Photopolymerization of Coating Materials for Protection against Carbon Steel Corrosion
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
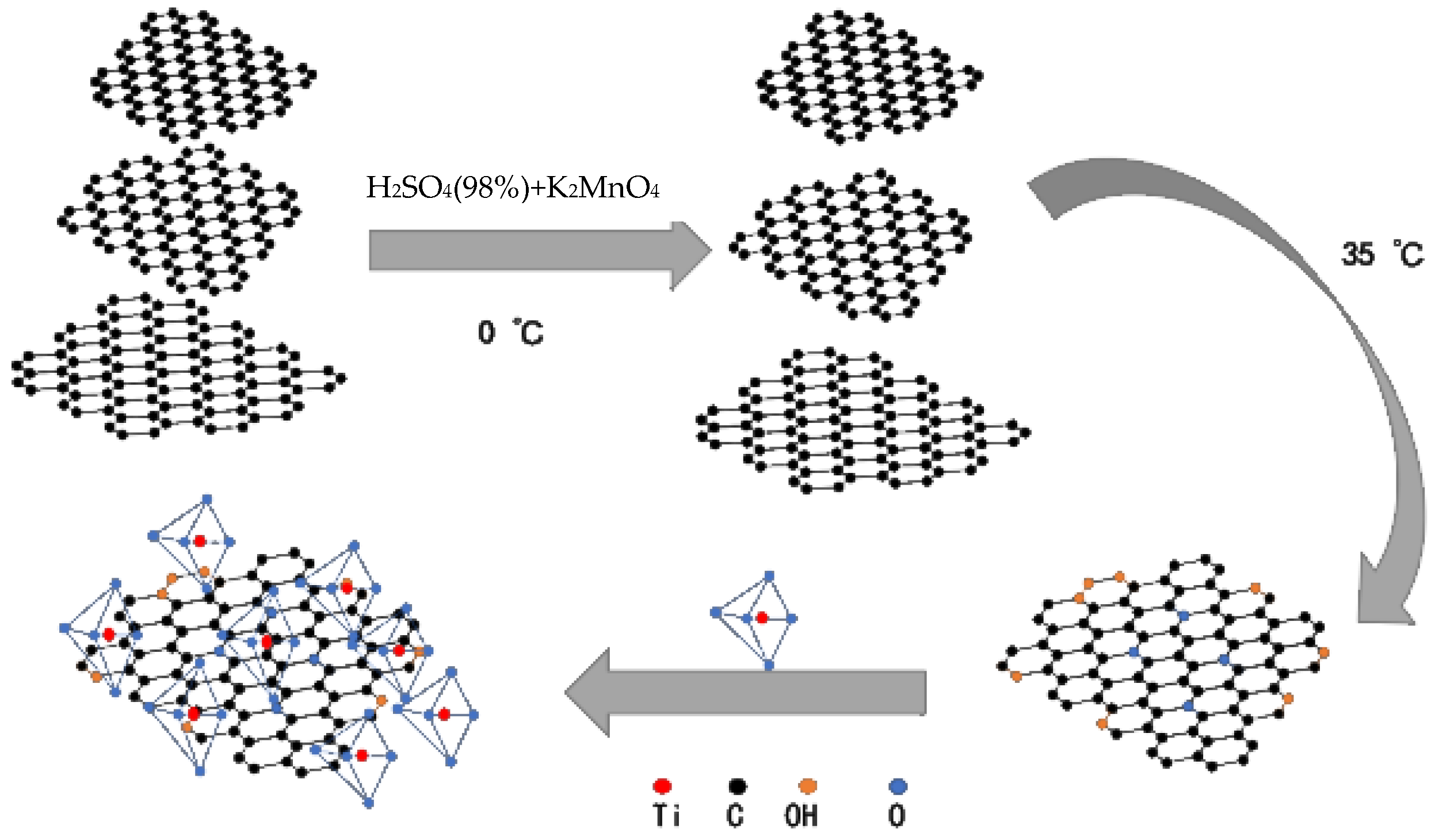
2.2. Composite Synthesis
2.3. Substrate Preparation
2.4. Film Deposition
2.5. Electrochemical Assessments
2.6. Polarization
3. Results and Discussion
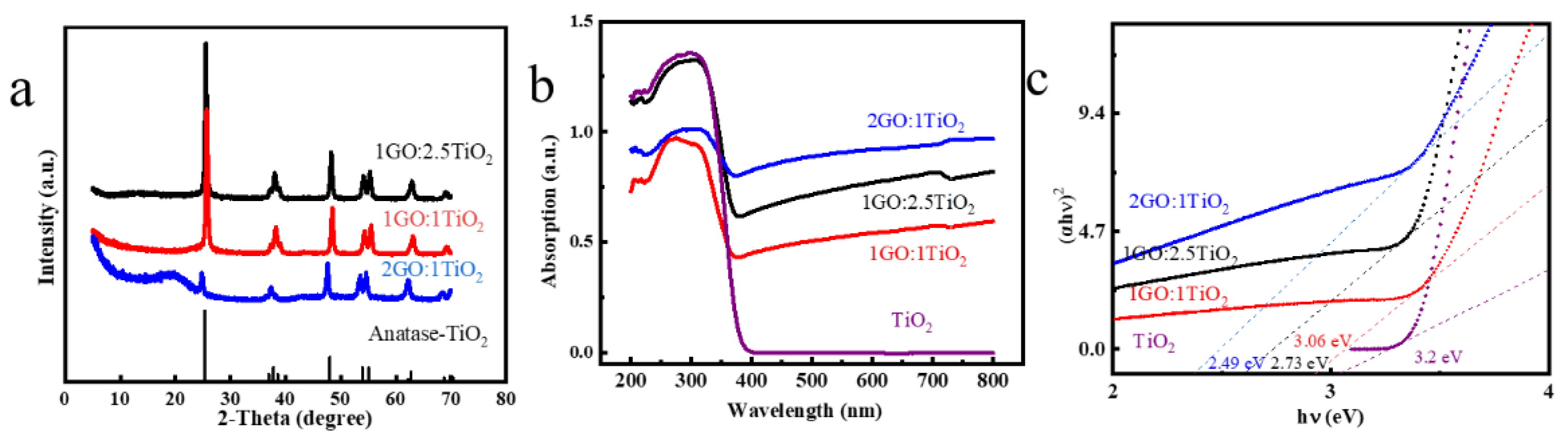
3.1. Structure and Optical Performance Investigation of Modified Photocatalyst
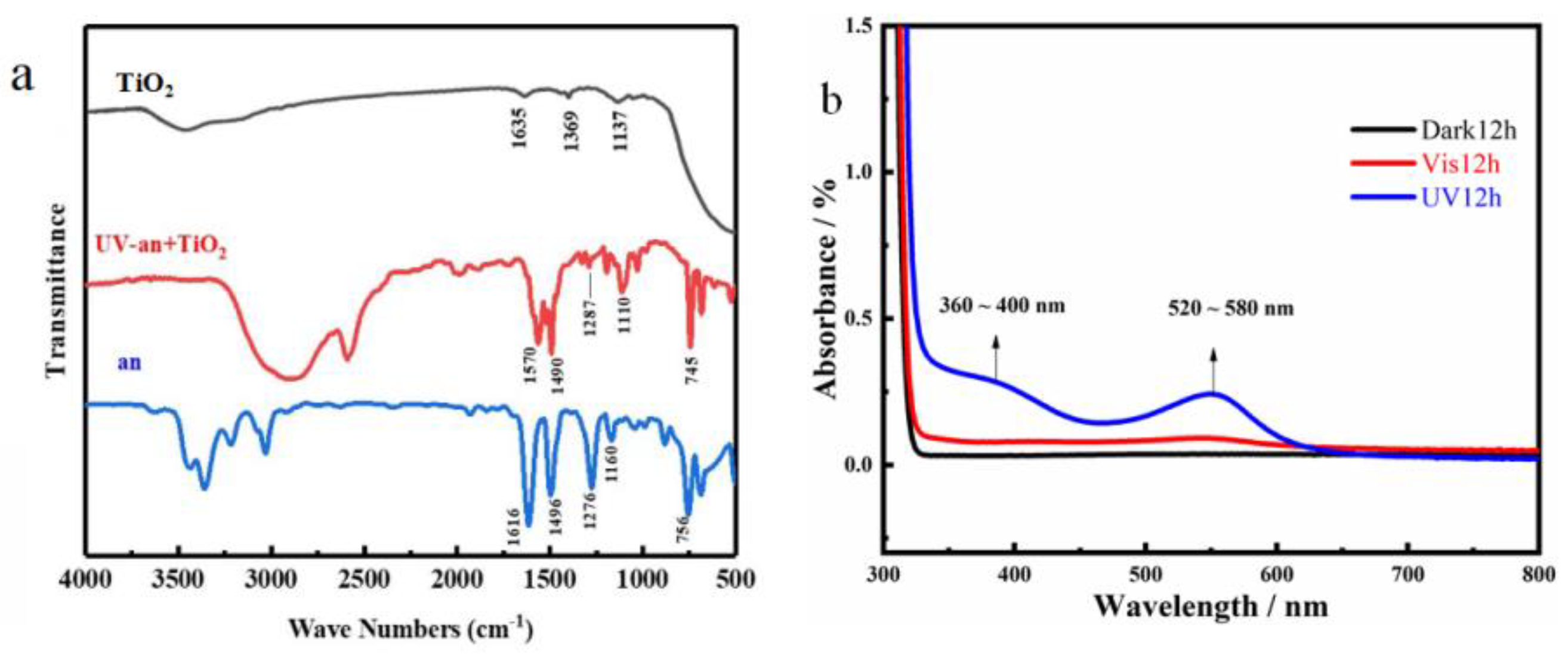
3.2. Structural Analysis of PANI
3.3. Surface Characterization of the Coatings
3.4. Corrosion Protection Evaluation of the Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Koch, G. Cost of corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; Woodhead Publishing: Sawston, UK, 2017; pp. 3–30. [Google Scholar]
- Koch, G.; Varney, F.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion; OAPUS310GKOCH (AP110272); NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Zhou, Y.; Ma, Y.; Sun, Y.; Xiong, Z.; Qi, C.; Zhang, Y.; Liu, Y. Robust Superhydrophobic Surface Based on Multiple Hybrid Coatings for Application in Corrosion Protection. ACS Appl. Mater. Interfaces 2019, 11, 6512–6526. [Google Scholar] [CrossRef]
- Attarchi, M.; Brenna, A.; Ormellese, M. Cathodic protection and DC non-stationary anodic interference. J. Nat. Gas Sci. Eng. 2020, 82, 103497. [Google Scholar] [CrossRef]
- Chen, X.; Wen, S.F.; Feng, T.; Yuan, X. High solids organic-inorganic hybrid coatings based on silicone-epoxy-silica coating with improved anticorrosion performance for AA2024 protection. Prog. Org. Coat. 2020, 139, 105374. [Google Scholar] [CrossRef]
- Sun, T.; Shen, X.J.; Peng, C.; Fan, H.Y.; Liu, M.J.; Wu, Z.J. A novel strategy for the synthesis of self-healing capsule and its application. Compos. Sci. Technol. 2019, 171, 13–20. [Google Scholar] [CrossRef]
- Li, H.Y.; Cui, Y.X.; Li, Z.K.; Zhu, Y.J.; Wang, H.Y. Fabrication of microcapsules containing dual-functional tung oil and properties suitable for self-healing and self-lubricating coatings. Prog. Org. Coat. 2018, 115, 164–171. [Google Scholar] [CrossRef]
