Shortwave Ultraviolet Persistent Luminescence of Sr2MgSi2O7: Pr3+
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Li, Y.; Ding, W.; Xu, L.; Ma, Y.; Zhang, L. Recent Advances of Persistent Luminescence Nanoparticles in Bioapplications. Nano-Micro Lett. 2020, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Song, L.; Liu, N.; Shi, J.; Zhang, Y. Chromium-Doped Zinc Gallate Near-Infrared Persistent Luminescence Nanoparticles in Autofluorescence-Free Biosensing and Bioimaging: A Review. ACS Appl. Nano Mater. 2021, 4, 6497–6514. [Google Scholar] [CrossRef]
- Ding, D.; Li, S.; Xu, H.; Zhu, L.; Meng, S.; Liu, J.; Lin, Q.; Leung, S.W.; Sun, W.; Li, Y.; et al. X-ray-Activated Simultaneous Near-Infrared and Short-Wave Infrared Persistent Luminescence Imaging for Long-Term Tracking of Drug Delivery. ACS Appl. Mater. Interfaces 2021, 13, 16166–16172. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Wei, Y.; Wang, Y.; Zhang, J.; Li, H.; Ci, Z. Long Persistent Phosphor CdSiO3:Gd3+,Bi3+and Potential Photocatalytic Application of CdSiO3:Gd3+,Bi3+@TiO2in Dark. J. Am. Ceram. Soc. 2016, 99, 2368–2375. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Li, Z.-Y.; Zhang, J.-Y.; Lu, Y.; Guo, S.-Q.; Zhao, Q.; Wang, X.; Yong, Z.-J.; Li, H.; Ma, J.-P.; et al. X-ray-activated long persistent phosphors featuring strong UVC afterglow emissions. Light. Sci. Appl. 2018, 7, 88. [Google Scholar] [CrossRef]
- Cai, H.; Song, Z.; Liu, Q. Infrared-Photostimulable and long-persistent ultraviolet-emitting phosphor LiLuGeO4:Bi3+,Yb3+ for biophotonic applications. Mater. Chem. Front. 2021, 5, 1468–1476. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, J.; Zhao, Y.; Han, K.; Xia, Z. Multi-responsive deep-ultraviolet emission in praseodymium-doped phosphors for microbial sterilization. Sci. China Mater. 2022, 65, 1103–1111. [Google Scholar] [CrossRef]
- Xiong, P.; Peng, M. (INVITED) Recent advances in ultraviolet persistent phosphors. Opt. Mater. X 2019, 2, 100022. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Y. Emerging Ultraviolet Persistent Luminescent Materials. Adv. Opt. Mater. 2022, 10, 2201466. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Liu, F.; Pan, Z. Solar-blind ultraviolet-C persistent luminescence phosphors. Nat. Commun. 2020, 11, 2040. [Google Scholar] [CrossRef]
