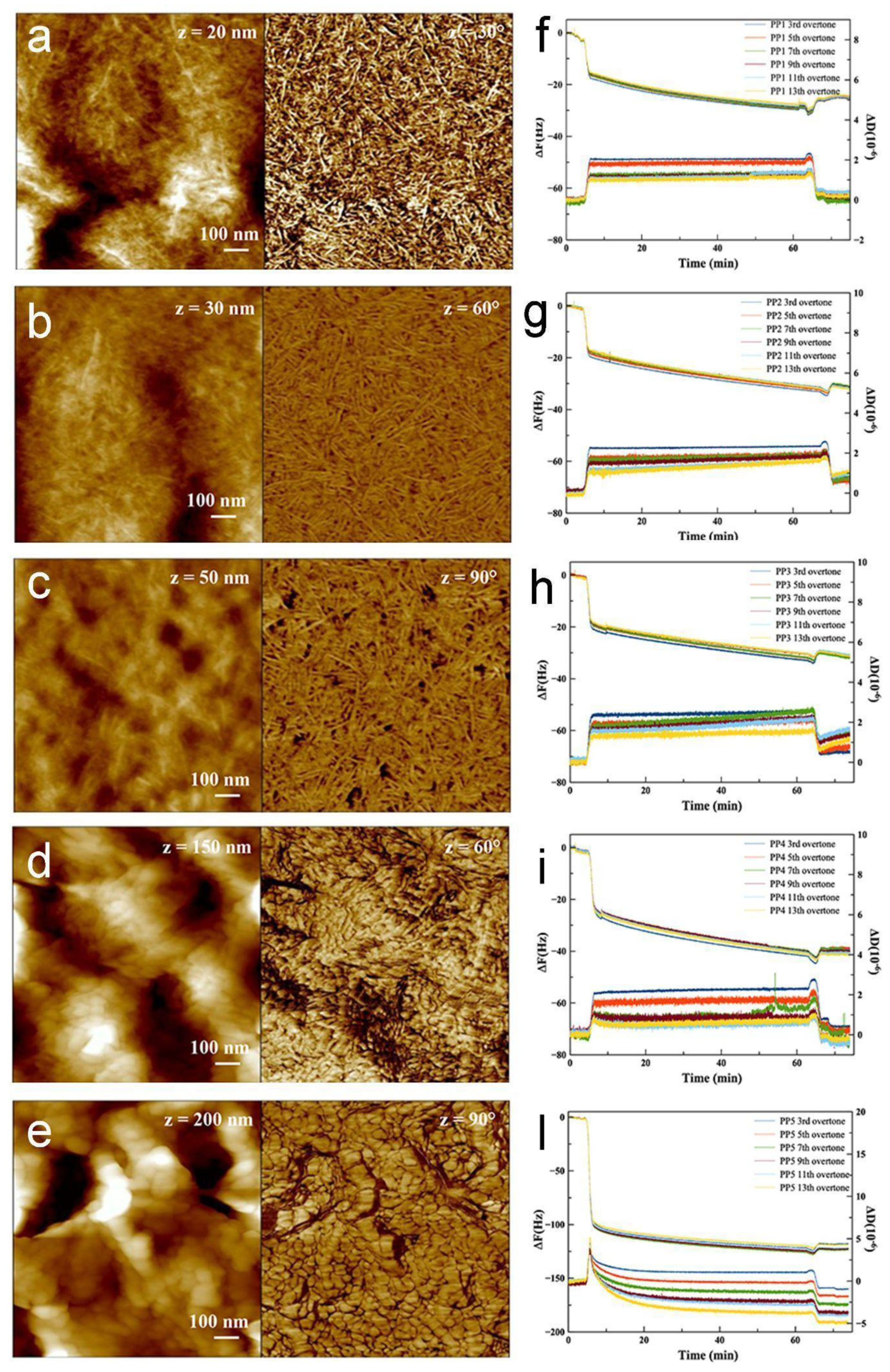
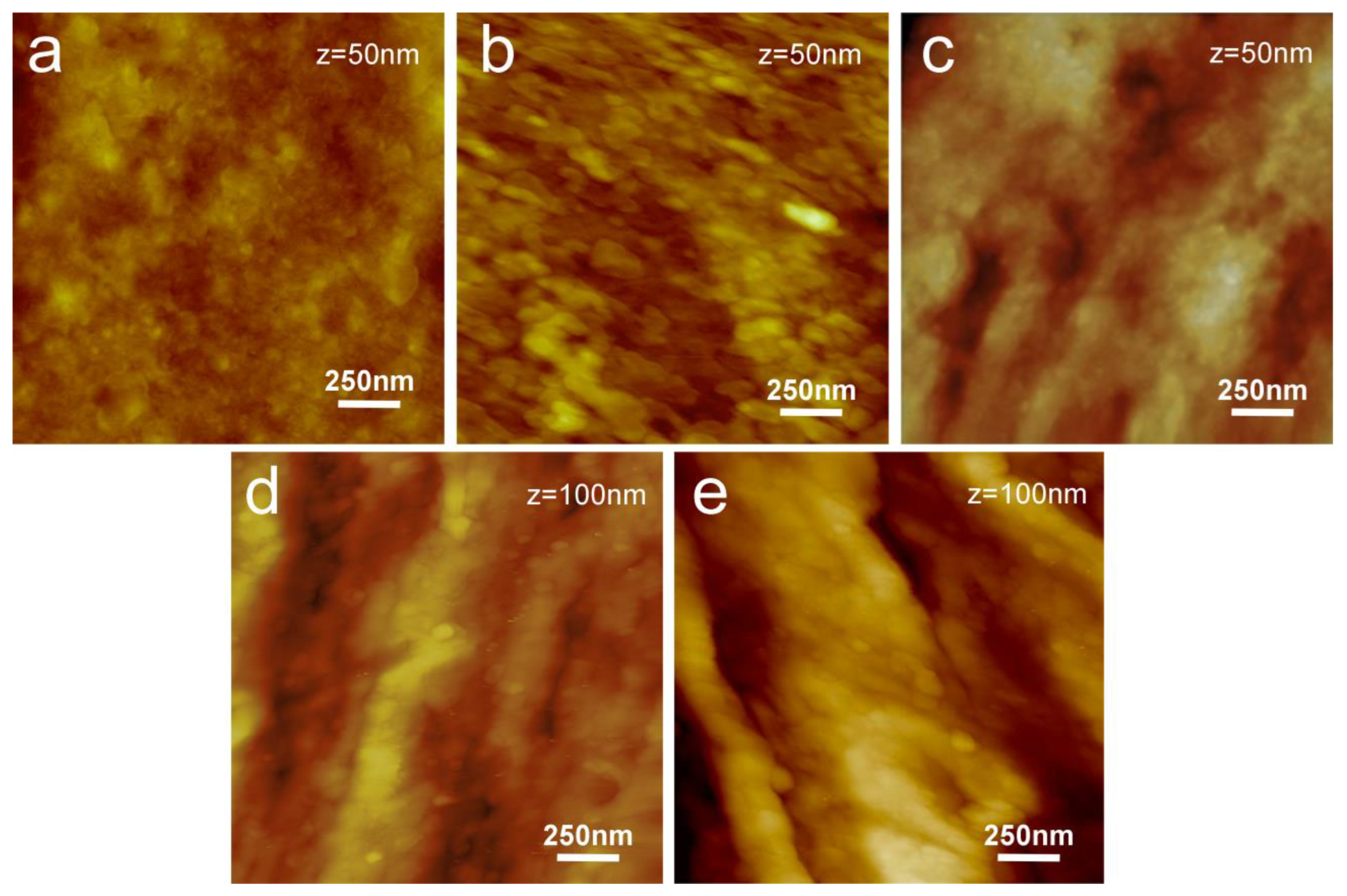
3.1. Protein Adsorption Analysis on PP Model Surfaces
3.2. Characterization of PP Injection Molded Specimens
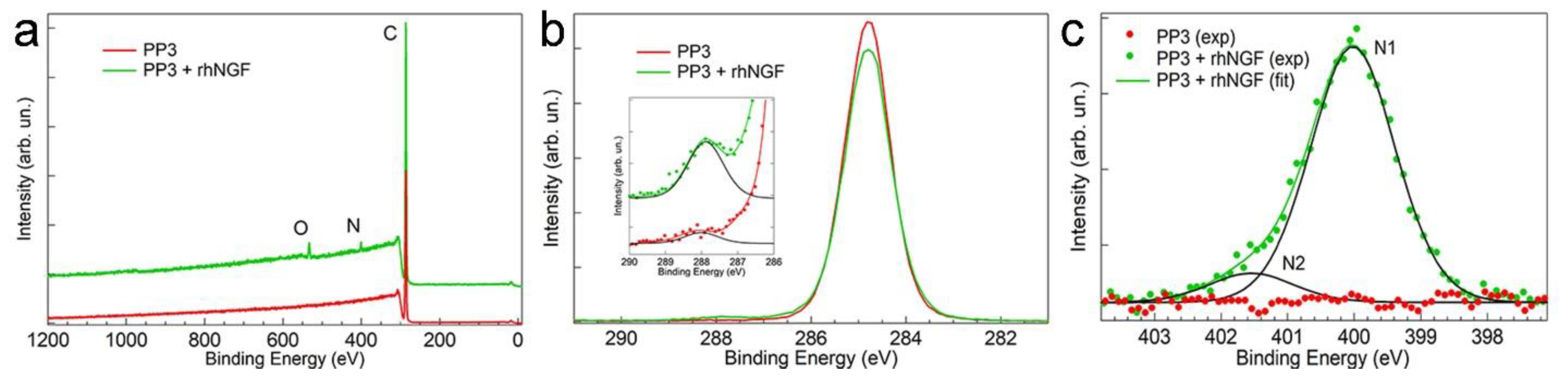
3.3. Measurement of the Adsorption of rhNGF on the Surface of PP3 Bottle by X-ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, X.-D.; Shuai, Y. Macromolecules and Antibody-Based Drugs. In Regulation of Cancer Immune Checkpoints: Molecular and Cellular Mechanisms and Therapy; Xu, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; pp. 485–530. ISBN 9789811532665. [Google Scholar]
- Shen, J.; Gao, H.; Chen, L.; Jiang, Y.; Li, S.; Chao, Y.; Liu, N.; Wang, Y.; Wei, T.; Liu, Y.; et al. Eyedrop-based macromolecular ophthalmic drug delivery for ocular fundus disease treatment. Sci. Adv. 2023, 9, eabq3104. [Google Scholar] [CrossRef]
- Krishnaswami, V.; Kandasamy, R.; Alagarsamy, S.; Palanisamy, R.; Natesan, S. Biological macromolecules for ophthalmic drug delivery to treat ocular diseases. Int. J. Biol. Macromol. 2018, 110, 7–16. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Huckle, J.; Newman, J.; Reid, D.L. Trends in small molecule drug properties: A developability molecule assessment perspective. Drug Discov. Today 2022, 27, 103366. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Atala, A. Small molecules and small molecule drugs in regenerative medicine. Drug Discov. Today 2014, 19, 801–808. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Srinivasan, P.; Hu, Z.; Wang, R.; Saragih, A.; Repka, M.A.; Murthy, S.N. Interactions Between Biological Products and Product Packaging and Potential Approaches to Overcome Them. AAPS PharmSciTech 2018, 19, 3681–3686. [Google Scholar] [CrossRef]
- Rodríguez Fernández, M.J.; Serrano Lopez, D.R.; Torrado, J.J. Effect of Primary Packaging Material on the Stability Characteristics of Diazepam and Midazolam Parenteral Formulations. Pharmaceutics 2022, 14, 2061. [Google Scholar] [CrossRef]