- Souzandeh, H.; Netravali, A.N. Self-healing of ‘green’ thermoset zein resins with irregular shaped waxy maize starch-based/poly(D,L-lactic-co-glycolic acid) microcapsules. Compos. Sci. Technol. 2019, 183, 107831. [Google Scholar] [CrossRef]
- Kong, F.H.; Xu, W.C.; Zhang, X.L.; Wang, X.; Zhang, Y.; Wu, J.L. High-efficiency self-repairing anticorrosion coatings with controlled assembly microcapsules. J. Mater. Sci. 2018, 53, 12850–12859. [Google Scholar] [CrossRef]
- Brown, E.N.; White, S.R.; Sottos, N.R. Microcapsule induced toughening in a self-healing polymer composite. J. Mater. Sci. 2004, 39, 1703–1710. [Google Scholar] [CrossRef]
- Hager, M.D. Self-healing materials. In Handbook of Solid State Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 201–225. [Google Scholar]
- Hager, M.D.; Greil, P.; Leyens, C.; van der Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef]
- Wang, L.T.; Deng, L.P.; Zhang, D.W.; Qian, H.C.; Du, C.W.; Li, X.G.; Mol, J.M.C.; Terryn, H.A. Shape memory composite (SMC) self-healing coatings for corrosion protection. Prog. Org. Coat. 2016, 97, 261–268. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, L.; Ju, P.; Huang, L.; Qian, H.; Zhang, D.; Li, X.; Terryn, H.A.; Mol, J.M.C. Triple-Action Self-Healing Protective Coatings Based on Shape Memory Polymers Containing Dual-Function Microspheres. ACS Appl. Mater Interfaces 2018, 10, 23369–23379. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.F.; Mather, P.T. Shape Memory Assisted Self-Healing Coating. ACS Macro Lett. 2013, 2, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Odarczenko, M.; Thakare, D.; Li, W.; Venkateswaran, S.P.; Sottos, N.R.; White, S.R. Sunlight-Activated Self-Healing Polymer Coatings. Adv. Eng. Mater. 2020, 22, 1901223. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; de Souza, T.A.C.; Magnani, M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Effective corrosion protection by eco-friendly self-healing PMMA-cerium oxide coatings. Chem. Eng. J. 2020, 383, 123219. [Google Scholar] [CrossRef]