- Yan, S.; Liu, F.; Zhang, J.; Wang, X.-J.; Liu, Y. Persistent Emission of Narrowband Ultraviolet-B Light upon Blue-Light Illumination. Phys. Rev. Appl. 2020, 13, 044051. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Yi, X.; Ming, H.; Ma, Z.; Peng, M. Rechargeable and sunlight-activated Sr3Y2Ge3O12:Bi3+ UV–Visible-NIR persistent luminescence material for night-vision signage and optical information storage. Chem. Eng. J. 2020, 421, 127820. [Google Scholar] [CrossRef]
- Peng, S.; Liu, L.; Wang, L.; Rong, R.; Song, L.; You, W.; Shi, J.; Zhang, Y. A novel self-activated ultraviolet persistent luminescence material and its anti-counterfeiting application based on intensity and time resolution from persistent luminescence. J. Rare Earths 2022, 40, 1417–1423. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Y.; Zhang, R.; Yuan, L.; Li, Z.; Wu, H.; Chen, L.; Hu, Y. Tunable ultraviolet-B full-spectrum delayed luminescence of bismuth-activated phosphors for high-secure data encryption and decryption. J. Alloy. Compd. 2022, 902, 163776. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Y. Achieving Ultraviolet C and Ultraviolet B Dual-Band Persistent Luminescence by Manipulating the Garnet Structure. Adv. Opt. Mater. 2022, 10, 2102157. [Google Scholar] [CrossRef]
- Das, S.; Sharma, S.; Manam, J. Near infrared emitting Cr3+ doped Zn3Ga2Ge2O10 long persistent phosphor for night vision surveillance and anti-counterfeit applications. Ceram. Int. 2022, 48, 824–831. [Google Scholar] [CrossRef]
- Liu, J.; Liang, Y.; Yan, S.; Chen, D.; Miao, S.; Wang, W.; Bi, J. Sunlight-activated long persistent luminescence in the ultraviolet-B spectral region from Bi3+-doped garnet phosphors for covert optical tagging. J. Mater. Chem. C 2021, 9, 9692–9701. [Google Scholar] [CrossRef]
- Yan, S.; Liang, Y.; Chen, Y.; Liu, J.; Chen, D.; Pan, Z. Ultraviolet-C persistent luminescence from the Lu2SiO5:Pr3+ persistent phosphor for solar-blind optical tagging. Dalton Trans. 2021, 50, 8457–8466. [Google Scholar] [CrossRef]
- Yan, S.; Liang, Y.; Liu, J.; Chen, D.; Miao, S.; Bi, J.; Sun, K. Development of ultraviolet-B long-lived persistent phosphors in Pr3+-doped garnets. J. Mater. Chem. C 2021, 9, 14730–14739. [Google Scholar] [CrossRef]
- Poelman, D.; Van Der Heggen, D.; Du, J.; Cosaert, E.; Smet, P.F. Persistent phosphors for the future: Fit for the right application. J. Appl. Phys. 2020, 128, 240903. [Google Scholar] [CrossRef]