- Cho, D.H.; Hahm, J. Protein–Polymer Interaction Characteristics Unique to Nanoscale Interfaces: A Perspective on Recent Insights. J. Phys. Chem. B 2021, 125, 6040–6057. [Google Scholar] [CrossRef] [PubMed]
- Poncin-Epaillard, F.; Vrlinic, T.; Debarnot, D.; Mozetic, M.; Coudreuse, A.; Legeay, G.; El Moualij, B.; Zorzi, W. Surface Treatment of Polymeric Materials Controlling the Adhesion of Biomolecules. J. Funct. Biomater. 2012, 3, 528–543. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, P.; Brunsteiner, M.; Khinast, J. Investigation of migrant-polymer interaction in pharmaceutical packaging material using the linear interaction energy algorithm. J. Pharm. Sci. 2014, 103, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Matsushima’, H.; Bogenmann, E. Nerve Growth Factor (NGF) Induces Neuronal Differentiation in Neuroblastoma Cells Transfected with the NGF Receptor cDNA. Mol. Cell. Biol. 1990, 10, 6. [Google Scholar]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Rocco, M.L.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Latina, V.; Balzamino, B.O.; Squitti, R.; Varano, M.; Calissano, P.; Micera, A. Nerve Growth Factor-Based Therapy in Alzheimer’s Disease and Age-Related Macular Degeneration. Front. Neurosci. 2021, 15, 735928. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.; Lambiase, A.; Rama, P.; Sinigaglia, F.; Allegretti, M.; Chao, W.; Mantelli, F.; Bonini, S.; Lambiase, A.; Rama, P.; et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology 2018, 125, 1332–1343. [Google Scholar] [CrossRef]
- Bruscolini, A.; Marenco, M.; Albanese, G.M.; Lambiase, A.; Sacchetti, M. Long-term clinical efficacy of topical treatment with recombinant human nerve growth factor in neurotrophic keratopathy: A novel cure for a rare degenerative corneal disease? Orphanet J. Rare Dis. 2022, 17, 57. [Google Scholar] [CrossRef]
- Schaut, R.A.; Weeks, W.P. Historical Review of Glasses Used for Parenteral Packaging. PDA J. Pharm. Sci. Technol. 2017, 71, 279–296. [Google Scholar] [CrossRef]
- Raina, H.; Jindal, A. Packaging of Non-Injectable Liquid Pharmaceuticals: A Review. J. Appl. Pharm. Sci. 2017, 7, 248–257. [Google Scholar] [CrossRef]
