Characterization of AZ31/HA Biodegradable Metal Matrix Composites Manufactured by Rapid Microwave Sintering
Abstract
1. Introduction
2. Materials and Experimental Procedure
2.1. Material Details
2.2. Development of AZ31/HA Composites
3. Characterization Details
3.1. Evaluation of Physical and Metallurgical Properties
3.2. Evaluation of Mechanical Properties
3.3. Evaluation of Corrosion Behavior of BMMCs
3.3.1. Immersion Test for Measurement of Weight Loss
3.3.2. Measurement of pH and Mg2+ Ion Concentration in HBSS
3.3.3. Electrochemical Analysis
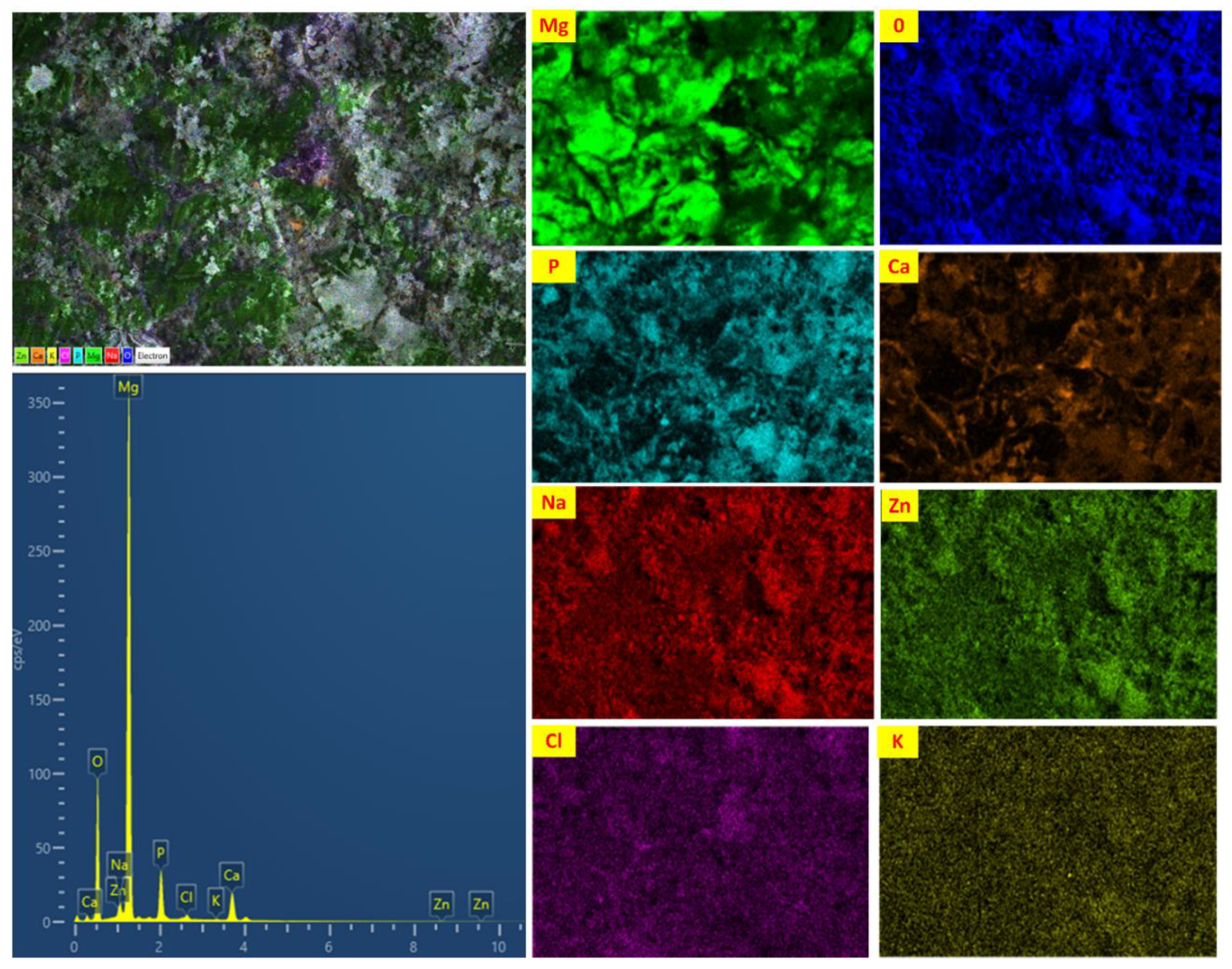
3.3.4. Observation of Surface Morphology
4. Results and Discussion
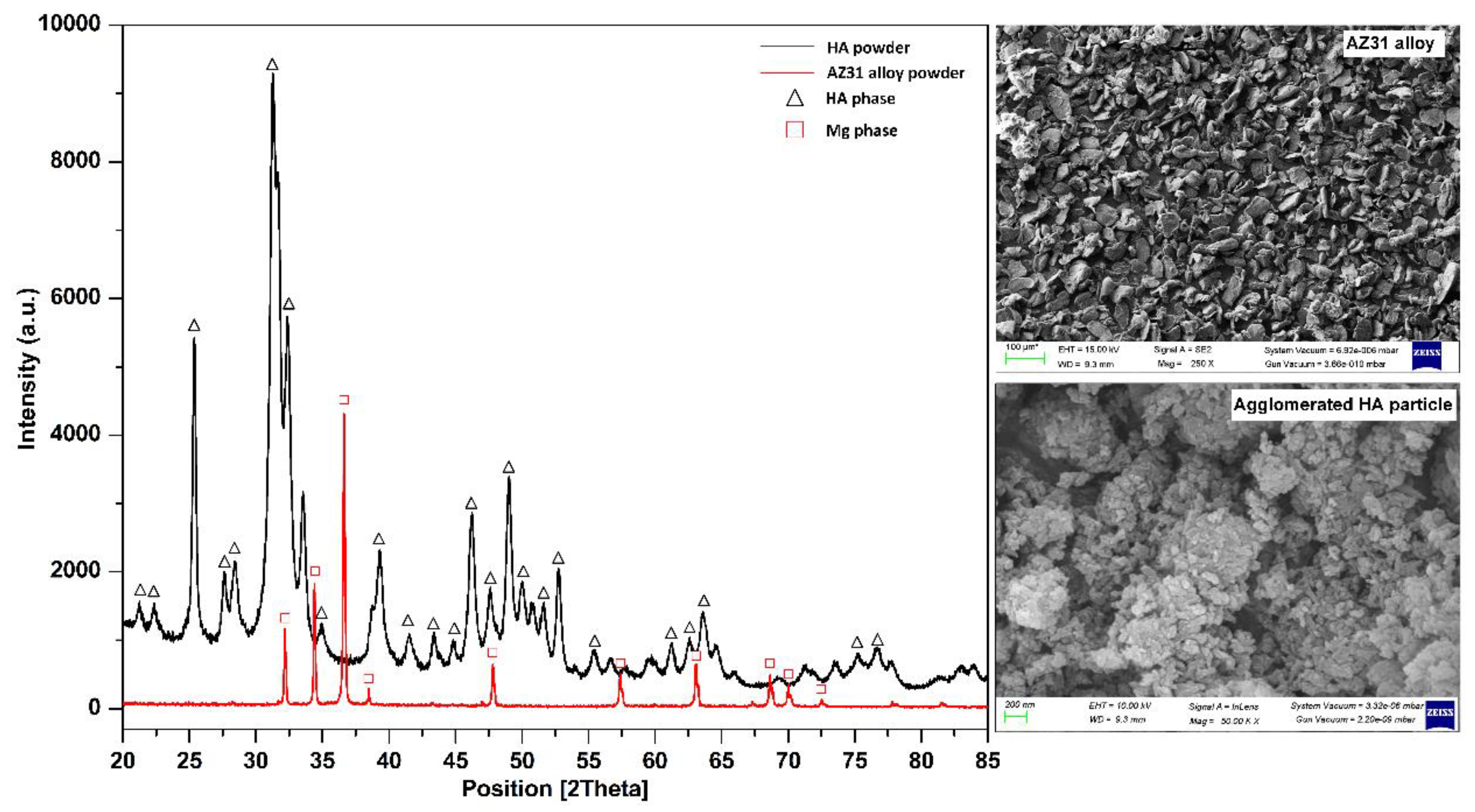
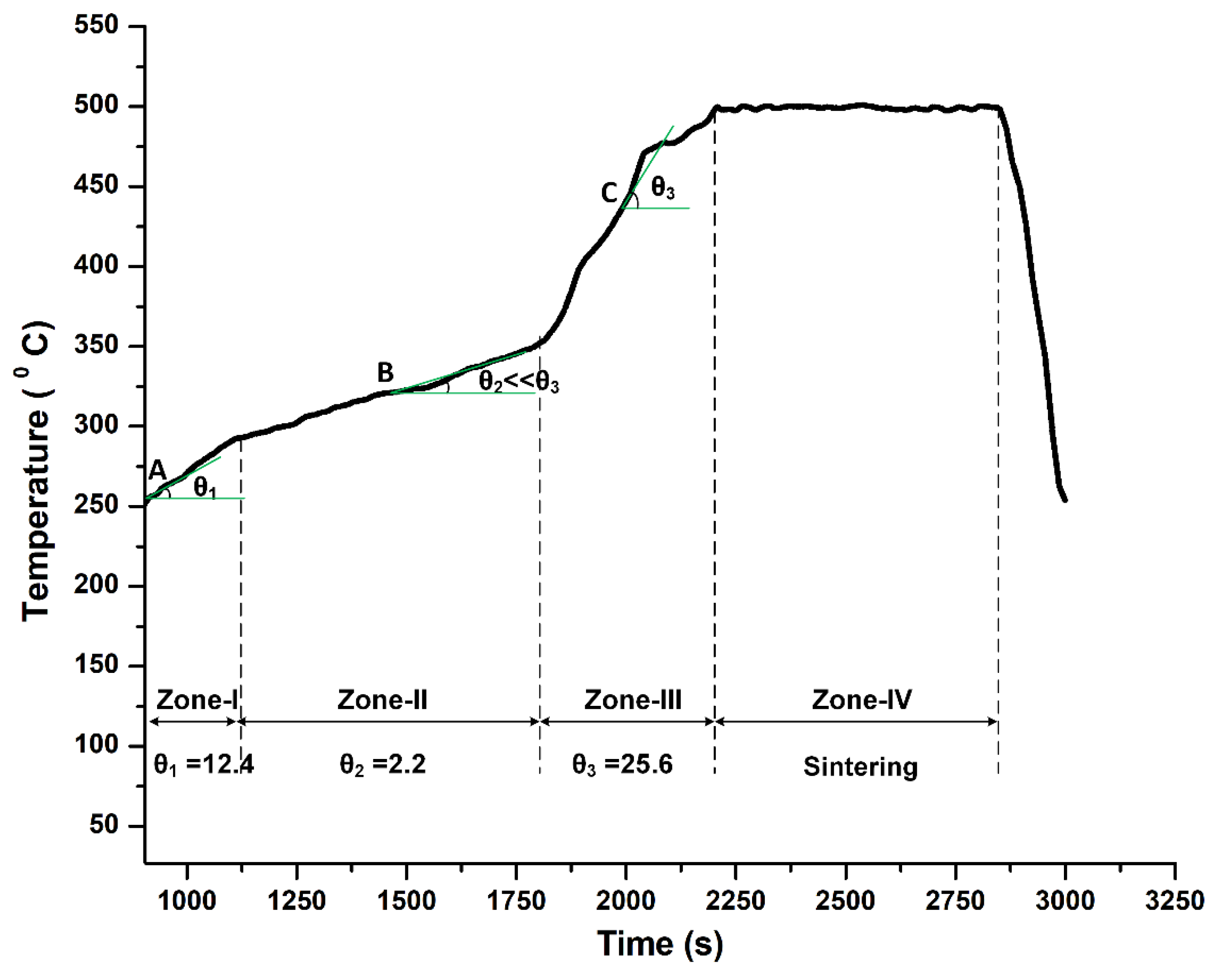
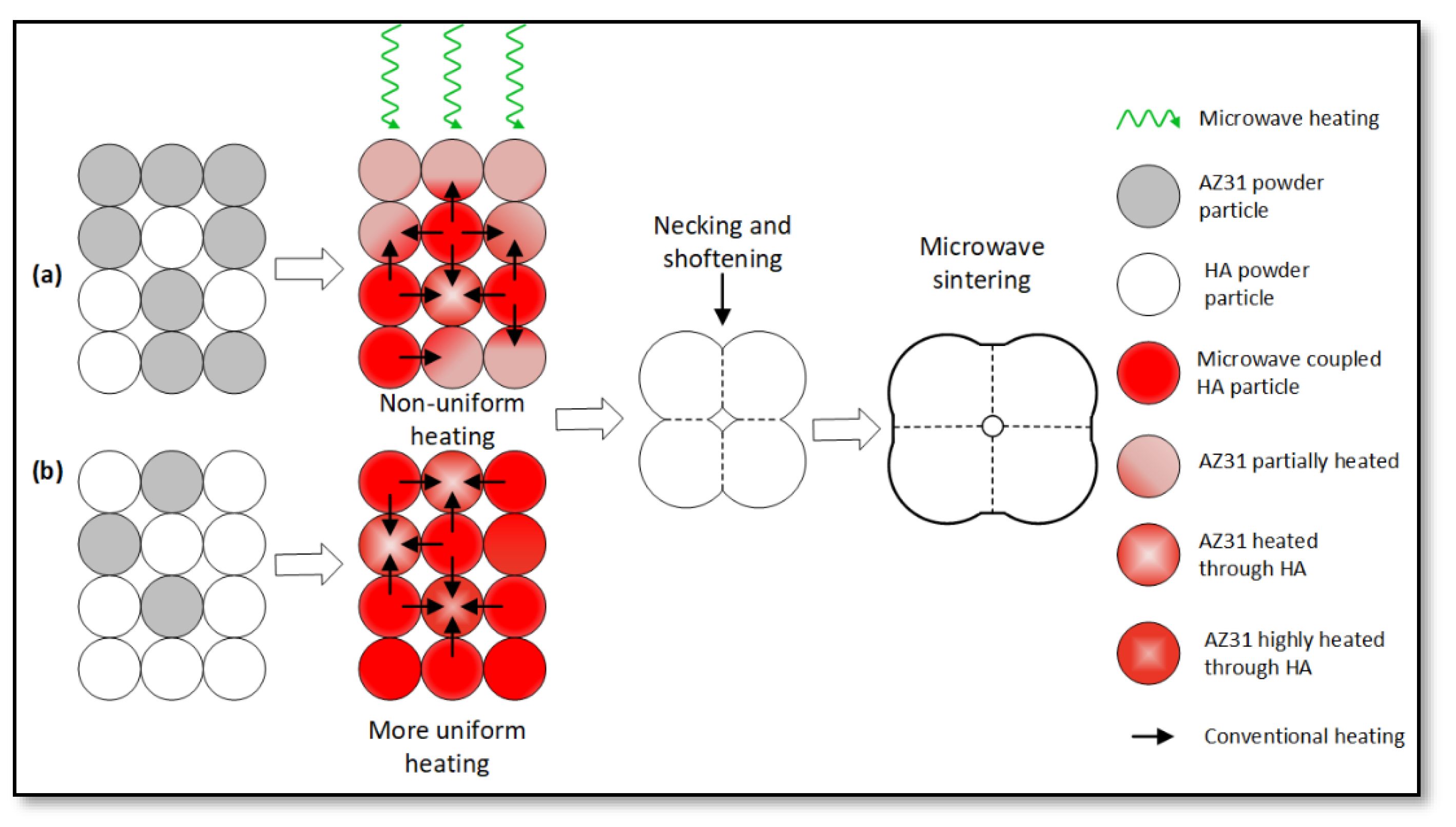
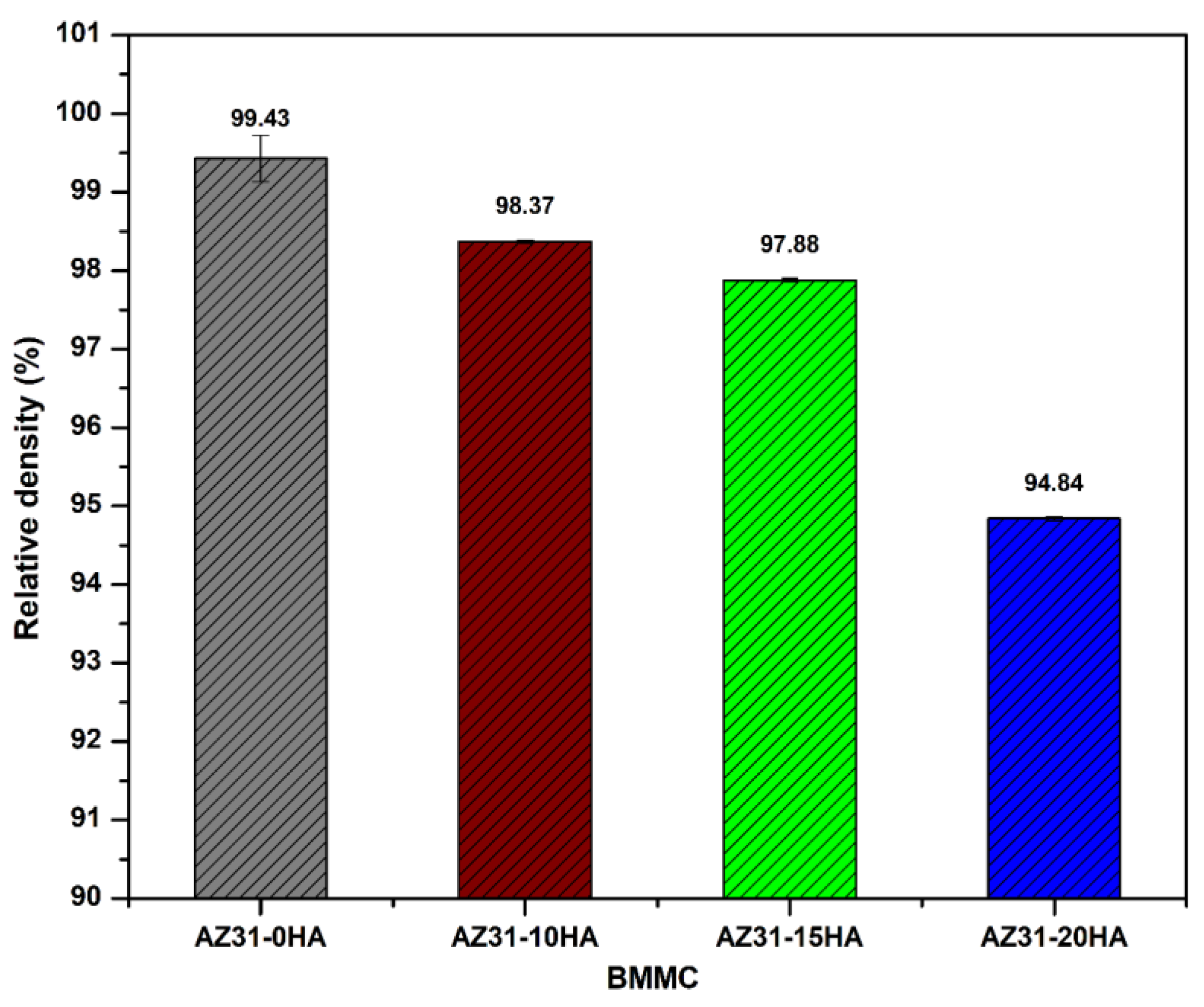
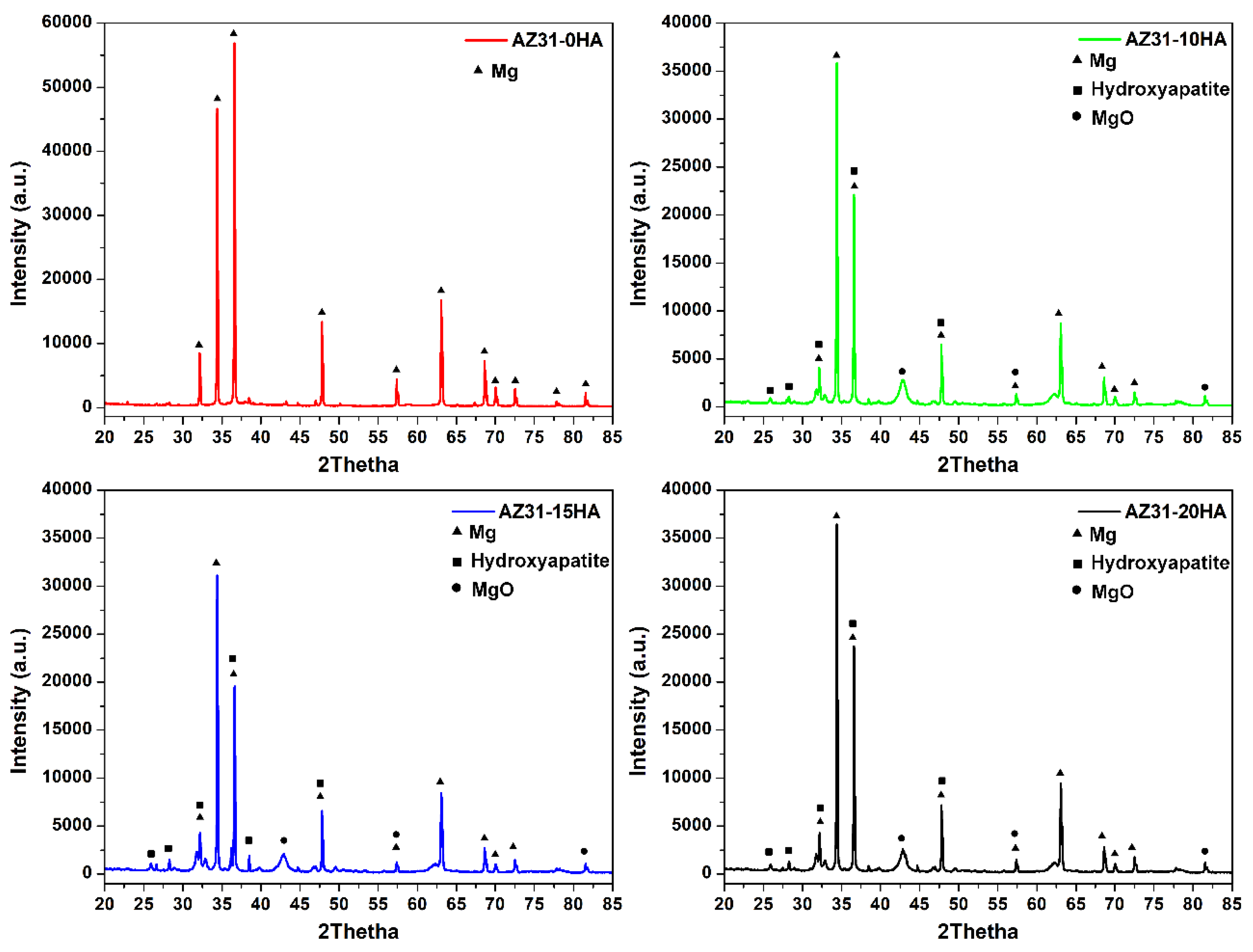
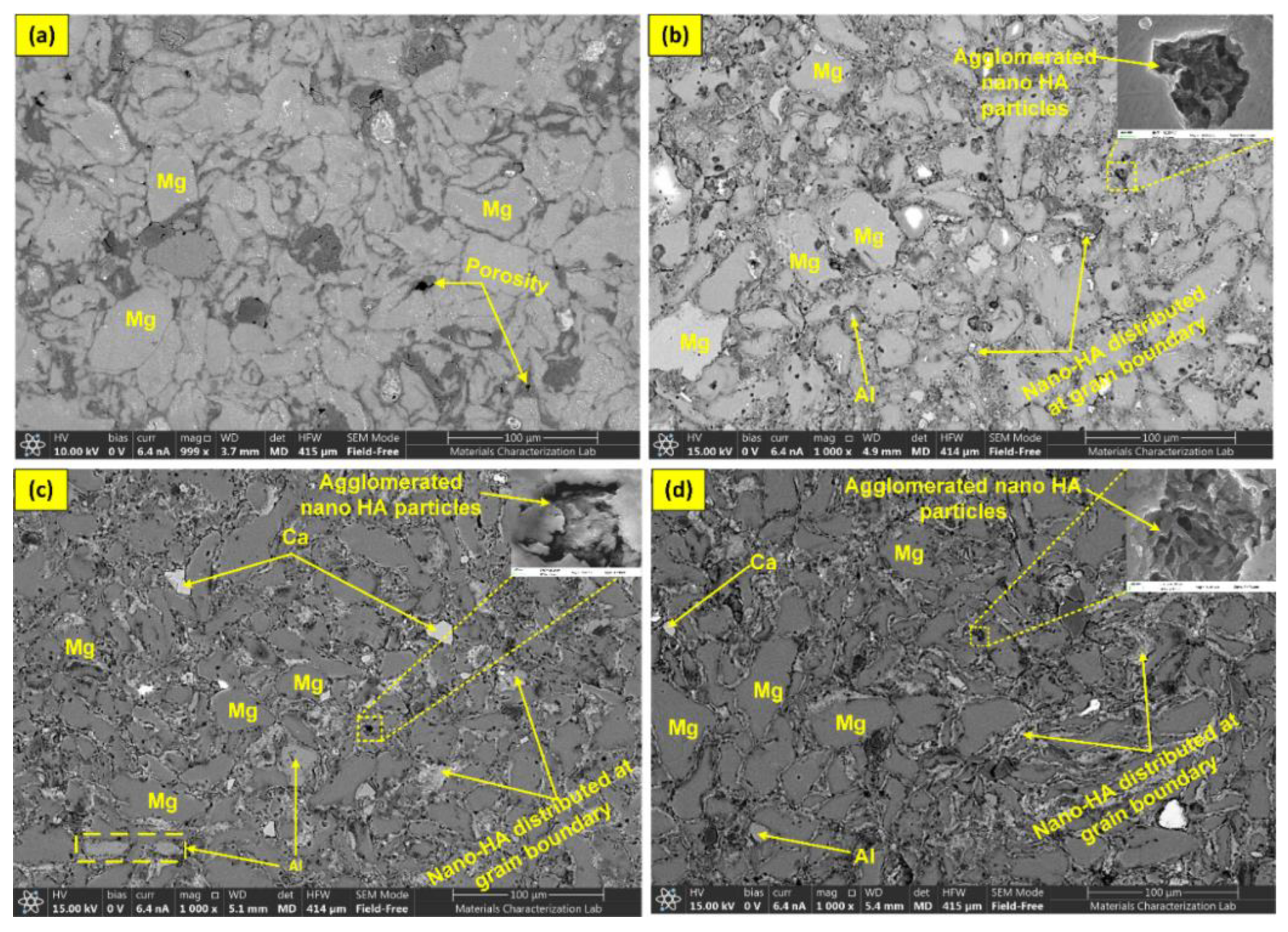
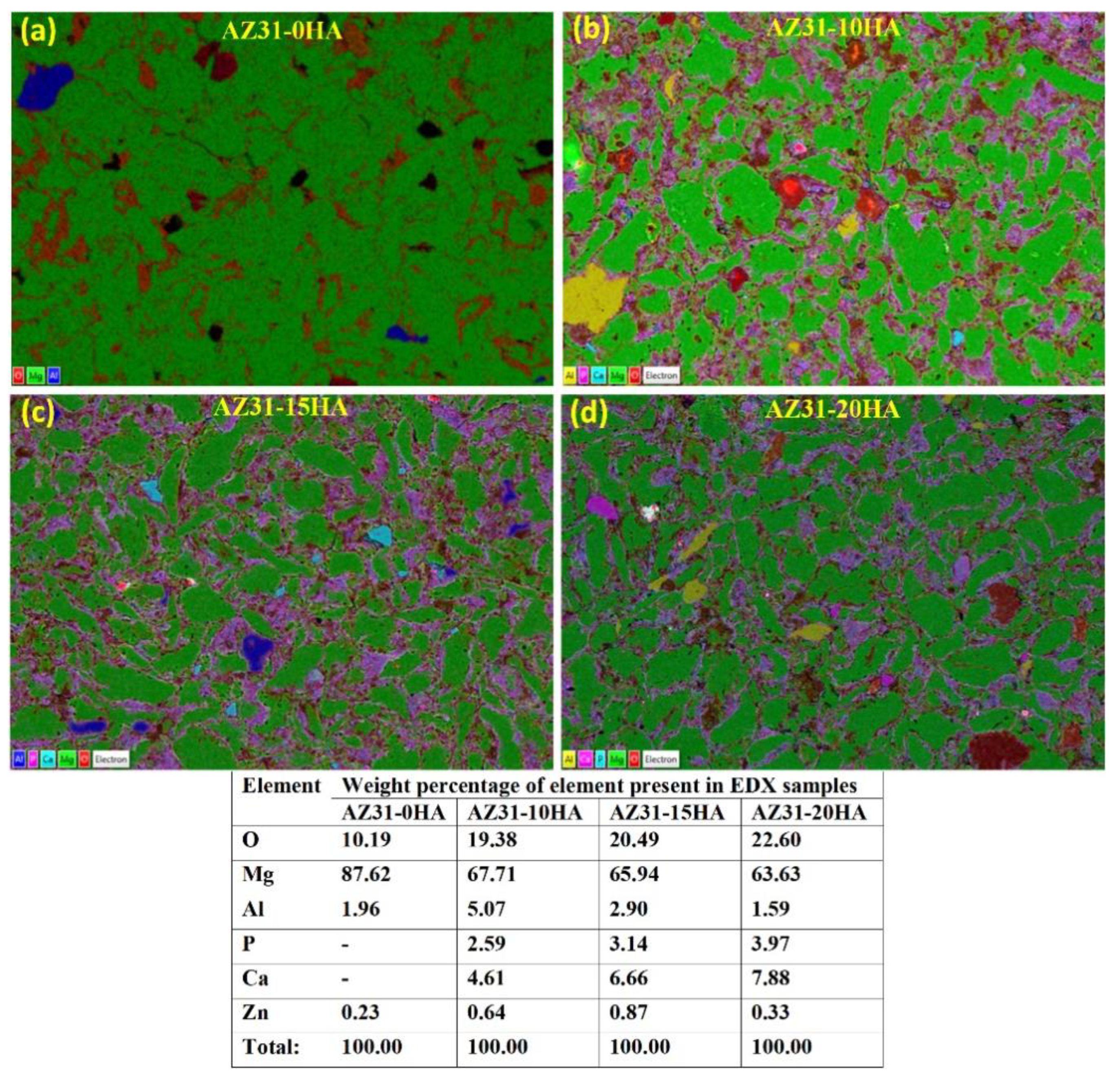
4.1. Density and Metallurgical Properties
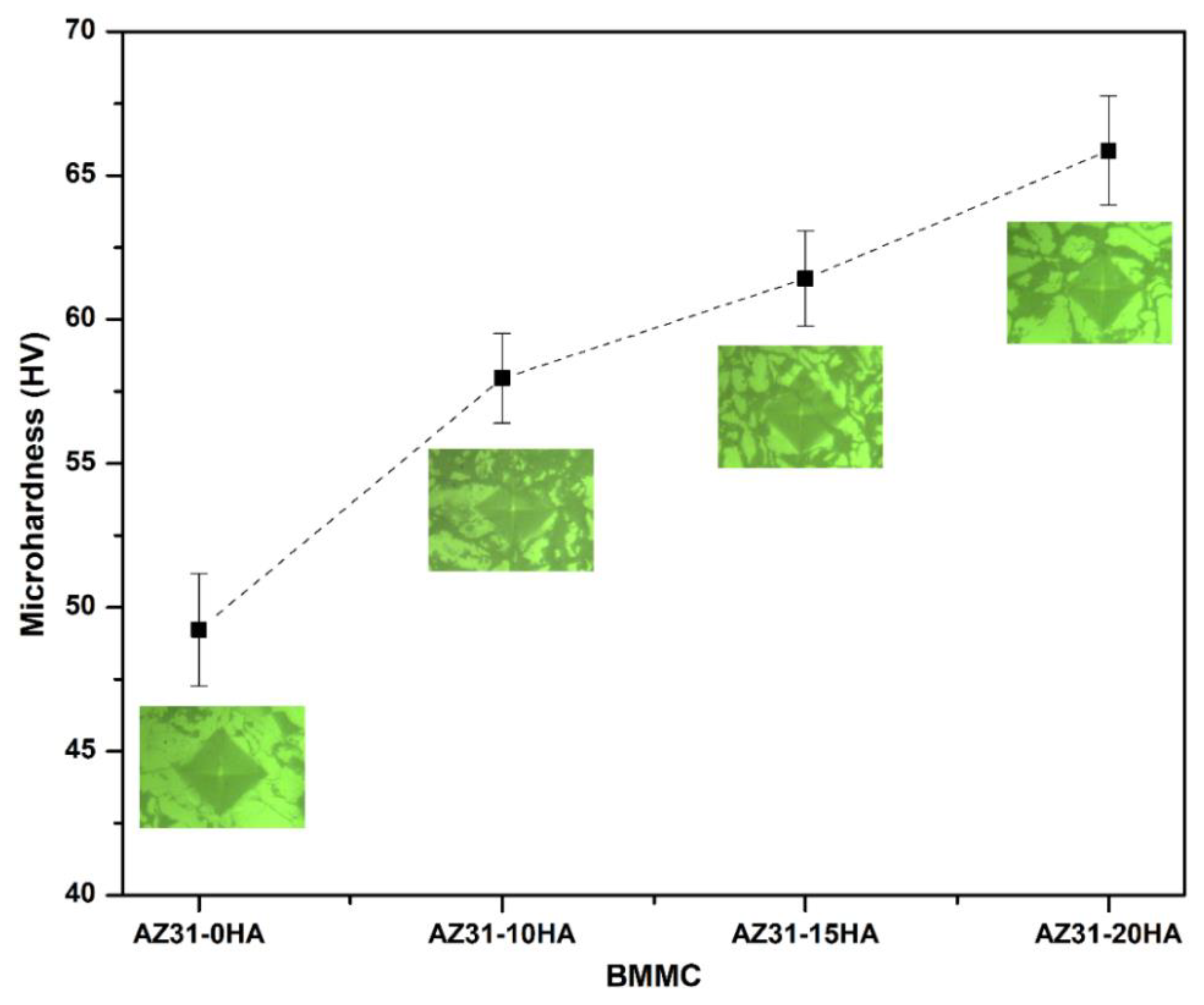
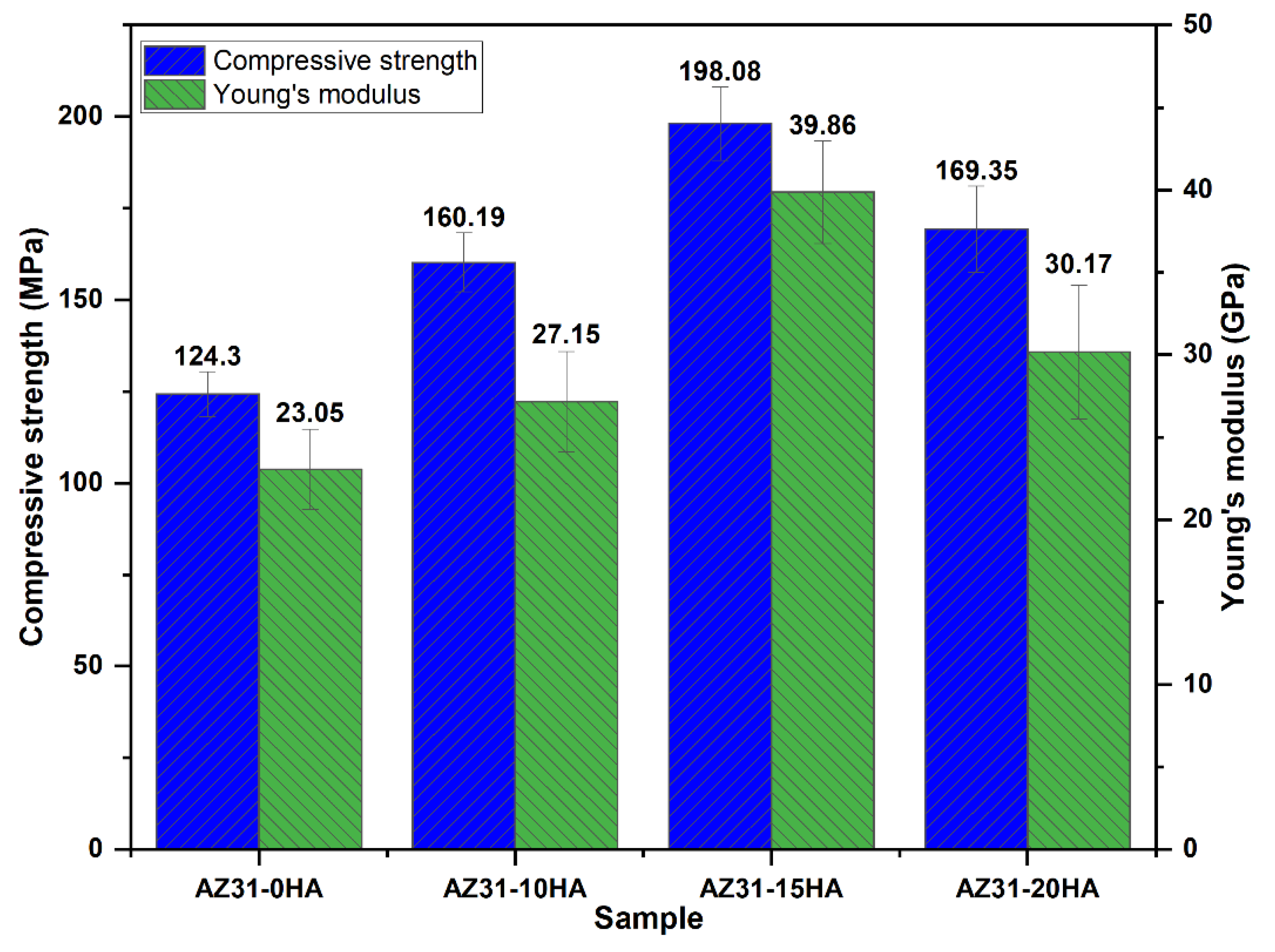
4.2. Mechanical Properties
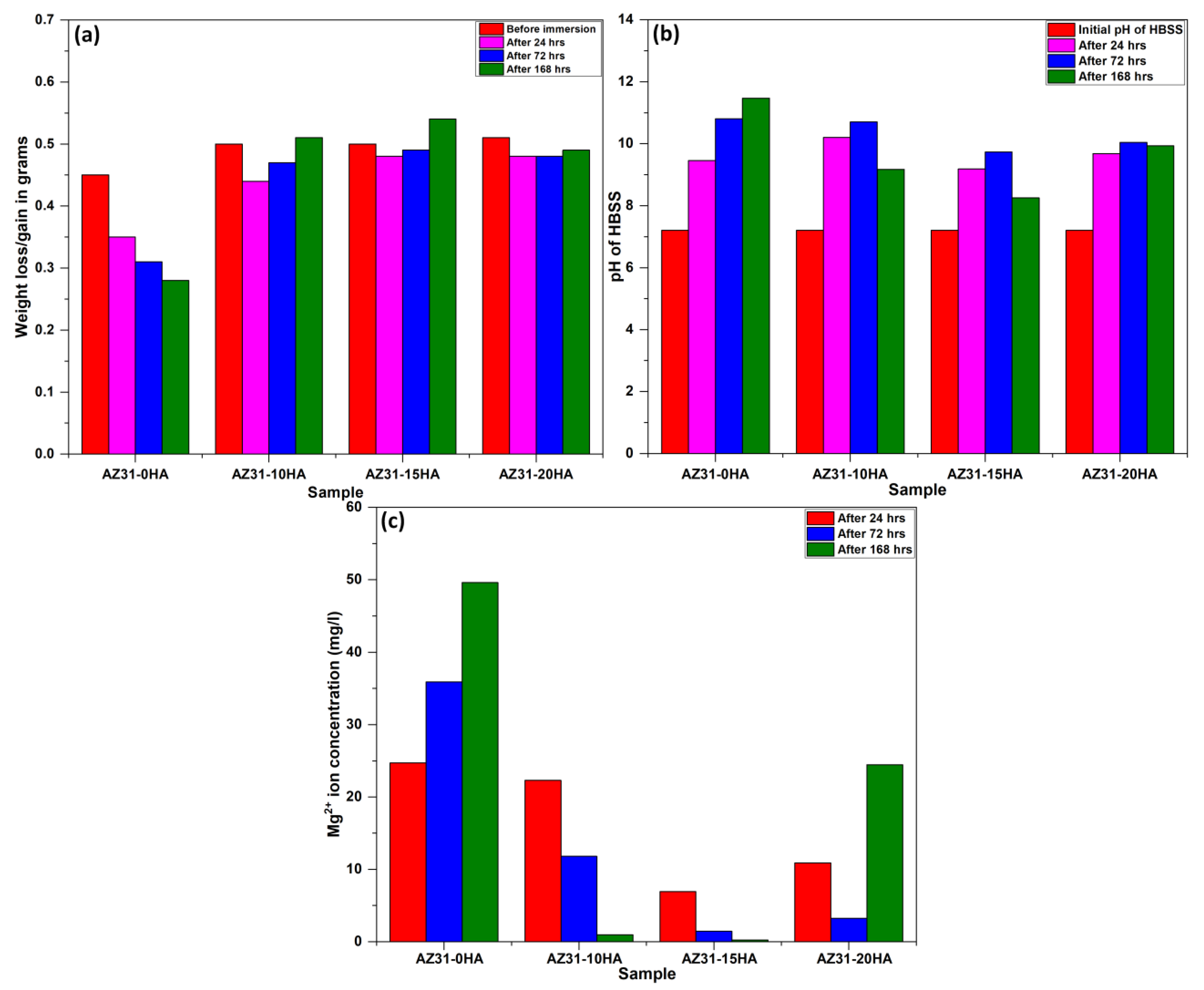
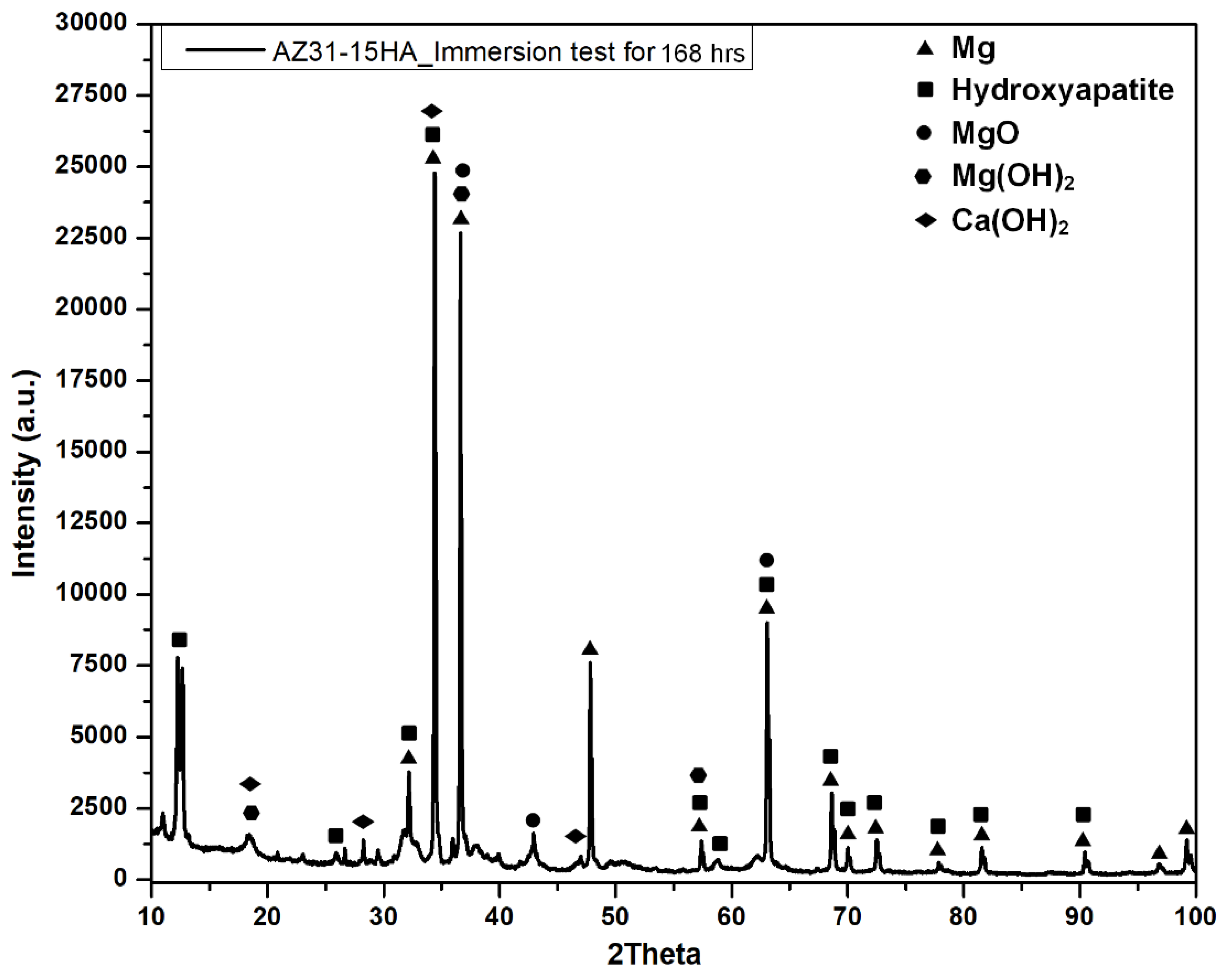
4.3. Biodegradation Behavior
5. Conclusions
- AZ31-0HA showed the highest relative density. It decreased with increased HA content. The addition of hard HA particles hindered the densification of the BMMCs as it was difficult to press during compaction and with a large difference in the sintering temperature of AZ31 metal alloy and HA.
- The microhardness of the BMMCs increased with increasing HA content owing to the presence of HA hard phase in the composite. It was uniformly distributed in the composite volume that was observed in microstructures.
- The highest compressive strength and Young’s modulus were recorded with the AZ31-15HA. Further HA addition decreased the compressive strength and Young’s modulus. It is attributed to a decrease in the density and agglomeration of HA particles.