- Xu, J.; Tanabe, S. Persistent luminescence instead of phosphorescence: History, mechanism, and perspective. J. Lumin 2019, 205, 581–620. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Rubio-Marcos, F.; Rodriguez, M.; Fernandez, J.F. Long lasting phosphors: SrAl2O4:Eu, Dy as the most studied material. Renew. Sustain. Energy Rev. 2018, 81, 2759–2770. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; James, J.; Gupta, S.K.; Hussain, S. UV-A,B,C Emitting Persistent Luminescent Materials. Materials 2022, 16, 236. [Google Scholar] [CrossRef]
- Sharma, S.K.K.; Bettinelli, M.; Carrasco, I.; Beyer, J.; Gloaguen, R.; Heitmann, J. Dynamics of Charges in Superlong Blacklight-Emitting CaB2O4:Ce3+ Persistent Phosphor. J. Phys. Chem. C 2019, 123, 14639–14646. [Google Scholar] [CrossRef]
- Li, H.; Liu, Q.; Ma, J.; Feng, Z.; Liu, J.; Zhao, Q.; Kuroiwa, Y.; Moriyoshi, C.; Ye, B.; Zhang, J.; et al. Theory-Guided Defect Tuning through Topochemical Reactions for Accelerated Discovery of UVC Persistent Phosphors. Adv. Opt. Mater. 2020, 8, 1901727. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Kner, P.A.; Pan, Z. Gd3+-activated narrowband ultraviolet-B persistent luminescence through persistent energy transfer. Dalton Trans. 2021, 50, 3499–3505. [Google Scholar] [CrossRef]
- Yuan, W.; Tan, T.; Wu, H.; Pang, R.; Zhang, S.; Jiang, L.; Li, D.; Wu, Z.; Li, C.; Zhang, H. Intense UV long persistent luminescence benefiting from the coexistence of Pr3+/Pr4+ in a praseodymium-doped BaLu2Al2Ga2SiO12 phosphor. J. Mater. Chem. C 2021, 9, 5206–5216. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Sun, H.-T. X-ray-activated UVA long persistent luminescence from defective fluoride elpasolites. J. Rare Earths 2020, 38, 124–129. [Google Scholar] [CrossRef]
- Fu, J. Orange- and Violet-Emitting Long-Lasting Phosphors. J. Am. Ceram. Soc. 2004, 85, 255–257. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, F.; Chen, Y.; Sun, K.; Pan, Z. Long persistent luminescence in the ultraviolet in Pb2+-doped Sr2MgGe2O7 persistent phosphor. Dalton Trans. 2016, 45, 1322–1326. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, J.; Wang, T.; Wu, H.; Qiu, J.; Song, Z.; Zhou, D. Ultraviolet long afterglow emission iin Bi3+ doped CdSiO3 phosphors. Mater. Express 2014, 4, 172–176. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Zheng, S.; Fu, X.; Yang, Y.; Wang, J.; Zhang, H. Super-Long Persistent Luminescence in the Ultraviolet A Region from a Bi 3+ -Doped LiYGeO4 Phosphor. Adv. Opt. Mater. 2019, 7, 1900526. [Google Scholar] [CrossRef]