- Yan, D.S.; Wang, Y.L.; Liu, J.L.; Song, D.L.; Zhang, T.; Liu, J.Y.; He, F.; Zhang, M.; Wang, J. Self-healing system adapted to different pH environments for active corrosion protection of magnesium alloy. J. Alloys Compd. 2020, 824, 153918. [Google Scholar] [CrossRef]
- Cruz, M.; Rodil, S.E. Improving the corrosion resistance of aluminum alloy (AA7075) using amorphous chromium oxide coatings. Mater. Lett. 2020, 278, 128459. [Google Scholar] [CrossRef]
- Wang, J.P.; Song, X.; Wang, J.K.; Cui, X.; Zhou, Q.; Qi, T.; Li, G.L. Smart-Sensing Polymer Coatings with Autonomously Reporting Corrosion Dynamics of Self-Healing Systems. Adv. Mater. Interfaces 2019, 6, 1900055. [Google Scholar] [CrossRef]
- Taryba, M.; Lamaka, S.V.; Snihirova, D.; Ferreira, M.G.S.; Montemor, M.F.; Wijting, W.K.; Toews, S.; Grundmeier, G. The combined use of scanning vibrating electrode technique and micro-potentiometry to assess the self-repair processes in defects on “smart” coatings applied to galvanized steel. Electrochim. Acta 2011, 56, 4475–4488. [Google Scholar] [CrossRef]
- Malucelli, G.; Di Gianni, A.; Deflorian, F.; Fedel, M.; Bongiovanni, R. Preparation of ultraviolet-cured nanocomposite coatings for protecting against corrosion of metal substrates. Corros. Sci. 2009, 51, 1762–1771. [Google Scholar] [CrossRef]
- Khudyakov, I.V. Fast photopolymerization of acrylate coatings: Achievements and problems. Prog. Org. Coat. 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tri, P.N.; Azizi, S.; Dang, T.C.; Hoang, D.M.; Hoang, T.H.; Nguyen, T.L.; Bui, T.T.L.; Dang, V.H.; Nguyen, N.L.; et al. The role of organic and inorganic UV-absorbents on photopolymerization and mechanical properties of acrylate-urethane coating. Mater. Today Commun. 2020, 22, 100780. [Google Scholar] [CrossRef]
- de Barros, R.A.; Areias, M.C.C.; de Azevedo, W.M. Conducting polymer photopolymerization mechanism: The role of nitrate ions (NO3−). Synth. Met. 2010, 160, 61–64. [Google Scholar] [CrossRef]