- Puleo, D.A.; Bizios, R. (Eds.) Biological Interactions on Materials Surfaces: Understanding and Controlling Protein, Cell, and Tissue Responses; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-0-387-98160-4. [Google Scholar]
- Rashid, S.; Ward-Bond, J.; Krupin, O.; Berini, P. Non-specific adsorption of protein to microfluidic materials. Colloids Surf. B Biointerfaces 2021, 208, 112138. [Google Scholar] [CrossRef]
- Yoneda, S.; Maruno, T.; Mori, A.; Hioki, A.; Nishiumi, H.; Okada, R.; Murakami, M.; Zekun, W.; Fukuhara, A.; Itagaki, N.; et al. Influence of Protein Adsorption on Aggregation in Prefilled Syringes. J. Pharm. Sci. 2021, 110, 3568–3579. [Google Scholar] [CrossRef]
- Bordi, F.; Prato, M.; Cavalleri, O.; Cametti, C.; Canepa, M.; Gliozzi, A. Azurin Self-Assembled Monolayers Characterized by Coupling Electrical Impedance Spectroscopy and Spectroscopic Ellipsometry. J. Phys. Chem. B 2004, 108, 20263–20272. [Google Scholar] [CrossRef]
- Dahal, Y.R.; Olvera de la Cruz, M. Controlling protein adsorption modes electrostatically. Soft Matter 2020, 16, 5224–5232. [Google Scholar] [CrossRef] [PubMed]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. QCM-D and XPS study of protein adsorption on plasma polymers with sulfonate and phosphonate surface groups. Colloids Surf. B Biointerfaces 2019, 173, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Komorek, P.; Wałek, M.; Jachimska, B. Mechanism of lysozyme adsorption onto gold surface determined by quartz crystal microbalance and surface plasmon resonance. Bioelectrochemistry 2020, 135, 107582. [Google Scholar] [CrossRef]
- Stachiv, I.; Kuo, C.-Y.; Li, W. Protein adsorption by nanomechanical mass spectrometry: Beyond the real-time molecular weighting. Front. Mol. Biosci. 2023, 9, 1058441. [Google Scholar] [CrossRef]
- Chang, Y.; Chu, W.-L.; Chen, W.-Y.; Zheng, J.; Liu, L.; Ruaan, R.-C.; Higuchi, A. A systematic SPR study of human plasma protein adsorption behavior on the controlled surface packing of self-assembled poly(ethylene oxide) triblock copolymer surfaces. J. Biomed. Mater. Res. A 2010, 93, 400–408. [Google Scholar] [CrossRef]