- The AZ31-15HA exhibited the least weight loss for 24, 72 and 168 h immersion tests, and lower pH change of HBSS and discharge of Mg2+ ions after immersion tests in HBSS.
- The corrosion resistance of AZ31-15HA is the best among the four compositions. It exhibited weight gain after 72 h, resulting in a negative (−3.25 mm/year) corrosion rate. A negative corrosion rate indicates passive corrosion because of the formation of protective layers of Mg(OH)2 and Ca(OH)2 layers at the surface. It was also observed in electrochemical impedance spectroscopy (EIS) results and these results will be reported in a future study.
- The developed biodegradable composites in this study can be considered for human body fixation aids as they show favorable properties for orthopedic application. The BMMCs in this study did not show any toxicity during the in vitro cytotoxicity test.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, F.; Iotti, S.; Frassineti, C. Magnesium is a key regulator of the balance between osteoclast and osteoblast differentiation in the presence of vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef]
- Chan, C.Y.; Subramaniam, S.; Mohamed, N.; Ima-Nirwana, S.; Muhammad, N.; Fairus, A.; Ng, P.Y.; Jamil, N.A.; Aziz, N.A.; Chin, K.Y. Determinants of bone health status in a multi-ethnic population in Klang Valley, Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 384. [Google Scholar] [CrossRef] [PubMed]
- Mendia, S.; Luis, E.; Sahebkar, A.; Moran, M.R.; Romero, F.G. A systematic review and meta-analysis of randomized controlled trials on the effects of magnesium supplementation on insulin sensitivity and glucose control. Pharm. Res. 2016, 111, 272–282. [Google Scholar] [CrossRef]
- Christian, D.; Cardot, J.M.; Joanny, F.; Trzeciakiewicz, A.; Martin, E.; Pickering, G.; Dubray, C. An advanced formulation of a magnesium dietary supplement adapted for a long-term use supplementation improves magnesium bioavailability: In vitro and clinical comparative studies. Bio. Trace. Elem. Res. 2018, 186, 1–8. [Google Scholar]
