Abstract
Calcium-based binders, such as ordinary Portland cement (OPC) and lime (CaO), are the most common artificial cementitious materials used worldwide for concrete and soil improvement. However, using cement and lime has become one of the main concerns for engineers because they negatively affect the environment and economy, prompting research into alternative materials. The energy consumption involved in producing cementitious materials is high, and the subsequent CO2 emissions account for 8% of the total CO2 emissions. In recent years, an investigation into cement concrete’s sustainable and low-carbon characteristics has become the industry’s focus, achieved by using supplementary cementitious materials. This paper aims to review the problems and challenges encountered when using cement and lime. Calcined clay (natural pozzolana) has been used as a possible supplement or partial substitute to produce low-carbon cement or lime from 2012–2022. These materials can improve the concrete mixture’s performance, durability, and sustainability. Calcined clay has been utilized widely in concrete mixtures because it produces a low-carbon cement-based material. Owing to the large amount of calcined clay used, the clinker content of cement can be lowered by as much as 50% compared with traditional OPC. It helps conserve the limestone resources used in cement manufacture and helps reduce the carbon footprint associated with the cement industry. Its application is gradually growing in places such as Latin America and South Asia.
1. Introduction
Soil stabilization is a very useful technique for civil engineering work. Soil stabilization is the modification of one or more soil characteristics through chemical or mechanical methods to generate better soil material containing the desired engineering properties. Soils may also be stabilized to enhance durability and strength or to limit dust production and erosion.
Regardless of the aim for the stabilization, the desired result is the formation of a soil system or soil material that will remain and sustain in place under the design use conditions for the design life of the construction [1]. Traditional stabilization methods include the use of cement, lime, and waste materials. By enhancing various soil engineering properties, these stabilization methods generate an improved construction material [2]. Limestone is one of the most common crushed rocks and is an important part of building materials such as cement, lime, and building stones [3]. Using hydraulic binders in soil improvement is a widespread practice in foundation work [4].
Lime additive provides better index properties, increases the unconfined compression strength, increases the California bearing ratio value, reduces dispersity with an increasing lime quantity and curing period, and decreases the hydraulic conductivity [4,5,6,7]. Adding lime to soil also decreases the liquid limit, plasticity, and maximum dry density while slightly increasing the plastic limit and optimum moisture content [5]. Researchers [8] also showed that as the proportion of lime increased, the density decreased and the optimum moisture content increased. For lime-stabilized soils, clay minerals are considered the primary targets of chemical attack, and the cementitious products (C-A-S-H and C-S-H), which affect pore size distribution and the gains in strength, are related to the progressive creation of these new phases [9,10]. For the cement additive, the cementation effect improved the shear strength, stiffness, and significantly increased the pre-consolidation pressure of the soft soil and decreased the compressibility parameters and settlement of the treated soft soil [11,12,13]. The cement component material causes a decrease in the sensitivity to the moisture of the expansive soil, and the cement-induced hydration reaction decreases swelling and shrinkage while raising unconfined compressive strength and resilient modulus but lowering strain at failure [14].
Regardless of the improvements in the soil characteristics achieved by using cement or lime, these calcium-based binders have shown some shortcomings specific to the environment. This is a problem because, at present, the global trend from different approaches is to reduce environmental pollution, as shown in many studies [15,16]. For example, traditional binder cement generates as much as 5% of artificial CO2 emissions [17,18]. The OPC industry is under tight security due to the emission of huge volumes of CO2 in manufacturing clinker. In terms of global anthropogenic CO2 emissions, the cement sector may be responsible for as much as 5%, according to some estimations [19]. Every ton of cement produces one-quarter ton equivalent of CO2 released [20]. Cement manufacturing is recognized as being responsible for around 7.4% of the world’s carbon dioxide emissions (2.9 Gt in 2016) [21]. In 2016, the global cement production was roughly 4.65 Gt, to which China contributing 52% and the rest of Asia contributed 28.5%. Europe (The European Cement Association (CEMBUREAU) members) only makes 5.3% of the world’s cement. The cumulative global process emissions of carbon dioxide from 1928 and 2018 were 38.3 ± 2.4 Gt CO2, 71% of which have occurred since 1990 [22]. Therefore, China is considered to be the largest producer and consumer of cement, worldwide [23]. The cement industry produces approximately one-eighth of China’s total anthropogenic carbon dioxide emissions, and emissions of CO2 increased 5.8 times in 2008 [24]. Fossil fuel production and clinkering jointly account for more than 70% of CO2 equivalent [25]. The emissions in the OPC sector are 0.662 t CO2/ton of produced cement [26]. The concentrations of carbon dioxide emissions in Nigeria are in the range of 2440–2600 mg/m3 [27].
In 2015, the gross CO2 emissions from cement were approximately 840 kg/ton (China–Korea–Japan), 863 kg/ton (Central America), 845 kg/ton (Middle East), 880 kg/ton (North America), 830 kg/ton (Africa), and 825 kg/ton (EU) [28].
The coefficient of CO2 emissions for lime was proposed to be 0.12 Mg C per Mg for CaCO3 [20], which explained that 100% of carbon in CaCO3, is ultimately released to the atmosphere as CO2. Major greenhouse emissions are implicated in global warming [29]. Another major problem that has been challenging for underground construction is corrosion due to sulfate attacks. In light of recent heaving and premature pavement failures in cement and lime-treated subgrades containing sulfates, the efficacy of calcium-based stabilization has been called into question. The stabilizers based on calcium react with soil sulfates and alumina to produce ettringite, a mineral that increases the expansive properties of sulfate-rich soils. In addition to this problem, the high energy demand and the high cost of cement used as a binder in mortar production have led to a search for alternatives to use as a partial replacement for lime and cement in concrete and soil stabilization. One of these alternatives is the clay slag binder activated with sodium carbonate, sodium silicate, and calcium hydroxide solutions, which has shown improved strength and durability [30]. Therefore, this paper primarily aims to review the major problems of using calcium-based binders in soft soil stabilization, to propose solutions to the problems by using calcined clay as a partial replacement, and to compare the efficiency of the stabilization performance achieved by calcium-based binders alone as stabilizers and calcium-based binders with calcined clay as a primary stabilizer.
2. Cement–Soil Stabilization
One of the most important and common techniques of chemical stabilization is mixing the soft soil with cement material to produce a soil–cement mixture, which contains soil, water, and measured amounts of cement and is compacted to the required density [31]. Many geotechnical problems are encountered when construction activities are carried out in soft soil deposits due to their high compressibility characteristics and low shear strength. Therefore, cement–soil stabilization has become a popular soft soil modification and stabilization technique in cement slurry or dry cement powder [32,33].
The modification of the soil–cement mixture occurs when Ca ions released from the cement during hydration and hydrolysis occupy the positions of exchangeable ions on the surface of the clay minerals, increasing stability and strength, controlling deformability, and reducing plasticity. At the same time, the stabilization of the soil–cement mixture occurs when cement is added to a reactive soil to generate long-term strength gain through cement reaction. This reaction generates stable cementitious products (calcium aluminate hydrates and calcium silicate hydrates) as the Ca from the cement reacts with the silicates and aluminates that are solubilized from the clay. As a result, cement treatment produces high and long-lasting strength gains [13]. Many researchers [9,12] have conducted experimental studies to identify how cement stabilization procedures can increase strength and compressibility in soft ground. Cement–soil stabilization has many benefits, such as decreased swelling and shrinking, increased strength and elastic modulus, and resistance to the damaging effects of moisture, freezing, and thawing. The cement additive decreased the maximum dry density and increased the optimum water content of sandy soils [34,35].Cement-treated soils are more brittle than untreated soils [36].
3. Lime–Soil Stabilization
Lime is made by heating limestone to very high temperatures. Three different forms of lime can be used to improve soil: hydrated lime (calcium hydroxide, Ca [OH]2), quicklime (calcium oxide, CaO), and hydrated lime slurry [37,38]. Lime is known to raise the soil’s shrinkage limit, optimum moisture content, and strength, while decreasing its liquid limit, plasticity index, swelling potential, and maximum dry density [4,5,19]. Lime also enhances the compatibility and workability of subgrade soils [39,40,41]. Soft soils benefit from lime stabilization in several ways, particularly in terms of its enhanced engineering properties, such as increased strength, less swelling, increased resilience, and resistance to the damaging effects of moisture. Clays with a range of plasticity, from medium to high, show the most improvement in these characteristics [42].
The optimum percentage of lime that increases the MDD, bearing capacity ratio, and strength, and decreases the plasticity indices is 5% lime by the dry mass of soil [43]. A total of 5% of lime is sufficient for cation exchange and pozzolanic reactions in soil and produces a new mineral (calcium aluminate hydrates) [44]. The optimum lime content for high strength has been shown to be 4–6% [45]. Lime-soil samples were prepared by many methods, such as injecting into the deep soil layers or mixing with soil in a dry state and adding water [46] (jet grouting, hydraulic, and deep soil mixing [47]), while lime columns were used for the shallow layers. Another method was lime slurry: A typical lime slurry for the stabilization of soil is made by mixing 1 kg of lime with 2.5 L of water, resulting in a 31% lime solution [48]. For example, according to the preceding guideline, 600 g of lime was combined with 1500 mL of tap water to make the lime slurry match the 6% dry soil weight previously compacted in the test mold.