- Pinto, G.; Parisse, P.; Solano, I.; Canepa, P.; Canepa, M.; Casalis, L.; Cavalleri, O. Functionalizing gold with single strand DNA: Novel insight into optical properties via combined spectroscopic ellipsometry and nanolithography measurements. Soft Matter 2019, 15, 2463–2468. [Google Scholar] [CrossRef]
- Toccafondi, C.; Prato, M.; Maidecchi, G.; Penco, A.; Bisio, F.; Cavalleri, O.; Canepa, M. Optical properties of Yeast Cytochrome c monolayer on gold: An in situ spectroscopic ellipsometry investigation. J. Colloid Interface Sci. 2011, 364, 125–132. [Google Scholar] [CrossRef]
- Pinto, G.; Canepa, P.; Canale, C.; Canepa, M.; Cavalleri, O. Morphological and Mechanical Characterization of DNA SAMs Combining Nanolithography with AFM and Optical Methods. Materials 2020, 13, 2888. [Google Scholar] [CrossRef]
- Hikosaka, M.; Seto, T. The Order of the Molecular Chains in lsotactic Polypropylene Crystals. Polym. J. 1973, 5, 17. [Google Scholar] [CrossRef]
- Keith, H.D.; Padden, F.J.; Walter, N.M.; Wyckoff, H.W. Evidence for a Second Crystal Form of Polypropylene. J. Appl. Phys. 1959, 30, 1485–1488. [Google Scholar] [CrossRef]
- Blangiardo, A.; Lagomarsino, G.; Basso, A.; Canepa, P.; Cavalleri, O.; Rossi, S.; Monticelli, O. Preparation, application and recycling of a catalytic microflow reactor based on polylactic acid. Appl. Surf. Sci. 2021, 569, 151019. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Pinto, G.; Dante, S.; Rotondi, S.M.C.; Canepa, P.; Cavalleri, O.; Canepa, M. Spectroscopic Ellipsometry Investigation of a Sensing Functional Interface: DNA SAMs Hybridization. Adv. Mater. Interfaces 2022, 9, 2200364. [Google Scholar] [CrossRef]
- Almeida, A.T.; Salvadori, M.C.; Petri, D.F.S. Enolase Adsorption onto Hydrophobic and Hydrophilic Solid Substrates. Langmuir 2002, 18, 6914–6920. [Google Scholar] [CrossRef]
- Kubiak, K.; Adamczyk, Z.; Oćwieja, M. Kinetics of Silver Nanoparticle Deposition at PAH Monolayers: Reference QCM Results. Langmuir 2015, 31, 2988–2996. [Google Scholar] [CrossRef]
