Effect of Carbon Nanotubes on the Na+ Intercalation Capacity of Binder Free Mn2V2O7-CNTs Electrode: A Structural Investigation
Abstract
1. Introduction
2. Materials and Methods
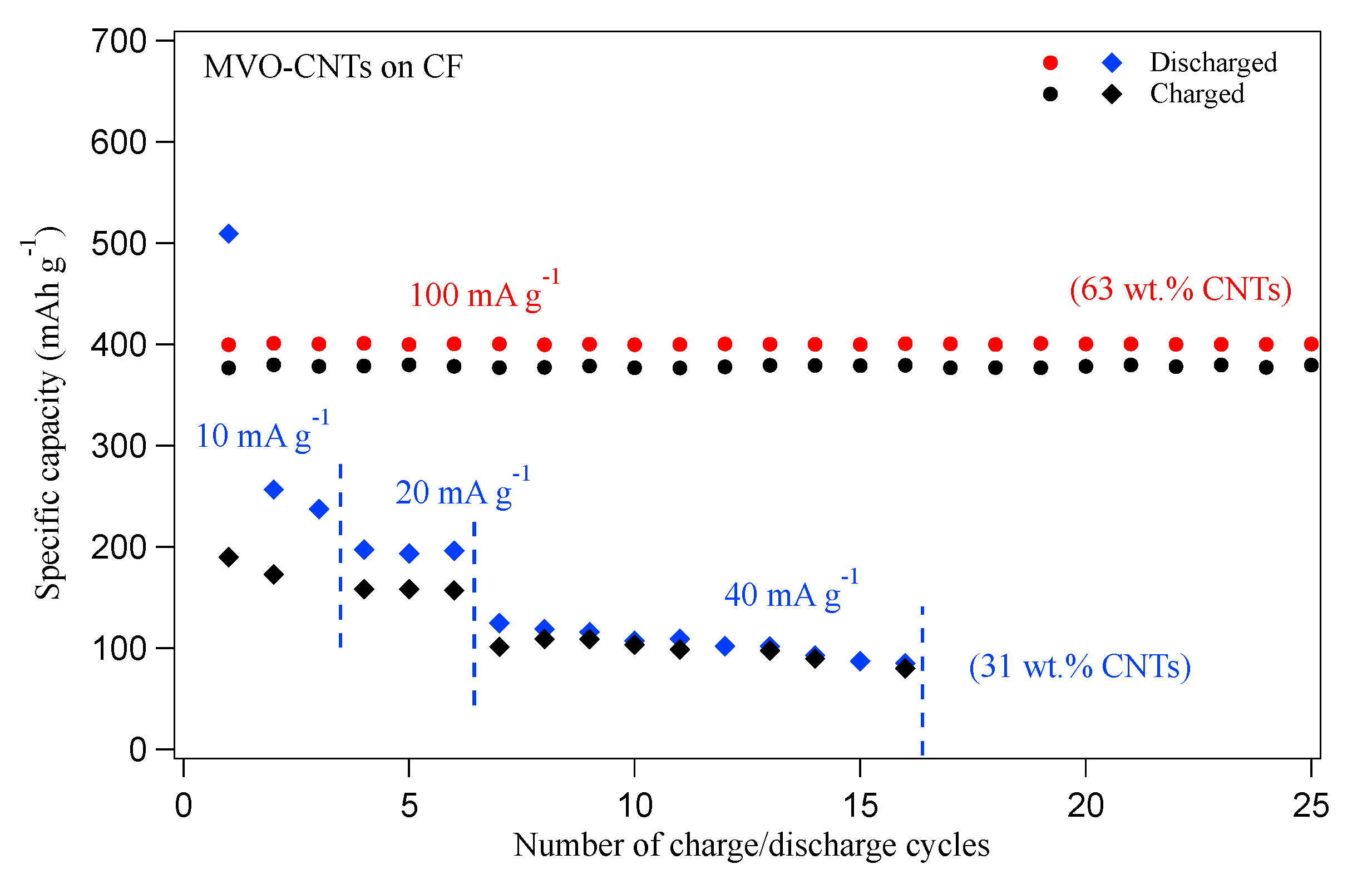
3. Results
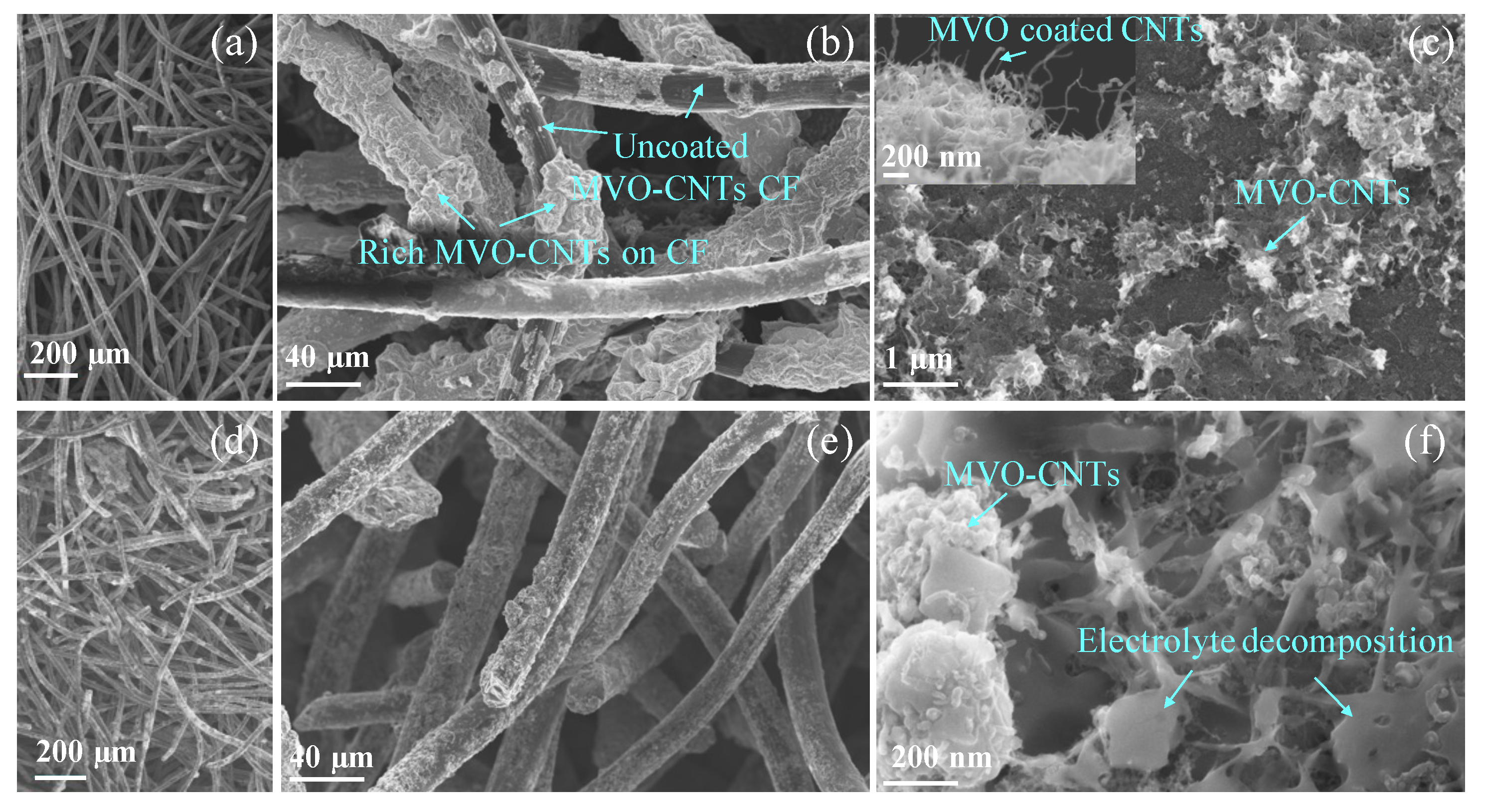
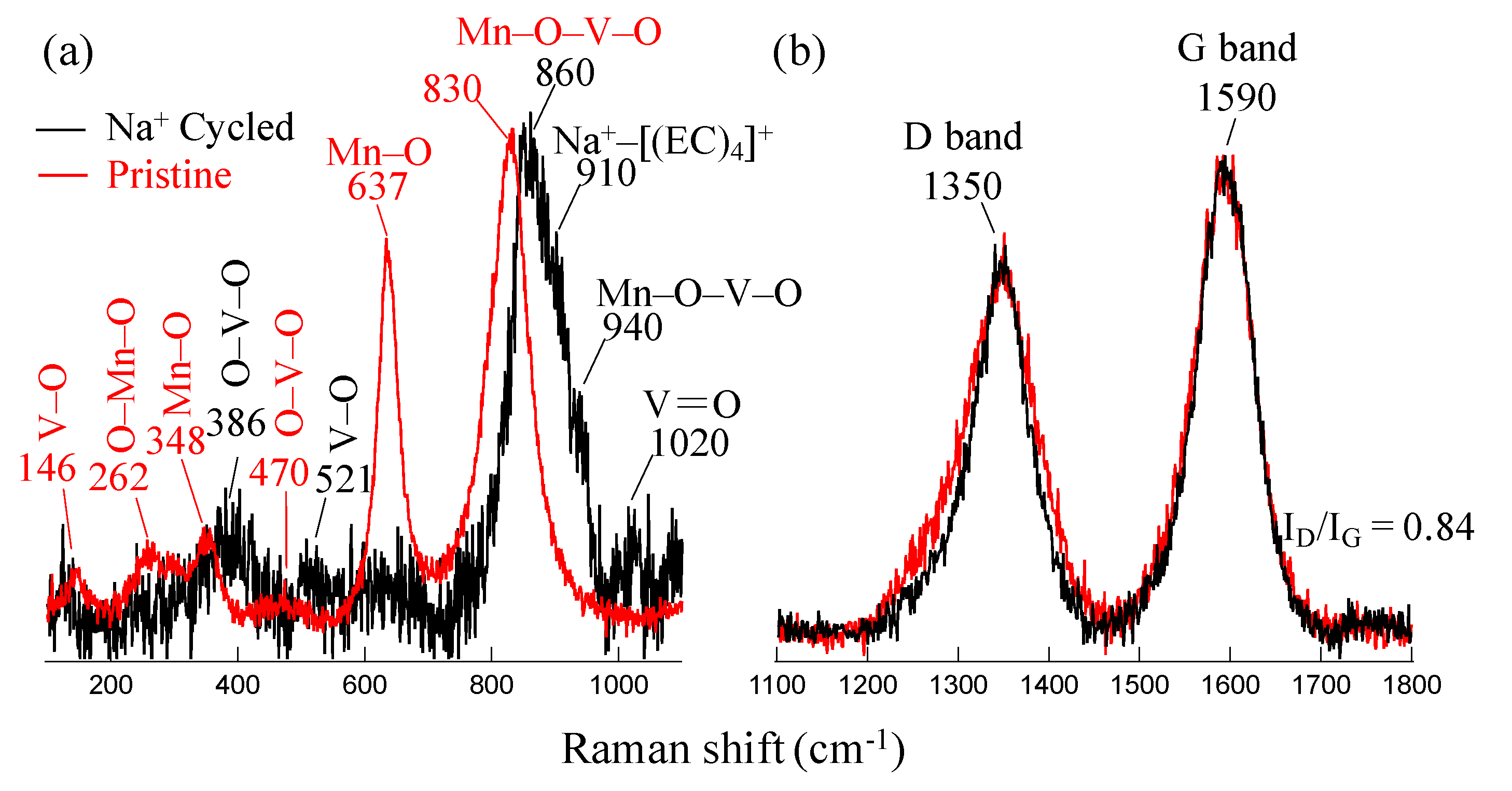
3.1. Morphological and Structural Phase Analysis
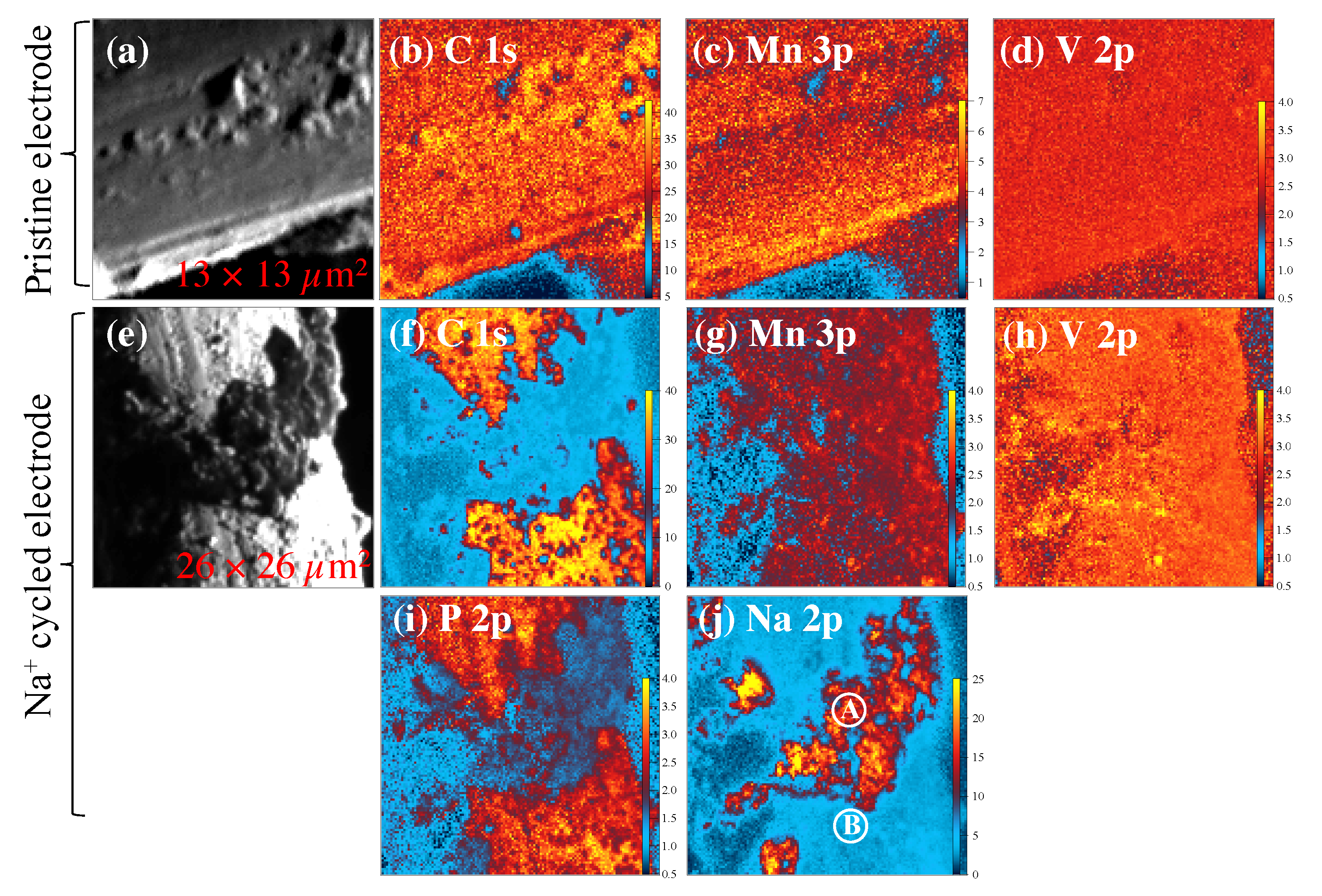
3.2. SPEM Maps and Electronic States Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNTs | Carbon Nanotubes |
| CF | Carbon Fiber |
| CEI | Cathode Electrolyte interphase |
| MVO | Manganese Vanadium Oxide |
| NIBs | Sodium-ion Batteries |
| SEI | Solid Electrolyte interphase |
| SPEM | Scanning Photoelectron Microscopy |
References
- Xiang, X.; Zhang, K.; Chen, J. Recent advances and prospects of cathode materials for sodium-ion batteries. Adv. Mater. 2015, 27, 5343–5364. [Google Scholar] [CrossRef]
- Dai, Z.; Mani, U.; Tan, H.T.; Yan, Q. Advanced Cathode Materials for Sodium-Ion Batteries: What Determines Our Choices? Small Methods 2017, 1, 1700098. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent progress in electrode materials for sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Ong, S.P.; Chevrier, V.L.; Hautier, G.; Jain, A.; Moore, C.; Kim, S.; Ma, X.; Ceder, G. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 2011, 4, 3680–3688. [Google Scholar] [CrossRef]
- Song, J.; Xiao, B.; Lin, Y.; Xu, K.; Li, X. Interphases in Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703082. [Google Scholar] [CrossRef]