- Sun, H.; Gao, Q.; Wang, A.; Liu, Y.; Wang, X.-J.; Liu, F. Ultraviolet-B persistent luminescence and thermoluminescence of bismuth ion doped garnet phosphors. Opt. Mater. Express 2020, 10, 1296. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Wang, W.; Yan, S.; Liu, J.; Liang, Y. Long-lasting ultraviolet-A persistent luminescence and photostimulated persistent luminescence in Bi3+-doped LiScGeO4 phosphor. Inorg. Chem. Front. 2020, 7, 3063–3071. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiong, P.; Liu, H.; Peng, M. Ultraviolet-A Persistent Luminescence of a Bi3+-Activated LiScGeO4 Material. Inorg. Chem. 2020, 59, 12920–12927. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, Z.; Li, H.; Zhao, Q.; Shirahata, N.; Kuroiwa, Y.; Moriyoshi, C.; Duan, C.; Sun, H. Non-Rare-Earth UVC Persistent Phosphors Enabled by Bismuth Doping. Adv. Opt. Mater. 2021, 9, 2002065. [Google Scholar] [CrossRef]
- Liu, B.-M.; Gan, W.-J.; Lou, S.-Q.; Zou, R.; Tang, Q.; Wang, C.-X.; Jiao, J.; Wang, J. X-ray-activated, UVA persistent luminescent materials based on Bi-doped SrLaAlO4 for deep-Seated photodynamic activation. J. Appl. Phys. 2021, 129, 120901. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Wang, C.; Liu, F.; Wang, X.-J. Ultraviolet glow of Lu3Ga5O12:Bi3+ phosphor in indoor lighting. J. Lumin. 2022, 248, 118932. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, L.; Peng, S.; Shi, J.; Sun, X.; Zhang, Y. Short wavelength persistent luminescence in the ultraviolet A region from a novel phosphor. J. Lumin. 2022, 251, 119103. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Y.; Zhang, J.; Li, X.; Wu, H.; Zhang, R.; Yao, Q.; Hu, Y. Linear charging-discharging of an ultralong UVA persistent phosphor for advanced optical data storage and wide-wavelength-range detector. Chem. Eng. J. 2023, 453, 139558. [Google Scholar] [CrossRef]
- Miao, S.; Liang, Y.; Chen, D.; Yan, S.; Liu, J.; Wang, W.; Bi, J. Enabling narrowband cyan photoluminescence and long-lasting ultraviolet-A persistent luminescence in Bi3+ single-doped Sr3Sc2Ge3O12 phosphors by selective site occupation. J. Mater. Chem. C 2022, 10, 14211–14219. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Zhang, J.; Wang, H.; Shi, J.; Gong, M. Luminescent properties of Sr2MgSi2O7:Eu2+ as blue phosphor for NUV light-emitting diodes. Powder Technol. 2010, 204, 263–267. [Google Scholar] [CrossRef]
