Electropolishing Stainless Steel Optimization Using Surface Quality, Dimensional Accuracy, and Electrical Consumption Criteria
Abstract
1. Introduction
2. Experimental Design
3. Results and Discussion
3.1. Identification of Significant Factors in the Electropolishing Process
3.2. Analysis of the Electropolishing Parameter Effects on the Polishing Rate
3.2.1. Initial Texture
3.2.2. Initial Texture
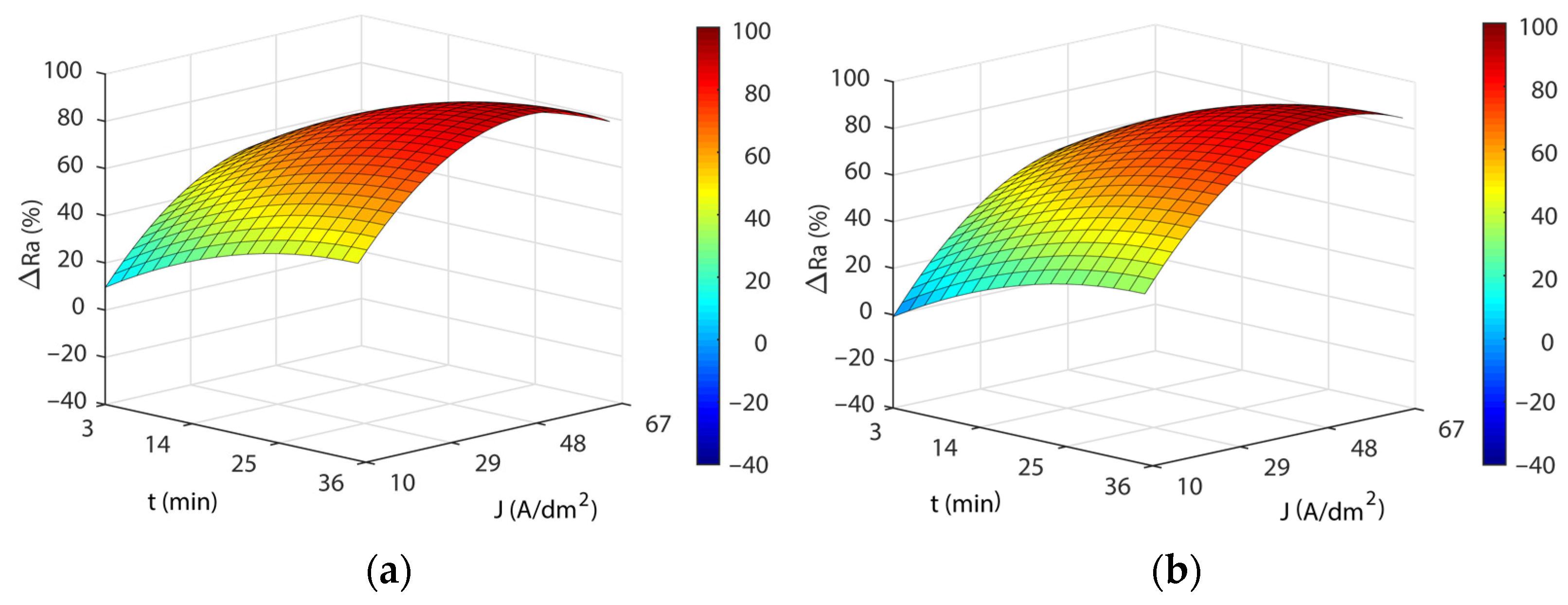
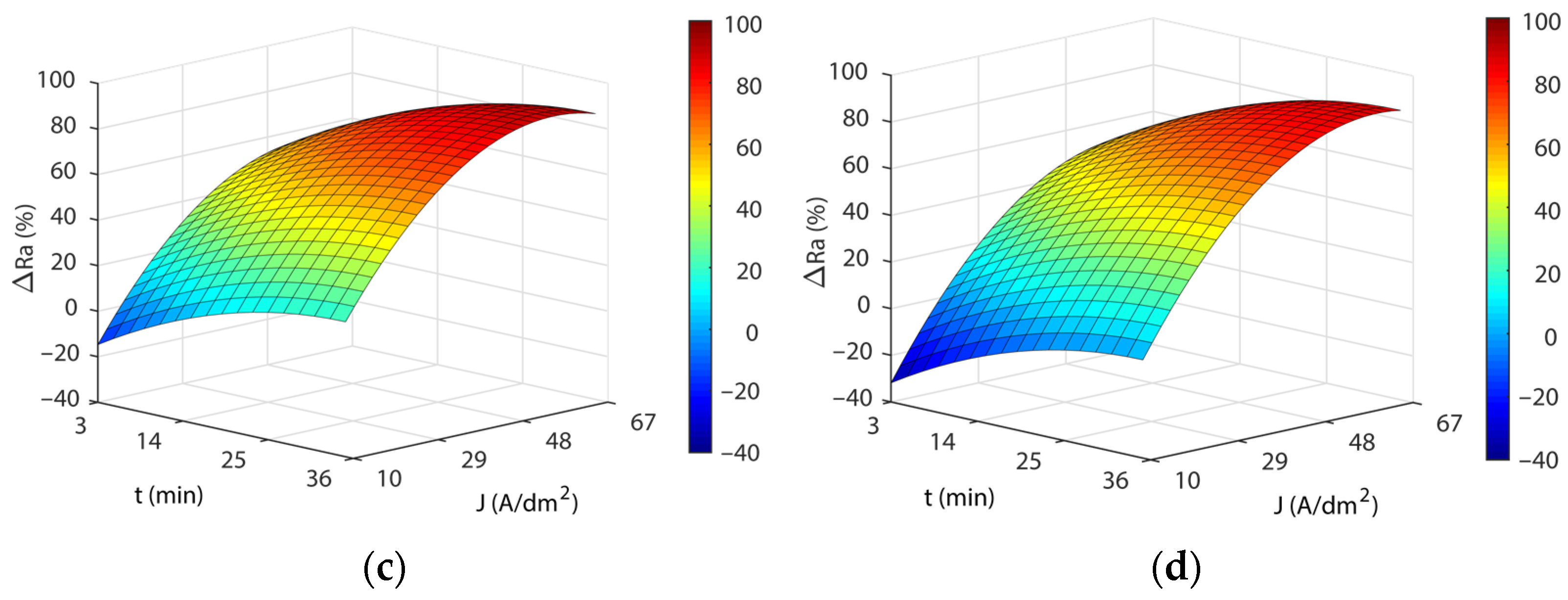
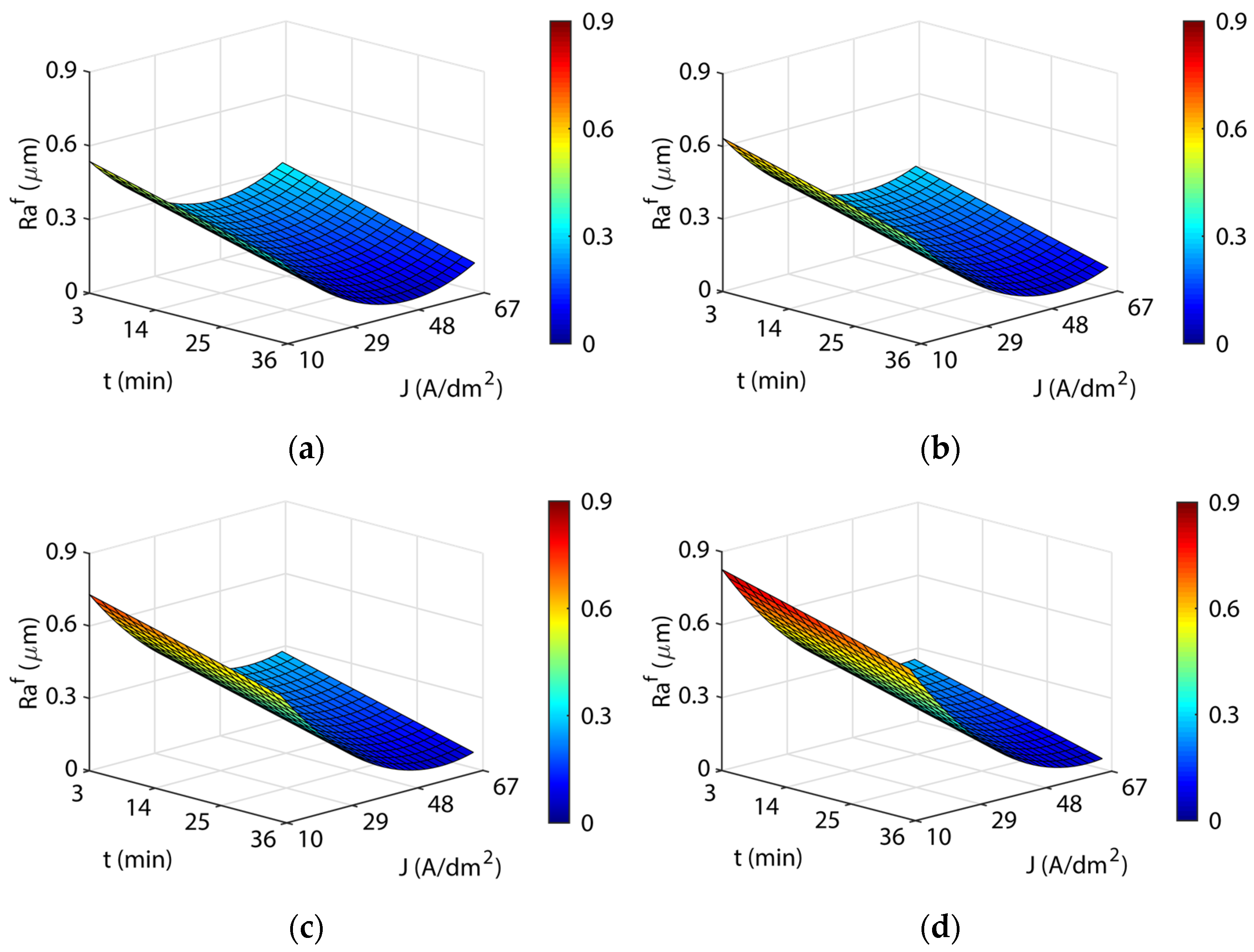
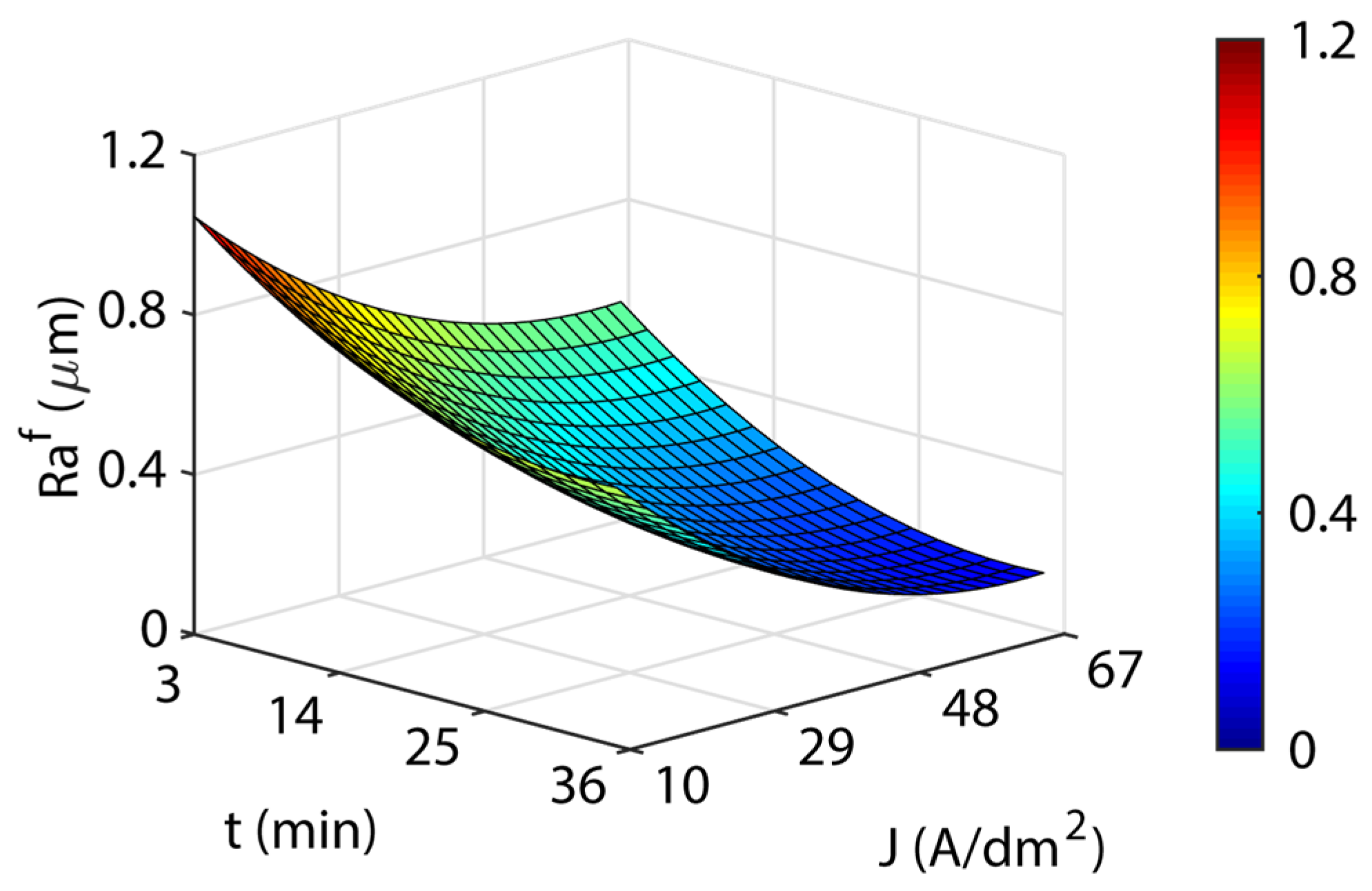
3.3. Analysis of the Electropolishing Factors Effects on Final Surface Roughness
3.3.1. Initial Texture
3.3.2. Initial Surface Texture
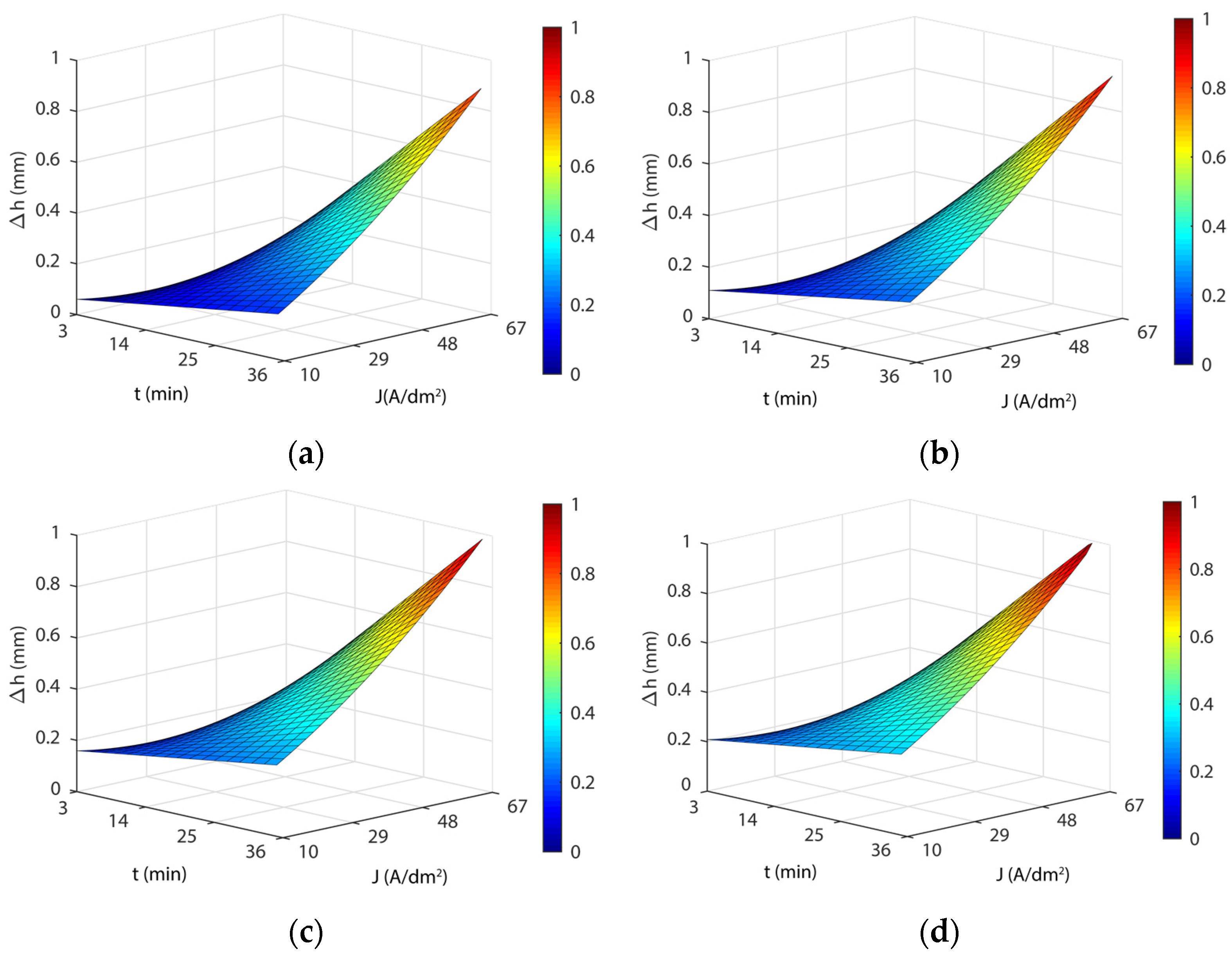
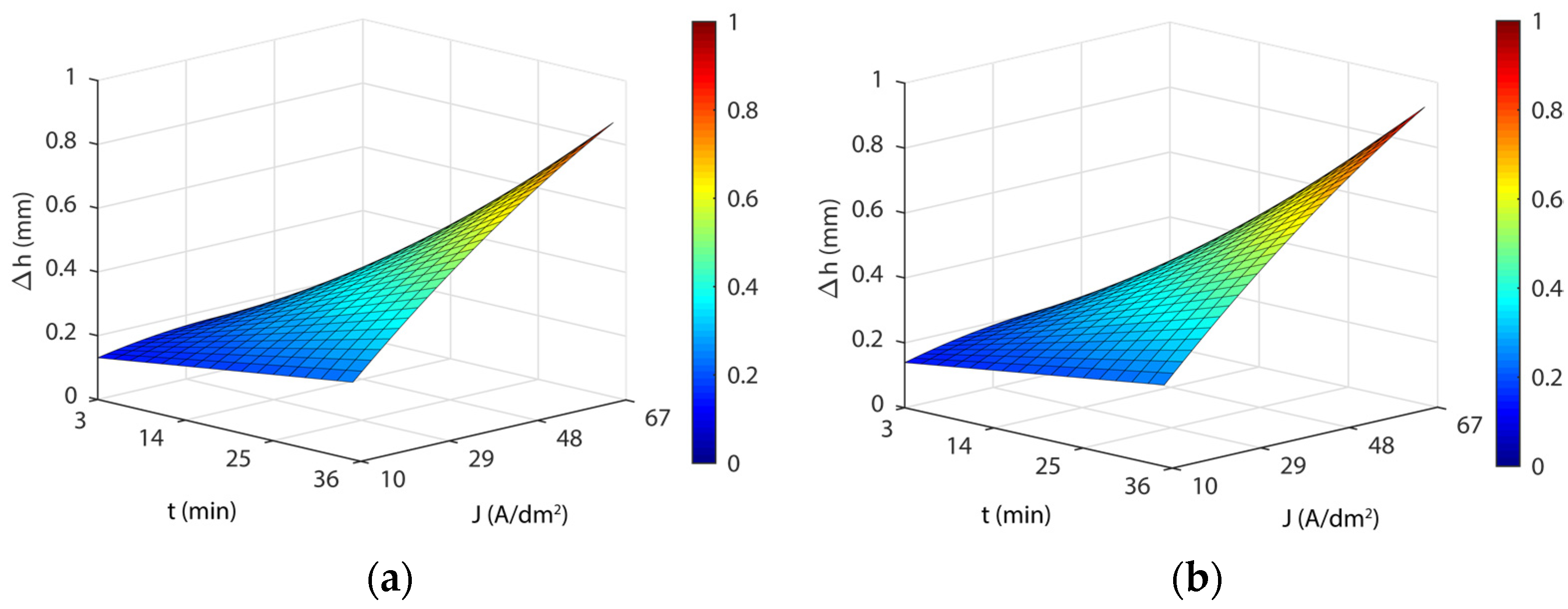
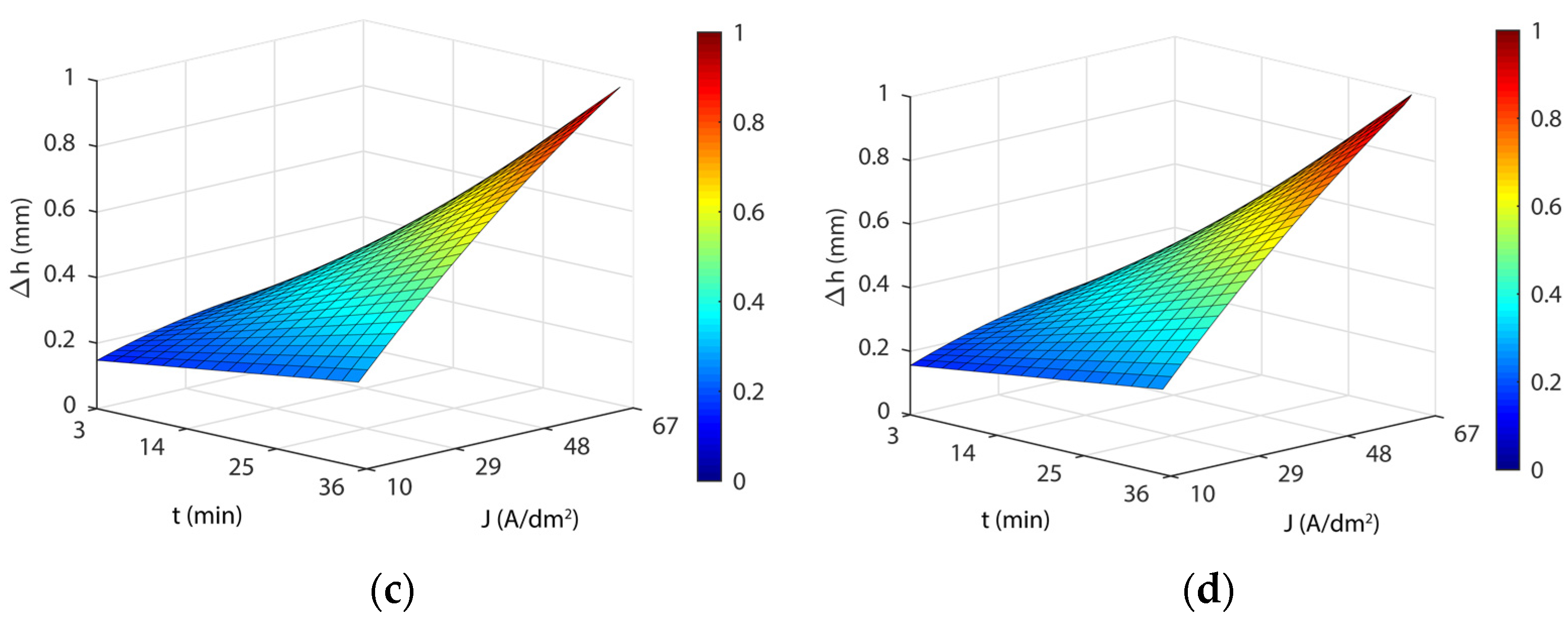
3.4. Analysis of the Effects of Electropolishing Factors on Dimensional Accuracy
3.4.1. Initial Texture
3.4.2. Initial Texture
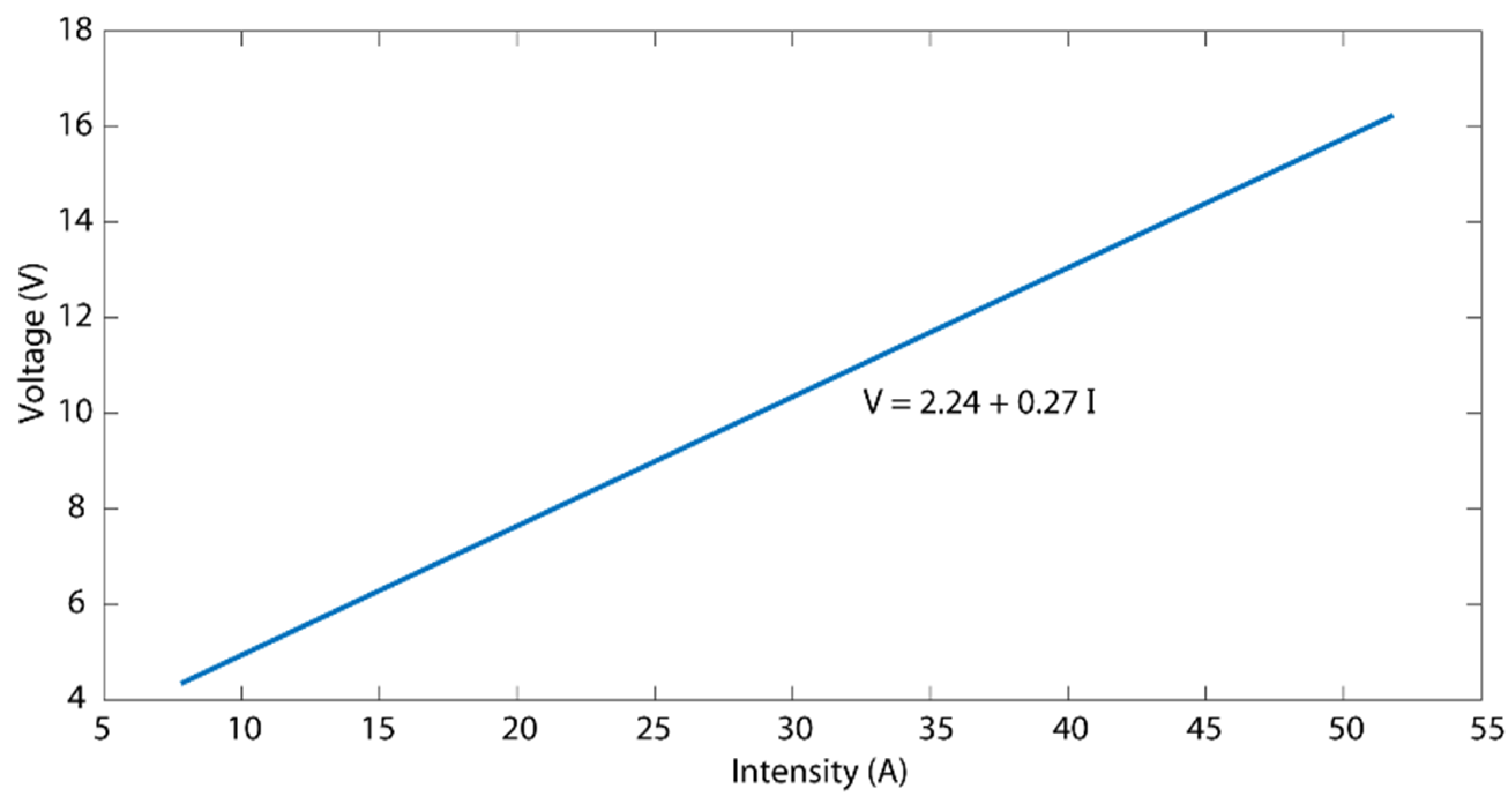
3.5. Analysis of the Effects of Electropolishing Factors on Electrical Consumption Cost
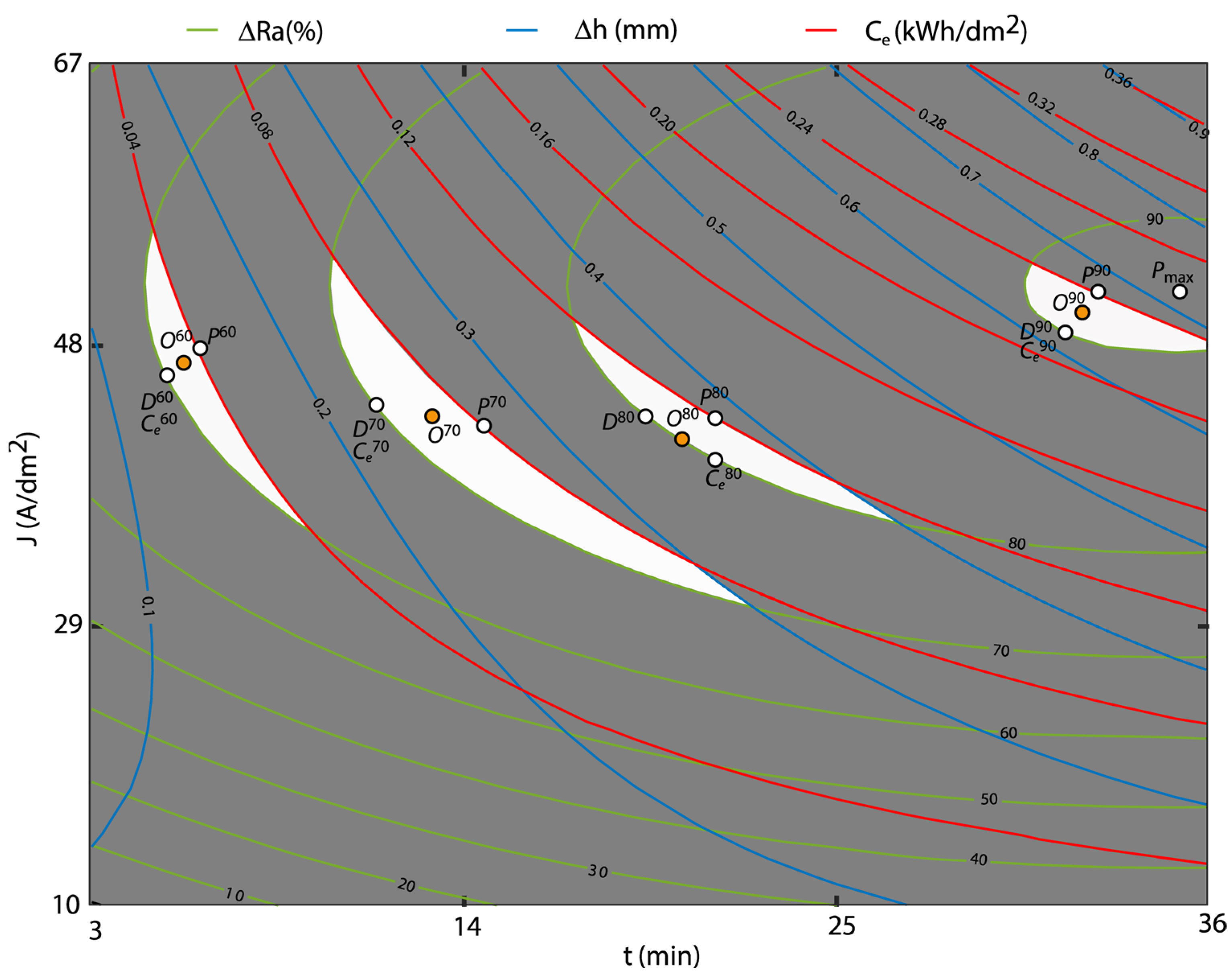
3.6. Multi-Objective Optimization by Overlaid Contour Plot
3.7. Multi-Objective Optimization Based on the Desirability Function