- Gazotti, W.A.; De Paoli, M.-A. High yield preparation of a soluble polyaniline derivative. Synth. Met. 1996, 80, 263–269. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Shao, Y.W.; Liu, X.L.; Shi, C.; Wang, Y.Q.; Meng, G.Z.; Zeng, X.G.; Yang, Y. A study on corrosion protection of different polyaniline coatings for mild steel. Prog. Org. Coat. 2017, 111, 240–247. [Google Scholar] [CrossRef]
- Sun, M.; Ma, Z.D.; Li, A.H.; Zhu, G.Y.; Zhang, Y. Anticorrosive performance of polyaniline/waterborne epoxy/poly (methylhydrosiloxane) composite coatings. Prog. Org. Coat. 2020, 139, 105462. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, L.; Wei, X.X.; Zhang, B.; Meng, F.D.; Wang, F.H. Oxide film formed on Al alloy beneath sulfosalicylic acid doped polyaniline incorporated into epoxy organic coating. Appl. Surf. Sci. 2020, 512, 144840. [Google Scholar] [CrossRef]
- Perrin, F.X.; Phan, T.A.; Nguyen, D.L. Synthesis and characterization of polyaniline nanoparticles in phosphonic acid amphiphile aqueous micellar solutions for waterborne corrosion protection coatings. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1606–1616. [Google Scholar] [CrossRef]
- Lu, W.-K.; Elsenbaumer, R.L.; Wessling, B. Corrosion protection of mild steel by coatings containing polyaniline. Synth. Met. 1995, 71, 2163–2166. [Google Scholar] [CrossRef]
- Kordas, G. Corrosion Barrier Coatings: Progress and Perspectives of the Chemical Route. Corros. Mater. Degrad. 2022, 3, 376–413. [Google Scholar] [CrossRef]
- Peltier, F.; Thierry, D. Review of Cr-Free Coatings for the Corrosion Protection of Aluminum Aerospace Alloys. Coatings 2022, 12, 518. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, L.; Leng, X.; Lang, X.; Lian, J. Synthesis of amorphous TiO2 modified ZnO nanorod film with enhanced photocatalytic properties. Appl. Surf. Sci. 2014, 299, 97–104. [Google Scholar] [CrossRef]
- Zhang, Y. Strengthening, Corrosion and Protection of High-Temperature Structural Materials. Coatings 2022, 12, 1136. [Google Scholar] [CrossRef]
