Significance of Melt Pool Structure on the Hydrogen Embrittlement Behavior of a Selective Laser-Melted 316L Austenitic Stainless Steel
Abstract
1. Introduction
2. Experimental Procedures
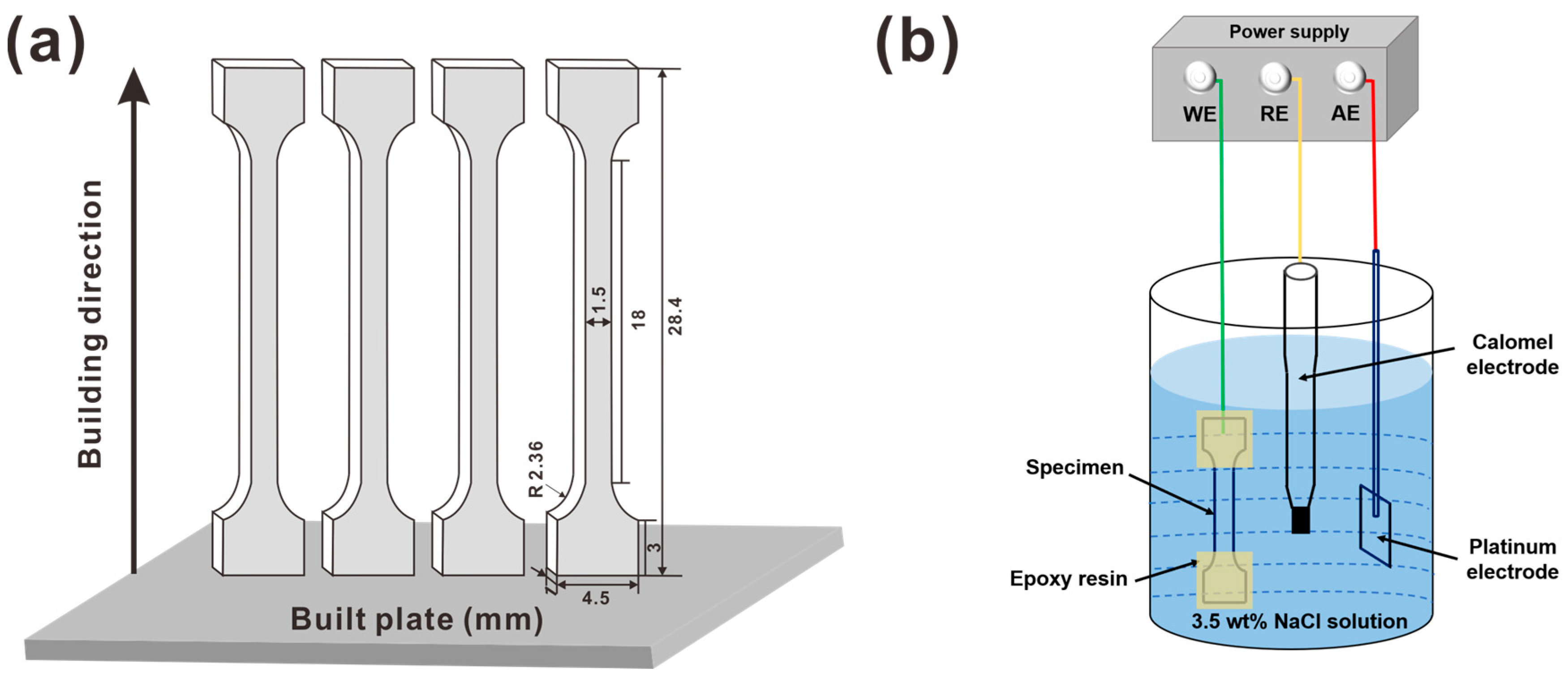
2.1. Material Fabrication
2.2. Electrochemical Hydrogen Charging and SSRT Test