4. Problems of Calcium-Based Binders
Environmental concerns pose a major threat to most countries worldwide, especially with the increasing infrastructure size. Cement is one of the most widely used construction materials. As a result, cement production and use have grown worldwide over time. In 1994, 1370 million tons (Mt) of cement was made worldwide, according to the United States Geological Survey (USGS) [49]. The USGS reports that global cement production has risen from 1370 Mt in 1994 to 4100 Mt in 2017, a more-than-threefold increase [50]. At the same time, the production of cement has received worldwide attention as one of the main sources of anthropogenic carbon dioxide emissions. The cement industry is a major cause of global warming [51]. It is considered the third largest industrial source of pollution, emitting more than 500,000 tons of nitrogen oxide, sulfur dioxide, and carbon monoxide, per year.
The associated CO2 emissions for clinker are between 849 and 868 kg CO2/ton. For OPC, the related CO2 emissions are between 802 and 855 kg CO2/ton [52]. The global process emissions in 2018 were 1.50 ± 0.12 Gt CO2, equivalent to approximately 4% of emissions from fossil fuels [22]. The cumulative carbon dioxide emissions from 1928 and 2018 were 38.3 ± 2.4 Gt CO2, 71% of which have occurred since 1990. Cement, hollow concrete blocks, and reinforcing bars (rebars) were the highest energy consumers and CO2 emitters in the study. They were accountable for 94% of the total embodied energy and 98% of the total CO2 emissions [53]. More information on CO2 emissions in specific regions was found by researchers of [54]. They studied the non-fossil fuel CO2 emissions from industrial processes in China between 2003–2018 for the production of lime, calcium carbide, plate glass, ethylene, aluminum, ferroalloys, soda ash, lead, and zinc. They showed that these industrial processes are equivalent to approximately 5% of China’s total CO2 emissions from cement production processes and fossil fuel combustion. In addition, a study on the CO2 emission factors for Chinese cement production based on organic and inorganic carbon from 2011 to 2015 [41] showed that the CO2 emission factor is 785.53–796.17 kgCO2/tcl.
Researchers [55] studied the CO2 emission factors of cement production in China and showed that the median values for the process, fuel, and direct emission factors are 525, 369, and 919 kg CO2/t clinker, respectively. However, the factor for electricity emissions is 74.9 kg CO2/t clinker. The final emission factor calculated from cement products is 761 kg CO2/t cement. Moreover, carbon dioxide emissions from 1574 cement factories in China were between 500 and 600 kg CO2/t clinker [56]. The carbon dioxide emissions from power plants varied among different enterprises, with an average level of 348 kg CO2/t clinker and a standard deviation of 233 kg CO2/t clinker. China’s cement companies, on average, emit 806 kg CO2/t clinker into the atmosphere. In 2009, the amounts of CO2 reached approximately 14.8% of the national CO2 emissions created by the cement industry in China [57]. Based on the current energy-related and emission-control policies, the researchers in [24] assessed the direct emissions of air pollutants from China’s cement industry, beginning in 1990, and forecasted future emissions through to 2020. The study showed that the cement industry produces approximately one-eighth of China’s total anthropogenic carbon dioxide emissions; emissions of CO2 increased 5.8 times in 2008.
Therefore, China is considered the largest producer and consumer of cement worldwide [23]. In 2010, China produced 1.87 billion metric tons of cement, about 57% of the total cement made worldwide. CO2 is released into the air in large amounts when fossil fuels are burned, and limestone is heated to create cement. In 2009, the cement industry released 1073 Mt of carbon dioxide into the air, representing 15% of China’s total greenhouse gas emissions. One study [58] compared different ways of calculating CO2 emissions from cement production to determine the uncertainties, finding that China’s cement-related CO2 emissions have a relative uncertainty of between 10% and 18%.
A study on the construction phase of a residential tower in Tehran Metropolitan City [59] found that the CO2 emissions were 6%, 78%, and 10% from cement mortar, concrete, and rebar, respectively. A study on the CO2 emissions in China [60] also showed that the CO2 emissions increased as cement production increased. Based on clinker output, raw material consumption (primarily limestone), fuel consumption (i.e., coal), and C/CR, the study displayed China’s cement CO2 emissions, by province, between 2005 and 2014. In 2005, cement production in China produced 641.31 Mt of CO2; in 2014, that number increased to 1246.04 Mt.
In Malaysia, the cement production is approximately 20 million tons per year [61]. The combustion of fossil fuels in pyro-handling units produces approximately 40% of the total emanations, while another 10% results from transporting crude materials and electricity. Finally, about 50% of carbon emissions are discharged in the decomposition of MgCO3 and CaCO3 to produce MgO and CaO and as the core chemical responses in the process. This study showed that, in 2006, Malaysia consumed 20 Mt of cement and had a clinker ratio of 0.89 t/t CO2, which is higher than the world average. Another study [25] analyzed the environmental impacts of the Brazilian cement industry, finding that fossil fuel production and clinkering together account for more than 70% of CO2 equivalent. Research on CO2 emissions in Poland’s cement industry [26] showed that the branch emissions index for Poland’s cement sector is 0.662 tons of CO2 per ton of produced cement. The concentrations of carbon dioxide emissions in Nigeria were recorded in the range of 2440–2600 mg/m3 [27].
The carbon dioxide (CO2) emission factor from lime applied in temperate upland soil was 0.026 mg C per mg of the CaCO3 emitted annually [62]. Furthermore, more than three billion metric tons of carbon dioxide are released into the earth’s atmosphere annually through cement, lime, and gypsum manufacturing enterprises [63]. The maximum global cumulative CO2 emissions related to the cement process would be 45.45 billion tons under the SSP3 scenario [64]. India, China, the United States (US), Nigeria, and Pakistan are responsible for the majority of the global total CO2 emissions from cement production processes between 2015 and 2000. In a new analysis of global process emissions in 2016 [18], the global CO2 emissions from cement production were demonstrated to be 1.45 0.20 Gt CO2, equivalent to about 4% of the emissions from fossil fuels. A total of 35% of the CO2 emissions come from fuel combustion to decompose and heat limestone to produce lime or clinker in an open atmosphere, and the remaining 65% comes from limestone rock itself [65]. An investigation of CO2 emissions [66] found that carbon emission levels in the cement industry range between 5% and 8%, with the plant producing approximately 900 kg of CO2 for every ton of OPC manufactured. This is similar to another study [67], where the manufacturing and use of the OPC used in concrete produced 810 kg CO2/ton of cement. Researchers [68] have shown that the carbon dioxide emissions and energy use from the worldwide cement industry were calculated to be 633 kg CO2/ton of cementitious product.
The researchers in [69,70,71,72] confirmed that 6–8% of the world’s ever-increasing anthropogenic CO2 emissions originate from the OPC industry. Approximately half of the 1435 Mt/ emissions are caused by the energy required to prepare the cement. The other half is unavoidable because it is a byproduct of converting CaO from CaCO3 and is intrinsic to binder chemistry.
Although lime is the second-largest source of CO2 emissions from industrial processes, after cement production in China’s lime industry, in general, about 800–850 kg of CO2 is released per ton of cement clinker. This represents about 5–8% of all CO2 emissions [73,74]. In total, the lime and cement industries were responsible for 8% of global carbon dioxide emissions between 2010 and 2011 [75]. Another study [76] showed that the process of emitting increased rapidly between 2001 and 2012, from 88.79 Mt to 141.72 Mt. The study’s emission factor and activity data have a relative uncertainty of 2.83 and 3.34 percent. Similar range of CO2 emissions from cement is given by [77]; cement plants account for approximately 5–7% of global CO2 emissions, with 900 kg of CO2 emitted into the atmosphere to produce one ton of cement. The cement industry produces about 5% of the global artificial carbon dioxide emissions, of which 40% is from burning fuel and 50% is from the chemical process [78]. The amount of carbon dioxide emitted by the cement industry is nearly 900 kg for every 1000 kg of cement produced. The high percentage of carbon dioxide produced in the chemical reaction leads to a large decrease in mass conversion from limestone to cement.
Carbon dioxide is emitted during the production process of non-metallic minerals, such as cement, plaster, lime, glass, and ceramics [79], which, in 2015, increased in the European Union (EU) by 2.5% compared with 2014. This is the result of widely varying trends in EU member states, with increases for the top seven emitters (with the exception of the decrease in Germany) being Germany (−0.2%), France (+2%), Italy, and Spain (+3.9%), Poland (+5.5%), Romania (+3.3%), and the United Kingdom (+3.1%). Carbon dioxide emissions are generated by the oxidation of carbonate in the cement clinker production process, which is considered the main constituent of cement and the highest of the non-combustion sources of carbon dioxide from industrial manufacturing, contributing to about 4.0% of the total global emissions in 2015. Fuel combustion related to cement production has a similar level for the emissions of CO2. Therefore, cement production accounts for about 8% of global CO2 emissions. Furthermore, in 2015, China produced 58% of the world’s cement, with India coming in second with 6.8% and the US coming in third with 2.7%. The EU is responsible for around 4.1% of the global CO2 output [80]. The carbon dioxide emissions for producing one ton of NaOH, lime, slag, and limestone used as raw materials in OPC were 2.987, 2.975, and 2.987 kg/CO2-e from limestone, slag, and various energies, respectively [81]. The emission factor for the carbon dioxide produced by cement manufacture is 0.82 kg CO2-e/kg [82].