- Sahu, I.P.; Bisen, D.P.; Brahme, N.; Ganjir, M. Enhancement of the photoluminescence and long afterglow properties of Sr2MgSi2O7:Eu2+phosphor by Dy3+co-doping. Luminescence 2015, 30, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Sahu, I.P.; Bisen, D.P.; Brahme, N.; Tamrakar, R.K. Enhanced luminescence performance of Sr2MgSi2O7:Eu2+ blue long persistence phosphor by co-doping with Ce3+ ions. J. Mater. Sci. Mater. Electron. 2016, 27, 554–569. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Ran, W.; Deng, Z.; Shi, J.; Ren, C. Tunable luminescence in Sr2MgSi2O7:Tb3+,Eu3+ phosphors Based on Energy Transfer. Materials 2017, 10, 227. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, L.; Levy, D.; Zayat, M.; Jiménez, J.; Mather, G.; Durán, A.; Pascual, M. Processing and luminescence of Eu/Dy-doped Sr2MgSi2O7 glass-ceramics. J. Eur. Ceram. Soc. 2021, 41, 811–822. [Google Scholar] [CrossRef]
- Liu, B.; Shi, C.; Yin, M.; Dong, L.; Xiao, Z. The trap states in the Sr2MgSi2O7 and (Sr,Ca)MgSi2O7 long afterglow phosphor activated by Eu2+ and Dy3+. J. Alloy. Compd. 2005, 387, 65–69. [Google Scholar] [CrossRef]
- Shi, C.; Fu, Y.; Liu, B.; Zhang, G.; Chen, Y.; Qi, Z.; Luo, X. The roles of Eu2+ and Dy3+ in the blue long-lasting phosphor Sr2MgSi2O7: Eu2+, Dy3+. J. Lumin- 2007, 122–123, 11–13. [Google Scholar] [CrossRef]
- Furusho, H.; Hölsä, J.; Laamanen, T.; Lastusaari, M.; Niittykoski, J.; Okajima, Y.; Yamamoto, A. Probing lattice defects in Sr2MgSi2O7:Eu2+,Dy3+. J. Lumin. 2008, 128, 881–884. [Google Scholar] [CrossRef]
- Brito, H.; Hassinen, J.; Hölsä, J.; Jungner, H.; Laamanen, T.; Lastusaari, M.; Malkamäki, M.; Niittykoski, J.; Novak, P.; Rodrigues, L.C.V. Optical energy storage properties of Sr2MgSi2O7:Eu2+,R3+ persistent luminescence materials. J. Therm. Anal. Calorim. 2011, 105, 657–662. [Google Scholar] [CrossRef]
- Brito, H.; Hölsä, J.; Jungner, H.; Laamanen, T.; Lastusaari, M.; Malkamäki, M.; Rodrigues, L. Persistent luminescence fading in a thermoluminescence study. Opt. Mat. Express. 2012, 2, 287–293. [Google Scholar] [CrossRef]
- Singh, V.; Sivaramaiah, G.; Singh, N.; Pathak, M.; Rao, J.; Jirimali, H.; Natarajan, V. Investigation of ultraviolet emitting Gd doped Sr2MgSi2O7 phosphors. Optik 2018, 169, 397–402. [Google Scholar] [CrossRef]
- Hölsä, J.; Laamanen, T.; Lastusaari, M.; Novák, P. Defect aggregates in the Sr2MgSi2O7 persistent luminescence material. J. Rare Earths 2011, 29, 1130–1136. [Google Scholar] [CrossRef]
- Duan, H.; Dong, Y.; Huang, Y.; Hu, Y.; Chen, X. The important role of oxygen vacancies in Sr2MgSi2O7 phosphor. Phys. Lett. A 2016, 380, 1056–1062. [Google Scholar] [CrossRef]