- Qin, X.Y.; Liu, S.; Lu, W.B.; Li, H.Y.; Chang, G.H.; Zhang, Y.W.; Tian, J.Q.; Luo, Y.L.; Asiri, A.M.; Al-Youbi, A.O.; et al. Submicrometre-scale polyaniline colloidal spheres: Photopolymerization preparation using fluorescent carbon nitride dots as a photocatalyst. Catal. Sci. Technol. 2012, 2, 711–714. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, P.; Tu, Y.; Liu, Z.; Dong, B.W.; Azad, A.; Ma, D.; Wang, D.; Zhang, X.; Yang, Y.; et al. Modification of TiO2 Nanoparticles with Organodiboron Molecules Inducing Stable Surface Ti(3+) Complex. iScience 2019, 20, 195–204. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.F.; Lei, B.Y.; Zhou, L.; Wang, S.Q. Visible light photocatalysis of pristine anatase TiO2 mesocrystals induced by largely exposed and stepped {001} surface. Green Chem. 2019, 21, 483–490. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M.; Ismail, A.A. Uniform dispersion of CuO nanoparticles on mesoporous TiO2 networks promotes visible light photocatalysis. Ceram. Int. 2020, 46, 8819–8826. [Google Scholar] [CrossRef]
- Zheng, P.; Zhou, W.; Wang, Y.B.; Ren, D.Z.; Zhao, J.; Guo, S.W. N-doped graphene-wrapped TiO2 nanotubes with stable surface Ti3+ for visible-light photocatalysis. Appl. Surf. Sci. 2020, 512, 144549. [Google Scholar] [CrossRef]
- Chang, M.S.; Kim, Y.S.; Kang, J.H.; Park, J.; Sung, S.J.; So, S.H.; Park, K.T.; Yang, S.J.; Kim, T.; Park, C.R. Guidelines for Tailored Chemical Functionalization of Graphene. Chem. Mater. 2017, 29, 307–318. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, e1901997. [Google Scholar] [CrossRef]
- Pan, Z.H.; Hisatomi, T.; Wang, Q.; Chen, S.S.; Nakabayashi, M.; Shibata, N.; Pan, C.S.; Takata, T.; Katayama, M.; Minegishi, T.; et al. Photocatalyst Sheets Composed of Particulate LaMg1/3Ta2/3O2N and Mo-Doped BiVO4 for Z-Scheme Water Splitting under Visible Light. ACS Catal. 2016, 6, 7188–7196. [Google Scholar] [CrossRef]
- Wang, Y.; Gilbertson, L.M. Informing rational design of graphene oxide through surface chemistry manipulations: Properties governing electrochemical and biological activities. Green Chem. 2017, 19, 2826–2838. [Google Scholar] [CrossRef]