Another major problem with calcium-based binders is the sulfate attack. When sulfates are in the soil or groundwater around a concrete structure, they seriously threaten its long-term durability. External sulfate can enter and cause a sulfate attack, one of the most well-known and studied chemical attacks. In OPC systems, sulfate attacks generally cause ettringite formation, accompanied by cracking, expansion, and a loss of strength. According to new theories, this expansion is driven by crystallization pressure when ettringite forms from an oversaturated solution in small pores. Near the material’s surface, gypsum and ettringite have been seen to form when a higher sulfate concentration is present. The temperature affected the durability of cement-based materials to sulfate attack, where the uniform surface crumbled at 5 °C and edges and corners scaled at 20 °C, and the damage was sharper at 20 °C than at 5 °C [83]. The researchers in [84] conducted a study to evaluate the effect of sodium and magnesium sulfate attacking OPC paste while an electric field was present. The results showed that ettringite was formed initially but broke down later to make gypsum. Thermodynamic modeling shows that the pore solution’s alkalinity dropped drastically during this process, causing ettringite to decompose. In addition to the sulfate access, decalcification occurred in this area, shown by the breakdown of portlandite and C-S-H. When the sample was exposed to MgSO4, the access to sulfate and decalcification occurred later and in a deeper area than when it was exposed to Na2SO4.
The results of thaumasite production by sulfosilicate clinker hydration [85] showed that the clinker’s belite and ternesite prefer turning into C-S-H gels, and the sulfate ions from the ternesite turn into gypsum. The chemical reaction between gypsum, carbon dioxide, and silica forms thaumasite. Thaumasite was clearly visible after 28 days of hydration, and its content in one sample reached approximately 34% in weight. The compressive strength first increased, and then decreased, within 56 days due to the sulfate attack [86,87]. When larger pores are filled with the products of erosion and develop into small pores in the early stage of erosion, in the later stage of erosion, the proportion of larger pores increases, and the cracks occur inside the specimen. Another study [88] showed that the acidic curing environment has a negative effect on the properties of concrete, where the strength decreased with an increase in the duration of the curing age and the proportion concentration of acid due to the sulfate attack.
Researchers [89] have investigated the degradation and mechanism of cast-in-situ concrete when immersed in sulfate-rich corrosive environments and found that corrosion in cast-in-situ concrete is much faster than the degradation of precast concrete due to the faster development of cracks in the cast-in-situ concrete. Sulfate attack leads to weight loss and great expansion in the later corrosion, putting concrete structures in great danger, particularly for cast-in-situ construction. The main products of corrosion induced by sulfate attack are gypsum and ettringite. A study [90] investigated the internal and external effects of sodium sulfate on the strength of soil–cement specimens and found that the internal attack decreased the strength of the soil–cement specimens by up to 70% compared with the prefabricated (about 40%) and cast-in-place (about 20%) samples.
This decrease in the trend of strength gain was seen after 28 days. The temperature and sulfate ion concentration in cement concrete sulfate attacks are highly significant, and the primary products of the erosion of sulfate attack on cement concrete are plate-like gypsum, rod-like ettringite with a larger slenderness ratio, incompletely corroded calcium hydroxide, and granular sulfate salt [91]. Under a seawater attack, the interaction between chloride ions and hydrates can form Kuzel’s and Friedel’s salts. Magnesium ion can replace the ion of Ca in portlandite, lowers the alkalinity of pore solution, and destabilizes the C–S–H gel. The change of phase primarily occurs on the surface of the concrete, weakening the structure and leading to delamination and spalling under the physical attack of the wave [92]. The production of gypsum and ettringite on the surface of the concrete causes a large tensile strength loss, which is the greatest threat to concrete structures in the field when subjected to a sulfate attack, particularly for OPC with a high C3A component [93]. Experimental studies on the paste of cement exposed to external sulfate attack (sodium sulfate solution Na2SO4) [94] found that the ettringite first precipitates in the largest pores without causing any expansion and then penetrates the gel and capillary pores, leading to accelerated swelling.
A further study [95] assessed the durability of a soil–cement mixture subjected to external sulfate attacks (a huge amount of sodium sulfates (25 g/L Na2SO4) was used to accelerate the degradation process). The results showed that for the most porous soil–cement specimens, an external sulfate attack could cause peeling on the surface of the test samples. Given the morphology of the needles seen by SEM analysis, this might be clarified through ettringite crystallization. On the one hand, the precipitation of these minerals results in a mass gain of up to 2.25% for some specimens after two years of sulfate exposure. The researchers in [96] studied the effect of environmental conditions on OPC structures and found that the cement with a higher amount of tricalcium aluminate (C3A) showed a more obvious deterioration. Visual changes, such as the crystallization of expansive products, cracking, and complete disintegration, were also observed. Furthermore, resistance was lost in specimens with low slag content. The loss of strength is a direct outcome of the sulfate attack because it causes a loss of cohesion due to the C-S-H decalcification. The cement with a lower percentage of CaO showed a better performance in resisting a sulfate attack. The generated stresses and the expansions in sulfate exposure conditions increased continuously with the increasing immersion time [97]. The effects of sulfate exposure on the pore network formation of different OPC matrices after two days of casting were investigated in [98]. The results indicate that the patterns of expansive product precipitation are related to the degree of refinement of the pore network. Large pores concentrate a greater proportion of the expanding product generated during the early phases of exposure. Later stages of precipitation result in finer pore sizes.
The production of gypsum and ettringite in the presence of sulfate ions had a beneficial effect on the evolution of the characteristics of OPC during the initial stage of a sodium sulfate attack [99]. Subsequently, these samples displayed a decline in characteristics due to the growth of expansive products and the development of microcracks. The mortar’s compressive strength and static elastic modulus grew during the initial immersion phase, and then plateaued as the immersion time increased. After an initial period of immersion (150 and 120 days), the static elastic modulus and compressive strength decreased as the immersion time increased. Similar behavior for strength was shown by another study [100]. The sodium sulfate solution-soaked mortar expanded and hardened to a higher density than the water-soaked type. The results also demonstrated that increasing the concentration of the sodium sulfate solution shortened the time required to achieve peak properties and accelerated the deterioration of the properties in the late stage.
A sulfate attack can generally be categorized into four phases [101]. In the first phase, ettringite forms at the expense of monosulfate (monosulfate dissolves continuously and is replaced by a greater volume of ettringite). The ettringite grows in stage two, at the expense of the carboaluminate phases (monocarbonate is destabilized and starts dissolving when the hemicarbonate is consumed, leading the ettringite growth to continue). Ettringite grows in stage three, at the expense of some hydrotalcite (hydrotalcite gradually dissolves in stages one and two, but in stage three it will be the only remaining solid that can contribute the aluminates to the solution). In the fourth phase, gypsum replaces portlandite. In addition, the calcium sulfoaluminate products form cement-stabilized clay as a result of the sulfate attack [102]. These products cause volumetric expansion and lead to the formation of microcracks in the specimens. Another study [103] found a sudden reduction in the small-strain shear modulus and a gradual increase in the hydraulic conductivity of a cement-admixed clay due to the degradation of the interparticle cementation in the specimens as a result of the sulfate attack. The damage is produced by ettringite production in pores as small as 10–50 nm, creating stresses of up to 8 MPa, exceeding the tensile strength of the binder matrix. Stresses increase with the C3A level and sulfate concentration [104]. In addition, the production of ettringite in micropores causes expansion due to the interaction of sulfate solutions with mortar, with the expansion reaching the crystallization pressure [97]. Crystals expand due to the pressure created by their growth within limited pores. The mortar bars exposed to a sulfate solution generated gypsum and ettringite, causing some expansion [105]. However, the bars eventually expanded and decomposed due to the development of thaumasite.
Cement clinker samples submerged in a sodium sulfate solution primarily revealed dicalcium silicate, tricalcium silicate, brownmillerite, and tricalcium aluminate as the primary mineral components. Ettringite is primarily formed when calcium aluminate reacts with sulfate ions. A sulfate attack, caused by ettringite expansion, manifests as microscopic fissures in the concrete. Sulfate ions can rapidly enter a solution at larger concentrations [106]. Gypsum forms in both high- and low-concentration solutions of MgSO4 and Na2SO4, and these compounds damage the C–S–H gel [107]. Under partial soaking conditions, cement mortar can be separated into four zones: the soaking zone, the wet zone, the crystallization zone, and the dry zone [108]. Corrosion products were studied in each zone. In the wet and soaking zones, ettringite is the most common by-product of corrosion. Gypsum and crystals of Na2SO4.10H2O and Na2SO4 are the corrosion products in the crystallization zone. Table 1 explains the major problems of calcium-based binders (cement and lime).