- Aitasalo, T.; Hassinen, J.; Hölsä, J.; Laamanen, T.; Lastusaari, M.; Malkamäki, M.; Niittykoski, J.; Novák, P. Synchrotron radiation investigations of the Sr2MgSi2O7:Eu2+,R3+ persistent luminescence materials. J. Rare Earths 2009, 27, 529–538. [Google Scholar] [CrossRef]
- Hölsä, J.; Kirm, M.; Laamanen, T.; Lastusaari, M.; Niittykoski, J.; Novák, P. Electronic structure of the Sr2MgSi2O7:Eu2+ persistent luminescence material. J. Lumin- 2009, 129, 1560–1563. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Wei, S.; Wu, Y.; Mu, X.; Zhao, Z.; Wen, M.; Dong, H.; Zhang, X.; Wu, F.; et al. Photoluminescence properties of Sr2MgSi2O7:Pb2+ and tunable emission from UVB to UVC based on ion substitution. J. Lumin. 2020, 225, 117353. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program. BGMN J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Endo, T.; Doi, Y.; Wakeshima, M.; Hinatsu, Y. Crystal Structures and Magnetic Properties of New Europium Melilites Eu2MSi2O7 (M = Mg, Mn) and Their Strontium Analogues. Inorg. Chem. 2010, 49, 10809–10814. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, T.; Kamoshita, M.; Iguchi, H.; Wang, J.; Ishizawa, N. Apatite-type SrPr4(SiO4)3O. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, i68. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–766. [Google Scholar] [CrossRef]
- de Hair, J. The luminescence of Pr3+ in BaY4Si5O17. J. Solid State Chem. 1980, 33, 33–36. [Google Scholar] [CrossRef]
- Pustovarov, V.A.; Ivanovskikh, K.V.; Khatchenko, Y.E.; Bettinelli, M.; Shi, Q. Luminescence Spectroscopy and Decay Kinetics of Pr3+ Ions in K3LuSi2O7:Pr3+. Phys. Solid State 2019, 61, 752–757. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.; Wang, Y.; Li, F.; Sun, X. Separation of high-purity yttrium from ion-absorbed rare earth concentrate using (2,6-dimethylheptyl) phenoxy acetic/propanoic acid. Sep. Purif. Technol. 2017, 184, 280–287. [Google Scholar] [CrossRef]
- Pedreira, W.; Sarkis, J.; Rodrigues, C.; Tomiyoshi, I.; Queiroz, C.D.S.; Abrão, A. Determination of trace amounts of rare earth elements in highly pure praseodymium oxide by double focusing inductively coupled plasma mass spectrometry and high-performance liquid chromatography. J. Alloy. Compd. 2001, 323–324, 49–52. [Google Scholar] [CrossRef]
- Bos, A. Theory of thermoluminescence. Radiat. Meas. 2006, 41, S45–S56. [Google Scholar] [CrossRef]
- McKeever, S.W.S. On the analysis of complex thermoluminescence. Glow-Curves: Resolution into individual peaks. Phys. Status Solidi A 1980, 62, 331–340. [Google Scholar] [CrossRef]