- Rachel, N.; Howard, J.M.; Henein, M.Y. Cardiovascular calcification and bone: A comparison of the effects of dietary and serum calcium, phosphorous, magnesium and vitamin D. Int. Cardio. Forum J. 2014, 1, 209–218. [Google Scholar]
- Senthilkumar, A.; Gupta, M. Current and Emerging Bioresorbable Metallic Scaffolds: An Insight into Their Development, Processing and Characterisation. J. Indian Inst. Sci. 2022, 102, 585–598. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, D.; Jain, V.; Sharma, A.K. Microwave processing of materials and applications in manufacturing industries: A review. Mater. Manufac. Process. 2015, 30, 1–29. [Google Scholar] [CrossRef]
- Fang, Y.; Agrawal, D.K.; Roy, D.M.; Roy, R. Fabrication of porous hydroxyapatite ceramics by microwave processing. J. Mater. Res. 1992, 7, 490–494. [Google Scholar] [CrossRef]
- Asl, S.K.F.; Nemeth, S.; Tan, M.J. Improved corrosion protection of Magnesium by hydrothermally deposited biodegradable calcium phosphate coating. Mater. Chem. Phys. 2015, 161, 185–193. [Google Scholar]
- Zhang, M.; Cai, S.; Shen, S.; Xu, G.; Li, Y.; Ling, R.; Wu, X. In-situ defect repairing in hydroxyapatite/phytic acid hybrid coatings on AZ31 magnesium alloy by hydrothermal treatment. J. Alloy. Comp. 2016, 658, 649–656. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Li, Y.; Zhao, J.; Qin, L.; Lai, Y. Corrosion and biocompatibility improvement of magnesium-based alloys as bone implant materials: A review. Reg. Biomater. 2017, 4, 129–137. [Google Scholar] [CrossRef]
- Khalajabada, S.Z.; Ahmad, N.; Izman, S.; Abu, A.B.H.; Haider, W.; Kadir, M.R.A. In vitro biodegradation, electrochemical corrosion evaluations and mechanical properties of an Mg/HA/TiO2 nanocomposite for biomedical applications. J. Alloy. Compd. 2017, 696, 768–781. [Google Scholar] [CrossRef]