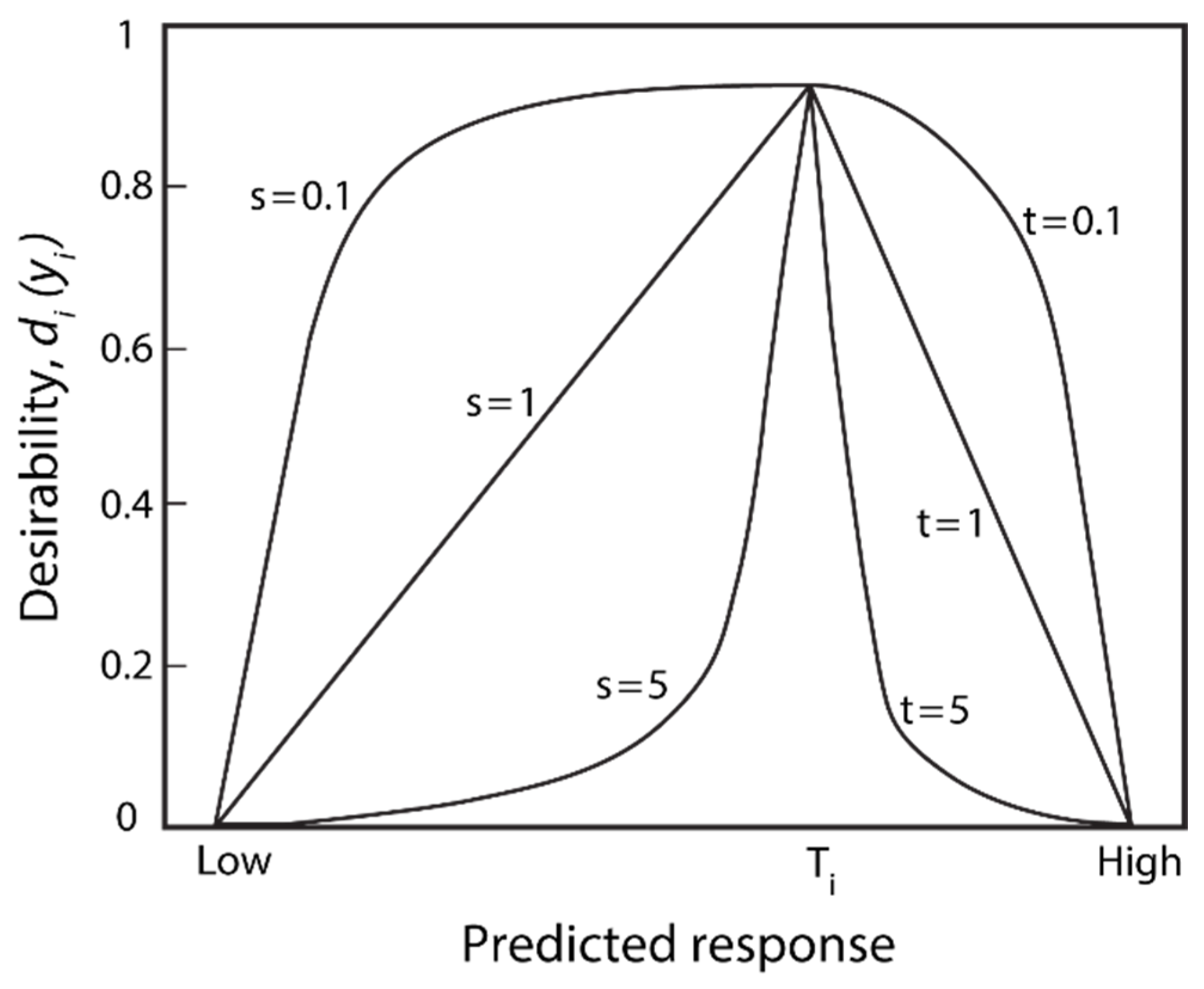
3.7.1. Fundamentals of the Desirability Function Method
- (a)
- If the objective was to maximize the response yi, the desirability function di(yi) was defined by Equation (17), where L was the minimum objective of the response, and H was the maximum objective:when the objective H of the response yi was its maximum value, then di(yi) = 1; and if the objective L of the response yi was its minimum value, then di(yi) = 0.
- (b)
- If the objective was to minimize the response yi, the desirability function di(yi) was defined by Equation (18):When the objective L of the response yi was its minimum value, then di(yi) =1; and if the objective H of the response yi was its maximum value, then di(yi) = 0.
- (c)
- If the response variable was to be maintained at the objective value T, the desirability function was defined by Equation (19):
3.7.2. Multi-Objective Optimization with the Desirability Function
3.8. Discussion
4. Conclusions
- Current density and EP time were the parameters having the greatest effects on the EP process, affecting polishing rate, final surface finish, dimensional accuracy, and power consumption cost. The increase in both