- Shen, Z.; Shi, Q.; Huang, W.; Huang, B.; Wang, M.; Gao, J.; Shi, Y.; Lu, T. Stabilization of microcrystal λ-Ti3O5 at room temperature by aluminum-ion doping. Appl. Phys. Lett. 2017, 111, 191902. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, Y.; Chen, Q.L. Synthesis of an Ag2WO4/Ti3C2 Schottky composite by electrostatic traction and its photocatalytic activity. Ceram. Int. 2019, 45, 22298–22307. [Google Scholar] [CrossRef]
- Hu, C.; Li, T.; Yin, H.; Hu, L.; Tang, J.; Ren, K. Preparation and corrosion protection of three different acids doped polyaniline/epoxy resin composite coatings on carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126069. [Google Scholar] [CrossRef]
- Drury, A.; Chaure, S.; Kröll, M.; Nicolosi, V.; Chaure, N.; Blau, W.J. Fabrication and Characterization of Silver/Polyaniline Composite Nanowires in Porous Anodic Alumina. Chem. Mater. 2007, 19, 4252–4258. [Google Scholar] [CrossRef]
- Pruneanu, S.; Veress, E.; Marian, I.; Oniciu, L. Characterization of polyaniline by cyclic voltammetry and UV-Vis absorption spectroscopy. J. Mater. Sci. 1999, 34, 2733–2739. [Google Scholar] [CrossRef]
- Heydarnezhad, H.R.; Pourabbas, B.; Tayefi, M. Conducting Electroactive Polymers via Photopolymerization: A Review on Synthesis and Applications. Polym. Plast. Technol. Eng. 2018, 57, 1093–1109. [Google Scholar] [CrossRef]
- Fischer, D.A.; Vargas, I.T.; Pizarro, G.E.; Armijo, F.; Walczak, M. The effect of scan rate on the precision of determining corrosion current by Tafel extrapolation: A numerical study on the example of pure Cu in chloride containing medium. Electrochim. Acta 2019, 313, 457–467. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Wu, P.; Shen, J. Photocathodic protection effect of TiO2 films for carbon steel in 3% NaCl solutions. Electrochim. Acta 2005, 50, 3401–3406. [Google Scholar] [CrossRef]
- Guo, L.; Yamaguchi, H.; Yamamoto, M.; Matsui, F.; Wang, G.; Liu, F.; Yang, P.; Batista, E.R.; Moody, N.A.; Takashima, Y.; et al. Graphene as reusable substrate for bialkali photocathodes. Appl. Phys. Lett. 2020, 116, 251903. [Google Scholar] [CrossRef]