- Horowitz, Y.; Satinger, D.; Yossian, D.; Brandan, M.; Buenfil, A.; Gamboa-Debuen, I.; Rodriguez-Villafuerte, M.; Ruiz, C. Ionisation Density Effects in the Thermoluminescence of TLD-100:Computerised Tm-Tstop Glow Curve Analysis. Radiat. Prot. Dosim. 1999, 84, 239–242. [Google Scholar] [CrossRef]
- Akın, A.; Ekdal, E.; Arslanlar, Y.T.; Ayvacıklı, M.; Karalı, T.; Can, N. Thermally stimulated luminescence glow curve structure of β-irradiated CaB4O7:Dy. Luminescence 2015, 30, 830–834. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Lin, Z.; Li, H.; Hou, D.; Song, J.; Lin, S.; Song, C.; Lin, H.; Lin, Z. Positive effect of codoping Yb3+ on the super-long persistent luminescence of Cr3+-doped zinc aluminum germanate. Ceram. Int. 2018, 44, 17377–17382. [Google Scholar] [CrossRef]
- Eeckhout, K.V.D.; Bos, A.J.J.; Poelman, D.; Smet, P.F. Revealing trap depth distributions in persistent phosphors. Phys. Rev. B 2013, 87, 045126. [Google Scholar] [CrossRef]
- Gao, D.; Kuang, Q.; Gao, F.; Xin, H.; Yun, S.; Wang, Y. Achieving opto-responsive multimode luminescence in Zn1+xGa2−2xGexO4:Mn persistent phosphors for advanced anti-counterfeiting and information encryption. Mater. Today Phys. 2022, 27, 100765. [Google Scholar] [CrossRef]
- Liu, F.; Yan, W.; Chuang, Y.-J.; Zhen, Z.; Xie, J.; Pan, Z. Photostimulated near-infrared persistent luminescence as a new optical read-out from Cr3+-doped LiGa5O8. Sci. Rep. 2013, 3, 1554. [Google Scholar] [CrossRef]
- Misra, S.K. Multifrequency Electron Paramagnetic Resonance; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions. In Electron Paramagnetic Resonance of Transition Ions; Clarendon Press: Oxford, UK, 1970. [Google Scholar]
- Bennati, M. EPR Interactions—Hyperfine Couplings. eMagRes 2017, 6, 271–282. [Google Scholar] [CrossRef]
- Marfunin, A.S. Spectroscopy, luminescence and radiation centers in minerals. Earth-Science Rev. 1979, 16, 369–370. [Google Scholar] [CrossRef]
- Hughes, A.; Henderson, B. Color centers in simple oxides. In Point Defects in Solids; Springer: Boston, MA, USA, 1972; pp. 381–384. [Google Scholar] [CrossRef]
- Nikl, M.; Laguta, V.V.; Vedda, A. Complex oxide scintillators: Material defects and scintillation performance. Phys. Status Solidi B 2008, 245, 1701–1722. [Google Scholar] [CrossRef]