parameters improved performance, reaching maximum ΔRa values of 85–91% at current density intervals of 48–60 A/dm2 and a 31–35 min EP time. Above these maximum values, the increase in both parameters produced a stabilization and a subsequent worsening of the EP process performance.
- Electrolyte temperature was the EP parameter with the least effect on the polishing rate and final roughness. It was only significant in initial texture The maximum polishing rate obtained was 90.72% at 45 °C, but with variations below ~5% in the other temperatures analyzed. The 35 °C temperature was the best option owing to the excellent results in the EP process, i.e., polishing rate, final roughness, dimensional accuracy, and electrical consumption cost.
- The initial texture was significant on all criteria analyzed, but especially on the polishing rate and the final roughness obtained. The best polishing rates (89–91%) were obtained for initial texture , with less dimensional variation and cost in all of the temperatures analyzed. The initial texture obtained a maximum polishing rate of 87.9%, but with greater dimensional variation and higher electrical consumption cost. The initial texture obtained the best final roughness with a minimum of ~0.03 µm in comparison to the initial texture of ~0.15 µm.
- As for dimensional accuracy (Δh), a highly significant interaction was found between current density and EP time. At low values, the modulation of both parameters had a minor effect on Δh with variations below ~0.150 mm, but at high values, more than 1 mm in thickness variation was observed. The lowest Δh was obtained at 35 °C in all of the cases analyzed.
- Electrical consumption cost (Ce) also showed a significant interaction between current density and EP time. At low values, the modulation of both parameters had a negligible effect on Ce with consumption at the interval of 0.002–0.031 kWh/dm2; but high values reached a maximum consumption of 0.373 kWh/dm2. The lowest cost was obtained at a 35–45 °C temperature range.
- OCP methodology determined the optimum polishing areas per polishing ranges, where the optimum individual and multi-objectives were determined using a graphical method under EP process performance, dimensional accuracy and cost criteria. This methodology allows better control of EP process conditions by polishing ranges. The results obtained with OCP confirmed the results of the individual RSM analysis.