- Canepa, P.; Solano, I.; Uttiya, S.; Gemme, G.; Rolandi, R.; Canepa, M.; Cavalleri, O. Phosphonate molecular layers on TiO2 surfaces. MATEC Web Conf. 2017, 98, 03001. [Google Scholar] [CrossRef]
- Gruian, C.; Vanea, E.; Simon, S.; Simon, V. FTIR and XPS studies of protein adsorption onto functionalized bioactive glass. Biochim. Biophys. Acta BBA Proteins Proteomics 2012, 1824, 873–881. [Google Scholar] [CrossRef]
- Uvdal, K.; Bodö, P.; Liedberg, B. l-cysteine adsorbed on gold and copper: An X-ray photoelectron spectroscopy study. J. Colloid Interface Sci. 1992, 149, 162–173. [Google Scholar] [CrossRef]
- Canepa, P.; Gonella, G.; Pinto, G.; Grachev, V.; Canepa, M.; Cavalleri, O. Anchoring of Aminophosphonates on Titanium Oxide for Biomolecular Coupling. J. Phys. Chem. C 2019, 123, 16843–16850. [Google Scholar] [CrossRef]
| n. | Manufacturer | Commercia Code | Notes |
|---|---|---|---|
| PP1 | Borealis | BormedTM RD808CF | Polypropylene Random Copolymer; Melt Flow Index: 8.0 g/10 min (230 °C/2.16 kg, ISO1133); Melting temperature: 140 °C |
| PP2 | Borealis | BormedTM SB815MO | Polypropylene Random Copolymer; Melt Flow Index: 1.5 g/10 min (230 °C/2.16 kg, ISO 1133); Melting temperature: 145 °C |
| PP3 | Borealis | BormedTM SC876CF | Polypropylene Random Copolymer; Melt Flow Index: 3.8 g/10 min (230 °C/2.16 kg, ISO 1133); Melting temperature: 149 °C |
| PP4 | Borealis | BormedTM HD810MO | Polypropylene Homopolymer; Melt Flow index: 10 g/10 min (230 °C/2.16 kg, ISO 1133); Melting temperature: 164 °C |
| PP5 | LyondellBasell | Purell HP372P | Polypropylene Homopolymer; Melt Flow Index: 18 g/10 min (230 °C/2.16 kg, ISO 1133) |
| PP Sample | Thickness (nm) |
|---|---|
| PP1 | 163 ± 8 |
| PP2 | 280 ± 16 |
| PP3 | 200 ± 12 |
| PP4 | (40 ± 2) × 10 |
| PP5 | (40 ± 2) × 10 |
| PP Sample | Ra (nm) | Δf (Hz) | ΔD (10−6) | Mass (ng/cm2) | Molecular Density (Molecules/cm2) |
|---|---|---|---|---|---|
| PP1 | 4.7 ± 1.1 | −24.3 ± 0.7 | 0.2 ± 0.1 | 431 ± 13 | 1.0 × 1013 |
| PP2 | 4.6 ± 0.7 | −30.9 ± 0.7 | 0.8 ± 0.3 | 547 ± 12 | 1.2 × 1013 |