- Weadock, N.; Varongchayakul, N.; Wan, J.; Lee, S.; Seog, J.; Hu, L. Determination of mechanical properties of the SEI in sodium ion batteries via colloidal probe microscopy. Nano Energy 2013, 2, 713–719. [Google Scholar] [CrossRef]
- Rahman, M.M.; Glushenkov, A.M.; Ramireddy, T.; Chen, Y. Electrochemical investigation of sodium reactivity with nanostructured Co3O4 for sodium-ion batteries. Chem. Commun. 2014, 50, 5057–5060. [Google Scholar] [CrossRef]
- Wang, J.; Luo, C.; Gao, T.; Langrock, A.; Mignerey, A.C.; Wang, C. An Advanced MoS2/Carbon Anode for High-Performance Sodium-Ion Batteries. Small 2015, 11, 473–481. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the Solid Electrolyte Interphase (SEI) in Sodium Ion Batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Diemant, T.; Hekmatfar, M.; Grugeon, S.; Behm, R.J.; Laruelle, S.; Armand, M.; Passerini, S. Impact of the electrolyte salt anion on the solid electrolyte interphase formation in sodium ion batteries. Nano Energy 2019, 55, 327–340. [Google Scholar] [CrossRef]
- Park, J.; Ku, K.; Son, S.B.; Gim, J.; Kim, Y.; Lee, E.; Johnson, C. Effect of Electrolytes on the Cathode-Electrolyte Interfacial Stability of Fe-Based Layered Cathodes for Sodium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 030536. [Google Scholar] [CrossRef]
- Wang, E.; Niu, Y.; Yin, Y.X.; Guo, Y.G. Manipulating Electrode/Electrolyte Interphases of Sodium-Ion Batteries: Strategies and Perspectives. ACS Mater. Lett. 2021, 3, 18–41. [Google Scholar] [CrossRef]
