Hetero-Bimetallic Ferrocene-Containing Zinc(II)-Terpyridyl-Based Metallomesogen: Structural and Electrochemical Characterization
Abstract
1. Introduction
2. Results
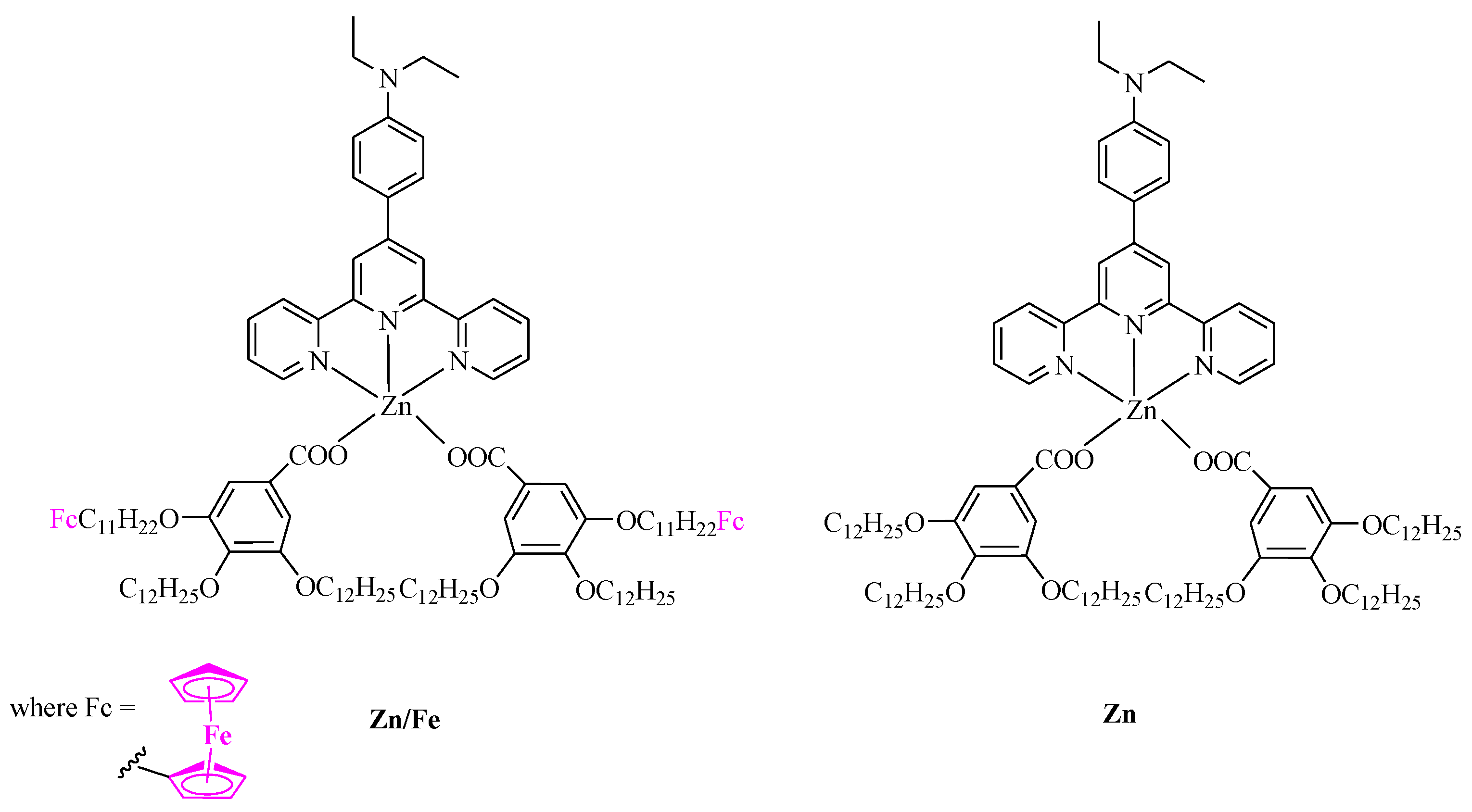
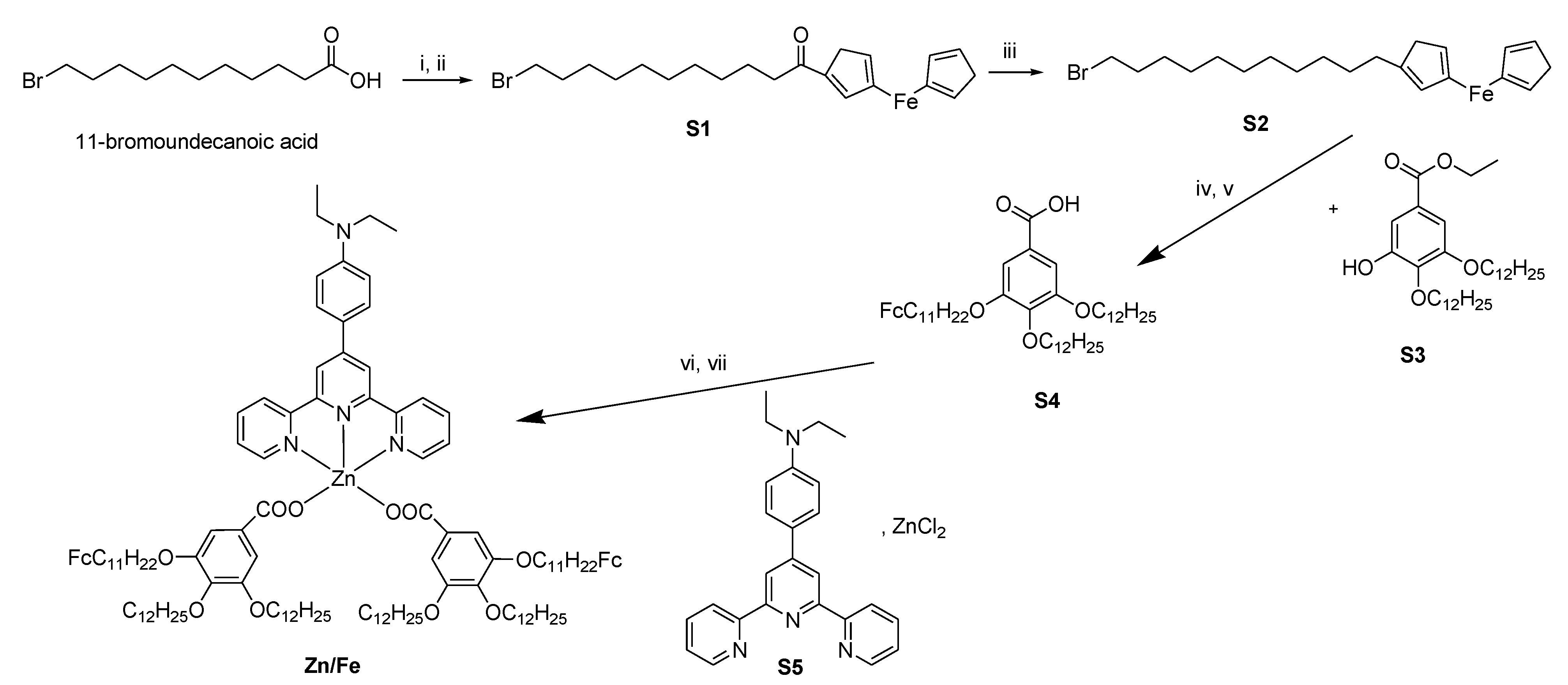
2.1. Synthesis
2.2. Mesomorphism
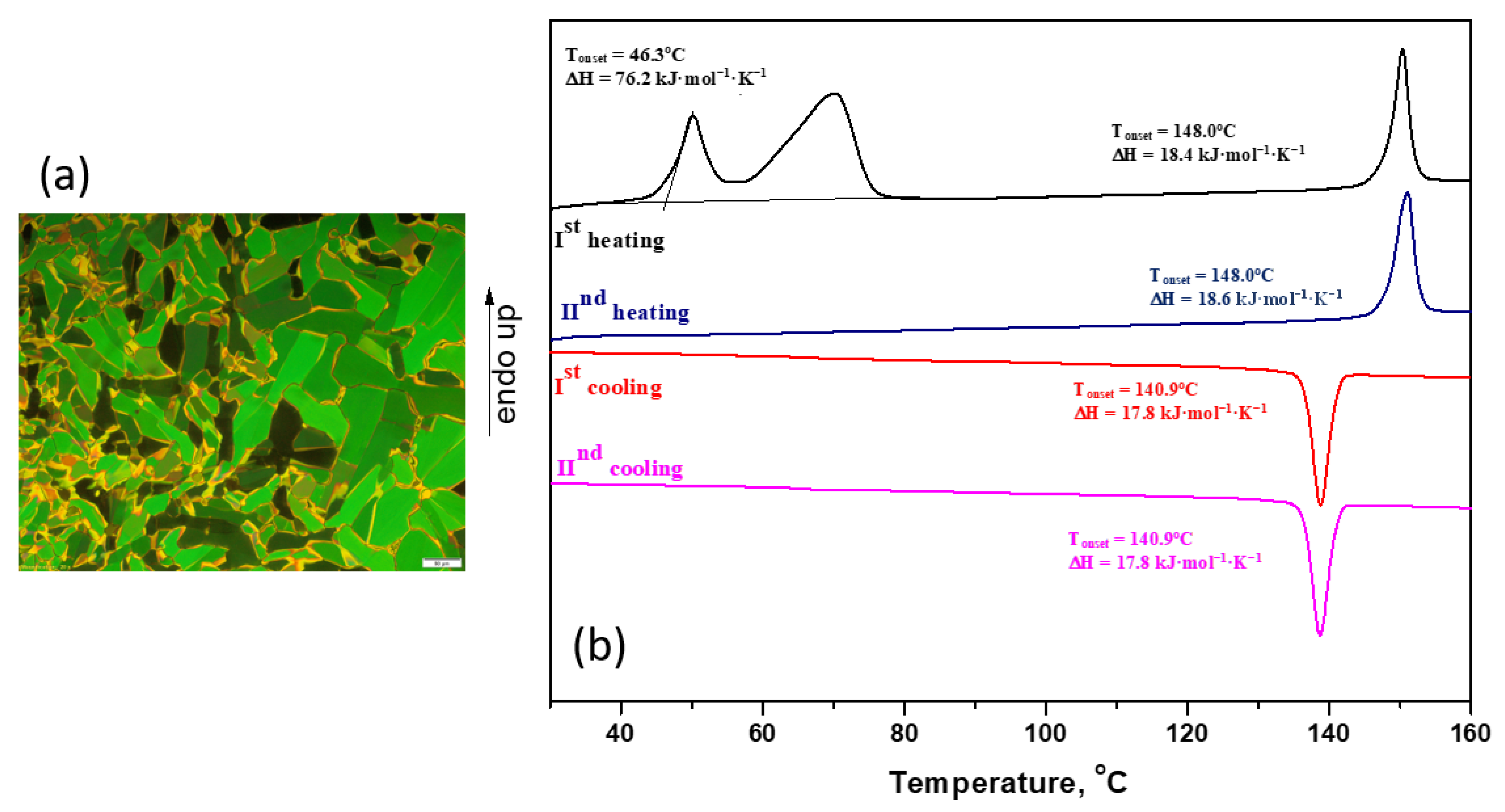
2.2.1. POM and DSC Studies
2.2.2. PXRD Studies
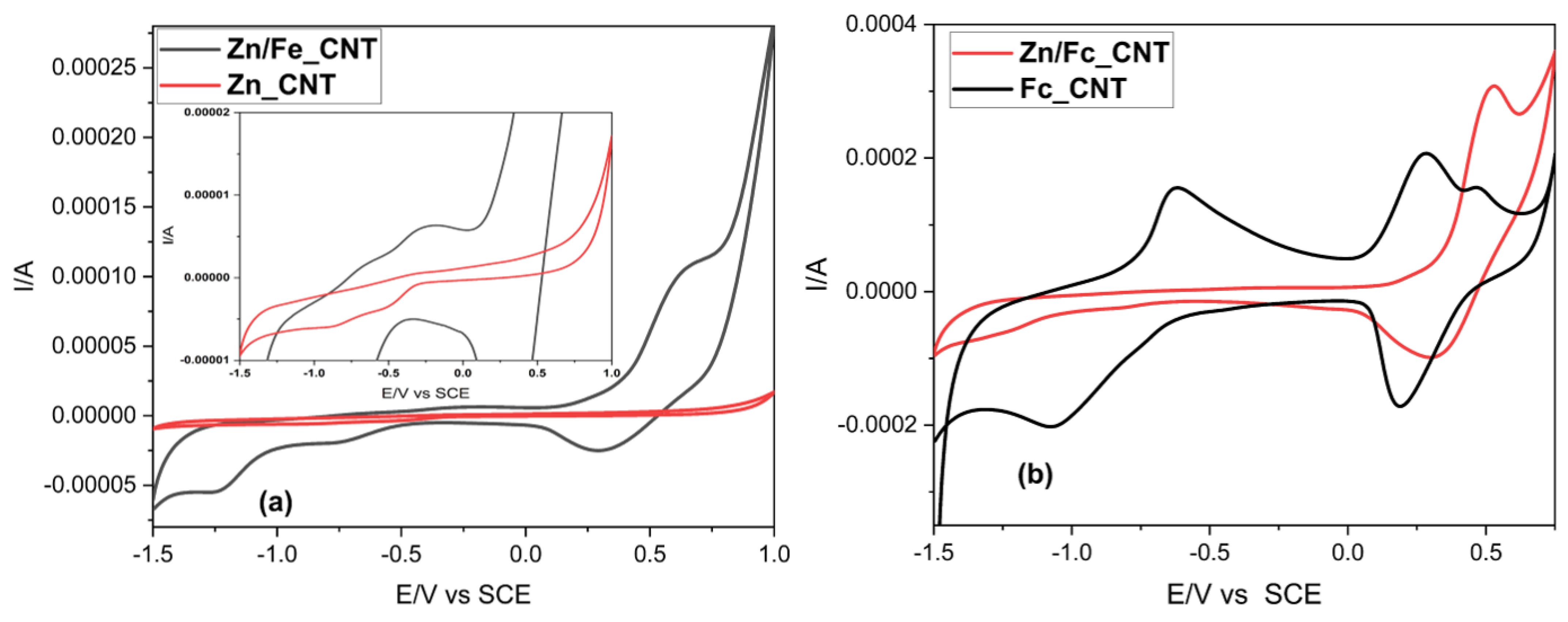
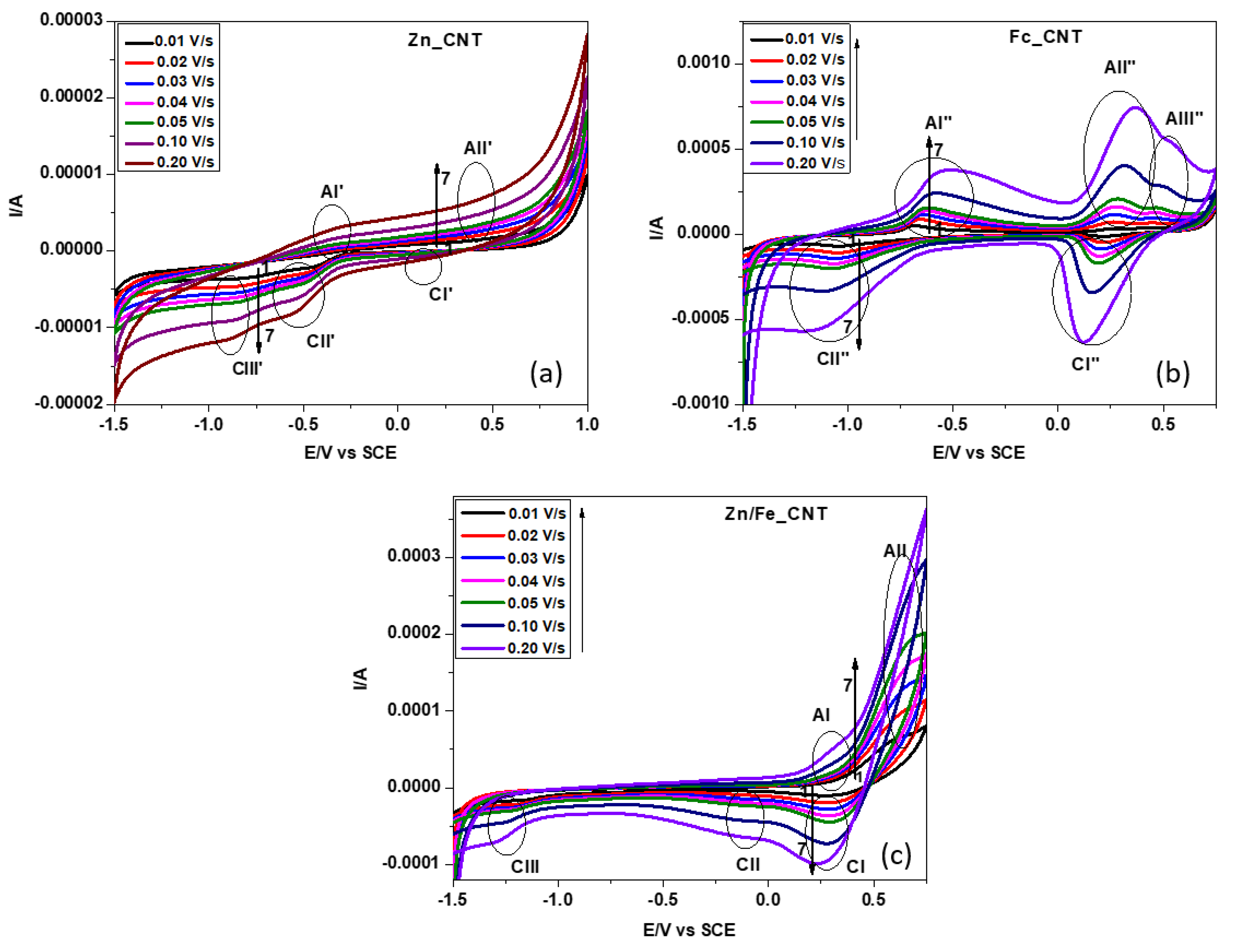
2.3. Electrochemistry
3. Materials and Methods
3.1. Synthesis
3.2. Optical and Thermal Studies
3.3. Powder X-ray Diffraction (PXRD) Analysis
3.4. Electrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szerb, E.I.; Crispini, A.; Aiello, I.; La Deda, M. Part XII: Inorganic Materials for Optoelectronics, 62: Liquid Crystals. In Springer Handbook of Inorganic Photochemistry; Bahnemann, D.W., Patrocinio, A.O.T., Zysman-Colman, E., Eds.; Springer: Cham, Switzerland, 2022; pp. 1811–1848. [Google Scholar]
- Cuerva, C.; Cano, M.; Lodeiro, C. Advanced Functional Luminescent Metallomesogens: The Key Role of the Metal Center. Chem. Rev. 2021, 121, 12966–13010. [Google Scholar] [CrossRef] [PubMed]
- La Deda, M.; Di Maio, G.; Candreva, A.; Heinrich, B.; Andelescu, A.-A.; Popa, E.; Voirin, E.; Badea, V.; Amati, M.; Costişor, O.; et al. Very intense polarized emission in self-assembled room temperature metallomesogens based on Zn(II) coordination complexes: An experimental and computational study. J. Mater. Chem. C 2022, 10, 115–125. [Google Scholar] [CrossRef]
- Yang, X.F.; Wu, X.G.; Zhou, D.; Yu, J.T.; Xie, G.H.; Bruce, D.W.; Wang, Y.F. Platinum-based metallomesogens bearing a Pt(4,6-dfppy)(acac) skeleton: Synthesis, photophysical properties and polarised phosphorescence application. Dalton Trans. 2018, 47, 13368–13377. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, L.F.; Ganea, P.C.; Manaila-Maximean, D.; Pasuk, I.; Circu, V. Synthesis and thermal, emission and dielectric properties of liquid crystalline Eu(III), Sm(III) and Tb(III) complexes based on mesogenic 4-pyridone ligands functionalized with cyanobiphenyl groups. J. Mol. Liq. 2019, 290, 111184. [Google Scholar] [CrossRef]
