Platinum-Functionalized Graphene Oxide: One-Pot Synthesis and Application as an Electrocatalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
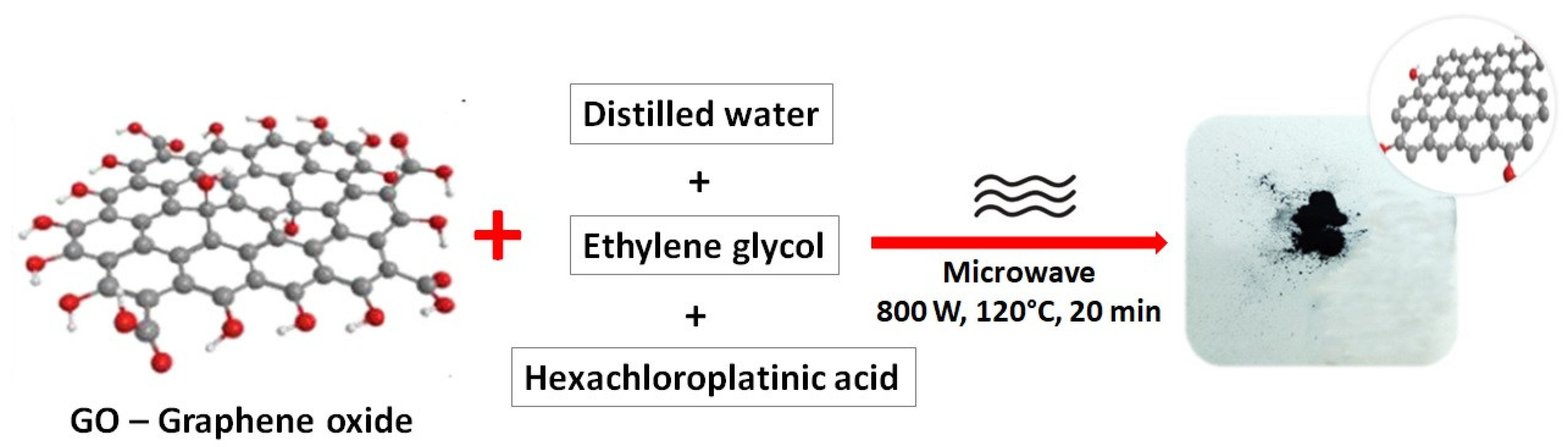
2.2. Synthesis
2.3. Instrumentation
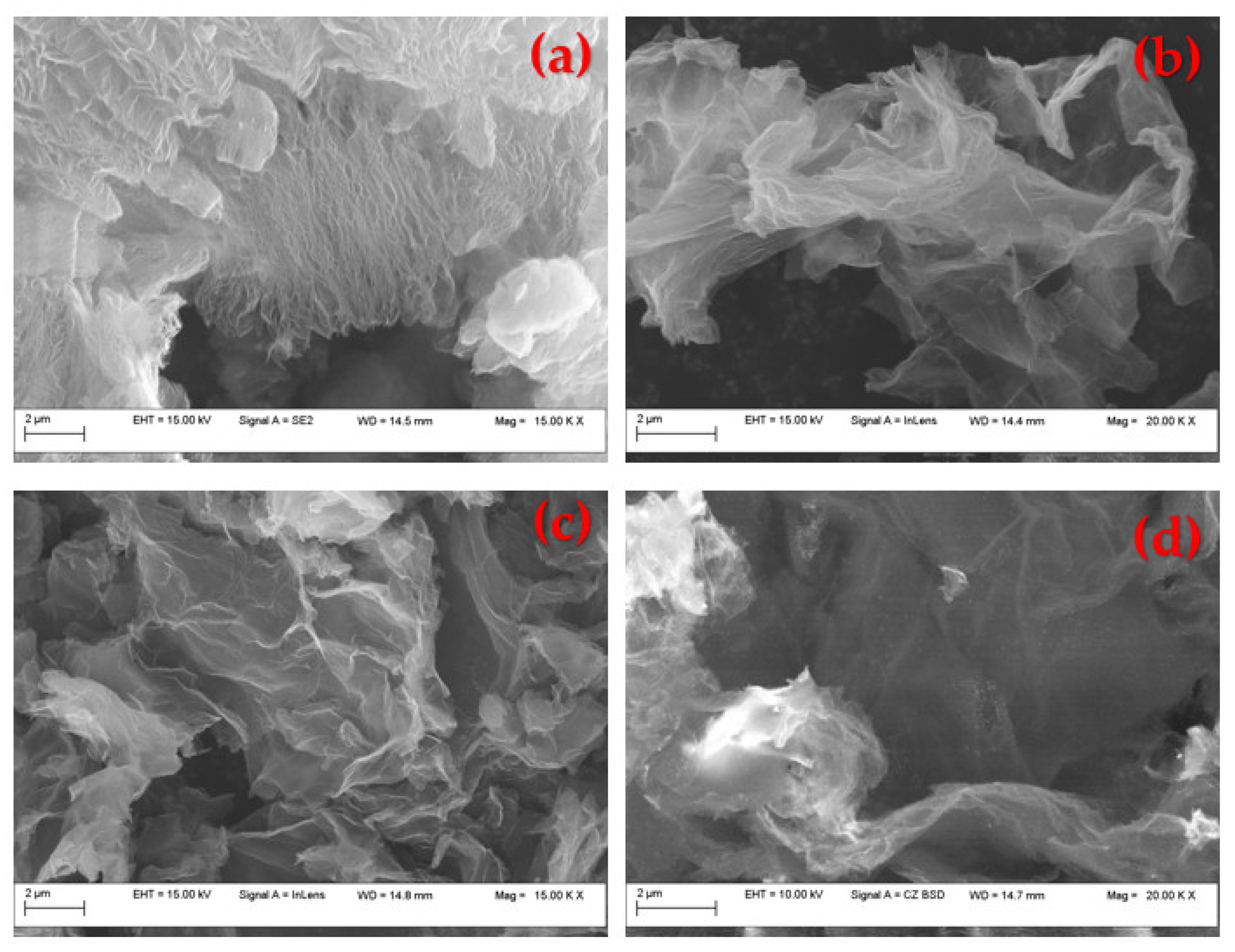
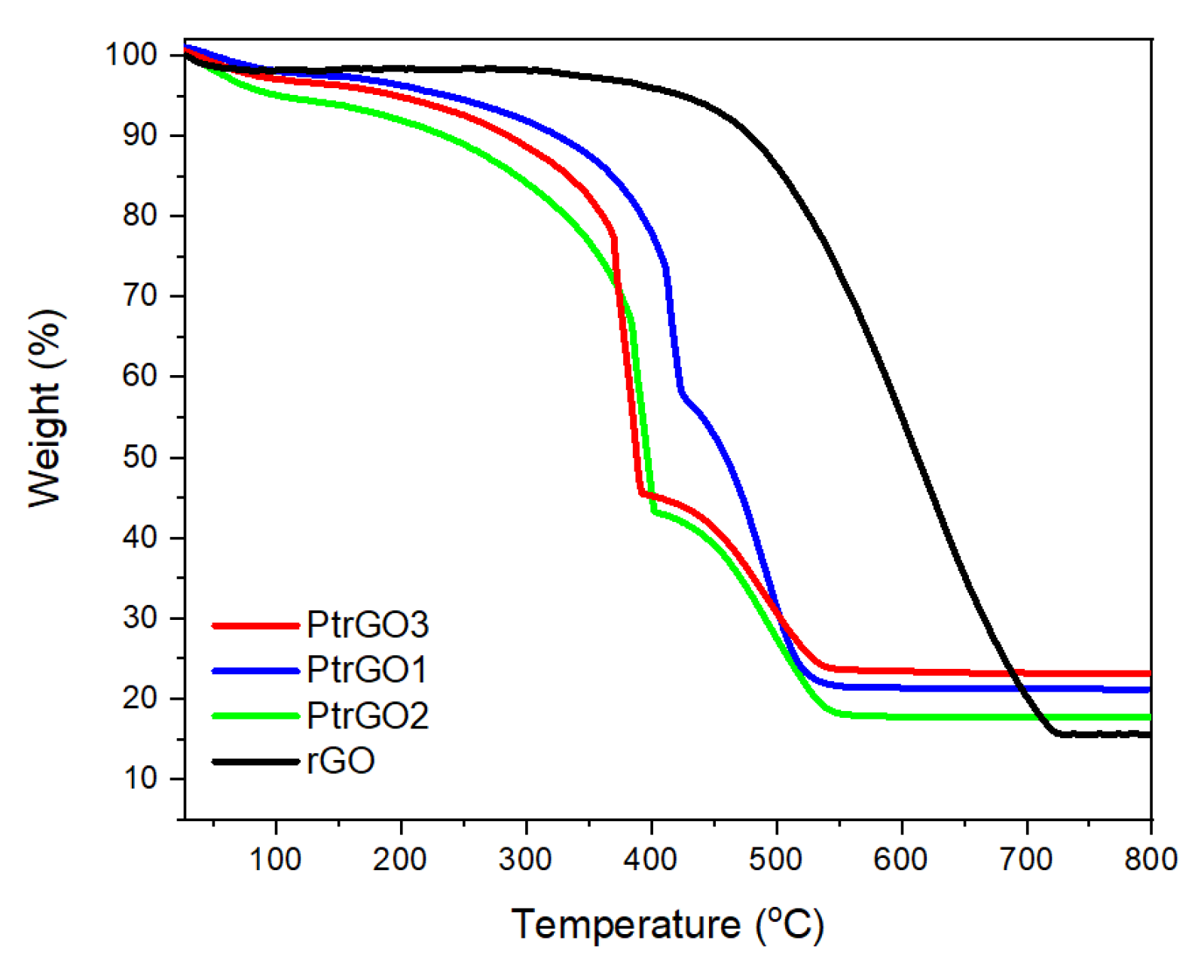
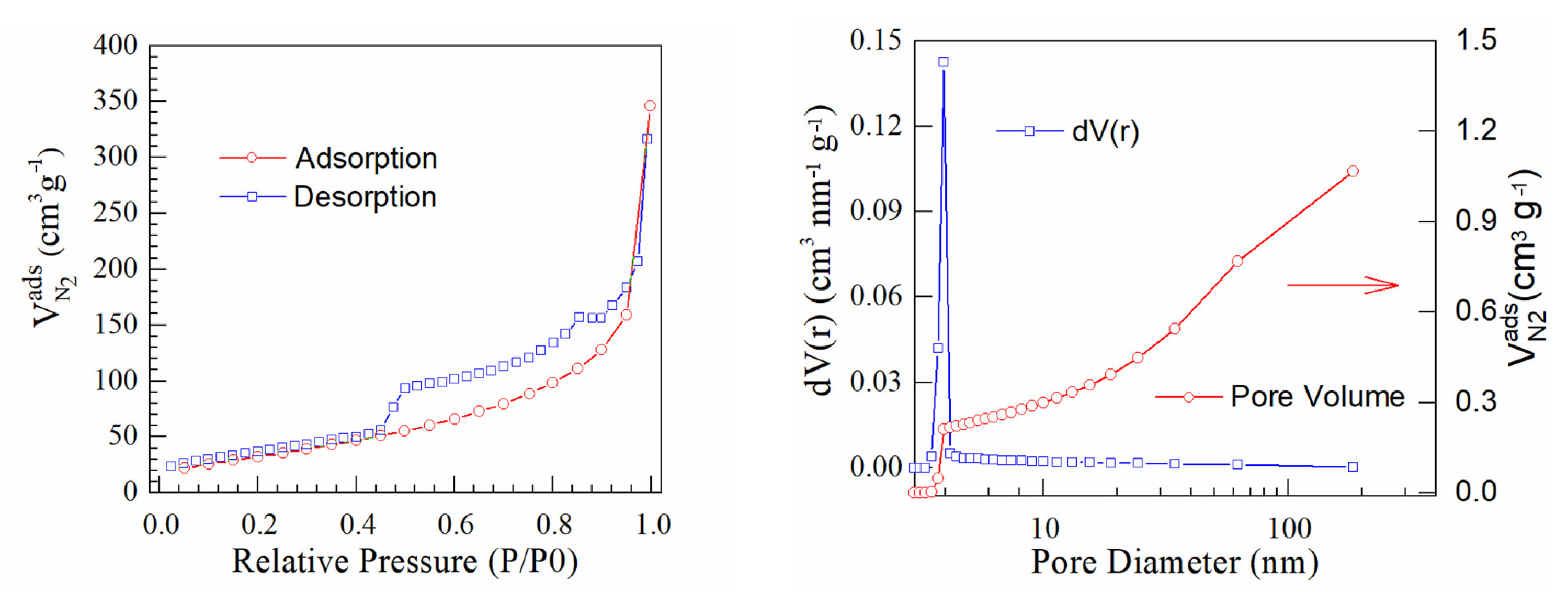
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cele, N.P.; Sinha-Ray, S.; Munda, J.; Jimoh, A.A. The state of the art proton exchange membrane fuel cells for clean energy. In 2010 IEEE International Energy Conference; IEEE: Piscataway, NJ, USA, 2010; pp. 865–869. [Google Scholar]
- Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Electrochemical behavior of the carbon black Vulcan XC-72R: Influence of the surface chemistry. Int. J. Hydrog. Energy 2018, 43, 7911–7922. [Google Scholar] [CrossRef]
- Mark, K. Debe, Electrocatalyst approaches and chanllenges for automotive fuel cell. Nature 2012, 486, 43. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanism for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Osman, S.H.; Kamarudin, S.K.; Karim, N.A.; Basri, S. Application of graphene in low-temperature fuel cell technology: An overview. Int. J. Energy Res. 2021, 45, 18318–18336. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene-based nanoarchitectures. Anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J. Phys. Chem. Lett. 2010, 1, 520–527. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.E.; Zhang, Y.; Dubonos, S.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Ovid’Ko, I.A. Mechanical properties of graphene. Rev. Adv. Mater. Sci. 2013, 34, 1–11. [Google Scholar]
- Woodford, C. Graphene—A Simple Introduction. Explain that Stuff. 2021. Available online: https://www.explainthatstuff.com/graphene.html (accessed on 5 July 2022).