Table 1.
Calcium-based Binders (Cement and Lime) Problems.
5. Alternatives and Partial Replacement by Calcined Clay
Cement in concrete is the most common artificial cementitious material worldwide, greatly impacting the world economy and environment. First, the energy consumption in producing cementitious material is high. An essential constituent of concrete releases a significant amount of CO2, a greenhouse gas. As illustrated above, 8% of the total carbon emissions come from cement material. Therefore, enhancing the sustainability of cement concrete has become a significant issue in recent decades to improve sustainable development. Globally, improving cement concrete’s sustainable characteristics and low-carbon content has been the focus of industry attention in recent decades.
Many methods are available to enhance cement concrete’s sustainability and reduce cement material’s impact on the economy or environment, such as using chemical and mineral admixtures in the concrete. One of these methods is the use of calcined clay (natural pozzolana) in concrete, which has developed rapidly in recent years, as demonstrated by the researchers reviewed in Table 2. The table summarizes the most recent research on using calcined clay as a partial replacement for cement or lime in cement and soil improvement. Figure 1 shows the effectiveness of calcined clay on compressive strength as an alternative for lime or cement in concrete. This calcined clay material can be found both artificially and naturally.

Table 2.
Calcined Clay as a Partial Replacement for Cement or Lime in Cement and Soil Improvement.
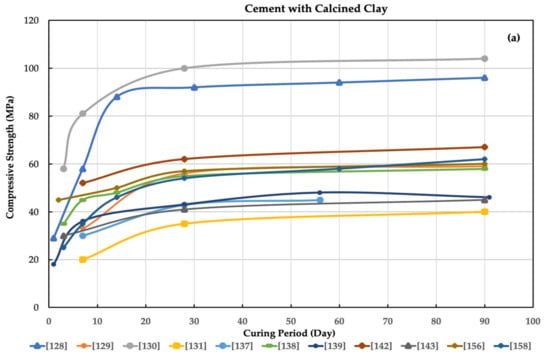
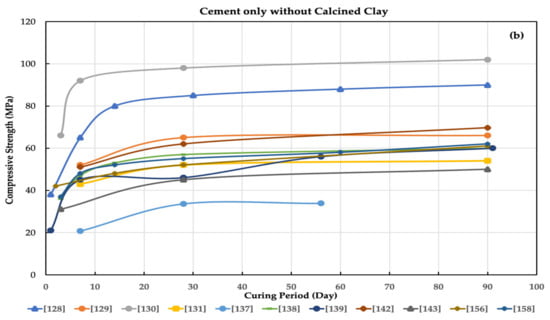
Figure 1.
Compressive strength for (a) cement only and (b) cement with calcined clay as alternatives for cement in concrete by many researchers.
Using calcined clay (CC) offers significant advantages as a cement replacement material and a low-cost alternative binder. Using CC could enhance the strength, which reached an approximate 10% increase in compressive strength relative to the control at 90 days [168]. Its use also reduces water adsorption [166], reduces the coefficients of chloride ions [161], and increases the durability of concrete [134,140]. Importantly, using CC is considered a good solution to reduce carbon dioxide emissions and produce an eco-friendly CC at a low cost [151]. The replacement proportion in cement clinker can reach as low as 50% [132,138]. Limestone–CC cement has exceptional durability and mechanical properties, such as a higher final compressive strength. In addition, it increases the C-S-H amount at 28 days of hydration [167].
As a result, CC can be considered a global alternative to a variety of traditional low-carbon OPC materials. This “pozzolanic calcined clay,” as it is also known, in addition to reducing CO2 emissions in industries, also brings several economic advantages for cement production. Its application in South Asia and Latin America is gradually growing.
6. Conclusions
This paper has focused on two main parts. The first deals with the problems of using cement and lime as supplementary cementitious materials (SCMs) cause for soil, concrete, and the environment. For example, where the energy consumption involved in producing cementitious materials is high, carbon dioxide emissions account for 8% of the total carbon emissions. The presence of sulfates in the soils or groundwater surrounding a concrete structure may also cause a serious threat to the long-term durability of concrete and soil due to the effect of a sulfate attack, which is considered one of the most widely admitted and well-studied chemical attacks.
Therefore, improving the sustainability of cement concrete has become a major issue in improving socially sustainable development. Internationally, in recent decades, improving cement concrete’s sustainable and low-carbon characteristics has become the focus of industry attention by using SCMs, which are industrial by-products or natural materials used to improve the durability, performance, and sustainability of concrete mixtures. One of these SCMs is calcined clay; therefore, the second part of this review paper focuses on using this material to reduce the use of cement or lime by comparing cement or lime alone and using calcined clay with cement or lime.
Based on the results of using calcined clay in concrete, which has developed rapidly in recent years, this material, can produces a low-carbon cement-based material, and it is recommended for use in concrete. Compared with traditional OPC, the clinker content in its cement can be as low as 50% because it uses a large amount of calcined clay and limestone as key components. It will conserve the limestone resources used in cement manufacturing and help to reduce the carbon footprint associated with the cement industry. Its application in places such as Latin America and South Asia is gradually growing.
Author Contributions
A.A.M.; methodology, writing original draft preparation; H.N.; N.A.M.N.; G.F.H.; A.H.S.; review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data reported in this study was originally generated and can be requested from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CC: Calcined Clay; OPC: Ordinary Portland Cement; LS: Limestone; SCMs: Supplementary cementitious materials; LC3: limestone calcined clay cement (LC3); CS: Calcined Shale.
References
- Onyelowe, K.; Okafor, F.O. A Comparative Review of Soil Modification Methods. ARPN J. Earth Sci. 2012, 1, 36–41. [Google Scholar]
- Ismail, M.A.; Joer, H.A.; Sim, W.H.; Randolph, M.F. Effect of Cement Type on Shear Behavior of Cemented Calcareous Soil. J. Geotech. Geoenviron. Eng. 2002, 128, 520–529. [Google Scholar] [CrossRef]
- Sizirici, B.; Fseha, Y.; Cho, C.S.; Yildiz, I.; Byon, Y.J. A Review of Carbon Footprint Reduction in Construction Industry, from Design to Operation. Materials 2021, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Kiecana, M.; Kanty, P.; Auyńska, K. Optimal Control Time Evaluation for Dry DSM Soil-Cement Composites. In Proceedings of the MATEC Web of Conferences, Moscow, Russia; 2018. [Google Scholar] [CrossRef]
- Gidday, B.G.; Mittal, S. Improving the Characteristics of Dispersive Subgrade Soils Using Lime. Heliyon 2020, 6, e03384. [Google Scholar] [CrossRef]
- Baldovino, J.A.; Moreira, E.B.; Teixeira, W.; Izzo, R.L.S.; Rose, J.L. Effects of Lime Addition on Geotechnical Properties of Sedimentary Soil in Curitiba, Brazil. J. Rock Mech. Geotech. Eng. 2018, 10, 188–194. [Google Scholar] [CrossRef]
- Locat, J.; Tremblay, H.; Leroueil, S. Mechanical and Hydraulic Behaviour of a Soft inorganic Clay Treated with Lime. Can. Geotech. J. 1996, 33, 654–669. [Google Scholar] [CrossRef]
- Arman, A.; Munfakh, G.A. Lime Stabilization of Organic Soils. Highw. Res. Rec. 1972, 37–45. [Google Scholar]
- Choquette, M.; Bérubé, M.A.; Locat, J. Mineralogical and Microtextural Changes Associated with Lime Stabilization of Marine Clays from Eastern Canada. Appl. Clay Sci. 1987, 2, 215–232. [Google Scholar] [CrossRef]
- Bhuria, N.R.; Sachan, A. Shear Strength and Constant Rate of Strain Consolidation Behaviour of Cement-Treated Slurry-Consolidated Soft Soil. Curr. Sci. 2014, 106, 972–979. [Google Scholar]
- Kang, G.; Tsuchida, T.; Athapaththu, A.M.R.G. Engineering Behavior of Cement-Treated Marine Dredged Clay during Early and Later Stages of Curing. Eng. Geol. 2016, 209, 163–174. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Phojan, W.; Suddeepong, A.; Chinkulkijniwat, A.; Liu, M.D. Strength Development in Blended Cement Admixed Saline Clay. Appl. Clay Sci. 2012, 55, 44–52. [Google Scholar] [CrossRef]