- Wu, X.G.; Xie, G.H.; Cabry, C.P.; Xu, X.Y.; Cowling, S.J.; Bruce, D.W.; Zhu, W.G.; Baranoff, E.; Wang, Y.F. Linearly polarized electroluminescence from ionic iridium complex-based metallomesogens: The effect of aliphatic-chain on their photophysical properties. J. Mater. Chem. C 2018, 6, 3298–3309. [Google Scholar] [CrossRef]
- Geng, H.; Luo, K.J.; Zou, G.; Zhao, L.; Wang, H.; Li, Q.; Ni, H.L. Have ambipolar carrier transmission property based on novel platinum(II) complexes: Synthesis, photophysical properties, liquid crystalline characteristics, polarized luminescence. Dyes Pigm. 2018, 149, 82–91. [Google Scholar] [CrossRef]
- Wang, Y.F.; Fan, J.; Shi, J.W.; Qi, H.R.; Baranoff, E.; Xie, G.H.; Li, Q.G.; Tan, H.; Liu, Y.; Zhu, W.G. Influence of integrated alkyl-chain length on the mesogenic and photophysical properties of platinum-based metallomesogens and their application for polarized white OLEDs. Dyes Pigm. 2016, 133, 238–247. [Google Scholar] [CrossRef]
- Akiyoshi, R.; Zenno, H.; Sekine, Y.; Nakaya, M.; Akita, M.; Kosumi, D.; Lindoy, L.F.; Hayami, S. A Ferroelectric Metallomesogen Exhibiting Field-Induced Slow Magnetic Relaxation. Chem. Eur. J. 2022, 28, e202103367. [Google Scholar] [CrossRef]
- Liu, S.T.; Zhu, Z.H.; Li, X.L.; Tang, J.K. New iron(II) spin-crossover metallomesogen with long aliphatic chain terminated by a C=C bond. Inorg. Chem. Front. 2022, 9, 267–274. [Google Scholar] [CrossRef]
- Fitzpatrick, A.J.; Martinho, P.N.; Gildea, B.J.; Holbrey, J.D.; Morgan, G.G. Robust Room Temperature Hysteresis in an Fe-III Spin Crossover Metallomesogen. Eur. J. Inorg. Chem. 2016, 2025–2029. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Kovshik, A.P.; Galyametdinov, Y.G. Effect of Magnetic and Electric Field on the Orientation of Rare-Earth-Containing Nematics. Inorg. Chem. 2021, 60, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, K.; Yoganandham, S.T.; Arumugam, S.; Arumugam, D.; Thananjeyan, K. An Overview of liquid crystalline mesophase transition and photophysical properties of “f block,” “d block,” and (SCO) spin-crossover metallomesogens in the optoelectronics. J. Mol. Liq. 2021, 321, 114793. [Google Scholar] [CrossRef]