- Gosling, J.H.; Makarovsky, O.; Wang, F.; Cottam, N.D.; Greenaway, M.T.; Patanè, A.; Fromhold, T.M. Universal mobility characteristics of graphene originating from charge scattering by ionised impurities. Commun. Phys. 2021, 4, 30. [Google Scholar] [CrossRef]
- Tani, H.; Izutani, Y.; Lu, R.; Koganezawa, S.; Tagawa, N. Electric field effect on friction coefficient of diamond-like carbon film heated by laser irradiation. Tribol. Lett. 2021, 69, 103. [Google Scholar] [CrossRef]
- Rao, C.E.E.; Sood, A.E.; Subrahmanyam, K.E.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Bong, S.; Kim, Y.R.; Kim, I.; Woo, S.; Uhm, S.; Lee, J.; Kim, H. Graphene supported electrocatalysts for methanol oxidation. Electrochem. Commun. 2010, 12, 129–131. [Google Scholar] [CrossRef]
- Tiliakos, A.; Trefilov, A.M.; Tanasă, E.; Balan, A.; Stamatin, I. Laser-induced graphene as the microporous layer in proton exchange membrane fuel cells. Appl. Surf. Sci. 2020, 504, 144096. [Google Scholar] [CrossRef]
- Tiliakos, A.; Trefilov, A.M.; Tanasă, E.; Balan, A.; Stamatin, I. Space-Filling Supercapacitor Carpets: Highly scalable fractal architecture for energy storage. J. Power Sources 2018, 384, 145–155. [Google Scholar] [CrossRef]
- Dong, L.; Gari, R.R.S.; Li, Z.; Craig, M.M.; Hou, S. Graphene-supported platinum and platinum–ruthenium nanoparticles with high electrocatalytic activity for methanol and ethanol oxidation. Carbon 2010, 48, 781–787. [Google Scholar] [CrossRef]
- Yoo, B.M.; Shin, J.E.; Lee, H.D.; Park, H.B. Graphene and graphene oxide membranes for gas separation applications. Curr. Opin. Chem. Eng. 2017, 16, 39–47. [Google Scholar] [CrossRef]
- Mendoza, J.J.; Ledezma, R.; Gallardo, C.A.; Elias, A.; Elizalde, L.E. Covalent surface functionalization of carbon nanostructures via [2+1] cycloaddition microwave-assisted reactions. J. Mater. Sci. 2021, 56, 13524–13539. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Yap, P.L.; Kabiri, S.; Auyoong, Y.L.; Tran, D.N.; Losic, D. Tuning the multifunctional surface chemistry of reduced graphene oxide via combined elemental doping and chemical modifications. ACS Omega 2019, 4, 19787–19798. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yung, T.Y.; Liu, L.K. The microwave-assisted ionic liquid nanocomposite synthesis: Platinum nanoparticles on graphene and the application on hydrogenation of styrene. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Xue, Q.; Li, S.; Chen, Y.; Lee, J.M. Reduced graphene oxide supported platinum nanocubes composites: One-pot hydrothermal synthesis and enhanced catalytic activity. Nanotechnology 2015, 26, 065603. [Google Scholar] [CrossRef]
- Nie, R.; Wang, J.; Wang, L.; Qin, Y.; Chen, P.; Hou, Z. Platinum supported on reduced graphene oxide as a catalyst for hydrogenation of nitroarenes. Carbon 2012, 50, 586–596. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Sahoo, S.; Satpati, A.K.; Bahadur, D. Solvothermal synthesis of reduced graphene oxide/Au nanocomposite-modified electrode for the determination of inorganic mercury and electrochemical oxidation of toxic phenolic compounds. Electrochim. Acta 2015, 180, 1023–1032. [Google Scholar] [CrossRef]
- Marinoiu, A.; Carcadea, E.; Sacca, A.; Carbone, A.; Sisu, C.; Dogaru, A.; Raceanu, M.; Varlam, M. One-step synthesis of graphene supported platinum nanoparticles as electrocatalyst for PEM fuel cells. Int. J. Hydrog. Energy 2021, 46, 12242–12253. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Modafferi, V.; Santangelo, S.; Fiore, M.; Fazio, E.; Triolo, C.; Patanè, S.; Ruffo, R.; Musolino, M.G. Transition metal oxides on reduced graphene oxide nanocomposites: Evaluation of physicochemical properties. J. Nanomater. 2019, 2019, 1703218. [Google Scholar] [CrossRef]
- Marinoiu, A.; Raceanu, M.; Carcadea, E.; Andrulevicius, M.; Tamuleviciene, A.; Tamulevicius, T.; Capris, C.; Varlam, M. Efficient method to obtain Platinum–Cobalt supported on graphene oxide and electrocatalyst development. Int. J. Hydrog. Energy 2020, 45, 26226–26237. [Google Scholar] [CrossRef]