- Di Cicco, A.; Giglia, A.; Gunnella, R.; Koch, S.L.; Mueller, F.; Nobili, F.; Pasqualini, M.; Passerini, S.; Tossici, R.; Witkowska, A. SEI Growth and Depth Profiling on ZFO Electrodes by Soft X-Ray Absorption Spectroscopy. Adv. Energy Mater. 2015, 5, 1500642. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, Y.; Xiao, B.; Engelhard, M.H.; Yi, R.; Vo, T.D.; Matthews, B.E.; Li, X.; Wang, C.; Le, P.M.L.; et al. Stabilizing Interfacial Reactions for Stable Cycling of High-Voltage Sodium Batteries. Adv. Funct. Mater. 2022, 32, 2204995. [Google Scholar] [CrossRef]
- Jin, Y.; Le, P.M.; Gao, P.; Xu, Y.; Xiao, B.; Engelhard, M.H.; Cao, X.; Vo, T.D.; Hu, J.; Zhong, L.; et al. Low-solvation electrolytes for high-voltage sodium-ion batteries. Nat. Energy 2022, 7, 718–725. [Google Scholar] [CrossRef]
- Parmar, R.; Rezvani, S.; Nobili, F.; Di Cicco, A.; Trapananti, A.; Minicucci, M.; Nannarone, S.; Giglia, A.; Maroni, F.; Gunnella, R. Electrochemical Response and Structural Stability of the Li+ Ion Battery Cathode with Coated LiMn2O4 Nanoparticles. ACS Appl. Energy Mater. 2020, 3, 8356–8365. [Google Scholar] [CrossRef]
- Rezvani, S.J.; Parmar, R.; Maroni, F.; Nobili, F.; Di Cicco, A.; Gunnella, R. Does Alumina Coating Alter the Solid Permeable Interphase Dynamics in LiMn2O4 Cathodes? ACS J. Phys. Chem. C 2020, 124, 26670–26677. [Google Scholar] [CrossRef]
- Moeez, I.; Susanto, D.; Chang, W.; Lim, H.D.; Chung, K.Y. Artificial cathode electrolyte interphase by functional additives toward long-life sodium-ion batteries. Chem. Eng. J. 2021, 425, 130547. [Google Scholar] [CrossRef]
- Zuo, W.; Innocenti, A.; Zarrabeitia, M.; Bresser, D.; Yang, Y.; Passerini, S. Layered Oxide Cathodes for Sodium-Ion Batteries: Storage Mechanism, Electrochemistry, and Techno-economics. Accounts Chem. Res. 2023, 56, 284–296. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Wu, F.; Bai, Y.; Wu, C. Boost sodium-ion batteries to commercialization: Strategies to enhance initial Coulombic efficiency of hard carbon anode. Nano Energy 2021, 82, 105738. [Google Scholar] [CrossRef]