- Gruzdev, M.S.; Chervonova, U.V.; Vorobeva, V.E.; Kolker, A.M. Highly branched mesomorphic iron(III) complexes with a long alkyl fragments on periphery. J. Mol. Liq. 2020, 320, 114505. [Google Scholar] [CrossRef]
- Wang, X.J.; Valldor, M.; Spielberg, E.T.; Heinemann, F.W.; Meyer, K.; Mudring, A.V. Paramagnetic iron-containing ionic liquid crystals. J. Mol. Liq. 2020, 304, 112583. [Google Scholar] [CrossRef]
- Park, M.; Kang, D.G.; Ko, H.; Rim, M.; Tran, D.T.; Park, S.; Kang, M.; Kim, T.W.; Kim, N.; Jeong, K.U. Molecular engineering of a porphyrin-based hierarchical superstructure: Planarity control of a discotic metallomesogen for high thermal conductivity. Mater. Horiz. 2020, 7, 2635–2642. [Google Scholar] [CrossRef]
- Rossi, L.; Huck-Iriart, C.; Giovanetti, L.; Antonel, P.S.; Marceca, E.; Cukiernik, F.D. Mesogenic Coordination Polymers Based on Ru-2(II,II)-Paddle-Wheel Units Exhibit High Electrical Conductivity. Eur. J. Inorg. Chem. 2022, 2022, e202100766. [Google Scholar] [CrossRef]
- Yang, B.; Ni, H.L.; Wang, H.F.; Hu, Y.H.; Luo, K.J.; Yu, W.H. Enhanced Synchronously Emission Dissymmetry Factor and Quantum Efficiency of Circularly Polarized Phosphorescence from Point-Chiral Cyclometalated Platinum(II) Liquid Crystal. J. Phys. Chem. C 2020, 124, 23879–23887. [Google Scholar] [CrossRef]
- Cuerva, C.; Campo, J.A.; Cano, M.; Sanz, J.; Sobrados, I.; Diez-Gómez, V.; Rivera-Calzada, A.; Schmidt, R. Water-Free Proton Conduction in Discotic Pyridylpyrazolate-based Pt(II) and Pd(II) Metallomesogens. Inorg. Chem. 2016, 55, 6995–7002. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, S.R.; Feng, S.S.; Li, Q.H.; Yang, B.; Zhao, Y.; Luo, K.J.; Wen, T.B. Cyclometalated Platinum(II) Metallomesogens Based on Half-Disc-Shaped beta-Diketonate Ligands with Hexacatenar: Crystal Structures, Mesophase Properties, and Semiconductor Devices. Inorg. Chem. 2022, 61, 11702–11714. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, B.; Zeng, L.W.; Luo, K.J.; Wang, H.F.; Ni, H.L.; Yang, C.L.; Li, Q. Tetradentate platinum(II) complexes: Synthesis, photophysical properties, liquid crystalline characteristics and charge transport behaviour. Dyes Pigm. 2019, 164, 398–406. [Google Scholar] [CrossRef]