- Marinoiu, A.; Raceanu, M.; Carcadea, E.; Varlam, M.; Stefanescu, I. Iodinated carbon materials for oxygen reduction reaction in proton exchange membrane fuel cell. Scalable synthesis and electrochemical performances. Arab. J. Chem. 2019, 12, 868–880. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Wan, C.; Zhao, Z.; Xu, X.; Lin, Z.; Wang, Y.; Liu, H.; Zang, K.; Luo, J.; et al. Microwave-assisted rapid synthesis of graphene-supported single atomic metals. Adv. Mater. 2018, 30, 1802146. [Google Scholar] [CrossRef]
- Oubraham, A.; Ion-Ebrasu, D.; Vasut, F.; Soare, A.; Sorlei, I.-S.; Marinoiu, A. pH-Dependence and Electrocatalytic Activity of Platinum-Functionalized Reduced Graphene Oxide Synthesized By Microwave Methods. In Proceedings of the 5th International Conference Emerging Technologies in Materials Engineering, Bucharest, Romania, 27–28 October 2022. [Google Scholar]
- Yilbas, B.S.; Ibrahim, A.; Ali, H.; Khaled, M.; Laoui, T. Hydrophobic and optical characteristics of graphene and graphene oxide films transferred onto functionalized silica particles deposited glass surface. Appl. Surf. Sci. 2018, 442, 213–223. [Google Scholar] [CrossRef]
- Delgado, A.V.; Ahualli, S.; Fernández, M.M.; González, M.A.; Iglesias, G.R.; Vivo-Vilches, J.F.; Jiménez, M.L. Geometrical properties of materials for energy production by salinity exchange. Environ. Chem. 2017, 14, 279–287. [Google Scholar] [CrossRef]
- Baturina, O.A.; Aubuchon, S.R.; Wynne, K.J. Thermal Stability in Air of Pt/C Catalysts and PEM Fuel Cell Catalyst Layers. Chem. Mater. 2006, 18, 1498–1504. [Google Scholar] [CrossRef]
- Davey, W.P. Precision measurements of the lattice constants of twelve common metals. Phys. Rev. 1925, 25, 753. [Google Scholar] [CrossRef]
- Sun, L.; Luo, Y.; Li, M.; Hu, G.; Xu, Y.; Tang, T.; Wen, J.; Li, X.; Wang, L. Role of Pyridinic-N for Nitrogen-doped graphene quantum dots in oxygen reaction reduction. J. Colloid Interface Sci. 2017, 508, 154–158. [Google Scholar] [CrossRef]