2.3. Microstructure Characterizations
2.4. Hydrogen Distribution Analysis by TOF-SIMS
3. Results
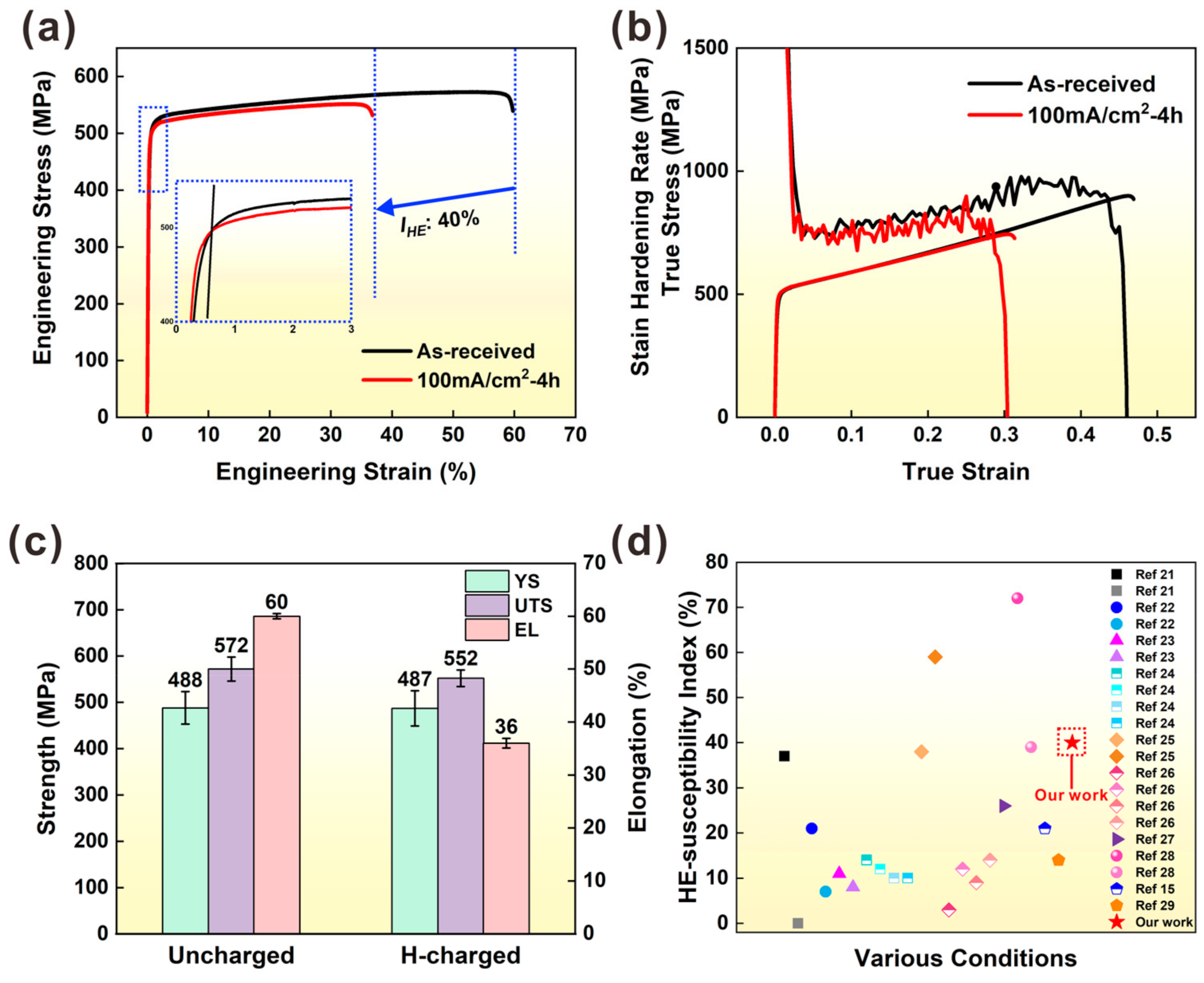
3.1. Tensile Properties of Uncharged and H-Charged Samples
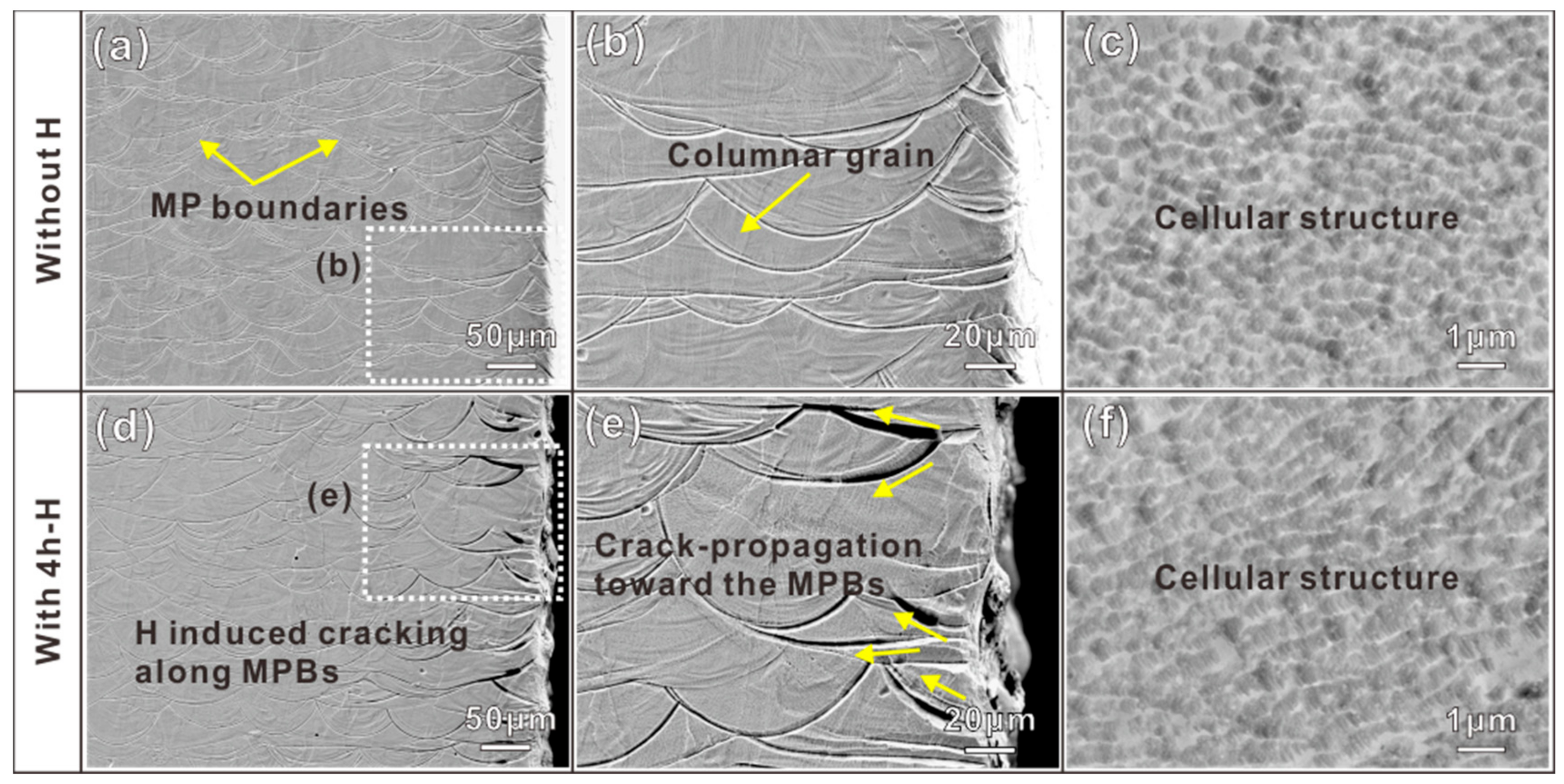
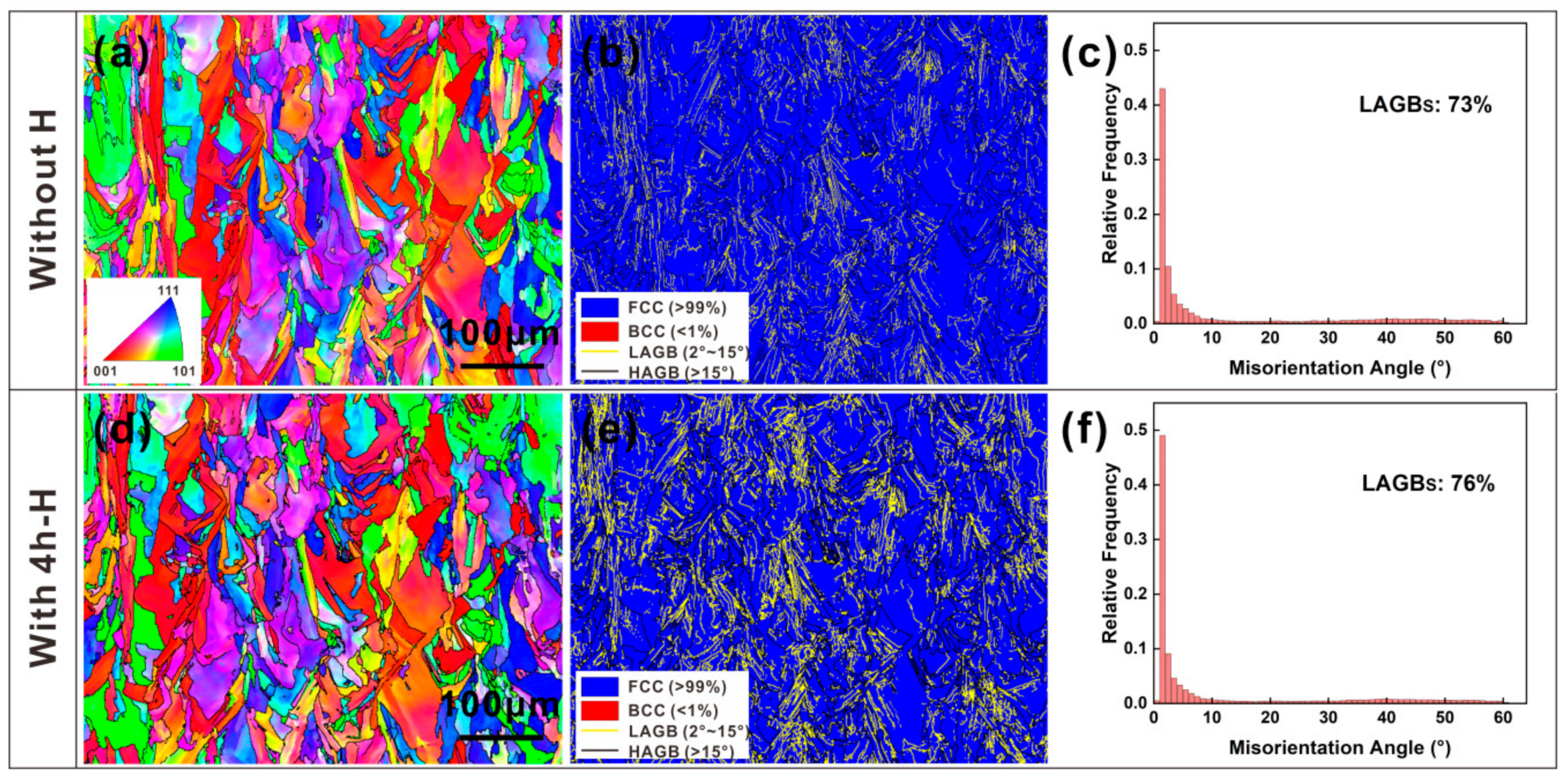