- Cuerva, C.; Campo, J.A.; Cano, M.; Schmidt, R. Lamellar columnar liquid-crystalline mesophases as a 2D platform for anhydrous proton conduction. J. Mater. Chem. C 2019, 7, 10318–10330. [Google Scholar] [CrossRef]
- Cuerva, C.; Campo, J.A.; Cano, M.; Schmidt, R. Nanostructured discotic Pd(II) metallomesogens as one-dimensional proton conductors. Dalton Trans. 2017, 46, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Andelescu, A.A.; Ilies (b. Motoc), S.; Cretu, C.; Popa, E.; Marinescu, S.; Heinrich, B.; Manea, F.; Negrea, S.; Donnio, B.; Szerb, E.I. Pentacoordinated Liquid Crystalline Zn(II) Complex Organized in Smectic Mesophase: Synthesis, Structural and Electrochemical Properties. Appl. Sci. 2022, 12, 8306. [Google Scholar] [CrossRef]
- Negrea, S.; Andelescu, A.A.; Ilies (b. Motoc), S.; Cretu, C.; Cseh, L.; Rastei, M.; Donnio, B.; Szerb, E.I.; Manea, F. Design of Nanostructured Hybrid Electrodes Based on a Liquid Crystalline Zn(II) Coordination Complex-Carbon Nanotubes Composition for the Specific Electrochemical Sensing of Uric Acid. Nanomaterials 2022, 12, 4215. [Google Scholar] [CrossRef]
- Binnemans, K.; Lodewyckx, K.; Donnio, B.; Guillon, D. Mixed Copper—Lanthanide Metallomesogens. Chem. Eur. J. 2002, 8, 1101–1105. [Google Scholar] [CrossRef]
- Binnemans, K.; Lodewyckx, K.; Donnio, B.; Guillon, D. Mixed f-d Metallomesogens with an Extended Rigid Core. Eur. J. Inorg. Chem. 2005, 1506–1513. [Google Scholar] [CrossRef]
- Kadkin, O.N.; An, J.; Han, H.; Galyametdinov, Y.G. A Novel Series of Heteropolynuclear Metallomesogens: Organopalladium Complexes with Ferrocenophane-Containing Ligands. Eur. J. Inorg. Chem. 2008, 1682–1688. [Google Scholar] [CrossRef]
- Marcos, M.; Omenat, A.; Barberá, J.; Durán, F.; Serrano, J.L. Structural study of metallomesogens derived from tris-[2-(salicylideneamino)ethyl]amine. A molecular meccano. J. Mater. Chem. 2004, 14, 3321–3327. [Google Scholar] [CrossRef]
- Paschke, R.; Liebsch, S.; Tschierske, C.; Oakley, M.A.; Sinn, E. Synthesis and Mesogenic Properties of Binuclear Copper(II) Complexes Derived from Salicylaldimine Schiff Bases. Inorg. Chem. 2003, 42, 8230–8240. [Google Scholar] [CrossRef]