- DFM methodology determined the optimum global multi-objective with excellent composite desirability values for both textures and enabled the weighting of the responses. The optimum multi-objective with DFM obtained better dimensional accuracy and cost than that obtained with OCP. The results obtained with DFM using independent models confirmed the results of the individual RSM analysis and the multi-response OCP analysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Núñez, P.; García-Plaza, E.; Hernando, M.; Trujillo, R. Characterization of Surface Finish of Electropolished Stainless Steel AISI 316L with Varying Electrolyte Concentrations. Procedia Eng. 2013, 63, 771–778. [Google Scholar] [CrossRef]
- Hernando, M.; López, P.J.N.; Plaza, E.G.; Trujillo, R. Effect of Electrolyte on the Surface Smoothness Obtained by Electropolishing of Stainless Steel. Mater. Sci. Forum 2012, 713, 55–60. [Google Scholar] [CrossRef]
- Beamud, E.; Núñez, P.; García-Plaza, E.; Rodríguez, D.; González, A.; García, J. Impact of electrolyte concentration on surface gloss in electropolished stainless steel. Procedia Manuf. 2017, 13, 663–670. [Google Scholar] [CrossRef]
- Han, W.; Fang, F. Fundamental aspects and recent developments in electropolishing. Int. J. Mach. Tools Manuf. 2019, 139, 1–23. [Google Scholar] [CrossRef]
- Lin, C.-C.; Hu, C.-C.; Lee, T.-C. Electropolishing of 304 stainless steel: Interactive effects of glycerol content, bath temperature, and current density on surface roughness and morphology. Surf. Coat. Technol. 2009, 204, 448–454. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lai, J.-J. The effects of electropolishing (EP) process parameters on corrosion resistance of 316L stainless steel. J. Mater. Process. Technol. 2003, 140, 206–210. [Google Scholar] [CrossRef]
- Lochynski, P.; Kowalski, M.; Szczygiel, B.; Kuczewski, K. Improvement of the stainless steel electropolishing process by organic additives. Pjct 2016, 18, 76–81. [Google Scholar] [CrossRef]
- Ahmed, A.; Darweesh, M.; Agour, Y.; Elzayett, M.; Hammad, W. Electropolishing of steel in presence of some amines. Prog. Org. Coat. 2019, 133, 255–266. [Google Scholar] [CrossRef]
- Barnes, P.; Savva, A.; Dixon, K.; Bull, H.; Rill, L.; Karsann, D.; Croft, S.; Schimpf, J.; Xiong, H. Electropolishing valve metals with a sulfuric acid-methanol electrolyte at low temperature. Surf. Coat. Technol. 2018, 347, 150–156. [Google Scholar] [CrossRef]
- Abouzeid, F.M.; Abubshait, H.A. A Study of Vitamin B Influence on the Morphology, Roughness, and Reflectance of Elec-tropolished Aluminum in H3PO4–H2SO4 Mixture. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Kwon, G.D.; Kim, Y.W.; Moyen, E.; Keum, D.H.; Lee, Y.H.; Baik, S.; Pribat, D. Controlled electropolishing of copper foils at elevated temperature. Appl. Surf. Sci. 2014, 307, 731–735. [Google Scholar] [CrossRef]
- Andrade, L.S.; Xavier, S.C.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Electropolishing of AISI-304 stainless steel using an oxidizing solution originally used for electrochemical coloration. Electrochim. Acta 2005, 50, 2623–2627. [Google Scholar] [CrossRef]
- Hou, Y.; Li, R.; Liang, J.; Su, P.; Ju, P. Electropolishing of Al and Al alloys in AlCl 3/trimethylamine hydrochloride ionic liquid. Surf. Coat. Technol. 2018, 335, 72–79. [Google Scholar] [CrossRef]
- Zhang, Y. Electropolishing Mechanism of Ti-6Al-4V Alloy Fabricated by Selective Laser Melting. Int. J. Electrochem. Sci. 2018, 13, 4792–4807. [Google Scholar] [CrossRef]
- Shim, H.-S.; Seo, M.J.; Hur, D.H. Effect of electropolishing on general corrosion of Alloy 690TT tubes in simulated primary coolant of pressurized water reactors. Appl. Surf. Sci. 2018, 467–468, 467–476. [Google Scholar] [CrossRef]
- Lin, C.-C.; Hu, C.-C. Electropolishing of 304 stainless steel: Surface roughness control using experimental design strategies and a summarized electropolishing model. Electrochim. Acta 2008, 53, 3356–3363. [Google Scholar] [CrossRef]
- Liu, M.; Meng, Y.; Zhao, Y.; Li, F.; Gong, Y.; Feng, L. Electropolishing parameters optimization for enhanced performance of nickel coating electroplated on mild steel. Surf. Coat. Technol. 2016, 286, 285–292. [Google Scholar] [CrossRef]
- Gomez-Gallegos, A.; Mill, F.; Mount, A. Surface finish control by electrochemical polishing in stainless steel 316 pipes. J. Manuf. Process. 2016, 23, 83–89. [Google Scholar] [CrossRef]
- Awad, A.; Ghany, N.A.; Dahy, T. Removal of tarnishing and roughness of copper surface by electropolishing treatment. Appl. Surf. Sci. 2010, 256, 4370–4375. [Google Scholar] [CrossRef]
- Yu, C.-U.; Hu, C.-C.; Bai, A.; Yang, Y.-F. Pore-size dependence of AAO films on surface roughness of Al-1050 sheets controlled by electropolishing coupled with fractional factorial design. Surf. Coat. Technol. 2007, 201, 7259–7265. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokicki, R.; Rokosz, K. Surface characterization of AISI 316L biomaterials obtained by electropolishing in a magnetic field. Surf. Coat. Technol. 2008, 202, 1668–1673. [Google Scholar] [CrossRef]
- Awad, A.; Ghazy, E.; El-Enin, S.A.; Mahmoud, M. Electropolishing of AISI-304 stainless steel for protection against SRB biofilm. Surf. Coat. Technol. 2012, 206, 3165–3172. [Google Scholar] [CrossRef]
- Ponto, L.; Datta, M.; Landolt, D. Electropolishing of iron-chromium alloys in phosphoric acid-sulphuric acid electrolytes. Surf. Coat. Technol. 1987, 30, 265–276. [Google Scholar] [CrossRef]
- Lopez-Ruiz, P.; Garcia-Blanco, M.B.; Vara, G.; Fernández-Pariente, I.; Guagliano, M.; Bagherifard, S. Obtaining tailored surface characteristics by combining shot peening and electropolishing on 316L stainless steel. Appl. Surf. Sci. 2019, 492, 1–7. [Google Scholar] [CrossRef]
- Xiao, Q.; Lu, Z.; Chen, J.; Ma, J.; Xiong, Q.; Li, H.; Xu, J.; Shoji, T. Magnetoelectropolishing treatment for improving the oxidation resistance of 316L stainless steel in pressurized water reactor primary water. J. Nucl. Mater. 2019, 518, 357–369. [Google Scholar] [CrossRef]
- Kityk, A.; Protsenko, V.; Danilov, F.; Kun, O.; Korniy, S. Electropolishing of aluminium in a deep eutectic solvent. Surf. Coat. Technol. 2019, 375, 143–149. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, Q.; Yin, F.; Cui, C. Special corrosion behavior of an inoculant refined Cu-Al-Mn shape memory alloy during electropolishing process. Mater. Charact. 2019, 153, 348–353. [Google Scholar] [CrossRef]
- Rokosz, K.; Lahtinen, J.; Hryniewicz, T.; Rzadkiewicz, S. XPS depth profiling analysis of passive surface layers formed on austenitic AISI 304L and AISI 316L SS after high-current-density electropolishing. Surf. Coat. Technol. 2015, 276, 516–520. [Google Scholar] [CrossRef]
- Haïdopoulos, M.; Turgeon, S.; Laroche, G.; Mantovani, D. Surface modifications of 316 stainless steel for the improvement of its interface properties with RFGD-deposited fluorocarbon coating. Surf. Coat. Technol. 2005, 197, 278–287. [Google Scholar] [CrossRef]
- Rotty, C.; Doche, M.-L.; Mandroyan, A.; Hihn, J.-Y.; Montavon, G.; Moutarlier, V. Comparison of electropolishing behaviours of TSC, ALM and cast 316L stainless steel in H3PO4/H2SO4. Surf. Interfaces 2017, 6, 170–176. [Google Scholar] [CrossRef]
- Mingear, J.; Zhang, B.; Hartl, D.; Elwany, A. Effect of process parameters and electropolishing on the surface roughness of interior channels in additively manufactured nickel-titanium shape memory alloy actuators. Addit. Manuf. 2019, 27, 565–575. [Google Scholar] [CrossRef]
- Jiménez-Pichardo, R.; Regalado, C.; Castaño-Tostado, E.; Meas-Vong, Y.; Santos-Cruz, J.; García-Almendárez, B.E. Evaluation of electrolyzed water as cleaning and disinfection agent on stainless steel as a model surface in the dairy industry. Food Control 2016, 60, 320–328. [Google Scholar] [CrossRef]
- Astakhov, V.P.; Abellan-Nebot, J.V.; Liu, J.; Romero-Subiron, F.; Priyadarshini, A.; Surjya, K.; Pal, S.K.; Samantaray, A.K.; Nandi, A.K. Statistical and Computational Techniques in Manufacturing; Davim, J.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-25858-9. [Google Scholar]
- Fedot’ev, N.P.; Grilikhes, S.Y.; Myers, H.S. Electropolishing, Anodizing and Electrolytic Pickling of Metals. J. Electrochem. Soc. 1960, 108, 77C. [Google Scholar] [CrossRef]
- Łyczkowska-Widłak, E.; Lochyński, P.; Nawrat, G. Electrochemical Polishing of Austenitic Stainless Steels. Materials 2020, 13, 2557. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Design and Analysis of Experiments, 10th ed.; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-49244-3. [Google Scholar]
- Astakhov, V.P.; Markopoulos, A.P.; Habrat, W.; Lauro, C.H.; Robson, B.D.; Brandão, L.C.; Davim, J.P. Design of Experiments in Production Engineering; Davim, J.P., Ed.; Management and Industrial Engineering; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-23837-1. [Google Scholar]
- ISO 6344-2; Coated Abrasives—Determination and Designation of Grain Size Distribution—Part 2: Macrogrit Sizes P12 to P220. ISO: Geneva, Switzerland, 2021.
- UNE-EN ISO 3274; Geometrical Product Specification (GPS). Surface Texture: Profile Method. Nominal Characteristics of Contact (Stylus) Instruments. ISO: Geneva, Switzerland, 1998.
- ISO 4287; Geometrical Product Specifications (GPS)-Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parame-ters. ISO: Geneva, Switzerland, 1997.
- Todhunter, L.; Leach, R.; Lawes, S.; Blateyron, F. Industrial survey of ISO surface texture parameters. CIRP J. Manuf. Sci. Technol. 2017, 19, 84–92. [Google Scholar] [CrossRef]
- ISO 16610-21; Geometrical Product Specifications (GPS)-Filtration-Part 21: Linear Profile Filters: Gaussian Filters. ISO: Geneva, Switzerland, 2011.