3.2. Microstructures of Samples without and with H
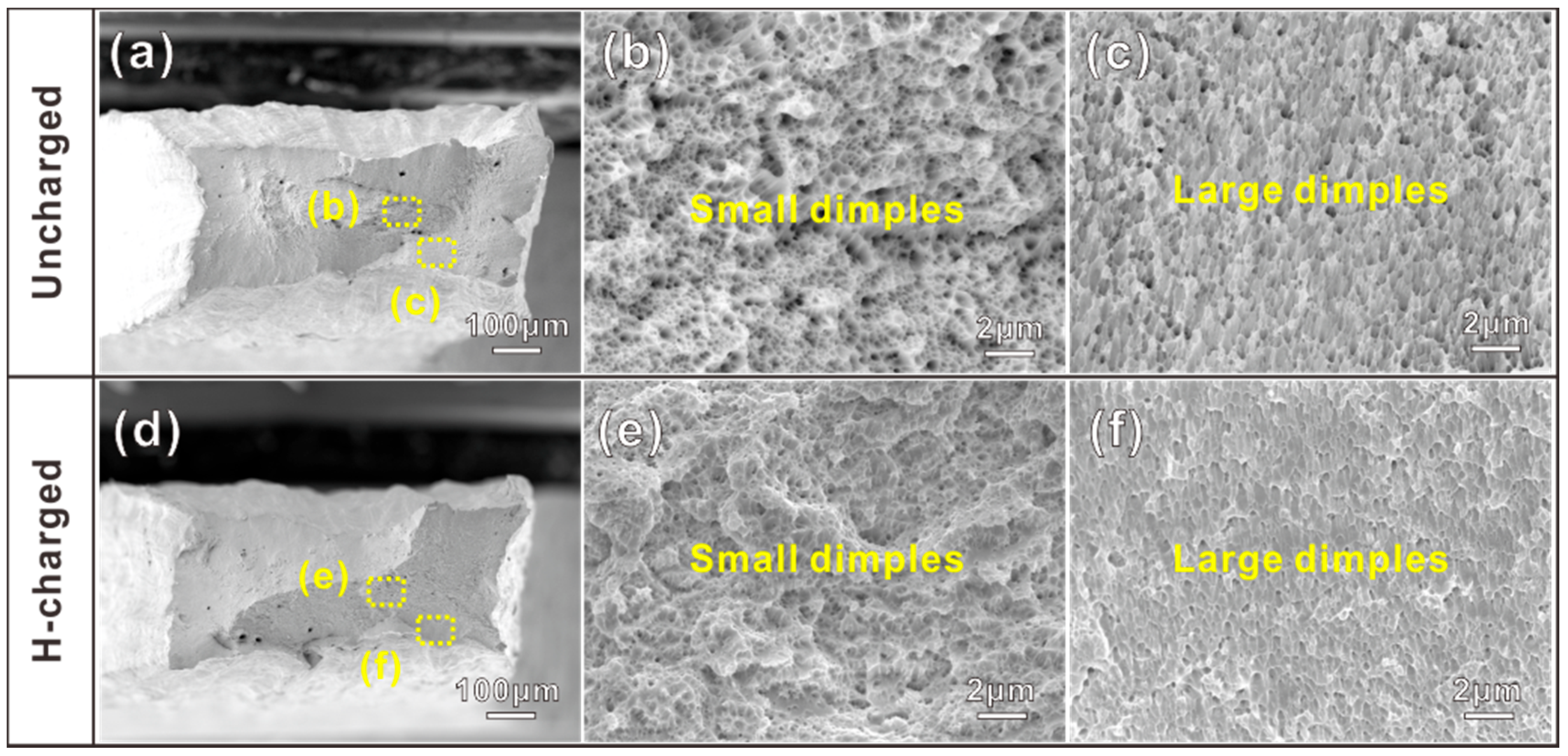
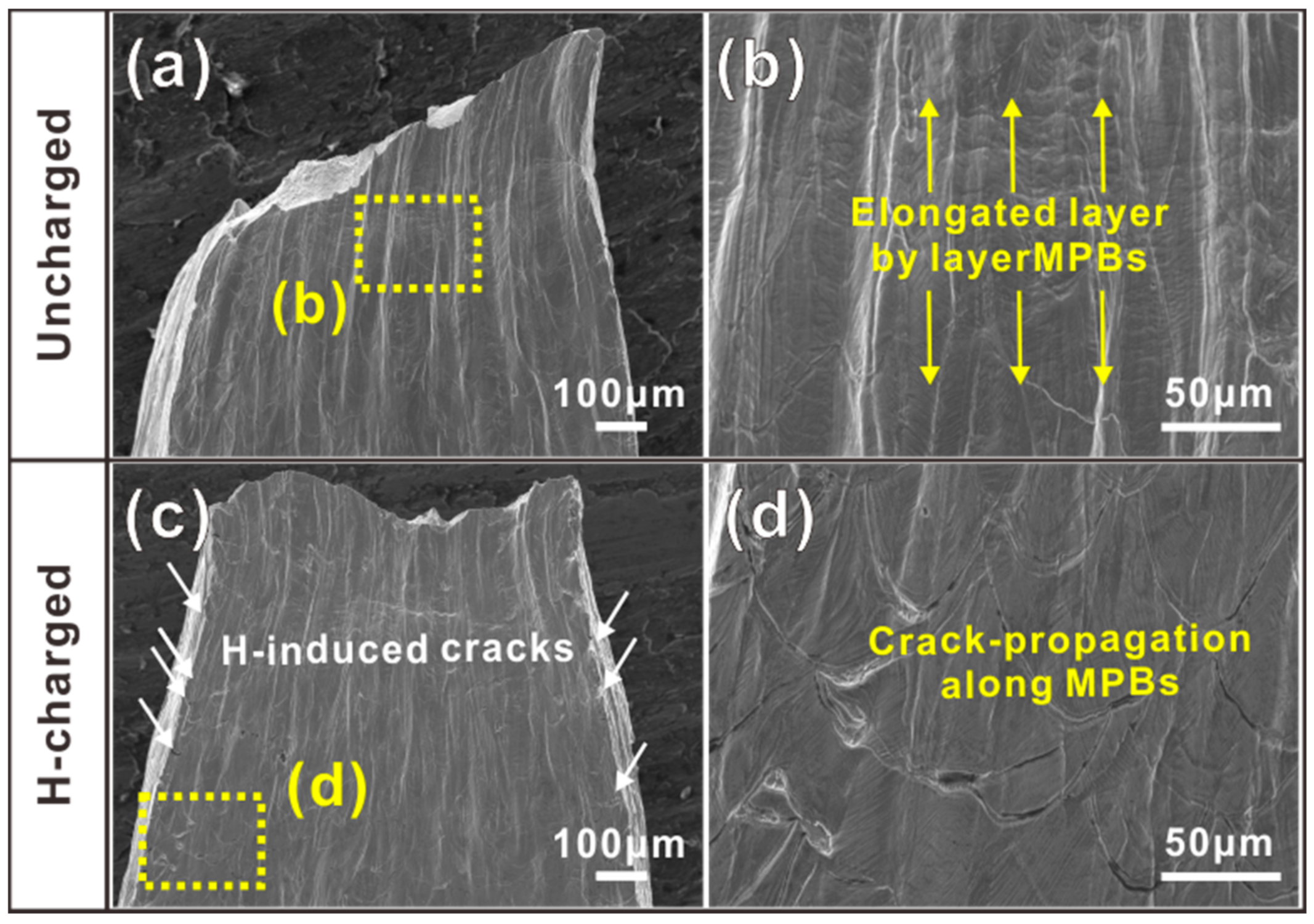
3.3. Fracture Analysis
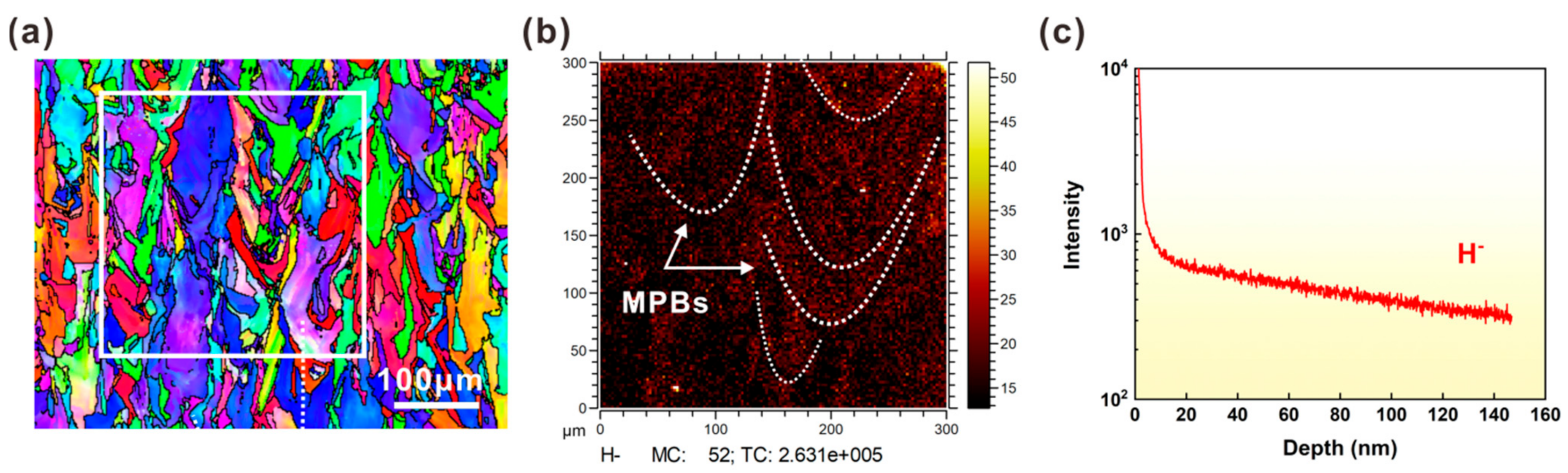
3.4. TOF-SIMS Results Analysis
4. Discussion
4.1. Effect of Hydrogen on Mechanical Properties