- Serrette, A.G.; Lai, C.K.; Swager, T.M. Complementary Shapes in Columnar Liquid Crystals: Structural Control in Homo- and Heteronuclear Bimetallic Assemblies. Chem. Mater. 1994, 6, 2252–2268. [Google Scholar] [CrossRef]
- Chico, R.; de Domingo, E.; Dominguez, C.; Donnio, B.; Heinrich, B.; Termine, R.; Golemme, A.; Coco, S.; Espinet, P. High One-Dimensional Charge Mobility in Semiconducting Columnar Mesophases of Isocyano-Triphenylene Metal Complexes. Chem. Mater. 2017, 29, 7587–7595. [Google Scholar] [CrossRef]
- Szydłowska, J.; Krówczyński, A.; Pociecha, D.; Szczytko, J.; Budzowski, P.; Twardowski, A.; Górecka, E. Dinuclear Mesogens with Antiferromagnetic Properties. ChemPhysChem 2010, 11, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Vulugundam, G.; Kondaiahb, P.; Bhattacharya, S. Co-liposomes of redox-active alkyl-ferrocene modified low MW branched PEI and DOPE for efficacious gene delivery in serum. J. Mater. Chem. B 2015, 3, 2318–2330. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, M.; Du, W.; Hu, L.; Li, F.; Tian, X.; Wang, A.; Zhang, Q.; Zhang, Z.; Wu, J.; et al. A Series of Zn(II) Terpyridine-Based Nitrate Complexes as Two-Photon Fluorescent Probe for Identifying Apoptotic and Living Cells via Subcellular Immigration. Inorg. Chem. 2018, 57, 7676–7683. [Google Scholar] [CrossRef]
- Kondratenko, Y.; Fundamensky, V.; Ignatyev, I.; Zolotarev, A.; Kochina, T.; Ugolkov, V. Synthesis and crystal structure of two zinc-containing complexes of triethanolamine. Polyhedron 2017, 130, 176–183. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Paul, S. Conducting polypyrrole modified with ferrocene for applications in carbon monoxide sensors. Sens. Actuators B 2007, 125, 60–65. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Aoki, R.; Sakamoto, R.; Toyoda, R.; Shimada, M.; Hattori, Y.; Asaoka, M.; Kitagawa, Y.; Nishibori, E.; Nakanoc, M.; et al. A simple zinc(II) complex that features multi-functional luminochromism induced by reversible ligand dissociation. Chem. Commun. 2017, 53, 3657–3660. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lu, W.; Liang, B.; Pombeiro, A.J.L. Synthesis, characterization, photoluminescent and thermal properties of zinc(II) 4′-phenyl-terpyridine compounds. New J. Chem. 2013, 37, 1529–1537. [Google Scholar] [CrossRef]