- Khalajabada, S.Z.; Kadir, M.R.A.; Izman, S.; Bakhsheshi, H.R.; Farahany, S. Effect of mechanical alloying on the phase evolution, microstructure and bio-corrosion properties of a Mg/HA/TiO2/MgO nanocomposite. Ceram. Int. 2014, 40, 16743–16759. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Kadir, M.R.A.; Izman, S.; Marvibaigi, M. The effect of MgO on the biodegradation, physical properties and biocompatibility of a Mg/HA/MgO nanocomposite manufactured by powder metallurgy method. J. Alloy. Comp. 2016, 655, 266–280. [Google Scholar] [CrossRef]
- Onoki, T.; Yamamoto, S.; Onodera, H.; Nakahira, A. New technique for bonding hydroxyapatite ceramics and magnesium alloy by hydrothermal hot-pressing method. Mater. Sci. Eng. C 2011, 31, 499–502. [Google Scholar] [CrossRef]
- Nazirah, R.; Zuhailawati, H.; Hazwani, M.R.S.N.; Abdullah, T.; Azzura, I.; Dhindaw, B. The Influence of Hydroxyapatite and Alumina Particles on the Mechanical Properties and Corrosion Behavior of Mg-Zn Hybrid Composites for Implants. Materials 2021, 14, 6246. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Liu, L. Corrosion properties of com- posite materials HA (beta-TCP)/Mg-Zn-Ca. Rare Metal Mater. Eng. 2014, 43, 205–209. [Google Scholar]
- Liu, D.; Zuo, Y.; Meng, W.; Chen, M.; Fan, Z. Fabrication of biodegradable nano-sized b-TCP/Mg composite by a novel melt shearing technology. Mater. Sci. Eng. C 2012, 32, 1253–1258. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Stormer, M.; Blawert, C.; Hort, N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef]
- Khanra, A.K.; Jung, H.C.; Yu, S.H.; Hong, K.S.; Shin, K.S. Microstructure and mechanical properties of Mg-HAP composites. Bull. Mater. Sci. 2010, 33, 43–47. [Google Scholar] [CrossRef]
- Ye, X.; Chen, M.; Yang, M.; Wei, J.; Liu, D. In vitro corrosion resistance and cytocompatibility of nano-hydroxyapatite reinforced Mg-Zn-Zr composites. J. Mater. Sci. Mater. Med. 2010, 21, 1321–1328. [Google Scholar] [CrossRef]