- Fan, M.; Zhu, C.; Yang, J.; Sun, D. Facile self-assembly N-doped graphene quantum dots/graphene for oxygen reduction reaction. Electrochim. Acta 2016, 216, 102–109. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Dhanasekaran, P.; Chetty, R.; Bhat, S.D. Microwave assisted poly (3, 4-ethylenedioxythiophene)–reduced graphene oxide nanocomposite supported Pt as durable electrocatalyst for polymer electrolyte fuel cells. N. J. Chem. 2018, 42, 10724–10732. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, S.I.; Lee, M.S.; Bak, S.J.; Lee, D.H.; Kwon, S.H.; Kim, T. Effect of uniformity and surface morphology of Pt nanoparticles to enhance oxygen reduction reaction in polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2022, 47, 29456–29466. [Google Scholar] [CrossRef]
- Nair, A.S.; Jafri, R.I. A facile one-step microwave synthesis of Pt deposited on N & P co-doped graphene intercalated carbon black-An efficient cathode electrocatalyst for PEM fuel cell. Int. J. Hydrog. Energy 2023, 48, 3653–3664. [Google Scholar]
- Wang, H.; Wang, W.; Xu, Y.Y.; Dong, S.; Xiao, J.; Wang, F.; Liu, H.; Xia, B.Y. Hollow nitrogen-doped carbon spheres with Fe3O4 nanoparticles encapsulated as a highly active oxygen-reduction catalyst. ACS Appl. Mater. Interfaces 2017, 9, 10610–10617. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Shi Zhang Qiao, S.Z. Determination of Electron Transfer Number for Oxygen Reduction Reaction: From Theory to Experiment. ACS Appl. Mater. Interfaces 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Talukder, N.; Wang, Y.; Nunna, B.B.; Lee, E.S. An In-Depth Exploration of the Electrochemical Oxygen Reduction Reaction (ORR) Phenomenon on Carbon-Based Catalysts in Alkaline and Acidic Mediums. Catalysts 2022, 12, 791. [Google Scholar] [CrossRef]
- Ruiz-Camacho, B.; Palafox-Segoviano, J.A.; P_erez-Díaz, P.J.; Medina-Ramírez, A. Synthesis of supported Pt nanoparticles by sonication for ORR: Effect of the graphene oxide carbon composite. Int. J. Hydrog. Energy 2021, 46, 26027–26039. [Google Scholar] [CrossRef]