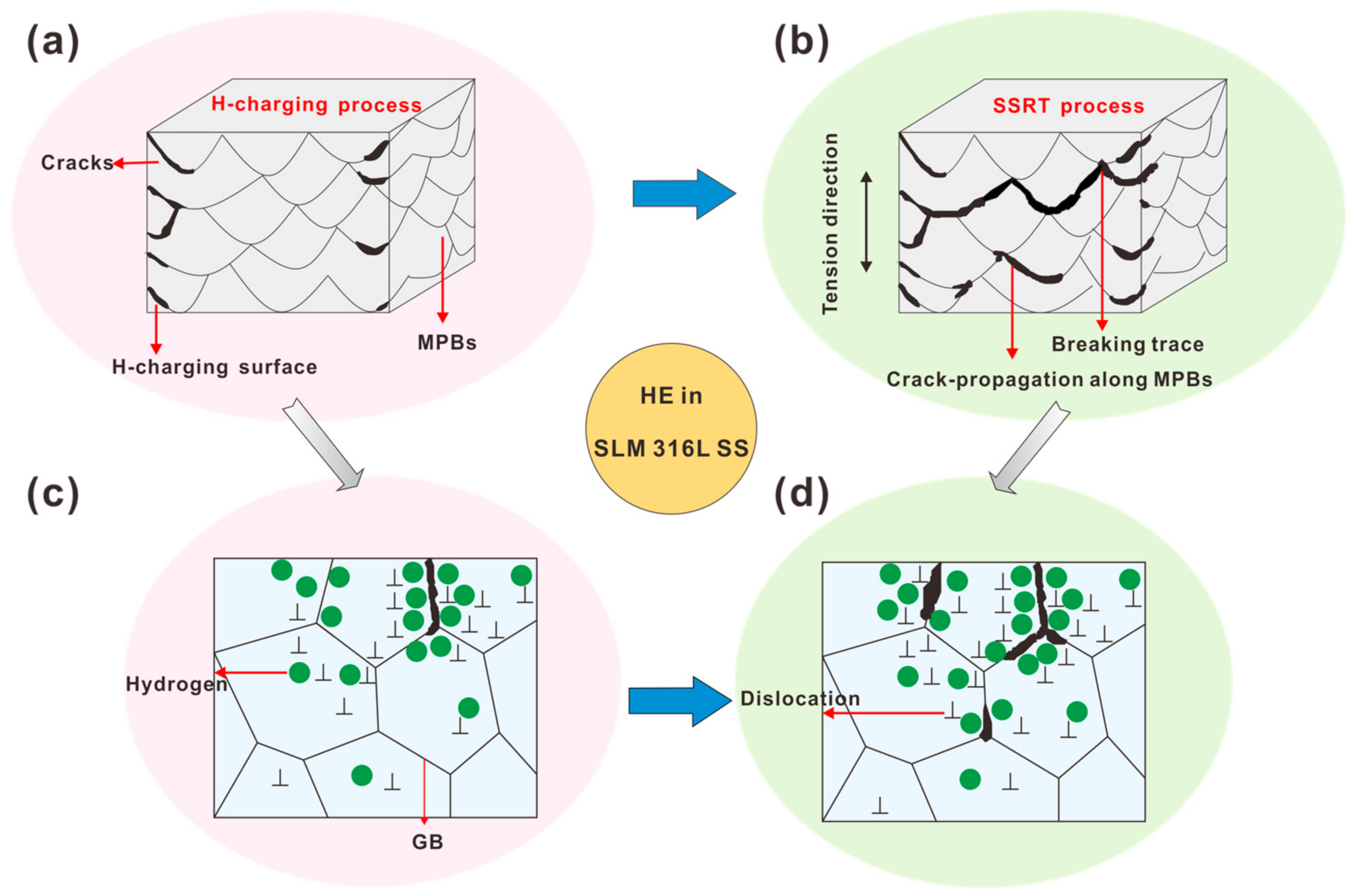
4.2. H-Induced MPB Cracking
5. Conclusions
- Hydrogen pre-charging caused cracking along the MPBs. Therefore, stress concentrations occurred around the cracks, which eventually led to the degradation of mechanical properties. In particular, tensile ductility decreased significantly from 60% to 36% in samples after 4 h of hydrogen charging.
- The hydrogen level rose at the MPBs of the SLM 316L SS sample after H-charging. This was mostly distributed on the surface of the sample, and the MPBs acted as hydrogen traps during hydrogen charging.
- The γ (austenite) to α’ (martensite) phase transition in the SLM 316L SS did not occur during hydrogen charging at room temperature because of the high austenite stability, which suggests that the martensite transition is not the reason for the reduction in the mechanical properties of the samples.