- Rezvani, S.J.; Gunnella, R.; Witkowska, A.; Mueller, F.; Pasqualini, M.; Nobili, F.; Passerini, S.; Cicco, A.D. Is the Solid Electrolyte Interphase an Extra-Charge Reservoir in Li-Ion Batteries? ACS Appl. Mater. Interfaces 2017, 9, 4570–4576. [Google Scholar] [CrossRef]
- Krasnenko, T.; Rotermel, M. Structural modification of Mn2V2O7: Thermal expansion and solid solutions. Russ. J. Gen. Chem. 2013, 83, 1640–1644. [Google Scholar] [CrossRef]
- Zheng, C.; Jian, B.; Zhong, J.; Huang, S. Single-crystalline Mn2V2O7 anodes with high rate and ultra-stable capability for sodium-ion batteries. J. Alloys Compd. 2023, 934, 168018. [Google Scholar] [CrossRef]
- Huong Le, T.X.; Bechelany, M.; Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon 2017, 122, 564–591. [Google Scholar] [CrossRef]
- Parmar, R.; Rezvani, S.; Neto, D.F.; Rosolen, J.; Kazim, S.; Mattiello, S.; Rajak, P.; Ciancio, R.; Thakur, M.; Minicucci, M.; et al. Structural phase stability and homogeneity enhancement of electrochemically synthesized Mn2V2O7 by nanocarbon networks. Carbon Trends 2022, 9, 100218. [Google Scholar] [CrossRef]
- Parmar, R.; de Freitas Neto, D.B.; Matsubara, E.Y.; Gunnella, R.; Rosolen, J.M. Electro-insertion of Mn2+ ions into V2O5·nH2O on MWCNTs coated carbon felt for binder-free Na+ ion battery electrodes. Sustain. Energy Fuels 2020, 4, 3951–3962. [Google Scholar] [CrossRef]