- Chan, C.; Abdullah, S.H. Settlement behaviour of a Cement—Stabilised Malaysian Clay. In Proceedings of the 6th international Conference on Case Histories in Geotechnical Engineering, Alington, VA, USA, 11–16 August 2006; pp. 1–6. [Google Scholar]
- Lu, Y.; Liu, S.; Zhang, Y.; Li, Z.; Xu, L. Freeze-thaw performance of a cement-treated expansive soil. Cold Reg. Sci. Technol. 2020, 170, 102926. [Google Scholar] [CrossRef]
- Sharma, T.S.K.; Hwa, K.Y. Architecting Hierarchal Zn3V2O8/P-RGO Nanostructure: Electrochemical Determination of Anti-Viral Drug Azithromycin in Biological Samples Using SPCE. Chem. Eng. J. 2022, 439, 135591. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, A.; Vashistha, V.K.; Das, D.K. Phyto-Assisted Synthesis and Characterization of V2 O5 Nanomaterial and Their Electrochemical and Antimicrobial Investigations. Nano Life 2020, 10, 2050003. [Google Scholar] [CrossRef]
- Gartner, E. Industrially Interesting Approaches to “Low-CO2 ” Cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 Emissions from Cement Production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Yang, J.; Hua, Y.; Ye, J.; Xu, S.; Liu, Z. CO2 Emissions Accounting and Carbon Peak Prediction of China’s Papermaking Industry. Forests 2022, 13, 1856. [Google Scholar] [CrossRef]
- Klein, C.D.; Novoa, R.S.A.; Ogle, S.; Smith, K.A.; Rochette, P.; Wirth, T.C. N2O Emission from Managed Soils, and CO2 Emissions from and urea application. Intergov. Panel Clim. Chang. (IPCC) 2006, 4, 1–54. [Google Scholar]
- The European Cement Association (CEMBUREAU). Activity Report, 1st ed.; CEMBUREAU: Brussels, Belgium, 2017. [Google Scholar]
- Andrew, R.M. Global CO2 Emissions from Cement Production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef]
- United States Geological Survey (USGS). Mineral Commodity Summaries 2011; U.S. Geological Survey: Reston, VA, USA, 2011. [Google Scholar]
- Lei, Y.; Zhang, Q.; Nielsen, C.; He, K. An Inventory of Primary Air Pollutants and CO2 Emissions from Cement Production in China, 1990–2020. Atmos. Environ. 2011, 45, 147–154. [Google Scholar] [CrossRef]
- Stafford, F.N.; Raupp-Pereira, F.; Labrincha, J.A.; Hotza, D. Life Cycle Assessment of the Production of Cement: A Brazilian Case Study. J. Clean. Prod. 2016, 137, 1293–1299. [Google Scholar] [CrossRef]
- Deja, J.; Uliasz-Bochenczyk, A.; Mokrzycki, E. CO2 Emissions from Polish Cement Industry. Int. J. Greenh. Gas Control 2010, 4, 583–588. [Google Scholar] [CrossRef]
- Kabir, G.; Madugu, A.I. Assessment of Environmental Impact on Air Quality by Cement Industry and Mitigating Measures: A Case Study. Environ. Monit. Assess. 2010, 160, 91–99. [Google Scholar] [CrossRef]
- Cement Sustainability Initiative. Cement Industry Energy and CO2 Performance: Getting the Numbers Right (GNR); World Business Council for Sustainable Development wbcsd: Geneva, Switzerland, 2016. [Google Scholar]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary Cementitious Materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Darange, R.; Adesina, A.; Das, S. Feasibility Study on the Sustainable Utilization of Uncalcined Clay Soils as Low-Cost Binders. Constr. Build. Mater. 2022, 340, 127724. [Google Scholar] [CrossRef]
- Croft, J.B. The Influence of Soil Mineralogical Composition on Cement Stabilization. Geotechnique 1967, 17, 119–135. [Google Scholar] [CrossRef]
- Bushra, I.; Robinson, R.G. Consolidation Behaviour of a Stabilised Marine Clay Soil. Proc Indian Geotech. Conf. 2009, 5, 431–434. [Google Scholar]
- Amed, K.; Ouedraogo, J.; Aubert, J.; Tribout, C.; Escadeillas, G. Is Stabilization of Earth Bricks using Low Cement or Lime Contents Relevant ? Constr. Build. Mater. 2020, 236, 117578. [Google Scholar] [CrossRef]
- Khalid, U.; Liao, C.C.; Ye, G.-L.; Yadav, S.K. Sustainable Improvement of Soft Marine Clay using Low Cement Content: A multi-scale Experimental Investigation. Constr. Build. Mater. 2018, 191, 469–480. [Google Scholar] [CrossRef]
- Deng, S.P.; Tabatabai, M.A. Effect of Tillage and Residue Management on Enzyme Activities in Soils: III. Phosphatases and Arylsulfatase. Biol. Fertil. Soils 1997, 24, 141–146. [Google Scholar] [CrossRef]
- Al-Rawas, A.A.; Hago, A.W.; Al-Sarmi, H. Effect of Lime, Cement and Sarooj (Artificial Pozzolan) on the Swelling Potential of an Expansive Soil from Oman. Build. Environ. 2005, 40, 681–687. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, F.; Wu, W.; Liang, S. Physicochemical and Index Properties of Loess Stabilized with Lime and Fly Ash Piles. Appl. Clay Sci. 2015, 114, 77–84. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Cui, Y.J.; Ferber, V.; Herrier, G.; Ozturk, T.; Plier, F.; Puiatti, D.; Salager, S.; Tang, A.M.; Tang, A.M. Effect of Freeze-thaw Cycles on Mechanical Strength of Lime-treated Fine-grained Soils. Transp. Geotech. 2019, 21, 100281. [Google Scholar] [CrossRef]
- El Shinawi, A. Instability Improvement of the Subgrade Soils by Lime addition at Borg El-Arab, Alexandria, Egypt. J. Afr. Earth Sci. 2017, 130, 195–201. [Google Scholar] [CrossRef]
- Zhang, X.; Mavroulidou, M.; Gunn, M.J. Mechanical Properties and Behaviour of a Partially Saturated Lime-treated, High Plasticity Clay. Eng. Geol. 2015, 193, 320–336. [Google Scholar] [CrossRef]
- Janosik, S.M. Post-construction evaluation of Lime treated Soils. NASPA J. 2005, 42, 231. [Google Scholar] [CrossRef]
- Little, N. Evaluation of Structural Properties of Lime Stabilized Soils and Aggregates; NLA: Arlington, VA, USA, 1999; Volume 1. [Google Scholar]
- Olaniyi, D. Evaluation of the Effect of Lime and Cement on the Engineering Properties of Selected Soil in a University in Southwestern Nigeria. J. Adv. Eng. Technol. 2017, 5, 1–7. [Google Scholar]
- Al-Mukhtar, M.; Lasledj, A.; Alcover, J.-F. Behaviour and Mineralogy Changes in Lime-Treated Expansive Soil at 50 °C. Appl. Clay Sci. 2010, 50, 199–203. [Google Scholar] [CrossRef]
- Bell, F.G. Lime Stabilization of Clay Minerals and Soils F.G. Eng. Geol. 1996, 42, 223–237. [Google Scholar] [CrossRef]
- Egorova, A.A.; Rybak, J.; Stefaniuk, D.; Zajączkowski, P. Basic Aspects of Deep Soil Mixing Technology Control. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 022019. [Google Scholar] [CrossRef]
- Kanty, P.; Rybak, J.; Stefaniuk, D. Some Remarks on Practical Aspects of Laboratory Testing of Deep Soil Mixing Composites Achieved in Organic Soils. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 022018. [Google Scholar] [CrossRef]
- Rao, S.M.; Thyagaraj, T. Lime Slurry Stabilisation of an Expansive Soil. Geotech. Eng. 2003, 156, 139–146. [Google Scholar] [CrossRef]
- Solomon, C. Minerals Yearbook—Cement; United States Geological Survey: Reston, VA, USA, 1994. [Google Scholar]
- United States Geological Survey (USGS). Mineral Commodity Summaries—Cement; United States Geological Survey: Reston, VA, USA, 2013. [Google Scholar]
- Ali, N.; Jaffar, A.; Anwer, M.; Khurram Khan Alwi, S.; Naeem Anjum, M.; Ali, N.; Riaz Raja, M.; Hussain, A.; Ming, X.; Author, C. The Greenhouse Gas Emissions Produced by Cement Production and Its Impact on Environment A Review of Global Cement Processing. Int. J. Res. 2015, 2, 488–500. [Google Scholar]
- Prakasan, S.; Palaniappan, S.; Gettu, R. Study of Energy Use and CO2 Emissions in the Manufacturing of Clinker and Cement. J. Inst. Eng. Ser. A 2020, 101, 221–232. [Google Scholar] [CrossRef]
- Taffese, W.Z.; Abegaz, K.A. Embodied Energy and CO2 Emissions of Widely Used Building Materials: The Ethiopian Context. Buildings 2019, 9, 136. [Google Scholar] [CrossRef]
- Cui, D.; Deng, Z.; Liu, Z. China’s Non-Fossil Fuel CO2 Emissions from Industrial Processes. Appl. Energy 2019, 254, 113537. [Google Scholar] [CrossRef]