- Under a slow strain rate of 10−5 s−1 tensile loading conditions, H-assisted cracks first appeared on the sample MPBs’ surface, and then H diffused mainly along the MPBs, finally leading to the fracture of the sample.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choo, H.; Sham, K.-L.; Bohling, J.; Ngo, A.; Xiao, X.; Ren, Y.; Depond, P.J.; Matthews, M.J.; Garlea, E. Effect of laser power on defect, texture, and microstructure of a laser powder bed fusion processed 316L stainless steel. Mater. Des. 2019, 164, 107534. [Google Scholar] [CrossRef]
- Ben, D.D.; Yang, H.J.; Gao, J.B.; Yang, B.Y.; Dong, Y.A.; Liu, X.Y.; Wang, X.G.; Duan, Q.Q.; Zhang, P.; Zhang, Z.F. Rapid Microstructure Homogenization of a Laser Melting Deposition Additive Manufactured Ti-6.5Al-3.5Mo-1.5Zr-0.3Si Alloy by Electropulsing. Materials 2022, 15, 7103. [Google Scholar] [CrossRef]
- Wang, Z.T.; Yang, S.L.; Huang, Y.B.; Fan, C.; Peng, Z.; Gao, Z.H. Microstructure and Fatigue Damage of 316L Stainless Steel Manufactured by Selective Laser Melting (SLM). Materials 2021, 14, 7544. [Google Scholar] [CrossRef]
- Gorsse, S.; Hutchinson, C.; Goune, M.; Banerjee, R. Additive manufacturing of metals: A brief review of the characteristic microstructures and properties of steels, Ti-6Al-4V and high-entropy alloys. Sci. Technol. Adv. Mater. 2017, 18, 584–610. [Google Scholar] [CrossRef] [PubMed]
- Sabooni, S.; Chabok, A.; Feng, S.C.; Blaauw, H.; Pijper, T.C.; Yang, H.J.; Pei, Y.T. Laser powder bed fusion of 17–4 PH stainless steel: A comparative study on the effect of heat treatment on the microstructure evolution and mechanical properties. Addit. Manuf. 2021, 46, 102176. [Google Scholar] [CrossRef]
- Xu, Z.W.; Liu, A.; Wang, X.S.; Liu, B.; Guo, M.H. Fatigue limit prediction model and fatigue crack growth mechanism for selective laser melting Ti6Al4V samples with inherent defects. Int. J. Fatigue 2021, 143, 106008. [Google Scholar] [CrossRef]
- Kumar, P.; Jayaraj, R.; Suryawanshi, J.; Satwik, U.R.; McKinnell, J.; Ramamurty, U. Fatigue strength of additively manufactured 316L austenitic stainless steel. Acta Mater. 2020, 199, 225–239. [Google Scholar] [CrossRef]
- Hu, X.X.; Lach, T.G.; Terrani, K.A. Deuterium permeation and retention in 316L Stainless Steel Manufactured by Laser Powder Bed Fusion. J. Nucl. Mater. 2021, 548, 152871. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Carvalho, O.; Silva, F.S.; Miranda, G. 316L stainless steel mechanical and tribological behavior—A comparison between selective laser melting, hot pressing and conventional casting. Addit. Manuf. 2017, 16, 81–89. [Google Scholar] [CrossRef]
- Lee, D.-H.; Sun, B.; Lee, S.; Ponge, D.; Jägle, E.A.; Raabe, D. Comparative study of hydrogen embrittlement resistance between additively and conventionally manufactured 304L austenitic stainless steels. Mater. Sci. Eng. A 2021, 803, 140499. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Hwang, J.-S.; Kim, M.-S.; Kim, J.-H.; Kim, S.-K.; Lee, J.-M. Charpy Impact Properties of Hydrogen-Exposed 316L Stainless Steel at Ambient and Cryogenic Temperatures. Metals 2019, 9, 625. [Google Scholar] [CrossRef]
- Man, C.; Duan, Z.W.; Cui, Z.Y.; Dong, C.F.; Kong, D.C.; Liu, T.T.; Chen, S.G.; Wang, X. The effect of sub-grain structure on intergranular corrosion of 316L stainless steel fabricated via selective laser melting. Mater. Lett. 2019, 243, 157–160. [Google Scholar] [CrossRef]
- Sánchez-Molina, M.; Amores, E.; Rojas, N.; Kunowsky, M. Additive manufacturing of bipolar plates for hydrogen production in proton exchange membrane water electrolysis cells. Int. J. Hydrog. Energy 2021, 46, 38983–38991. [Google Scholar] [CrossRef]
- Khaleghifar, F.; Razeghi, K.; Heidarzadeh, A.; Taherzadeh Mousavian, R. Effect of Hydrogen on the Tensile Behavior of Austenitic Stainless Steels 316L Produced by Laser-Powder Bed Fusion. Metals 2021, 11, 586. [Google Scholar] [CrossRef]
- Bertsch, K.M.; Nagao, A.; Rankouhi, B.; Kuehl, B.; Thoma, D.J. Hydrogen embrittlement of additively manufactured austenitic stainless steel 316 L. Corros. Sci. 2021, 192, 109790. [Google Scholar] [CrossRef]