- Neto, D.F.; Xavier, F.; Matsubara, E.; Parmar, R.; Gunnella, R.; Rosolen, J. The role of nanoparticle concentration and CNT coating in high-performance polymer-free micro/nanostructured carbon nanotube-nanoparticle composite electrode for Li intercalation. J. Electroanal. Chem. 2020, 858, 113826. [Google Scholar] [CrossRef]
- Bouibes, A.; Takenaka, N.; Kubota, K.; Komaba, S.; Nagaoka, M. Development of advanced electrolytes in Na-ion batteries: Application of the Red Moon method for molecular structure design of the SEI layer. RSC Adv. 2022, 12, 971–984. [Google Scholar] [CrossRef]
- Parmar, R.; de Freitas Neto, D.; Matsubara, E.; Gunnella, R.; Rosolen, J. Electrochemical synthesis and structural characterization of nanostructured V2O5.nH2O on CNTs coated/uncoated carbon felt composite. Nano-Struct. Nano-Objects 2020, 24, 100538. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, J.; Shek, C. Influence of grain size on the vibrational properties in Mn2O3 nanocrystals. J. Non-Cryst. Solids 2006, 352, 3285–3289. [Google Scholar] [CrossRef]
- Jiang, J.; Kucernak, A. Electrochemical supercapacitor material based on manganese oxide: Preparation and characterization. Electrochim. Acta 2002, 47, 2381–2386. [Google Scholar] [CrossRef]
- Wei, S.; Chen, S.; Su, X.; Qi, Z.; Wang, C.; Ganguli, B.; Zhang, P.; Zhu, K.; Cao, Y.; He, Q.; et al. Manganese buffer induced high-performance disordered MnVO cathodes in zinc batteries. Energy Environ. Sci. 2021, 14, 3954–3964. [Google Scholar] [CrossRef]
- Ma, M.; Cai, H.; Xu, C.; Huang, R.; Wang, S.; Pan, H.; Hu, Y.S. Engineering Solid Electrolyte Interface at Nano-Scale for High-Performance Hard Carbon in Sodium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2100278. [Google Scholar] [CrossRef]