- Cao, Z.; Shen, L.; Zhao, J.; Liu, L.; Zhong, S.; Sun, Y.; Yang, Y. Toward a Better Practice for Estimating the CO2 Emission Factors of Cement Production: An Experience from China. J. Clean. Prod. 2016, 139, 527–539. [Google Scholar] [CrossRef]
- Cai, B.; Wang, J.; He, J.; Geng, Y. Evaluating CO2 Emission Performance in China’s Cement Industry: An Enterprise Perspective. Appl. Energy 2016, 166, 191–200. [Google Scholar] [CrossRef]
- Chen, W.; Hong, J.; Xu, C. Pollutants Generated by Cement Production in China, Their Impacts, and the Potential for Environmental Improvement. J. Clean. Prod. 2015, 103, 61–69. [Google Scholar] [CrossRef]
- Ke, J.; McNeil, M.; Price, L.; Khanna, N.Z.; Zhou, N. Estimation of CO2 Emissions from China’s Cement Production: Methodologies and Uncertainties. Energy Policy 2013, 57, 172–181. [Google Scholar] [CrossRef]
- Nasab, T.J.; Monavari, S.M.; Jozi, S.A.; Majedi, H. Assessment of Carbon Footprint in the Construction Phase of High-Rise Constructions in Tehran. Int. J. Environ. Sci. Technol. 2020, 17, 3153–3164. [Google Scholar] [CrossRef]
- Wei, J.; Cen, K. Empirical Assessing Cement CO2 Emissions Based on China’s Economic and Social Development during 2001–2030. Sci. Total Environ. 2019, 653, 200–211. [Google Scholar] [CrossRef]
- Bakhtyar, B.; Kacemi, T.; Nawaz, M.A. A Review on Carbon Emissions in Malaysian Cement Industry. Int. J. Energy Econ. Policy 2017, 7, 282–286. [Google Scholar]
- Rae, S.; Tak, S.; Yeob, G.; Gu, J.; Joo, P.; Won, G. Geoderma Evaluation of the Carbon Dioxide (CO2) Emission Factor from Lime Applied in Temperate Upland Soil. Geoderma 2019, 337, 742–748. [Google Scholar] [CrossRef]
- Vinnichenko, V.; Ryazanov, A.; Krot, O. Construction Binders and Environmental Indicators of Their Production. MATEC Web Conf. 2018, 230, 03020. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Han, R.; Yu, B.; Wei, Y.M. Accounting Process-Related CO2 Emissions from Global Cement Production under Shared Socioeconomic Pathways. J. Clean. Prod. 2018, 184, 451–465. [Google Scholar] [CrossRef]
- Villachica, C.A.; Villachica, J.G. Technology Development for Strong Reduction of Energy Consumption and CO2 Emission in Lime and Cement Manufacture. Geosci. Eng. 2017, 63, 1–7. [Google Scholar] [CrossRef]
- Suganya, O.M.; Himasaikiranreddy, D.; Srinidhi, S.V. Case Study on Environmental Impacts by Cement Industry and Minimization Using Alternate Cementitious Materials. Int. J. Civ. Eng. Technol. 2018, 9, 910–917. [Google Scholar]
- Nayana, A.Y.; Kavitha, S. Evaluation of CO2 Emissions for Green Concrete with High Volume Slag, Recycled Aggregate, Recycled Water to Build Eco Environment. Int. J. Civ. Eng. Technol. 2017, 8, 703–708. [Google Scholar]
- Klee, H.; Hunziker, R.; van der Meer, R.; Westaway, R. Getting the Numbers Right: A Database of Energy Performance and Carbon Dioxide Emissions for the Cement Industry. Greenh. Gas Meas. Manag. 2011, 1, 109–118. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Garrtner, E.M. Eco-Efficient Cements: Potential, Economically Viable Solutions for a Low-CO2 Cement Based Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Kajaste, R.; Hurme, M. Cement Industry Greenhouse Gas Emissions—Management Options and Abatement cost. J. Clean. Prod. 2016, 112, 4041–4052. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Cement a Review. Geopolym. Sci. Tech. 2013, 0, 1–11. Available online: https://www.geopolymer.org (accessed on 16 January 2013).
- Rodrigues, F.A.; Joekes, I. Cement industry: Sustainability, Challenges and Perspectives. Environ. Chem. Lett. 2011, 9, 151–166. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.K.; Hussein, A.A. Geopolymer Mortars as Sustainable Repair Material: A Comprehensive Review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Kline, J.; Barcelo, L. Cement and CO2—A Victim of Success. Cem. Int. 2012, 10, 62–71. [Google Scholar]
- Olivier, J.G.J.; Janssens-Maenhout, G.; Peters, J.A.H.W. Trends in Global CO2 Emissions, 2012 Report. 2012. Available online: https://www.pbl.nl/en/publications/trends-in-global-co2-emissions-2012-report (accessed on 21 November 2022).
- Winnefeld, F.; Andreas, L.; Alexander, G.; Barbara, L. CO2 Storage in Cement and Concrete by Mineral Carbonation. Curr. Opin. Green Sustain. Chem. 2022, 38, 100672. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global Strategies and Potentials to Curb CO2 Emissions in Cement Industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Dubey, A. Studies on the Air Pollution Around Cement and Lime Factories. J. Environ. Earth Sci. 2013, 3, 191–195. [Google Scholar]
- Olivier, J.G.J.; Janssens-Maenhout, G.; Muntean, M.; Peters, J. Trends in Global CO2 Emissions. 2016. Available online: https://www.pbl.nl/en/trends-in-global-co2-emissions (accessed on 21 November 2022).
- Pardo, N.; Moya, J.A.; Mercier, A. Prospective on the Energy Efficiency and CO2 Emissions in the EU Cement Industry. Energy 2011, 36, 3244–3254. [Google Scholar] [CrossRef]
- Chan, C.C.S.; Thorpe, D.; Islam, M. An Evaluation Carbon Footprint in Fly Ash based Geopolymer Cement and Ordinary Portland Cement Manufacture. IEEE Int. Conf. Ind. Eng. Eng. Manag. 2016, 1, 254–259. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Jiang, X.; Mu, S.; Yang, Z.; Tang, J.; Li, T. Effect of Temperature on Durability of Cement-Based Material to Physical Sulfate Attack. Constr. Build. Mater. 2021, 266, 120936. [Google Scholar] [CrossRef]
- Li, C.; Jiang, Z.; Myers, R.J.; Chen, Q.; Wu, M.; Li, J.; Monteiro, P.J.M. Understanding the Sulfate Attack of Portland Cement–Based Materials Exposed to Applied Electric Fields: Mineralogical Alteration and Migration Behavior of Ionic Species. Cem. Concr. Compos. 2020, 111, 103630. [Google Scholar] [CrossRef]
- Dvořák, K.; Všianský, D.; Gazdič, D.; Fridrichová, M.; Vaičiukynienė, D. Thaumasite Formation by Hydration of Sulphosilicate Clinker. Mater. Today Commun. 2020, 25, 101449. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, T.; Luo, T.; Zhou, M.; Zhang, K.; Ma, W. Study on the Deterioration of Concrete under Dry-wet Cycle and Sulfate Attack. Materials 2020, 13, 4095. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, C.; Qi, C.; Zhang, B.; Song, K.I. A Microstructural Hydration Model for Cemented Paste Backfill Considering Internal Sulfate Attacks. Constr. Build. Mater. 2019, 211, 99–108. [Google Scholar] [CrossRef]
- Naseeruddin, S.; Venkateswarlu, D.; Kumar, A.S. Acid Attack on Concrete. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 2339–2343. [Google Scholar]
- Zhao, G.; Shi, M.; Guo, M.; Fan, H. Degradation Mechanism of Concrete Subjected to External Sulfate Attack: Comparison of Different Curing Conditions. Materials 2020, 13, 3179. [Google Scholar] [CrossRef]
- Atashband, S.; Sabermahani, M.; Elahi, H. Internal and External Effects of Sodium Sulfate on the Strength of Soil Cement. Iran. J. Sci. Technol. Trans. Civ. Eng. 2021, 45, 2595–2610. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Yu, Z.; Chen, L.; Zheng, Y. Research on Sulfate Attack Mechanism of Cement Concrete Based on Chemical Thermodynamics. Adv. Mater. Sci. Eng. 2020, 5, 6916039. [Google Scholar] [CrossRef]
- Yi, Y.; Zhu, D.; Guo, S.; Zhang, Z.; Shi, C. A Review on the Deterioration and Approaches to Enhance the Durability of Concrete in the Marine Environment. Cem. Concr. Compos. 2020, 113, 103695. [Google Scholar] [CrossRef]
- Haufe, J.; Vollpracht, A. Tensile Strength of Concrete Exposed to Sulfate Attack. Cem. Concr. Res. 2019, 116, 81–88. [Google Scholar] [CrossRef]