- Ma, Z.; Cao, Y.; Li, Q.; Guedes da Silva, M.F.C.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Synthesis, characterization, solid-state photo-luminescence and anti-tumor activity of zinc(II) 4′-phenyl-terpyridine compounds. J. Inorg. Biochem. 2010, 104, 704–711. [Google Scholar] [CrossRef]
- Andelescu, A.-A.; Heinrich, B.; Spirache, M.A.; Voirin, E.; La Deda, M.; Di Maio, G.; Szerb, E.I.; Donnio, B.; Costisor, O. Playing with PtII and ZnII Coordination to Obtain Luminescent Metallomesogens. Chem. Eur. J. 2020, 26, 4850–4860. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Godbert, N.; Crispini, A.; Ghedini, M.; Carini, M.; Chiaravalloti, F.; Ferrise, A.J. LCDiXRay: A user-friendly program for powder diffraction indexing of columnar liquid crystals. Appl. Cryst. 2014, 47, 668–679. [Google Scholar] [CrossRef]
- Bean, L.S.; Heng, L.Y.; Yamin, B.M.; Ahmad, M. The electrochemical behaviour of ferrocene in a photocurable poly(methyl methacrylate-co-2-hydroxylethyl methacrylate) film for a glucose biosensor. Bioelectrochem. 2005, 65, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Millare, B.; Xia, W.; Steyer, B.G.; Gerasimenko, A.A.; Ferreira, A.; Contreras, A.; Vullev, V.I. Electrochemical Oxidation of Ferrocene: A Strong Dependence on the Concentration of the Supporting Electrolyte for Nonpolar Solvents. J. Phys. Chem. A 2009, 113, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L.; Abbasi-Azad, M.; Habibi, B.; Rouhani, F.; Moghanni-Bavil-Olyaei, H.; Liu, K.-G.; Morsali, A. Electrochemical Applications of Ferrocene-Based Coordination Polymers. ChemPlusChem 2020, 85, 2397. [Google Scholar] [CrossRef]
- Eckermann, A.L.; Feld, D.J.; Shaw, J.A.; Meade, T.J. Electrochemistry of redox-active self-assembled monolayers. Coord. Chem. Rev. 2010, 254, 1769–1802. [Google Scholar] [CrossRef]
- Wu, H.; Lin, Q.; Batchelor-McAuley, C.; Compton, R.G. Nanoimpacts Reveal the Electron-Transfer Kinetics of the Ferrocene/Ferrocenium Couple Immobilised on Graphene Nanoplatelets. ChemElectroChem 2016, 3, 1478–1483. [Google Scholar] [CrossRef]
- Hampson, A.; Latham, R.J.; Marshall, A.; Giles, R.D. Some aspects of the electrochemical behaviour of the iron electrode in alkaline solutions. Electrochim. Acta 1974, 19, 397–401. [Google Scholar]