- Feng, A.; Han, Y. The microstructure, mechanical and corrosion properties of calcium polyphosphate reinforced ZK60A magnesium alloy composites. J. Alloy. Compd. 2010, 504, 585–593. [Google Scholar] [CrossRef]
- Del Campo, R.; Savoini, B.; Munoz, A.; Monge, M.A.; Pareja, R. Processing and mechanical characteristics of magnesium-hydroxyapatite metal matrix biocomposites. J. Mech. Behav. Biomed. Mater. 2017, 69, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sunil, B.R.; Kumar, T.S.S.; Chakkingal, U.; Nandakumar, V.; Doble, M.; Prasad, V.D.; Raghunath, M. In vitro and in vivo studies of biodegradable fine grained AZ31 magnesium alloy produced by equal channel angular pressing. Mater. Sci. Eng. C 2016, 59, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, A.K. Sintering of biomaterials for arthroplasty: A comparative study of microwave and conventional sintering techniques. Appl. Mech. Mater.-Tran. Technol. Pub. 2019, 895, 83–89. [Google Scholar] [CrossRef]
- Jaiswal, S.; Kumar, R.M.; Gupta, P.; Kumaraswamy, M.; Roy, P.; Lahiri, D. Mechanical, corrosion and biocompatibility behaviour of Mg-3Zn-HA biodegradable composites for orthopaedic fixture accessories. J. Mech. Behav. Biomed. Mater. 2018, 78, 442–454. [Google Scholar] [CrossRef]
- Khalil, K.A.; Almajid, A.A. Effect of high-frequency induction heat sintering conditions on the microstructure and mechanical properties of nanostructured magnesium/hydroxyapatite nanocomposites. Mater. Des. 2012, 36, 58–68. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, W.; Zheng, Y.; Dong, L.; Xi, Y.; Chai, D. Microstructure, mechanical property, bio-corrosion and cytotoxicity evaluations of Mg/HA composites. Mater. Sci. Eng. C 2010, 30, 827–832. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.H.; Meratian, M. Microstructure, mechanical properties and bio-corrosion evaluation of biodegradable AZ91-FA nanocomposites for biomedical applications. Mat. Sci. Eng. 2010, A527, 6938–6944. [Google Scholar] [CrossRef]