- Gu, Y.; Martin, R.P.; Omikrine Metalssi, O.; Fen-Chong, T.; Dangla, P. Pore Size Analyses of Cement Paste Exposed to External Sulfate Attack and Delayed Ettringite Formation. Cem. Concr. Res. 2019, 123, 105766. [Google Scholar] [CrossRef]
- Helson, O.; Eslami, J.; Beaucour, A.L.; Noumowe, A.; Gotteland, P. Durability of Soil Mix Material Subjected to Wetting/Drying Cycles and External Sulfate Attacks. Constr. Build. Mater. 2018, 192, 416–428. [Google Scholar] [CrossRef]
- Costa, L.C.B.; Escoqui, J.M.R.; Oliveira, T.M.; da Fonseca, L.G.; Farage, M.C.R. Sodium Sulfate Attack on Portland Cement Structures: Experimental and Analytical Approach. Rev. Esc. Minas 2018, 71, 531–542. [Google Scholar] [CrossRef]
- Ma, X.; Çopuroğlu, O.; Schlangen, E.; Han, N.; Xing, F. Expansion and Degradation of Cement Paste in Sodium Sulfate Solutions. Constr. Build. Mater. 2018, 158, 410–422. [Google Scholar] [CrossRef]
- Ikumi, T.; Segura, I.; Cavalaro, S.H.P. Inlfuence of Early Sulfate Exposure on the Pore Network Development of Mortars. Constr. Build. Mater. 2017, 143, 33–47. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, Y.; Liao, Y.; Chen, D. Study of the Evolution of Properties of Mortar under Sulfate Attack at Different Concentrations. Adv. Cem. Res. 2016, 28, 617–629. [Google Scholar] [CrossRef]
- Rendon, L.E.; Rendon, M.; Ramirez, N. The Effect on Concrete Resistivity of Sulfate Content in Water. MRS Online Proc. Libr. 2012, 1488, 134–137. [Google Scholar] [CrossRef]
- Feng, P.; Garboczi, E.J.; Miao, C.; Bullard, J.W. Microstructural Origins of Cement Paste Degradation by External Sulfate Attack. Constr. Build. Mater. 2015, 96, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Mardani-Aghabaglou, A.; Kalipcilar, I.; Inan Sezer, G.; Sezer, A.; Altun, S. Freeze-Thaw Resistance and Chloride-Ion Penetration of Cement-Stabilized Clay Exposed to Sulfate Attack. Appl. Clay Sci. 2015, 115, 179–188. [Google Scholar] [CrossRef]
- Verástegui-Flores, R.D.; Di Emidio, G. Impact of Sulfate Attack on Mechanical Properties and Hydraulic Conductivity of a Cement-Admixed Clay. Appl. Clay Sci. 2014, 101, 490–496. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulfate Attack Expansion Mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]
- Ramezanianpour, A.M.; Hooton, R.D. Thaumasite Sulfate Attack in Portland and Portland-Limestone Cement Mortars Exposed to Sulfate Solution. Constr. Build. Mater. 2013, 40, 162–173. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Zhu, J.; Zhang, M.; Ye, J. A New Diffusion Model of Sulfate Ions in Concrete. Constr. Build. Mater. 2013, 39, 39–45. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Wei, Q. Investigation of Sulphate Attack on C-S-H Gel. Adv. Mater. Res. 2011, 287, 1116–1120. [Google Scholar] [CrossRef]
- Ma, K.; Wang, W.; Long, G.; Xie, Y. Corrosion Products in Cement-Based Materials under Sulfate Partial Soaking Attack. Adv. Mater. Res. 2012, 368, 415–418. [Google Scholar] [CrossRef]
- Abdi, M.R.; Askarian, A.; Mahdi, S.S.G. Effects of Sodium and Calcium Sulphates on Volume Stability and Strength of Lime-Stabilized Kaolinite. Bull. Eng. Geol. Environ. 2020, 79, 941–957. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 Emissions from OPC and Recycled Cement Production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Borrachero, M.V. Ultrasonic and Impact Spectroscopy Monitoring on Internal Sulphate Attack of Cement-Based Materials. Mater. Des. 2017, 125, 46–54. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, Z.; Guan, D. CO2 Emissions from China’s Lime Industry. Appl. Energy 2016, 166, 245–252. [Google Scholar] [CrossRef]
- Olivier, P. Trends in Global CO2 and Total Greenhouse Gas 2021 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2022. [Google Scholar]
- Çelİk, E. Evaluation of Hydrated Lime Stabilization of Sulfate Bearing Expansive Soils. Ph.D. Thesis, Eastern Mediterranean University, Gazimağusa, North Cyprus, 2014. [Google Scholar]
- Buttress, A.J. Physicochemical Behaviour of Artificial Lime Stabilised Sulfate Bearing Cohesive Soils. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2013. [Google Scholar]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and Developments in Green Cement and Concrete Technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Cerato, A.B.; Miller, G.A.; Elwood-Madden, M.; Adams, A. Calcium-Based Stabilizer Induced Heave in Oklahoma Sulfate-Bearing Soils. 2011. Available online: https://rosap.ntl.bts.gov/view/dot/23202 (accessed on 21 December 2022).
- Rajasekaran, G.; Narasimha Rao, S. Sulphate Attack in Lime-Treated Marine Clay. Mar. Georesour. Geotechnol. 2005, 23, 93–116. [Google Scholar] [CrossRef]
- Sivapullaiah, P.V.; Sridharan, A.; Ramesh, H.N. Effect of Sulphate on the Shear Strength of Lime-Treated Kaolinitic Soil. Gr. Improv. 2006, 10, 23–30. [Google Scholar] [CrossRef]
- Sivapullaiah, P.V.; Sridharan, A.; Ramesh, H.N. Strength Behaviour of Lime-Treated Soils in the Presence of Sulphate. Can. Geotech. J. 2000, 37, 1358–1367. [Google Scholar] [CrossRef]
- Kinuthia, J.; Wild, S.; Jones, G. Effects of Monovalent and Divalent Metal Sulphates on Consistency and Compaction of Lime-Stabilised Kaolinite. Appl. Clay Sci. 1999, 14, 27–45. [Google Scholar] [CrossRef]
- Abdi, M.R.; Wild, S. Sulphate Expansion of Lime-Stabilized Kaolinite: I. Physical Characteristics. Clay Miner. 1993, 28, 555–567. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Dermatas, D. Clay Soil Heave Caused by Lime-Sulfate Reactions. Am. Soc. Test. Mater. 1992, 4, 41–64. [Google Scholar]
- Hunter, D. Lime—Induced Heave in Sulfate—Bearing Clay Soils. J. Geotech. Eng. 1988, 114, 150–167. [Google Scholar] [CrossRef]
- Heller, L.; Ben-Yair, M. Effect of Sulphate Solutions on Normal and Sulphate-Resisting Portland Cement. J. Appl. Chem. 2007, 14, 20–30. [Google Scholar] [CrossRef]
- Sherwood, P.T. Effect of Sulfates on Cement- and Lime-Stabilized Soils. In Proceedings of the 41st Annual Meeting of the Highway Research Board, Washington, DC, USA, 8–12 January 1962; Volume 353, pp. 98–107. [Google Scholar]
- Maier, M.; Sposito, R.; Beuntner, N.; Thienel, K.C. Particle Characteristics of Calcined Clays and Limestone and Their Impact on Early Hydration and Sulfate Demand of Blended Cement. Cem. Concr. Res. 2022, 154, 106736. [Google Scholar] [CrossRef]
- Ruan, Y.; Jamil, T.; Hu, C.; Gautam, B.P.; Yu, J. Microstructure and Mechanical Properties of Sustainable Cementitious Materials with Ultra-High Substitution Level of Calcined Clay and Limestone Powder. Constr. Build. Mater. 2022, 314, 125416. [Google Scholar] [CrossRef]
- Yaraghi, A.H.Y.; Ramezanianpour, A.M.; Ramezanianpour, A.A.; Bahman-Zadeh, F.; Zolfagharnasab, A. Evaluation of Test Procedures for Durability and Permeability Assessment of Concretes Containing Calcined Clay. J. Build. Eng. 2022, 58, 105016. [Google Scholar] [CrossRef]
- Xuan, M.Y.; Bae, S.C.; Kwon, S.J.; Wang, X.Y. Sustainability Enhancement of Calcined Clay and Limestone Powder Hybrid Ultra-High-Performance Concrete Using Belite-Rich Portland Cement. Constr. Build. Mater. 2022, 351, 128932. [Google Scholar] [CrossRef]
- Luzu, B.; Trauchessec, R.; Lecomte, A. Packing Density of Limestone Calcined Clay Binder. Powder Technol. 2022, 408, 1–12. [Google Scholar] [CrossRef]
- Sposito, R.; Maier, M.; Beuntner, N.; Thienel, K.C. Physical and Mineralogical Properties of Calcined Common Clays as SCM and Their Impact on Flow Resistance and Demand for Superplasticizer. Cem. Concr. Res. 2022, 154, 106743. [Google Scholar] [CrossRef]
- Cardinaud, G.; Rozière, E.; Martinage, O.; Loukili, A.; Barnes-Davin, L.; Paris, M.; Deneele, D. Calcined Clay—Limestone Cements: Hydration Processes with High and Low-Grade Kaolinite Clays. Constr. Build. Mater. 2021, 277, 122271. [Google Scholar] [CrossRef]