- Mukai, K.; Inoue, T.; Kato, Y.; Shirai, S. Superior Low-Temperature Power and Cycle Performances of Na-Ion Battery over Li-Ion Battery. ACS Omega 2017, 2, 864–872. [Google Scholar] [CrossRef]
- Gregoratti, L.; Barinov, A.; Benfatto, E.; Cautero, G.; Fava, C.; Lacovig, P.; Lonza, D.; Kiskinova, M.; Tommasini, R.; Mähl, S.; et al. 48-Channel electron detector for photoemission spectroscopy and microscopy. Rev. Sci. Instruments 2004, 75, 64–68. [Google Scholar] [CrossRef]
- Arya, A.K.; Raman, R.S.; Parmar, R.; Amati, M.; Gregoratti, L.; Saxena, S. Spectroscopic investigation of improved corrosion resistance of nickel due to multilayer graphene coating developed with suitably tilted substrate during CVD. Carbon 2022, 200, 215–226. [Google Scholar] [CrossRef]
- Liu, Q.; Fujigaya, T.; Nakashima, N. Graphene unrolled from ‘cup-stacked’ carbon nanotubes. Carbon 2012, 50, 5421–5428. [Google Scholar] [CrossRef]
- Jang, I.Y.; Ogata, H.; Park, K.C.; Lee, S.H.; Park, J.S.; Jung, Y.C.; Kim, Y.J.; Kim, Y.A.; Endo, M. Exposed Edge Planes of Cup-Stacked Carbon Nanotubes for an Electrochemical Capacitor. J. Phys. Chem. Lett. 2010, 1, 2099–2103. [Google Scholar] [CrossRef]
- Lee, S.H.; Teshima, K.; Jang, I.Y.; Yubuta, K.; Kim, Y.J.; Kim, Y.A.; Shishido, T.; Endo, M.; Oishi, S. An environmentally friendly dispersion method for cup-stacked carbon nanotubes in a water system. Chem. Commun. 2010, 46, 2295–2297. [Google Scholar] [CrossRef]