- Sunil, B.R.; Kumar, T.S.S.; Chakkingal, U.; Nandakumar, V.; Doble, M. Nano-hydroxyapatite reinforced AZ31 magnesium alloy by friction stir processing: A solid state processing for biodegradable metal matrix composites. J. Mat. Sci Mat. Med. 2014, 25, 975–988. [Google Scholar] [CrossRef]
- Ho, Y.H.; Man, K.; Joshi, S.S.; Pantawane, M.V.; Wu, T.C.; Yang, Y.; Dahotre, N.B. In-vitro biomineralization and biocompatibility of friction stir additively manufactured AZ31B magnesium alloy-hydroxyapatite composites. Bio. Mater. 2020, 5, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Shamami, D.Z.; Rabiee, S.M.; Shakeri, M. Characterization of magnesium-hydroxyapatite functionally graded composites prepared by rapid microwave sintering technique. Cer. Int. 2022, 48, 12641–12653. [Google Scholar] [CrossRef]
- Parkhomei, O.R.; Pinchuk, N.D.; Sych, O.E.; Tomila, T.V.; Tovstonog, G.B.; Gorban, V.F.; Yevych, Y.I.; Kuda, O.A. Structural and mechanical properties of bioactive glass–ceramic composites. Powder Metall. Met. Cer. 2016, 55, 172–184. [Google Scholar] [CrossRef]
- Xiong, G.; Nie, Y.; Ji, D.; Li, J.; Li, C.; Li, W.; Zhu, Y.; Luo, H.; Wan, Y. Characterization of biomedical hydroxyapatite/magnesium composites prepared by powder metallurgy assisted with microwave sintering. Curr. Appl. Phy. 2016, 16, 830–836. [Google Scholar] [CrossRef]
- Acheson, J.G.; McKillop, S.; Lemoine, P.; Boyd, A.R.; Meenan, B.J. Control of magnesium alloy corrosion by bioactive calcium phosphate coating: Implications for resorbable orthopaedic implants. Materialia 2019, 6, 100291. [Google Scholar] [CrossRef]
- Hing, K.A. Bone repair in the twenty–first century: Biology, chemistry or engineering? Phys. Eng. Sci. 2004, 362, 2821–2850. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Shamami, D.Z.; Rabiee, S.M.; Shakeri, M. Use of rapid microwave sintering technique for the processing of magnesium-hydroxyapatite composites. Ceram. Int. 2021, 47, 13023–13034. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; Carvalho, S.M.; Silva, A.R.; Ciminelli, V.S.; Mansur, H.S. Niobium-Doped Hydroxyapatite Bioceramics: Synthesis, Characterization and In Vitro Cytocompatibility. Materials 2015, 8, 4191–4209. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, S. Microwave processing of biomaterials for orthopedic implants: Challenges and possibilities. JOM 2020, 72, 1211–1228. [Google Scholar] [CrossRef]
- Hansen, A.W.; Fuhr, L.T.; Antonini, L.M.; Villarinho, D.J.; Marino, C.E.B.; Malfatti, C.D.F. The electrochemical behavior of the NiTi alloy in different simulated body fluids. Mater. Res. 2015, 18, 184–190. [Google Scholar] [CrossRef]
- Del Campo, R.; Savoini, B.; Munoz, A.; Monge, M.A.; Garcés, G. Mechanical properties and corrosion behavior of Mg–HAP composites. J. Mech. Behav. Biomed. Mater. 2014, 39, 238–246. [Google Scholar] [CrossRef]
- Rodzi, S.N.H.M.; Zuhailawati, H.; Dhindaw, B.K. Mechanical and degradation behaviour of biodegradable magnesium-zinc/hydroxyapatite composite with different powder mixing techniques. J. Magnes. Alloy. 2019, 7, 566–576. [Google Scholar] [CrossRef]
- Tang, H.; Han, Y.; Wu, T.; Tao, W.; Jian, X.; Wu, Y.; Xu, F. Synthesis and properties of hydroxyapatite-containing coating on AZ31 magnesium alloy by micro-arc oxidation. Appl. Surf. Sci. 2017, 400, 391–404. [Google Scholar] [CrossRef]
- Morcillo, M.; Veleva, L. Degradation of AZ31 and AZ91 magnesium alloys in different physiological media: Effect of surface layer stability on electrochemical behaviour. J. Magnes. Alloy. 2020, 8, 667–675. [Google Scholar] [CrossRef]
- Hing, K.A. Bioceramic bone graft substitutes: Influence of porosity and chemistry. Int. J. Appl. Ceram. Technol. 2005, 2, 184–199. [Google Scholar] [CrossRef]
| Main Elements | Magnesium | Aluminum | Zinc | Manganese | Silicon |
|---|---|---|---|---|---|
| Weight percent (wt.%) | 95–97 | 2.5–3.5 | 0.6–1.4 | 0.2 | 0.1 |
| Parameter | Value |
|---|---|
| Frequency | 2.45 GHz |
| Microwave power | 330–650 W |