- Weinrich, H.; Come, J.; Tempel, H.; Kungl, H.; Eichel, R.-A.; Balke, N. Understanding the Nanoscale Redox- Behavior of Iron-Anodes for Rechargeable Iron-Air Batteries. Nano Energy 2017, 41, 706–716. [Google Scholar] [CrossRef]
- Hang, B.T. Electrochemical Properties of Fe2O3 Electrode in Alkaline Solution Containing K2S Additive. VNU J. Sci. Math. Phys. 2018, 34, 45–51. [Google Scholar]
- Geana, D.; El Miligy, A.A.; Lorenz, W.J. Electrochemical behaviour of iron in alkaline sulphate solutions. J. Appl. Electrochem. 1974, 4, 337–345. [Google Scholar] [CrossRef]
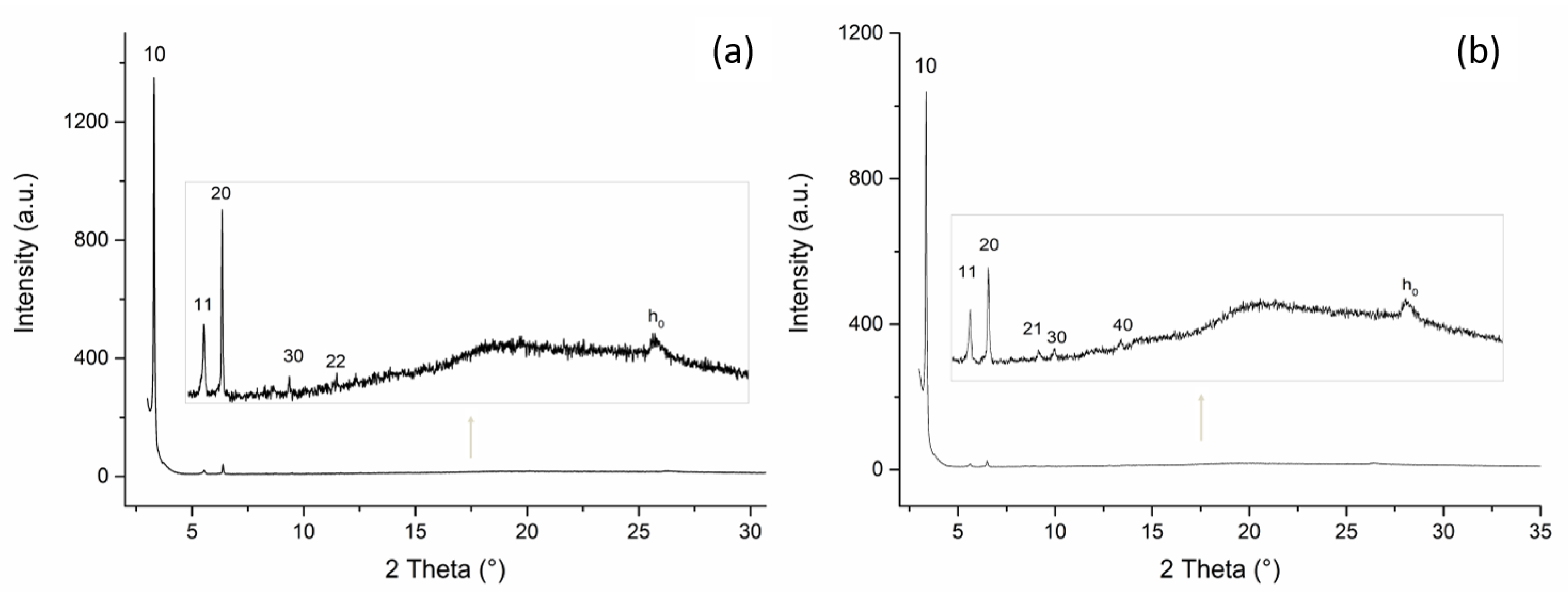
| T = 85 °C (Upon Cooling) | T = 25 °C (Upon Cooling) | ||||||
|---|---|---|---|---|---|---|---|
| dobs (Å) | hk | dcalcd (Å) * | Cell Parameter | dobs (Å) | hk | dcalcd (Å) * | Cell Parameter |
| 26.8 | 10 | 26.8 ** | 26.3 | 10 | 26.3 ** | ||
| 15.7 | 11 | 15.5 | 15.4 | 11 | 15.2 | ||
| 13.7 | 20 | 13.4 | a = 30.95 Å | 13.5 | 20 | 13.2 | a = 30.37 Å |
| 9.2 | 30 | 8.9 | 9.9 | 21 | 9.9 | ||
| 7.6 | 22 | 7.7 | 9.2 | 30 | 8.9 | ||
| 3.4 | 6.9 | 40 | 6.6 | ||||
| 3.4 | |||||||
| Modified Electrode | Γ/mol∙cm−2 | |
|---|---|---|
| Zn(II) Centres | Ferrocene Centres | |
| Zn_CNT | 2.20∙10−4 | - |
| Fc_CNT | - | 1.80∙10−3 |
| Zn/Fc_CNT | 6.66∙10−4 | 2.20∙10−4 |
| Electrode Type | Anodic | Cathodic | ||
|---|---|---|---|---|
| Process/Peak | E/V vs. SCE | Process/Peak | E/V vs. SCE | |
| Zn/Fe_CNT | ZnO dissolution /AI | +0.290 | Ferrocenium reduction/CI | +0.240 |
| Ferrocene oxidation/AII | +0.600 | Oxygen reduction/CII | −0.124 | |
| Zinc reduction/CIII | −1.300 | |||
| Zn_CNT | Zinc stripping/AI’ | −0.310 | Outer oxygen reduction reaction/CI’ | +0.135 |
| ZnO dissolution /AII’ | +0.450 | Reduction of inner O2 /CII’ | −0.560 | |
| Zn reduction/CIII’ | −0.900 | |||
| Fc_CNT | Fe oxidation /AI” | −0.680 | Ferrocenium reduction/CI” | +0.220 |
| Ferrocene oxidation/AII” | +0.260 | Fe(II) reduction /CII” | −1.000 | |
| Ferrocenium oxidation/AIII” | +0.550 | |||
| V1/2 | IpAII” | IpCI” | IpAII”/IpCI” | EpAII” | EpCI” | ΔEp |
|---|---|---|---|---|---|---|
| 0.100 | 26.46 | −14.86 | 1.78 | 0.240 | 0.220 | 0.020 |