- Terse-Thakoor, T.; Komori, K.; Ramnani, P.; Lee, I.; Mulchandani, A. Electrochemically Functionalized Seamless Three-Dimensional Graphene-Carbon Nanotube Hybrid for Direct Electron Transfer of Glucose Oxidase and Bioelectrocatalysis. Langmuir 2015, 31, 13054–13061. [Google Scholar] [CrossRef]
- Gómez, S.; Rendtorff, N.M.; Aglietti, E.F.; Sakka, Y.; Suárez, G. Surface modification of multiwall carbon nanotubes by sulfonitric treatment. Appl. Surf. Sci. 2016, 379, 264–269. [Google Scholar] [CrossRef]
- Parmar, R.; de Freitas Neto, D.; Kazim, S.; Rezvani, S.; Rosolen, J.; Gunnella, R.; Amati, M.; Gregoratti, L. Manganese vanadium sulfate-oxide cylindrical core-shell heterostructures by electro-spray deposition on graphite foil for Li+ ion intercalation. J. Alloys Compd. 2021, 888, 161483. [Google Scholar] [CrossRef]
- Ghosh, M.; Dilwale, S.; Vijayakumar, V.; Kurungot, S. Scalable Synthesis of Manganese-Doped Hydrated Vanadium Oxide as a Cathode Material for Aqueous Zinc-Metal Battery. ACS Appl. Mater. Interfaces 2020, 12, 48542–48552. [Google Scholar] [CrossRef]
- Parmar, R.; Amati, M.; Gregoratti, L.; Rezvani, S.J.; Banerjee, P.; Rajak, P.; Ciancio, R.; Gunnella, R. How the Environment Encourages the Natural Formation of Hydrated V2O5. ACS Omega 2022, 7, 31115–31119. [Google Scholar] [CrossRef]
- Källquist, I.; Le Ruyet, R.; Liu, H.; Mogensen, R.; Lee, M.T.; Edström, K.; Naylor, A.J. Advances in studying interfacial reactions in rechargeable batteries by photoelectron spectroscopy. J. Mater. Chem. A 2022, 10, 19466–19505. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Andrei, V.; Coaca, E.; Mihailescu, C.; Lungu, C.P.; Radulescu, C.; Dulama, I.D. Obtaining and characterization of PEO layers prepared on CP-Ti in sodium dihydrogen phosphate dihydrate acidic electrolyte solution. Surf. Coatings Technol. 