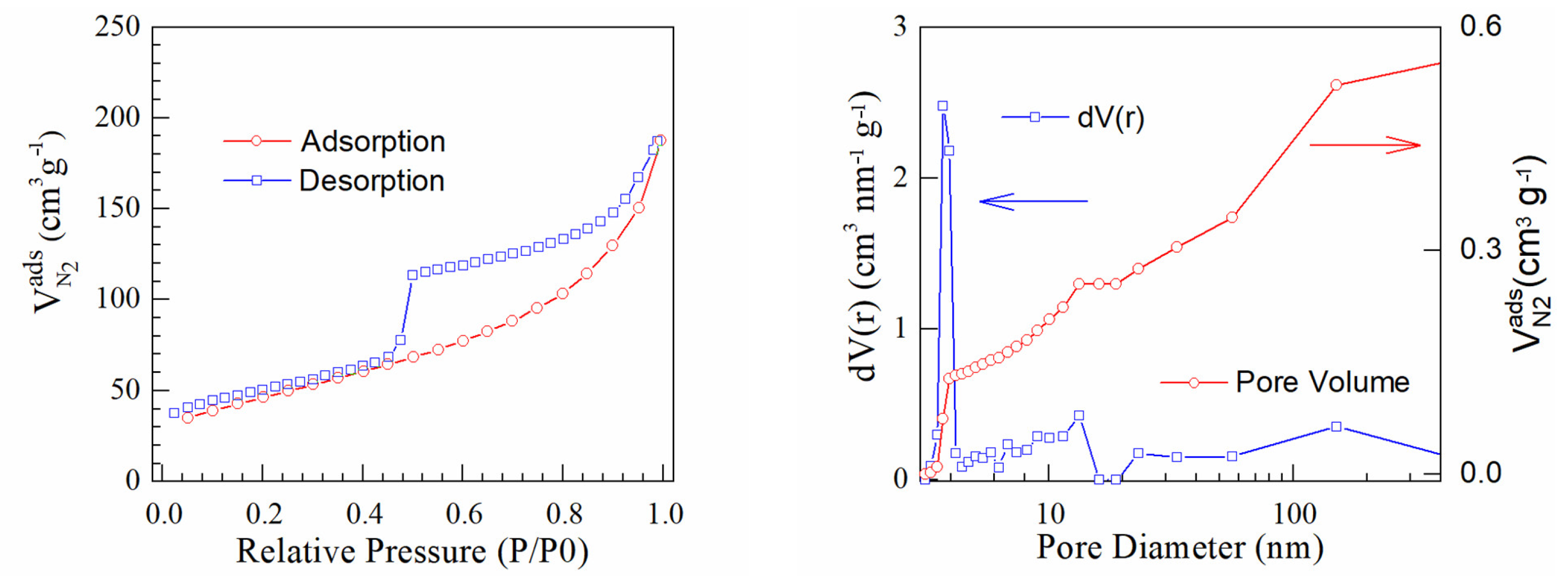



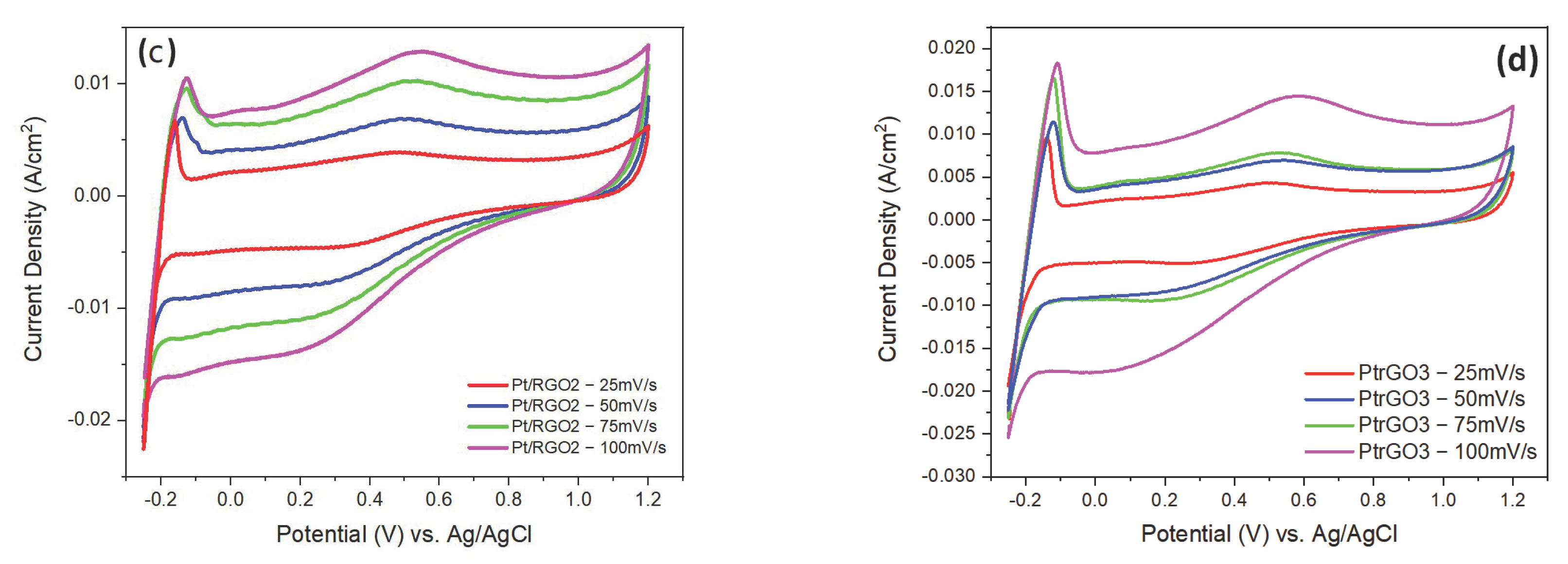
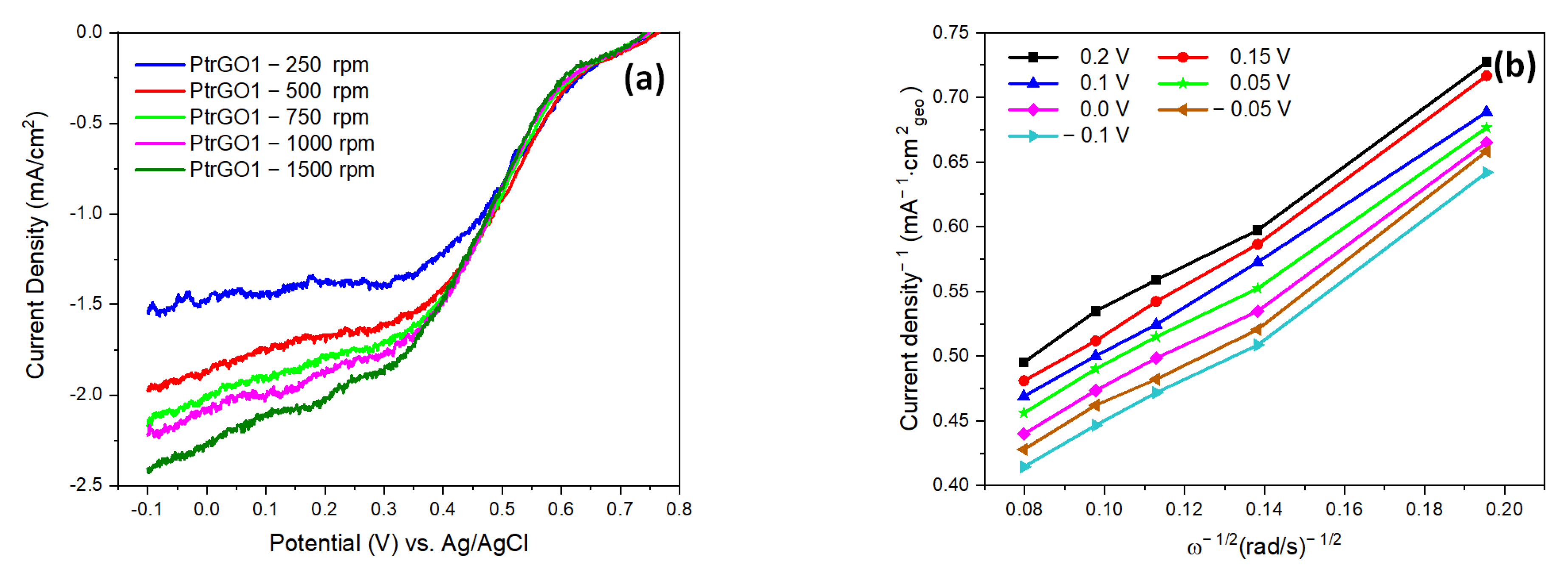
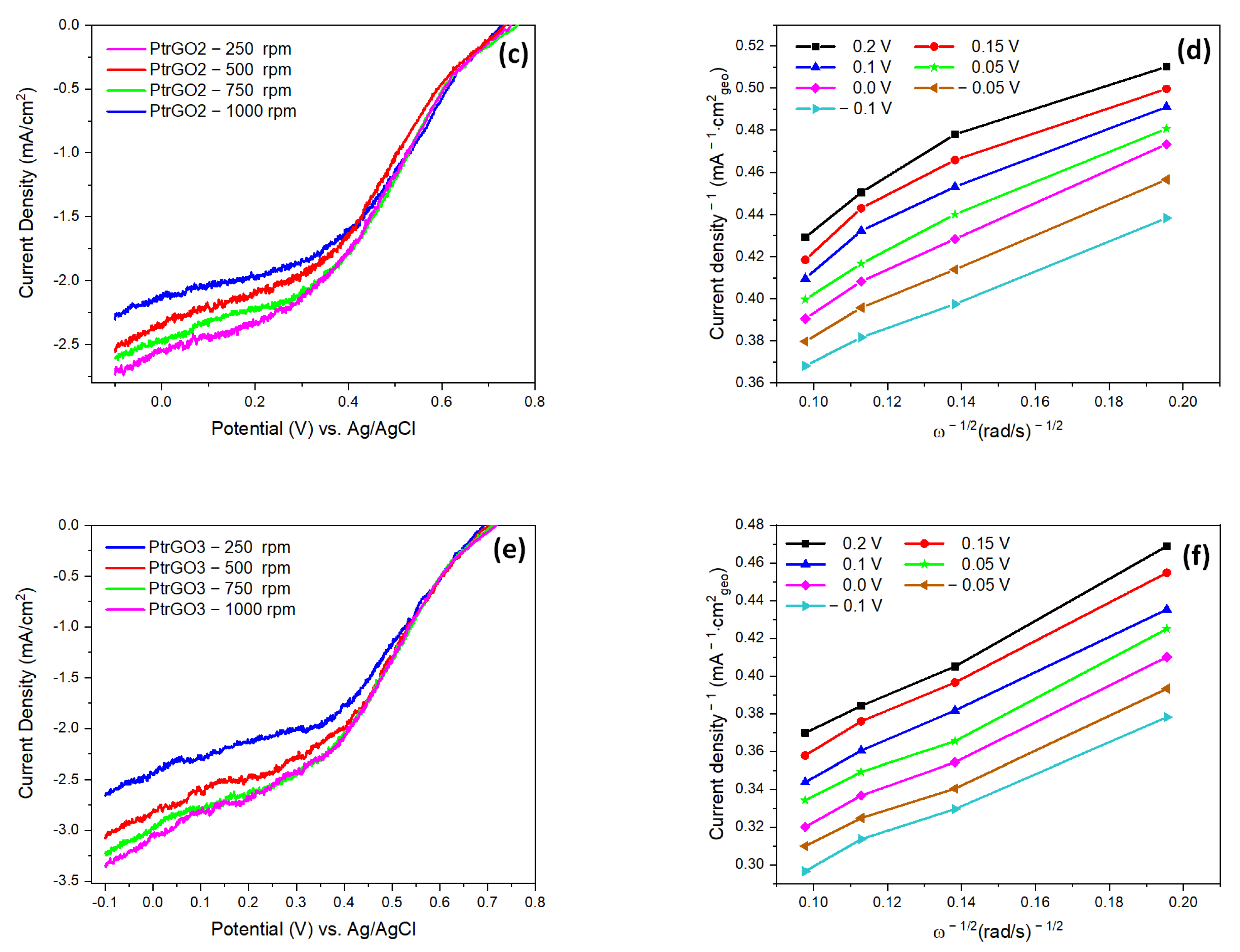
| Samples | pH | Pt (Wt.%) a by AAS | Pt (Wt.%) b by EDX |
|---|---|---|---|
| PtrGO1 | 3.3 | 4.1 | 4.3 |
| PtrGO2 | 11.9 | 2.0 | 2.2 |
| PtrGO3 | 7.2 | 6.5 | 5.7 |
| Element | Wt.% |
|---|---|
| C K | 73.2 |
| O K | 21.1 |
| Pt M | 5.7 |
| Totals | 100.0 |
| Samples | Specific Surface (m2/g) | Pore Volume (cc/g) | Pore Radius (Å) |
|---|---|---|---|
| PtrGO1 | 128 | 0.568 | 18.543 |
| PtrGO2 | 166 | 0.289 | 19.672 |
| PtrGO3 | 98 | 0.329 | 19.667 |
| a/a | 2ϑ (Deg.) | Miller (hkl) | d (Å) | FWHM (Deg.) | Size (Å) | Phase |
|---|---|---|---|---|---|---|
| 1 | 24.90 | 002 | 3.573 | 4.49 | 18.9 | rGO |
| 2 | 39.79 | 111 | 2.263 | 1.38 | 64.0 | Pt |
| 3 | 46.25 | 200 | 1.962 | 1.69 | 53.0 | Pt |
| 4 | 67.62 | 220 | 1.384 | 2.26 | 44.0 | Pt |
| 5 | 81.28 | 311 | 1.182 | 1.74 | 63.0 | Pt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oubraham, A.; Ion-Ebrasu, D.; Vasut, F.; Soare, A.; Sorlei, I.-S.; Marinoiu, A. Platinum-Functionalized Graphene Oxide: One-Pot Synthesis and Application as an Electrocatalyst. Materials 2023, 16, 1897. https://doi.org/10.3390/ma16051897
Oubraham A, Ion-Ebrasu D, Vasut F, Soare A, Sorlei I-S, Marinoiu A. Platinum-Functionalized Graphene Oxide: One-Pot Synthesis and Application as an Electrocatalyst. Materials. 2023; 16(5):1897. https://doi.org/10.3390/ma16051897
Chicago/Turabian StyleOubraham, Anisoara, Daniela Ion-Ebrasu, Felicia Vasut, Amalia Soare, Ioan-Sorin Sorlei, and Adriana Marinoiu. 2023. "Platinum-Functionalized Graphene Oxide: One-Pot Synthesis and Application as an Electrocatalyst" Materials 16, no. 5: 1897. https://doi.org/10.3390/ma16051897
APA StyleOubraham, A., Ion-Ebrasu, D., Vasut, F., Soare, A., Sorlei, I.-S., & Marinoiu, A. (2023). Platinum-Functionalized Graphene Oxide: One-Pot Synthesis and Application as an Electrocatalyst. Materials, 16(5), 1897. https://doi.org/10.3390/ma16051897