| 0.141 | 71.46 | −48.78 | 1.47 | 0.250 | 0.210 | 0.040 |
| 0.173 | 117.73 | −87.49 | 1.35 | 0.260 | 0.200 | 0.060 |
| 0.200 | 162.74 | −129.88 | 1.25 | 0.270 | 0.190 | 0.080 |
| 0.224 | 209.26 | −170.98 | 1.22 | 0.280 | 0.180 | 0.100 |
| 0.316 | 405.98 | −345.14 | 1.18 | 0.310 | 0.160 | 0.150 |
| 0.447 | 742.23 | −635.41 | 1.17 | 0.360 | 0.120 | 0.240 |
| V1/2 | IpAII | IpCI | IpAII/IpCI | EpAII | EpCI | ΔEp |
|---|---|---|---|---|---|---|
| 0.100 | 6.83 | −10.03 | 0.680 | 0.610 | 0.300 | 0.310 |
| 0.141 | 10.06 | −19.17 | 0.520 | 0.640 | 0.300 | 0.340 |
| 0.173 | 12.05 | −27.64 | 0.440 | 0.650 | 0.300 | 0.350 |
| 0.200 | 13.74 | −36.12 | 0.380 | 0.660 | 0.300 | 0.360 |
| 0.224 | 15.89 | −44.60 | 0.360 | 0.670 | 0.300 | 0.370 |
| 0.316 | 26.82 | −72.74 | 0.370 | 0.680 | 0.280 | 0.400 |
| 0.447 | 46.97 | −99.08 | 0.470 | 0.680 | 0.230 | 0.450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, E.; Andelescu, A.A.; Ilies, S.; Visan, A.; Cretu, C.; Scarpelli, F.; Crispini, A.; Manea, F.; Szerb, E.I. Hetero-Bimetallic Ferrocene-Containing Zinc(II)-Terpyridyl-Based Metallomesogen: Structural and Electrochemical Characterization. Materials 2023, 16, 1946. https://doi.org/10.3390/ma16051946
Popa E, Andelescu AA, Ilies S, Visan A, Cretu C, Scarpelli F, Crispini A, Manea F, Szerb EI. Hetero-Bimetallic Ferrocene-Containing Zinc(II)-Terpyridyl-Based Metallomesogen: Structural and Electrochemical Characterization. Materials. 2023; 16(5):1946. https://doi.org/10.3390/ma16051946
Chicago/Turabian StylePopa, Evelyn, Adelina A. Andelescu, Sorina Ilies (b. Motoc), Alexandru Visan, Carmen Cretu, Francesca Scarpelli, Alessandra Crispini, Florica Manea, and Elisabeta I. Szerb. 2023. "Hetero-Bimetallic Ferrocene-Containing Zinc(II)-Terpyridyl-Based Metallomesogen: Structural and Electrochemical Characterization" Materials 16, no. 5: 1946. https://doi.org/10.3390/ma16051946
APA StylePopa, E., Andelescu, A. A., Ilies, S., Visan, A., Cretu, C., Scarpelli, F., Crispini, A., Manea, F., & Szerb, E. I. (2023). Hetero-Bimetallic Ferrocene-Containing Zinc(II)-Terpyridyl-Based Metallomesogen: Structural and Electrochemical Characterization. Materials, 16(5), 1946. https://doi.org/10.3390/ma16051946



.png)