- Gionco, C.; Livraghi, S.; Maurelli, S.; Giamello, E.; Tosoni, S.; Di Valentin, C.; Pacchioni, G. Al- and Ga-Doped TiO2, ZrO2, and HfO2: The Nature of O 2p Trapped Holes from a Combined Electron Paramagnetic Resonance (EPR) and Density Functional Theory (DFT) Study. Chem. Mater. 2015, 27, 3936–3945. [Google Scholar] [CrossRef]
- Henderson, B.; Garrison, A. Hyperfine interaction of defects in insulators. Adv. Phys. 1973, 22, 423–528. [Google Scholar] [CrossRef]
- Unruh, W.P.; Chen, Y.; Abraham, M.M. 25Mg superhyperfine structure ofV-type defects in MgO. Phys. Rev. B 1977, 15, 4149–4156. [Google Scholar] [CrossRef]
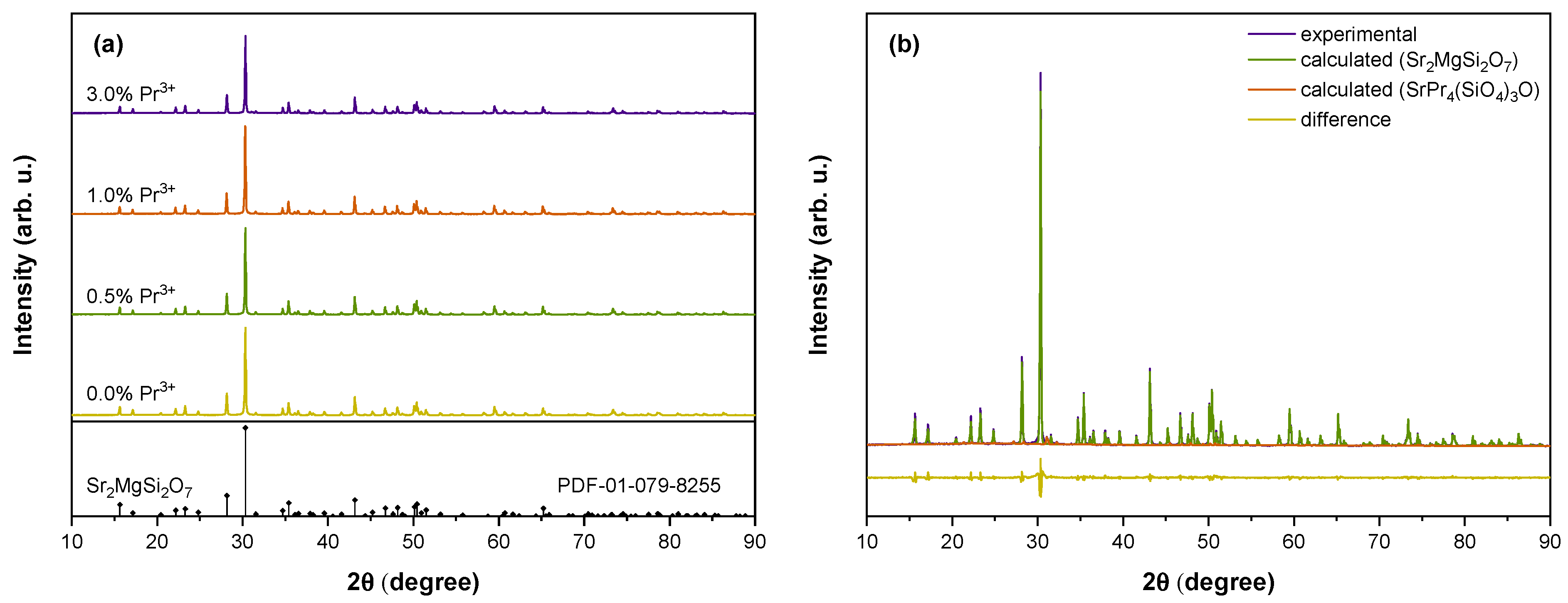

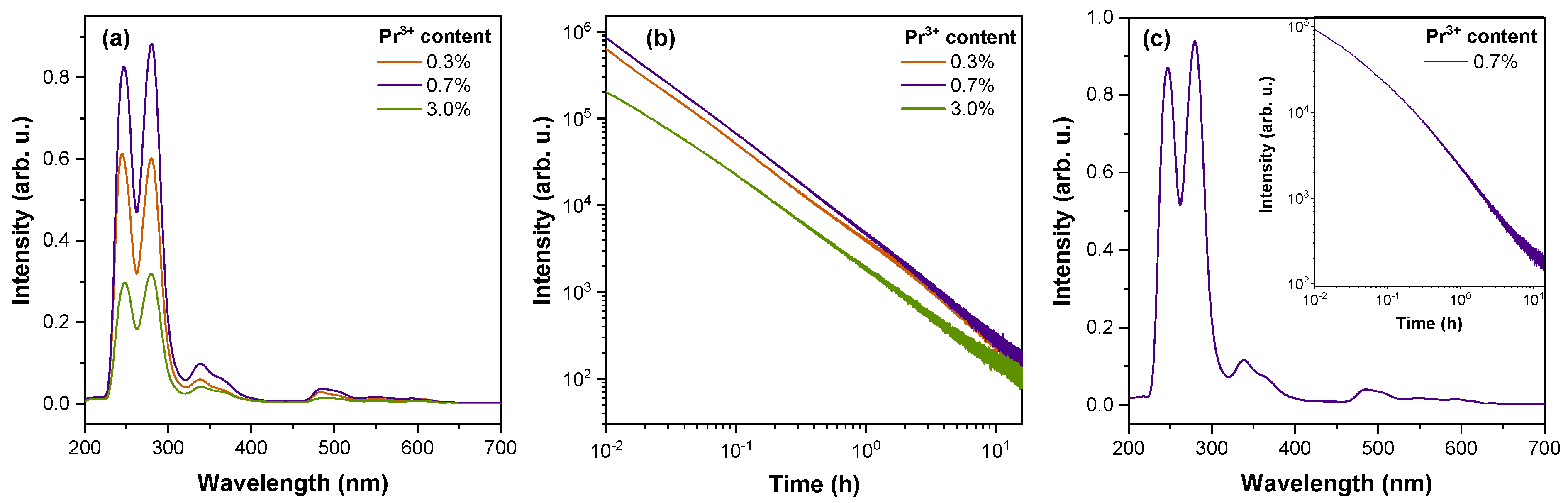
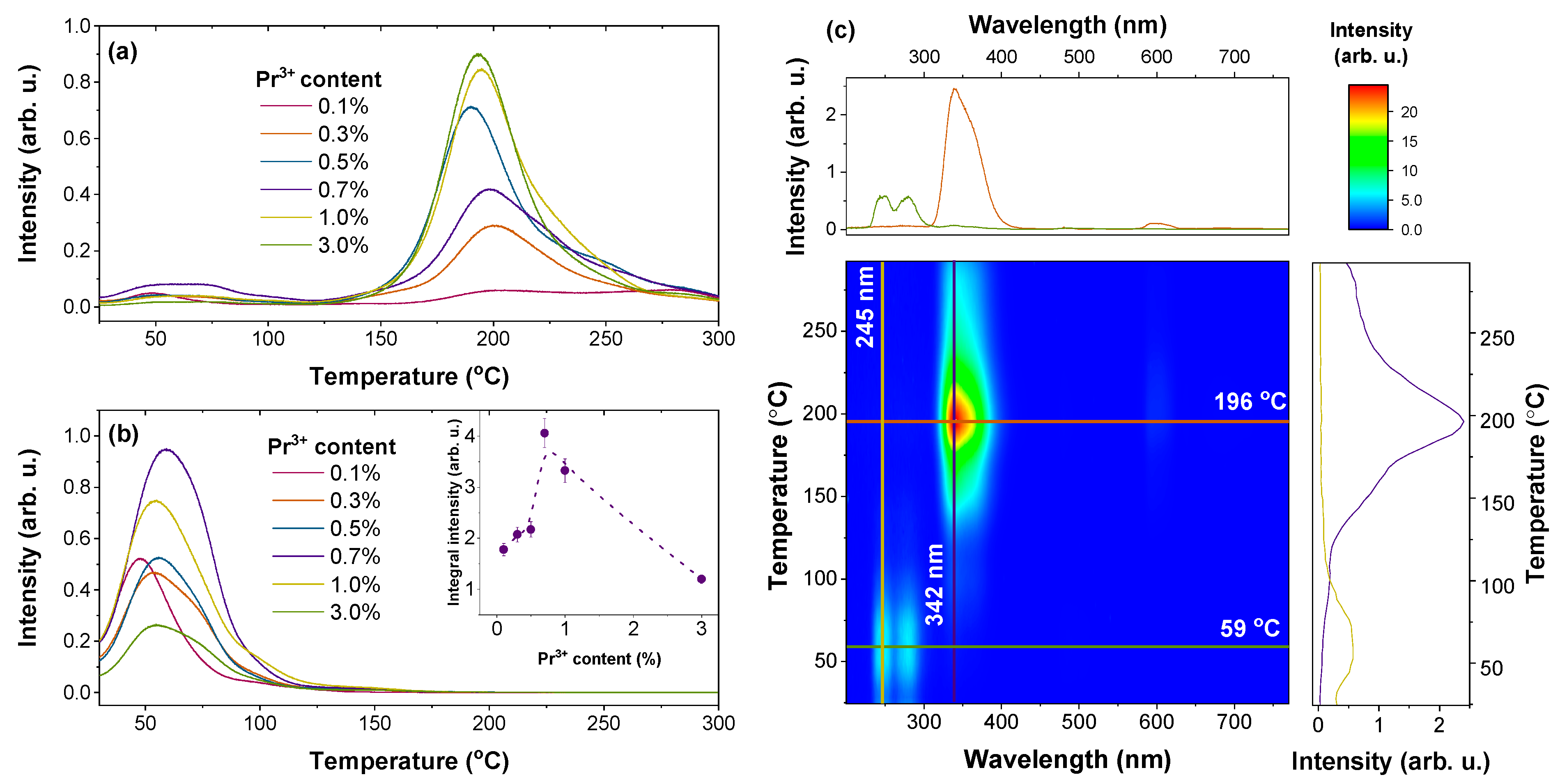


| Host | Emission Centre | Excitation | Maximum of Persistent Luminescence, nm | Reference |
|---|---|---|---|---|
| Lu2SiO5 | Pr3+ | UV (254 nm) | 270 | [10,18] |
| Ca2Al2SiO7 | Pr3+ | UV (254 nm) | 268 | [10] |
| Ca3Al2Si3O12 | Pr3+ | UV (254 nm) | 267 | [10] |
| LiYSiO4 | Pr3+ | UV (254 nm) | 267 | [10] |
| (Ca1.5Y1.5)(Al3.5Si1.5)O12 | Pr3+ | UV (254 nm) | 266 | [15] |
| Sr3Y2Si6O18 | Pr3+ | UV (254 nm) | 265 | [10] |
| Li2CaGeO4 | Pr3+ | UV (254 nm) X-rays | 252 | [7] |
| Cs2NaYF6 | Pr3+ | X-rays | 250 | [5] |
| Sr2MgSi2O7 | Pr3+ | X-rays UV (232 nm) | 243 | This work |
| YPO4 | Bi3+ | X-rays | 240 | [37] |
| LaPO4 | Pr3+ | X-rays | 231 | [26] |
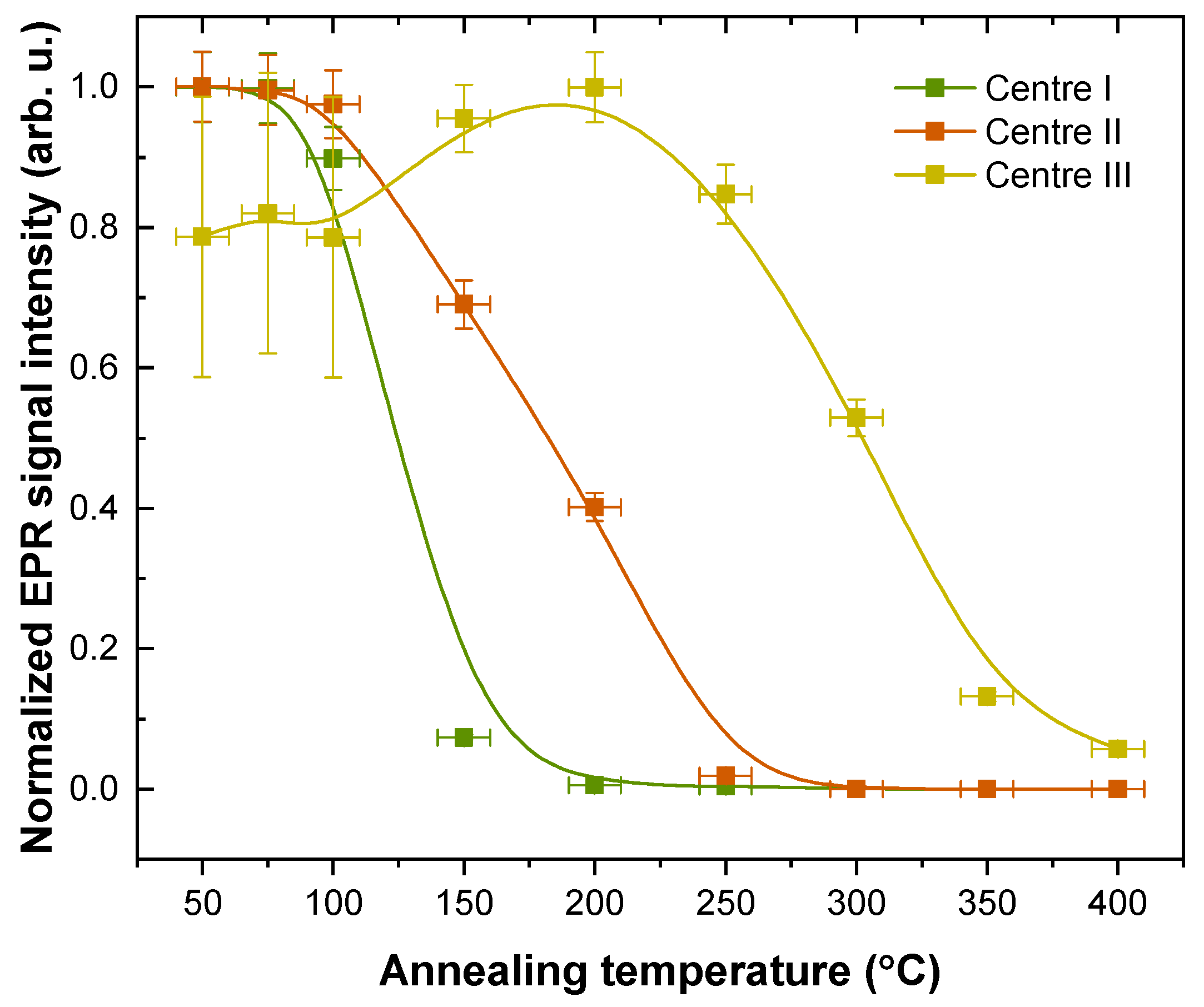
| Centre I | 2.0516 | 2.0120 | 2.0017 | 6.6 | 6.4 | |
| Centre II | 1.9803 | 1.9619 | 1.9111 | |||
| Centre III | 2.0132 | 2.0068 | 2.0028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antuzevics, A.; Doke, G.; Krieke, G.; Rodionovs, P.; Nilova, D.; Cirulis, J.; Fedotovs, A.; Rogulis, U. Shortwave Ultraviolet Persistent Luminescence of Sr2MgSi2O7: Pr3+. Materials 2023, 16, 1776. https://doi.org/10.3390/ma16051776
Antuzevics A, Doke G, Krieke G, Rodionovs P, Nilova D, Cirulis J, Fedotovs A, Rogulis U. Shortwave Ultraviolet Persistent Luminescence of Sr2MgSi2O7: Pr3+. Materials. 2023; 16(5):1776. https://doi.org/10.3390/ma16051776
Chicago/Turabian StyleAntuzevics, Andris, Guna Doke, Guna Krieke, Pavels Rodionovs, Dace Nilova, Jekabs Cirulis, Andris Fedotovs, and Uldis Rogulis. 2023. "Shortwave Ultraviolet Persistent Luminescence of Sr2MgSi2O7: Pr3+" Materials 16, no. 5: 1776. https://doi.org/10.3390/ma16051776
APA StyleAntuzevics, A., Doke, G., Krieke, G., Rodionovs, P., Nilova, D., Cirulis, J., Fedotovs, A., & Rogulis, U. (2023). Shortwave Ultraviolet Persistent Luminescence of Sr2MgSi2O7: Pr3+. Materials, 16(5), 1776. https://doi.org/10.3390/ma16051776