- Jamhiri, B. Evaluation of Pozzolan-Lime Stabilization on Physical Properties of Fine Sandy Engineering Fills. Konya J. Eng. Sci. 2020, 8, 80–90. [Google Scholar] [CrossRef]
- Admassu, K. Engineerinh Soil and the Need for Lime-natural Pozzolan Mixture. Ethiop. J. Sci. 2018, 41, 70–79. [Google Scholar]
- Lin, R.S.; Han, Y.; Wang, X.Y. Macro–Meso–Micro Experimental Studies of Calcined Clay Limestone Cement (LC3) Paste Subjected to Elevated Temperature. Cem. Concr. Compos. 2021, 116, 103871. [Google Scholar] [CrossRef]
- Chundawat, D.S.; Sharma, D.K.; Tomar, S. Durability Properties of Concrete Incorporating Calcined Phyllite. Preprint 2021, 2021010077. [Google Scholar] [CrossRef]
- Yu, J.; Wu, H.L.; Mishra, D.K.; Li, G.; Leung, C.K. Compressive Strength and Environmental Impact of Sustainable Blended Cement with High-Dosage Limestone and Calcined Clay (LC2). J. Clean. Prod. 2021, 278, 123616. [Google Scholar] [CrossRef]
- Dixit, A.; Du, H.; Pang, S.D. Performance of Mortar Incorporating Calcined Marine Clays with Varying Kaolinite Content. J. Clean. Prod. 2021, 282, 124513. [Google Scholar] [CrossRef]
- Msinjili, N.S.; Vogler, N.; Sturm, P.; Neubert, M.; Schröder, H.J.; Kühne, H.C.; Hünger, K.J.; Gluth, G.J.G. Calcined Brick Clays and Mixed Clays as Supplementary Cementitious Materials: Effects on the Performance of Blended Cement Mortars. Constr. Build. Mater. 2021, 266, 120990. [Google Scholar] [CrossRef]
- Lin, R.S.; Lee, H.S.; Han, Y.; Wang, X.Y. Experimental Studies on Hydration–Strength–Durability of Limestone-Cement-Calcined Hwangtoh Clay Ternary Composite. Constr. Build. Mater. 2021, 269, 121290. [Google Scholar] [CrossRef]
- Nair, N.; Mohammed Haneefa, K.; Santhanam, M.; Gettu, R. A Study on Fresh Properties of Limestone Calcined Clay Blended Cementitious Systems. Constr. Build. Mater. 2020, 254, 119326. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Santhanam, M. Investigation on the Microstructure-Related Characteristics to Elucidate Performance of Composite Cement with Limestone-Calcined Clay Combination. Cem. Concr. Res. 2020, 129, 105959. [Google Scholar] [CrossRef]
- Guo, F. Calcined Clay and Limestone as Partial Replacements of Portland Cement: Electrochemical Corrosion Behavior of Low Carbon Steel Rebar as Concrete Reinforcement in Corrosive Environment. Int. J. Electrochem. Sci. 2020, 15, 12281–12290. [Google Scholar] [CrossRef]
- Gobinath, R.; Awoyera, P.O.; Praveen, N.; Babu, V.A.; Sai, P.S.; Prathibha, K. Effects of calcined clay on the engineering properties of cementitious mortars. Mater. Today Proc. 2020, 39, 110–113. [Google Scholar] [CrossRef]
- Scrivener, K.; Favier, A. Calcined Clays for Sustainable Concrete: Proceedings of the 1st International Conference on Calcined Clays for Sustainable Concrete; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Du, H.; Pang, S.D. High-performance Concrete Incorporating Calcined Kaolin Clay and Limestone as Cement Substitute. Constr. Build. Mater. 2020, 264, 120152. [Google Scholar] [CrossRef]
- Jafari, K.; Rajabipour, F. Performance of Impure Calcined Clay as a Pozzolan in Concrete. Transp. Res. Rec. 2020, 2675, 98–107. [Google Scholar] [CrossRef]
- Murtaza, M.; Rahman, M.K.; Al-Majed, A.A. The application and comparative study of calcined clay and nanoclaymixed cement slurries at hpht conditions. In Proceedings of the Annual Offshore Technology Conference, Houston, TX, USA, 4–7 May 2020. [Google Scholar] [CrossRef]
- Akgün, Y. Behavior of Concrete Containing Alternative Pozzolan Calcined Marl Blended Cement. Period. Polytech. Civ. Eng. 2020, 64, 1087–1099. [Google Scholar] [CrossRef]
- Laidani, Z.E.A.; Benabed, B.; Abousnina, R.; Gueddouda, M.K.; Kadri, E.H. Experimental Investigation on Effects of Calcined Bentonite on Fresh, Strength and Durability Properties of Sustainable Self-Compacting Concrete. Constr. Build. Mater. 2020, 230, 117062. [Google Scholar] [CrossRef]
- Ston, J.; Scrivener, K. Basic Creep of Limestone–Calcined Clay Cements: An Experimental and Numerical Approach. Theor. Appl. Fract. Mech. 2019, 103, 102270. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; Castellano, C.C.; Trezza, M.A.; Rahhal, V.F.; Cordoba, G.; Lemma, R. Calcined Illite-Chlorite Shale as Supplementary Cementing Material: Thermal Treatment, Grinding, Color and Pozzolanic Activity. Appl. Clay Sci. 2019, 179, 105143. [Google Scholar] [CrossRef]
- Krishnan, S.; Emmanuel, A.C.; Bishnoi, S. Hydration and Phase Assemblage of Ternary Cements with Calcined Clay and Limestone. Constr. Build. Mater. 2019, 222, 64–72. [Google Scholar] [CrossRef]
- Pillai, R.G.; Gettu, R.; Santhanam, M.; Rengaraju, S.; Dhandapani, Y.; Rathnarajan, S.; Basavaraj, A.S. Service Life and Life Cycle Assessment of Reinforced Concrete Systems with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2019, 118, 111–119. [Google Scholar] [CrossRef]
- Trümer, A.; Ludwig, H.-M.; Schellhorn, M.; Diedel, R. Effect of a Calcined Westerwald Bentonite as Supplementary Cementitious Material on the Long-Term Performance of Concrete. Appl. Clay Sci. 2019, 168, 36–42. [Google Scholar] [CrossRef]
- Arjun, K.R.; Shaji, P.P. Partial Replecement of Cement Clinker with Limestone and low Quality Calcined Clay. Int. Res. J. Eng. Technol. 2019, 6, 1512–1515. [Google Scholar]
- Adekitan, O.A.; Popoola, M.O. Potentials of Calcined Clay as a Pozzolan. Epa. J. Silic. Based Compos. Mater. 2020, 72, 70–71. [Google Scholar] [CrossRef]
- Apsa; Ranga Rao, V. Performance of Limestone Calcined Clay Cement (Lc3). Int. J. Recent Technol. Eng. 2019, 7, 260–265. [Google Scholar]
- Qinfei, L.; Han, W.; Pengkun, H.; Heng, C.; Yang, W.; Xin, C. The Microstructure and Mechanical Properties of Cementitious Materials Comprised of Limestone, Calcined Clay and Clinker. Ceram.—Silikaty 2019, 63, 356–364. [Google Scholar] [CrossRef]
- Salem, N.; Ltifi, M.; Hassis, H. Mechanical and Durability Study of Tunisian Calcined Clay in Lightweight Concrete of Expanded Clay. Eur. J. Environ. Civ. Eng. 2021, 25, 2257–2276. [Google Scholar] [CrossRef]
- Shi, Z.; Ferreiro, S.; Lothenbach, B.; Geiker, M.R.; Kunther, W.; Kaufmann, J.; Herfort, D.; Skibsted, J. Sulfate Resistance of Calcined Clay—Limestone—Portland Cements. Cem. Concr. Res. 2019, 116, 238–251. [Google Scholar] [CrossRef]
- Mwiti, M.J.; Karanja, T.J.; Muthengia, W.J. Properties of Activated Blended Cement Containing High Content of Calcined Clay. Heliyon 2018, 4, e00742. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined Clay Limestone Cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Santhanam, M. Assessment of Pore Structure Evolution in the Limestone Calcined Clay Cementitious System and Its Implications for Performance. Cem. Concr. Compos. 2017, 84, 36–47. [Google Scholar] [CrossRef]
- Ayati, B.; Newport, D.; Wong, H.; Cheeseman, C. Low-Carbon Cements: Potential for Low-Grade Calcined Clays to Form Supplementary Cementitious Materials. Clean. Mater. 2022, 5, 100099. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Wang, Q.; Shah, S.P. The Effects of Nano-Calcined Kaolinite Clay on Cement Mortar Exposed to Acid Deposits. Constr. Build. Mater. 2016, 102, 486–495. [Google Scholar] [CrossRef]
- Taylor-Lange, S.C.; Lamon, E.L.; Riding, K.A.; Juenger, M.C.G. Calcined Kaolinite-Bentonite Clay Blends as Supplementary Cementitious Materials. Appl. Clay Sci. 2015, 108, 84–93. [Google Scholar] [CrossRef]
- Tironi, A.; Castellano, C.C.; Bonavetti, V.L.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Kaolinitic Calcined Clays—Portland Cement System: Hydration and Properties. Constr. Build. Mater. 2014, 64, 215–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).