| PP3 | 5.4 ± 1.1 | −29.0 ± 0.5 | 0.9 ± 0.3 | 514 ± 9 | 1.2 × 1013 |
| PP4 | 42 ± 9 | −38.5 ± 1.3 | 0.1 ± 0.3 | (68 ± 2) × 10 | 1.5 × 1013 |
| PP5 | 55 ± 7 | −121 ± 2 | 2.9 ± 1.5 | (214 ± 4) × 10 | 4.8 × 1013 |
| PP Sample | Tm Onset (°C) | Tm Peak (°C) | ΔHm (J/g) | XC (%) | Ra (nm) | Contact Angle (°) |
|---|---|---|---|---|---|---|
| PP1 | 120 | 144 | 61 | 29 | 8.8 ± 1.9 | 98 ± 2 |
| PP2 | 121 | 151 | 52 | 25 | 8.3 ± 1.1 | 102 ± 1 |
| PP3 | 127 | 152 | 40 | 19 | 7.8 ± 1.0 | 97 ± 2 |
| PP4 | 149 | 165 | 86 | 41 | 11.5 ± 1.4 | 74 ± 2 |
| PP5 | 148 | 163 | 84 | 40 | 12.2 ± 1.8 | 83 ± 1 |
| Time | N1s/C1s, 4 °C | N1s/C1s, 25 °C |
|---|---|---|
| 30 s | 0.04 | 0.03 |
| 7 days | 0.03 | 0.05 |
| 30 days | 0.05 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canepa, P.; Canale, C.; Cavalleri, O.; Marletta, G.; Messina, G.M.L.; Messori, M.; Novelli, R.; Mattioli, S.L.; Apparente, L.; Detta, N.; et al. Adsorption of the rhNGF Protein on Polypropylene with Different Grades of Copolymerization. Materials 2023, 16, 2076. https://doi.org/10.3390/ma16052076
Canepa P, Canale C, Cavalleri O, Marletta G, Messina GML, Messori M, Novelli R, Mattioli SL, Apparente L, Detta N, et al. Adsorption of the rhNGF Protein on Polypropylene with Different Grades of Copolymerization. Materials. 2023; 16(5):2076. https://doi.org/10.3390/ma16052076
Chicago/Turabian StyleCanepa, Paolo, Claudio Canale, Ornella Cavalleri, Giovanni Marletta, Grazia M. L. Messina, Massimo Messori, Rubina Novelli, Simone Luca Mattioli, Lucia Apparente, Nicola Detta, and et al. 2023. "Adsorption of the rhNGF Protein on Polypropylene with Different Grades of Copolymerization" Materials 16, no. 5: 2076. https://doi.org/10.3390/ma16052076
APA StyleCanepa, P., Canale, C., Cavalleri, O., Marletta, G., Messina, G. M. L., Messori, M., Novelli, R., Mattioli, S. L., Apparente, L., Detta, N., Romeo, T., & Allegretti, M. (2023). Adsorption of the rhNGF Protein on Polypropylene with Different Grades of Copolymerization. Materials, 16(5), 2076. https://doi.org/10.3390/ma16052076