| Crucible material | Alumina |
| Insulation material | Alumina wool |
| Sample compositions | AZ31-0HA, AZ31-10HA, AZ31-15HA and AZ31-20HA |
| Sintering holding time | 10 min |
| Sintering temperature | 500 °C |
| Processing condition | Forming gas environment (95% nitrogen + 5% hydrogen) |
| Solution | Ionic Concentration (mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | HPO42− | SO42− | |
| Blood plasma | 142.0 | 5.0 | 2.5 | 1.5 | 103.0 | 27 | 1.0 | 0.5 |
| HBSS | 141.6 | 5.81 | 1.26 | 0.81 | 144.8 | 4.09 | 0.78 | 0.81 |
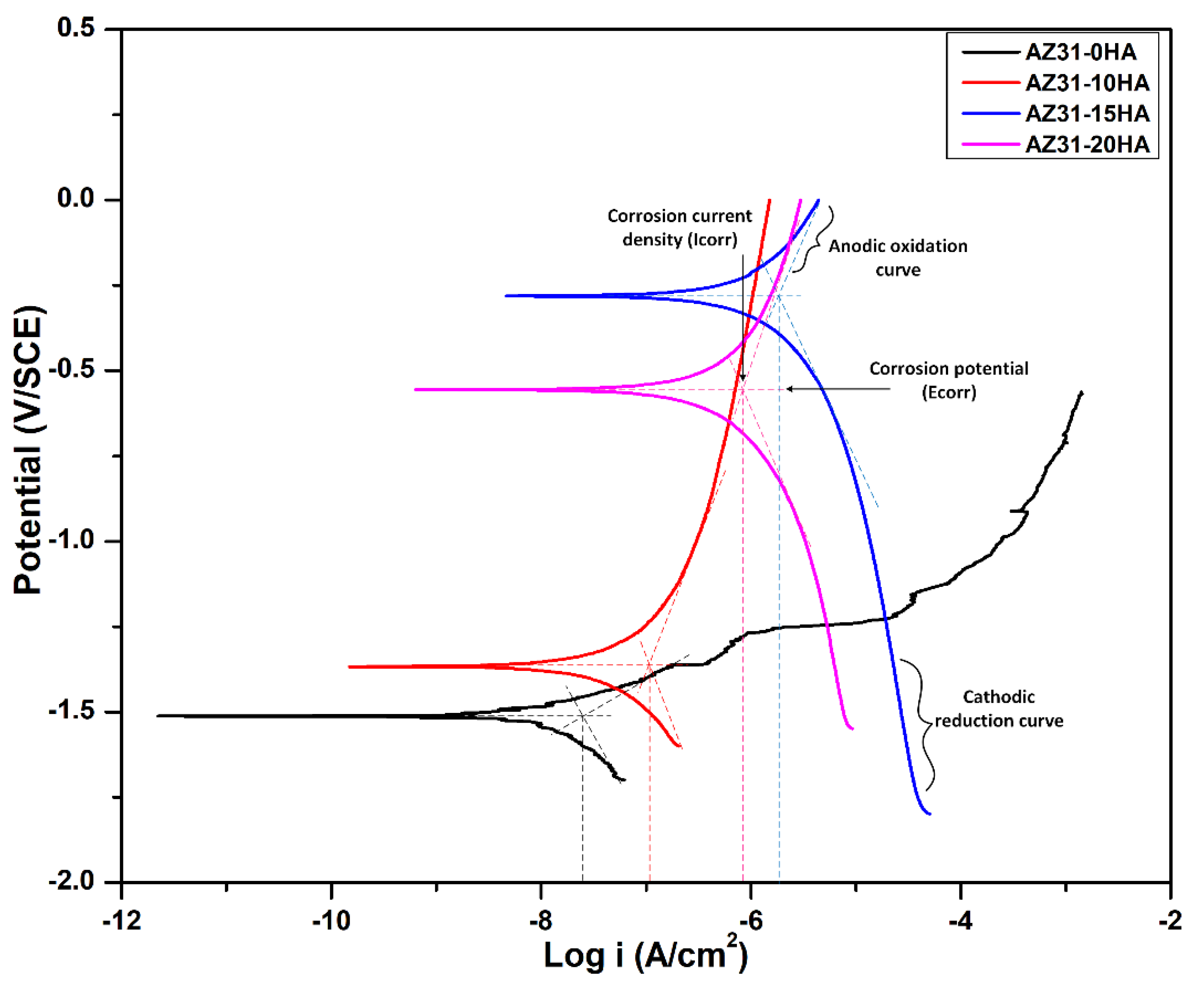
| Samples | Ecorr (V) | Icorr (A/cm2) | Corrosion Rate after 168 h Immersion Test (mm/Year) |
|---|---|---|---|
| AZ31-0HA | −1.51 | 2.9 × 10−8 | 8.48 |
| AZ31-10HA | −1.38 | 1.3 × 10−7 | (−)0.89 |
| AZ31-15HA | −0.28 | 1.4 × 10−6 | (−)3.25 |
| AZ31-20HA | −0.55 | 1.6 × 10−6 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Sharma, A.K.; Agrawal, D.; Lanagan, M.T.; Sikora, E.; Singh, I. Characterization of AZ31/HA Biodegradable Metal Matrix Composites Manufactured by Rapid Microwave Sintering. Materials 2023, 16, 1905. https://doi.org/10.3390/ma16051905
Gupta S, Sharma AK, Agrawal D, Lanagan MT, Sikora E, Singh I. Characterization of AZ31/HA Biodegradable Metal Matrix Composites Manufactured by Rapid Microwave Sintering. Materials. 2023; 16(5):1905. https://doi.org/10.3390/ma16051905
Chicago/Turabian StyleGupta, Shivani, Apurbba Kumar Sharma, Dinesh Agrawal, Michael T. Lanagan, Elzbieta Sikora, and Inderdeep Singh. 2023. "Characterization of AZ31/HA Biodegradable Metal Matrix Composites Manufactured by Rapid Microwave Sintering" Materials 16, no. 5: 1905. https://doi.org/10.3390/ma16051905
APA StyleGupta, S., Sharma, A. K., Agrawal, D., Lanagan, M. T., Sikora, E., & Singh, I. (2023). Characterization of AZ31/HA Biodegradable Metal Matrix Composites Manufactured by Rapid Microwave Sintering. Materials, 16(5), 1905. https://doi.org/10.3390/ma16051905