- Raymond, H.M.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 978-1-118-91601-8. [Google Scholar]
- Harrington, E.C. The Desirability Function. Ind. Qual. Control 1965, 21, 494–498. [Google Scholar]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
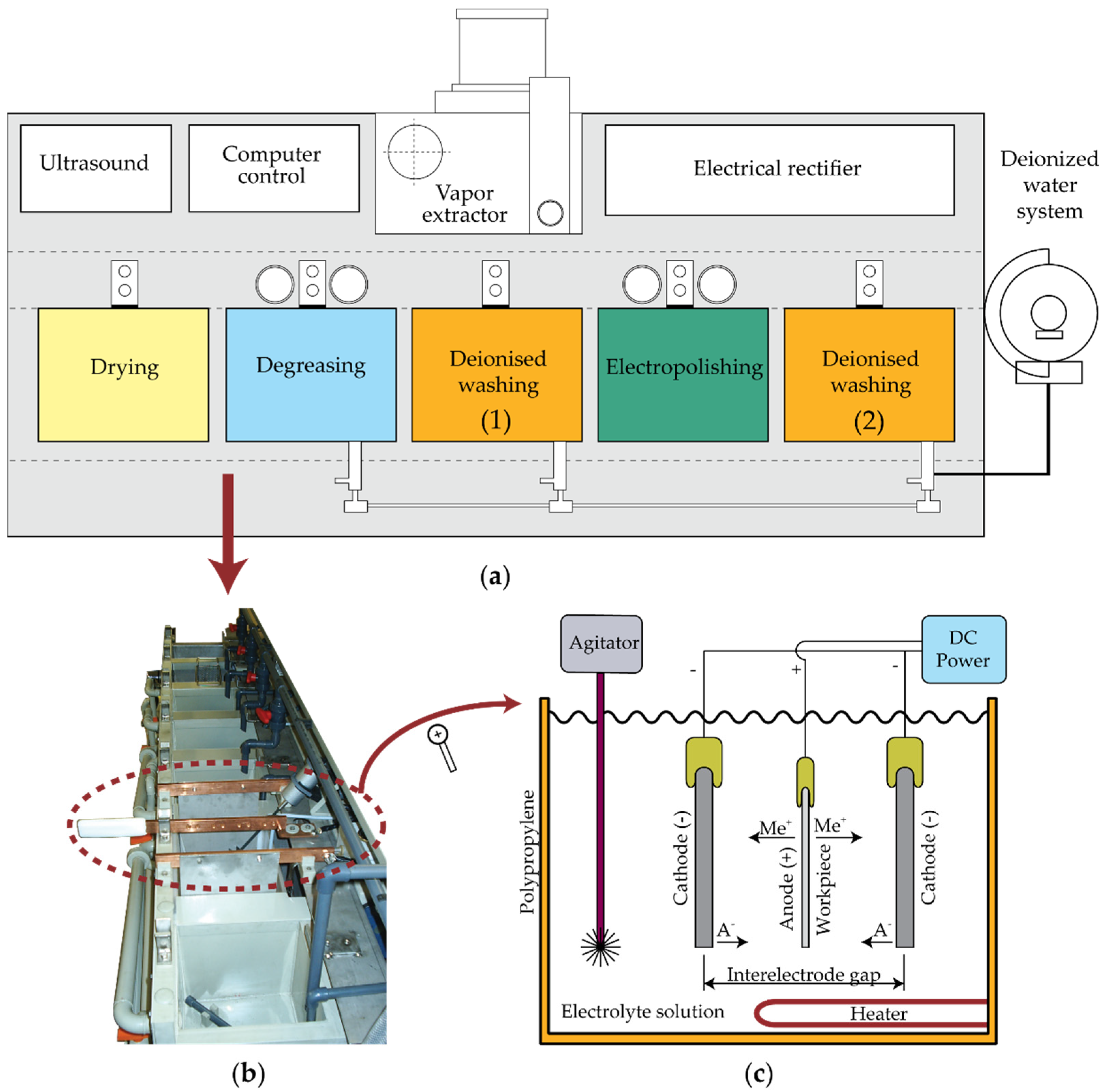
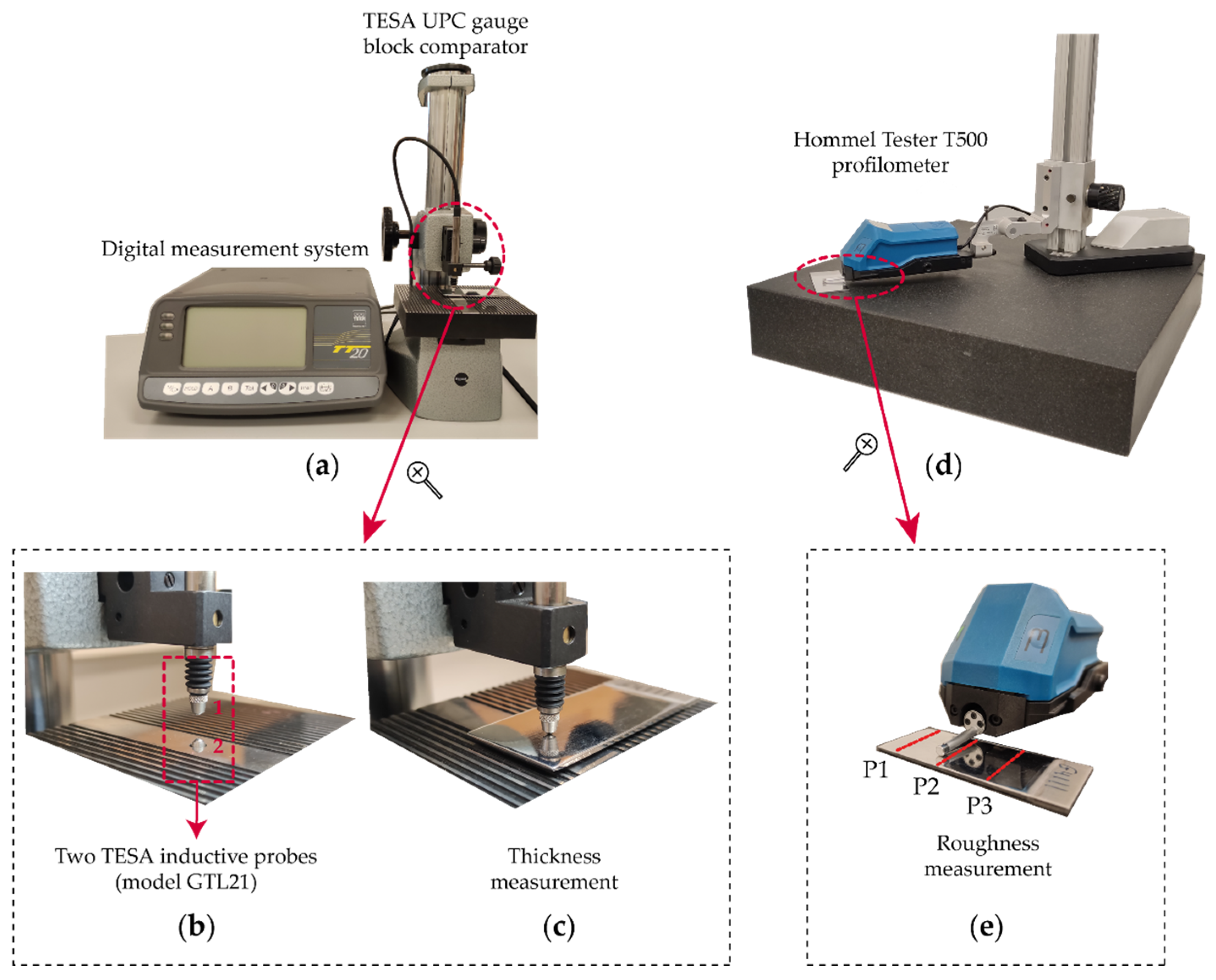



| Di (mm) | Ti (°C) | Ji (A/dm2) | ti (min) | |
|---|---|---|---|---|
| 0.5 ÷ 0.8 | 300 | 35 | 10 | 3 |
| 1.0 ÷ 1.3 | 150 | 65 | 67 | 36 |
| (µm) | Ti (°C) | Ji (A/dm2) | ti (min) |
|---|---|---|---|
| 0.5 ÷ 0.8 | 35 | 10 | 3 |
| 45 | 29 | 14 | |
| 1.0 ÷ 1.3 | 55 | 48 | 25 |
| 65 | 67 | 36 |
| Source | DF | Adj SS (Type III) | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Main effects | |||||
| A:D | 1 | 314.641 | 314.641 | 2.91 | 0.0895 |
| B:Ra0 | 1 | 1226.53 | 1226.53 | 11.35 | 0.0009 |
| C:T | 3 | 5434.81 | 1811.6 | 16.77 | 0.0000 |
| D:J | 3 | 116,275.0 | 38,758.2 | 358.69 | 0.0000 |
| E:t | 3 | 43,359.8 | 14,453.3 | 133.76 | 0.0000 |
| Interactions | |||||
| A×B | 1 | 4.25648 | 4.25648 | 0.04 | 0.8429 |
| A×C | 3 | 163.982 | 54.6607 | 0.51 | 0.6787 |
| A×D | 3 | 254.904 | 84.9681 | 0.79 | 0.5028 |
| A×E | 3 | 164.885 | 54.9616 | 0.51 | 0.6768 |
| B×C | 3 | 1201.83 | 400.609 | 3.71 | 0.0126 |
| B×D | 3 | 2139.52 | 713.173 | 6.60 | 0.0003 |
| B×E | 3 | 372.487 | 124.162 | 1.15 | 0.3305 |
| C×D | 9 | 14,686.0 | 1631.77 | 15.10 | 0.0000 |
| C×E | 9 | 739.626 | 82.1807 | 0.76 | 0.6530 |
| D×E | 9 | 5950.54 | 661.171 | 6.12 | 0.0000 |
| Error | 198 | 21,395.0 | 108.056 | ||
| Total | 255 | 213,683.0 |
| Source | DF | Adj SS (Type III) | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| J | 1 | 1859 | 1859.5 | 16.25 | 0.000 |
| t | 1 | 5583 | 5582.8 | 48.77 | 0.000 |
| T2 | 1 | 4889 | 4889.1 | 42.71 | 0.000 |
| J2 | 1 | 4964 | 4964.4 | 43.37 | 0.000 |
| t2 | 1 | 1560 | 1560.4 | 13.63 | 0.001 |
| T×J | 1 | 2824 | 2824.5 | 24.68 | 0.000 |
| J×t | 1 | 2032 | 2031.6 | 17.75 | 0.000 |
| Error | 56 | 6410 | 114.5 | ||
| Total | 63 | 45,798 |
| Temperature (°C) | Maximum ΔRa (%) | EP Time (min) | Current Density (A/dm2) |
|---|---|---|---|
| 35 | 89.98 | 35 | 48 |
| 45 | 90.72 | 35 | 52 |
| 55 | 89.34 | 35 | 56 |
| 65 | 85.85 | 35 | 60 |
| Source | DF | Adj SS (Type III) | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| J | 1 | 6084 | 6084.0 | 36.89 | 0.000 |
| t | 1 | 5496 | 5496.2 | 33.33 | 0.000 |
| J2 | 1 | 1903 | 1903.5 | 11.54 | 0.001 |
| t2 | 1 | 1499 | 1499.4 | 9.09 | 0.004 |
| J×t | 1 | 2124 | 2124.3 | 12.88 | 0.001 |
| Error | 57 | 9400 | 164.9 | ||
| Total | 62 | 36,896 |
| Source | DF | Adj SS (Type III) | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| J | 1 | 0.08481 | 0.084811 | 15.74 | 0.000 |
| t | 1 | 0.14125 | 0.141251 | 26.21 | 0.000 |
| T2 | 1 | 0.15370 | 0.153703 | 28.52 | 0.000 |
| J2 | 1 | 0.23281 | 0.232806 | 43.19 | 0.000 |
| t2 | 1 | 0.03331 | 0.033306 | 6.18 | 0.016 |
| T×J | 1 | 0.10078 | 0.100779 | 18.70 | 0.000 |
| J×t | 1 | 0.04774 | 0.047742 | 8.86 | 0.004 |
| Error | 56 | 0.30183 | 0.005390 | ||
| Total | 63 | 1.71170 |
| Temperature (°C) | EP Time (min) | Current Density (A/dm2) | Minimum Raf (µm) |
|---|---|---|---|
| 35 | 35 | 46 | 0.035 |
| 45 | 35 | 52 | 0.049 |