| Electrochemical Corrosion Measurements (SCE Was Employed as a Reference Electrode) | ||||
|---|---|---|---|---|
| Sample | Tafel Method | |||
| Ecorr (mV vs. SCE) | Icorr (A/cm2) | CR (mm/year) | PEs (%) | |
| carbon steel | −580 | 15.65 × 10−6 | 0.147 | / |
| composite | 35 | 11.94 × 10−6 | 0.139 | / |
| D-composite | −140 | 6.88 × 10−6 | 0.0803 | 73.5 |
| V-composite | 993 | 1.993 × 10−6 | 0.0232 | 83.3 |
| Sample | EIS Method | |||||
|---|---|---|---|---|---|---|
| Rs (Ω cm2) | CPEc (F/cm2) | Rc (Ω cm2) | CPEdl (F/cm2) | Rct (Ω cm2) | PEs (%) | |
| carbon steel | 4.017 | 4.002 × 10−4 | / | / | 0.7677 × 10−4 | / |
| composite | 8.686 | 2.009 × 10−4 | 2120 | 3.992 × 10−4 | 1.82 × 104 | / |
| D-composite | 10.95 | 4.662 × 10−4 | 2048 | 5.967 × 10−4 | 2.075 × 104 | 12.3 |
| V-composite | 12.46 | 2.357 × 10−4 | 6959 | 2.156 × 10−4 | 3.81 × 104 | 52.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Yang, H.; He, J.; Yu, S.; Xiao, R.; Luo, H.; Wen, Y.; Peng, S.; Liao, X.; Yang, D. Photopolymerization of Coating Materials for Protection against Carbon Steel Corrosion. Materials 2023, 16, 2015. https://doi.org/10.3390/ma16052015
Li B, Yang H, He J, Yu S, Xiao R, Luo H, Wen Y, Peng S, Liao X, Yang D. Photopolymerization of Coating Materials for Protection against Carbon Steel Corrosion. Materials. 2023; 16(5):2015. https://doi.org/10.3390/ma16052015
Chicago/Turabian StyleLi, Bo, Huibing Yang, Jinhang He, Siwu Yu, Rengui Xiao, Huanhu Luo, Yi Wen, Shengyan Peng, Xia Liao, and Daning Yang. 2023. "Photopolymerization of Coating Materials for Protection against Carbon Steel Corrosion" Materials 16, no. 5: 2015. https://doi.org/10.3390/ma16052015
APA StyleLi, B., Yang, H., He, J., Yu, S., Xiao, R., Luo, H., Wen, Y., Peng, S., Liao, X., & Yang, D. (2023). Photopolymerization of Coating Materials for Protection against Carbon Steel Corrosion. Materials, 16(5), 2015. https://doi.org/10.3390/ma16052015