2019, 375, 621–636. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, Y.; Song, B.; Wang, Z.; Wang, X.; Wan, F.; Ma, X. Manganese-ions and polyaniline co-intercalation into vanadium oxide for stable zinc-ion batteries. J. Power Sources 2022, 545, 231920. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmar, R.; Rezvani, J.; Amati, M.; Gregoratti, L.; Neto, D.B.d.F.; Rosolen, J.M.; Gunnella, R. Effect of Carbon Nanotubes on the Na+ Intercalation Capacity of Binder Free Mn2V2O7-CNTs Electrode: A Structural Investigation. Materials 2023, 16, 2069. https://doi.org/10.3390/ma16052069
Parmar R, Rezvani J, Amati M, Gregoratti L, Neto DBdF, Rosolen JM, Gunnella R. Effect of Carbon Nanotubes on the Na+ Intercalation Capacity of Binder Free Mn2V2O7-CNTs Electrode: A Structural Investigation. Materials. 2023; 16(5):2069. https://doi.org/10.3390/ma16052069
Chicago/Turabian StyleParmar, Rahul, Javad Rezvani, Matteo Amati, Luca Gregoratti, Decio Batista de Freitas Neto, Jose Mauricio Rosolen, and Roberto Gunnella. 2023. "Effect of Carbon Nanotubes on the Na+ Intercalation Capacity of Binder Free Mn2V2O7-CNTs Electrode: A Structural Investigation" Materials 16, no. 5: 2069. https://doi.org/10.3390/ma16052069
APA StyleParmar, R., Rezvani, J., Amati, M., Gregoratti, L., Neto, D. B. d. F., Rosolen, J. M., & Gunnella, R. (2023). Effect of Carbon Nanotubes on the Na+ Intercalation Capacity of Binder Free Mn2V2O7-CNTs Electrode: A Structural Investigation. Materials, 16(5), 2069. https://doi.org/10.3390/ma16052069