| 55 | 35 | 56 | 0.051 |
| 65 | 35 | 62 | 0.044 |
| Source | DF | Adj SS (Type III) | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| J | 1 | 0.6686 | 0.66861 | 31.37 | 0.000 |
| t | 1 | 0.6862 | 0.68621 | 32.20 | 0.000 |
| J2 | 1 | 0.1947 | 0.19470 | 9.13 | 0.004 |
| t2 | 1 | 0.1947 | 0.19470 | 9.13 | 0.004 |
| J×t | 1 | 0.2624 | 0.26240 | 12.31 | 0.001 |
| Error | 58 | 1.2362 | 0.02131 | ||
| Total | 63 | 4.3777 |
| Source | DF | Adj SS (Type III) | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| T | 1 | 0.19935 | 0.19935 | 24.77 | 0.000 |
| J | 1 | 0.03427 | 0.03427 | 4.26 | 0.043 |
| J2 | 1 | 0.03290 | 0.03290 | 4.09 | 0.048 |
| J×t | 1 | 2.58172 | 2.58172 | 320.78 | 0.000 |
| Error | 59 | 0.47484 | 0.00805 | ||
| Total | 63 | 4.68603 |
| Temperature (°C) | Δhmin (%) | Current Density (A/dm2) | EP Time (min) | Δhmax (%) | Current Density (A/dm2) | EP Time (min) |
|---|---|---|---|---|---|---|
| 35 | 0.031 | 32 | 3 | 0.887 | 67 | 36 |
| 45 | 0.081 | 32 | 3 | 0.937 | 67 | 36 |
| 55 | 0.130 | 32 | 3 | 0.986 | 67 | 36 |
| 65 | 0.180 | 32 | 3 | 1.036 | 67 | 36 |
| Source | DF | Adj SS (Type III) | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| J2 | 1 | 0.09376 | 0.09376 | 8.58 | 0.005 |
| T×J | 1 | 0.12918 | 0.12918 | 11.82 | 0.001 |
| J×t | 1 | 2.31068 | 2.31068 | 211.37 | 0.000 |
| Error | 60 | 0.65592 | 0.01093 | ||
| Total | 63 | 4.45284 |
| Temperature (°C) | Δhmin (%) | Current Density (A/dm2) | EP Time (min) | Δhmax (%) | Current Density (A/dm2) | EP Time (min) |
|---|---|---|---|---|---|---|
| 35 | 0.131 | 3 | 10 | 0.868 | 36 | 67 |
| 45 | 0.139 | 3 | 10 | 0.922 | 36 | 67 |
| 55 | 0.147 | 3 | 10 | 0.977 | 36 | 67 |
| 65 | 0.156 | 3 | 10 | 1.032 | 36 | 67 |
| Electropolishing Range | Optimal Points | J (A/dm2) | t (min) | P (%) | D (mm) | C (kWh/dm2) |
|---|---|---|---|---|---|---|
| 90% | P90 | 52 | 33.0 | 90.50 | 0.680 | 0.240 |
| D90 | 50 | 31.0 | 90.00 | 0.620 | 0.210 | |
| C90 | 50 | 31.0 | 90.00 | 0.620 | 0.210 | |
| O90 | 51 | 32.0 | 90.37 | 0.650 | 0.230 | |
| 80% | P80 | 44 | 21.0 | 82.06 | 0.380 | 0.120 |
| D80 | 44 | 19.0 | 80.00 | 0.350 | 0.100 | |
| C80 | 40 | 21.0 | 80.00 | 0.350 | 0.100 | |
| O80 | 42 | 20.0 | 80.00 | 0.350 | 0.100 | |
| 70% | P70 | 42 | 15.0 | 74.13 | 0.271 | 0.080 |
| D70 | 44 | 11.0 | 70.00 | 0.220 | 0.063 | |
| C70 | 44 | 11.0 | 70.00 | 0.220 | 0.063 | |
| O70 | 43 | 13.0 | 71.97 | 0.240 | 0.072 | |
| 60% | P60 | 48 | 6.3 | 62.50 | 0.150 | 0.040 |
| D60 | 46 | 5.0 | 60.00 | 0.127 | 0.031 | |
| C60 | 46 | 5.0 | 60.00 | 0.127 | 0.031 | |
| O60 | 47 | 5.6 | 61.34 | 0.141 | 0.036 |
| Electropolishing Range | Optimal Points | J (A/dm2) | t (min) | P (%) | D (mm) | C (kWh/dm2) |
|---|---|---|---|---|---|---|
| 80% | P80 | 47.00 | 25.00 | 81.50 | 0.570 | 0.160 |
| D80 | 51.50 | 21.00 | 80.00 | 0.531 | 0.156 | |
| C80 | 44.00 | 25.00 | 80.00 | 0.540 | 0.144 | |
| O80 | 47.75 | 23.00 | 80.47 | 0.539 | 0.150 | |
| 70% | P70 | 56.00 | 13.50 | 70.80 | 0.400 | 0.110 |
| D70 | 56.00 | 12.50 | 70.00 | 0.390 | 0.107 | |
| C70 | 44.00 | 15.50 | 70.00 | 0.400 | 0.088 | |
| O70 | 50.00 | 14.00 | 70.70 | 0.390 | 0.098 | |
| 60% | P60 | 60.00 | 7.50 | 62.00 | 0.300 | 0.073 |
| D60 | 60.00 | 7.00 | 60.00 | 0.280 | 0.064 | |
| C60 | 47.50 | 8.50 | 60.00 | 0.300 | 0.056 | |
| O60 | 53.75 | 7.75 | 60.82 | 0.289 | 0.061 |
| Response | Goal | Lower | Target | Upper | Weight | Importance |
|---|---|---|---|---|---|---|
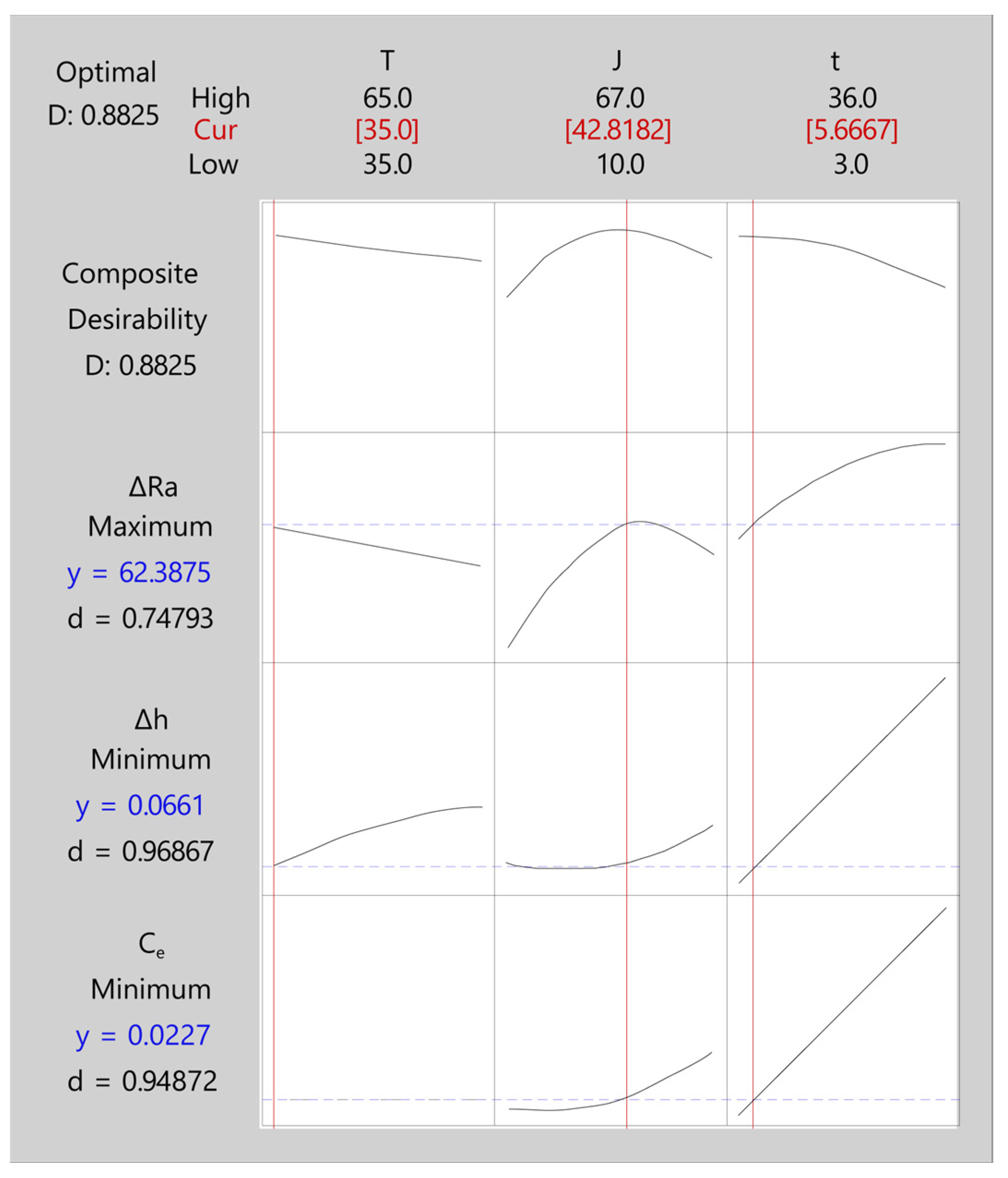
| ΔRa | Maximum | 0.0000 | 83.4133 | 83.4133 | 1 | 1 |
| Δh | Minimum | 0.0300 | 0.0300 | 1.18100 | 1 | 1 |
| Ce | Minimum | 0.0025 | 0.0025 | 0.39799 | 1 | 1 |
| Solution | T | J | t | ΔRa Fit | Δh Fit | Ce Fit | Composite Desirability |
|---|---|---|---|---|---|---|---|
| 1 | 35.0000 | 42.8182 | 5.66667 | 62.3875 | 0.066059 | 0.0227400 | 0.882521 |
| 2 | 35.0008 | 51.4402 | 3.00106 | 57.2971 | 0.039154 | 0.0151337 | 0.870483 |
| 3 | 35.0123 | 52.1628 | 3.00127 | 57.1020 | 0.040973 | 0.0160720 | 0.868321 |
| 4 | 35.0015 | 52.8449 | 3.00146 | 56.8909 | 0.042548 | 0.0169848 | 0.866159 |
| 5 | 49.9811 | 48.7552 | 3.00000 | 53.3307 | 0.140660 | 0.0119065 | 0.826252 |
| Response | Fit | SE Fit | 95% CI |
|---|---|---|---|
| ΔRa | 62.3875 | 3.34 (4%) | (55.71; 69.07) |
| Δh | 0.066059 | 0.0289 (2.44%) | (0.0082; 0.1239) |
| Ce | 0.02274 | 0.00164 (0.41%) | (0.01945; 0.02603) |
| Response | Goal | Lower | Target | Upper | Weight | Importance |
|---|---|---|---|---|---|---|
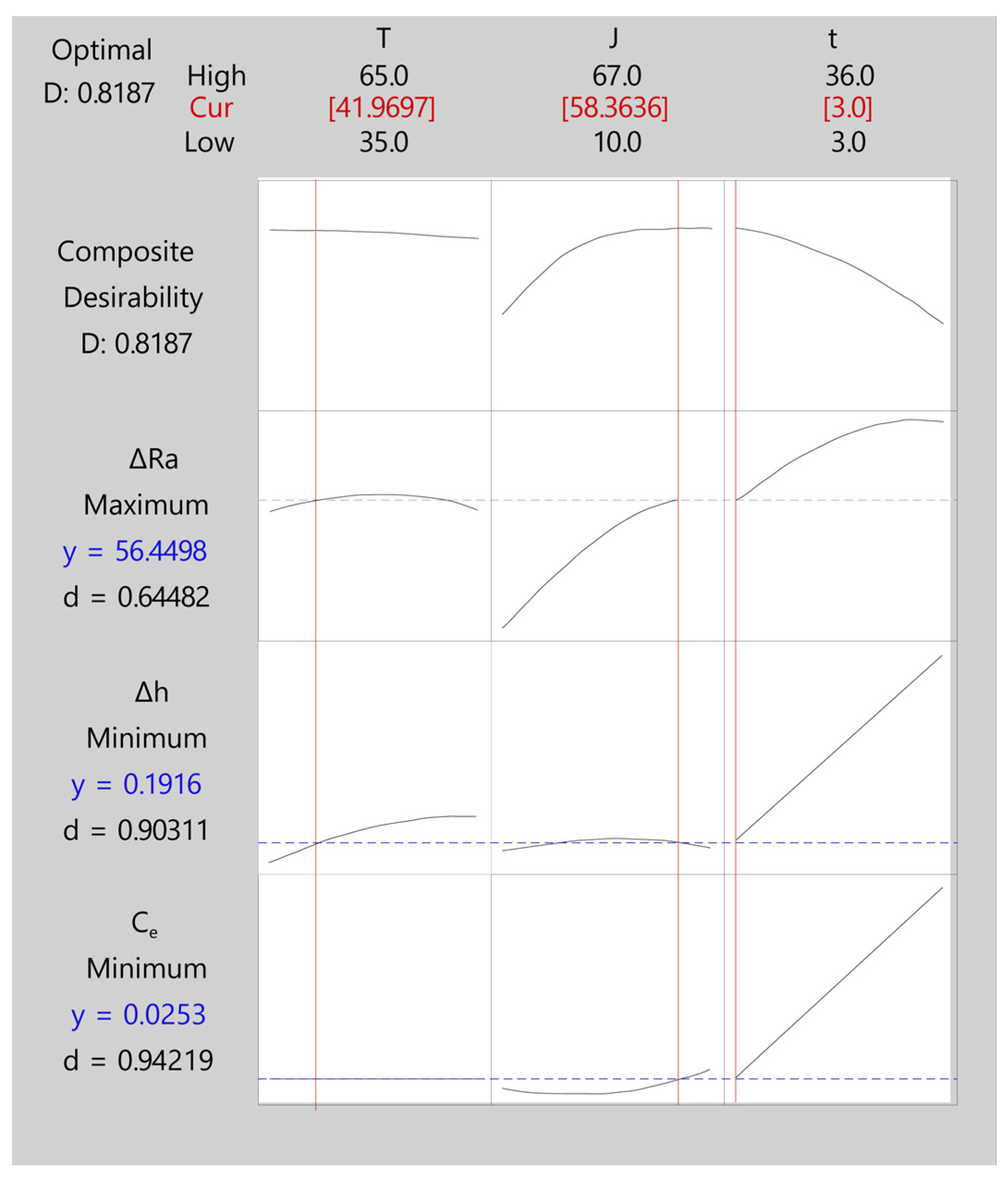
| ΔRa | Maximum | 0.0000 | 87.5433 | 87.5433 | 1 | 1 |
| Δh | Minimum | 0.0930 | 0.0930 | 1.11100 | 1 | 1 |
| Ce | Minimum | 0.0025 | 0.0025 | 0.39799 | 1 | 1 |
| Solution | T | J | t | ΔRa Fit | Δh Fit | Ce Fit | Composite Desirability |
|---|---|---|---|---|---|---|---|
| 1 | 41.9697 | 58.3636 | 3 | 56.4498 | 0.191636 | 0.0253191 | 0.818665 |
| 2 | 36.7169 | 66.9963 | 3 | 52.3271 | 0.117671 | 0.0418509 | 0.806792 |
| 3 | 35.1342 | 66.9962 | 3 | 50.7346 | 0.098369 | 0.0418508 | 0.803661 |
| 4 | 35.1003 | 66.9962 | 3 | 50.6988 | 0.097945 | 0.0418508 | 0.803584 |
| 5 | 35.0971 | 66.9962 | 3 | 50.6954 | 0.097905 | 0.0418508 | 0.803577 |
| Response | Fit | SE Fit | 95% CI |
|---|---|---|---|
| ΔRa | 56.45 | 4.88 (5.57%) | (46.66; 66.23) |
| Δh | 0.1916 | 0.0335 (3.01%) | (0.1245; 0.2588) |
| Ce | 0.02532 | 0.00194 (0.48%) | (0.02144; 0.02920) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beamud-González, E.M.; Núñez-López, P.J.; García-Plaza, E. Electropolishing Stainless Steel Optimization Using Surface Quality, Dimensional Accuracy, and Electrical Consumption Criteria. Materials 2023, 16, 1770. https://doi.org/10.3390/ma16051770
Beamud-González EM, Núñez-López PJ, García-Plaza E. Electropolishing Stainless Steel Optimization Using Surface Quality, Dimensional Accuracy, and Electrical Consumption Criteria. Materials. 2023; 16(5):1770. https://doi.org/10.3390/ma16051770
Chicago/Turabian StyleBeamud-González, Elena María, Pedro José Núñez-López, and Eustaquio García-Plaza. 2023. "Electropolishing Stainless Steel Optimization Using Surface Quality, Dimensional Accuracy, and Electrical Consumption Criteria" Materials 16, no. 5: 1770. https://doi.org/10.3390/ma16051770
APA StyleBeamud-González, E. M., Núñez-López, P. J., & García-Plaza, E. (2023). Electropolishing Stainless Steel Optimization Using Surface Quality, Dimensional Accuracy, and Electrical Consumption Criteria. Materials, 16(5), 1770. https://doi.org/10.3390/ma16051770