- Kong, D.C.; Dong, C.F.; Ni, X.Q.; Zhang, L.; Luo, H.; Li, R.X.; Wang, L.; Man, C.; Li, X.G. Superior resistance to hydrogen damage for selective laser melted 316L stainless steel in a proton exchange membrane fuel cell environment. Corros. Sci. 2020, 166, 108425. [Google Scholar] [CrossRef]
- Miller, J.T.; Martin, H.J.; Cudjoe, E. Comparison of the effects of a sulfuric acid environment on traditionally manufactured and additive manufactured stainless steel 316L alloy. Addit. Manuf. 2018, 23, 272–286. [Google Scholar] [CrossRef]
- Karnesky, R.A.; Chao, P.; Buchenauer, D.A. Hydrogen isotope permeation and trapping in additively manufactured steels. In Proceedings of the ASME Pressure Vessels and Piping Conference, Waikoloa, HI, USA, 16–20 July 2017. [Google Scholar]
- Lodhi, M.J.K.; Deen, K.M.; Greenlee-Wacker, M.C.; Haider, W. Additively manufactured 316L stainless steel with improved corrosion resistance and biological response for biomedical applications. Addit. Manuf. 2019, 27, 8–19. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Wan, J.F.; Zhao, Q.Y.; Liu, J.; Huang, F.; Huang, Y.H.; Li, X.G. Dual role of nanosized NbC precipitates in hydrogen embrittlement susceptibility of lath martensitic steel. Corros. Sci. 2020, 164, 108345. [Google Scholar] [CrossRef]
- Baek, S.-W.; Song, E.J.; Kim, J.H.; Lee, Y.-H.; Ryu, K.S.; Kim, S.-W. Hydrogen susceptibility of nano-sized oxide dispersed austenitic steel for fusion reactor. Fusion Eng. Des. 2017, 121, 105–110. [Google Scholar] [CrossRef]
- Huang, S.; Ma, D.H.; Sheng, J.; Agyenim-Boateng, E.; Zhao, J.X.; Zhou, J.Z. Effects of laser peening on tensile properties and martensitic transformation of AISI 316L stainless steel in a hydrogen-rich environment. Mater. Sci. Eng. A 2020, 788, 139543. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Kim, D.; Kim, S.; Nam, W.; Choe, H. Effects of Hydrogen Diffusion on the Mechanical Properties of Austenite 316L Steel at Ambient Temperature. Mater. Trans. 2011, 52, 507–513. [Google Scholar] [CrossRef]
- Huang, S.; Yuan, G.; Sheng, J.; Tan, W.S.; Agyenim-Boateng, E.; Zhou, J.Z.; Guo, H.F. Strengthening mechanism and hydrogen-induced crack resistance of AISI 316L stainless steel subjected to laser peening at different power densities. Int. J. Hydrog. Energy 2018, 43, 11263–11274. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Park, J.; Nahm, S.H.; Tak, N.; Baek, U.B. Ductility and fatigue properties of low nickel content type 316L austenitic stainless steel after gaseous thermal pre-charging with hydrogen. Int. J. Hydrog. Energy 2019, 44, 28031–28043. [Google Scholar] [CrossRef]
- Mati, D.; Takasaki, A.; Uematsu, S. Hydrogen Susceptibility of Pre-strained Type 316L Austenitic Stainless Steels in Aqueous Solutions. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 229. [Google Scholar]
- Pu, Z.; Chen, Y.; Dai, L.H. Strong resistance to hydrogen embrittlement of high-entropy alloy. Mater. Sci. Eng. A 2018, 736, 156–166. [Google Scholar] [CrossRef]
- Matsuoka, S.; Yamabe, J.; Matsunaga, H. Criteria for determining hydrogen compatibility and the mechanisms for hydrogen-assisted, surface crack growth in austenitic stainless steels. Eng. Fract. Mech. 2016, 153, 103–127. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, D.; Kim, Y.; Kim, D.W.; Kim, S.; Nam, W.; Kim, Y.-S.; Máthis, K.; Choe, H. Hydrogen Softening in the Thin Plate of Microcrystalline 316L Stainless Steel. Steel Res. Int. 2013, 84, 812–817. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.T.; Li, R.T.; Niu, P.D.; Wang, M.B.; Yuan, T.C.; Li, K. Hall-Petch relationship in selective laser melting additively manufactured metals: Using grain or cell size? J. Cent. South Univ. 2021, 28, 1043–1057. [Google Scholar] [CrossRef]
- Yu, C.F.; Zhong, Y.; Zhang, P.; Zhang, Z.J.; Zhao, C.C.; Zhang, Z.F.; Shen, Z.J.; Liu, W. Effect of Build Direction on Fatigue Performance of L-PBF 316L Stainless Steel. Acta Metall Sin-Engl. 2020, 33, 539–550. [Google Scholar] [CrossRef]
- Ben, D.D.; Ma, Y.R.; Yang, H.J.; Meng, L.X.; Shao, X.H.; Liu, H.Q.; Wang, S.G.; Duan, Q.Q.; Zhang, Z.F. Heterogeneous microstructure and voids dependence of tensile deformation in a selective laser melted AlSi10Mg alloy. Mater. Sci. Eng. A 2020, 798, 140109. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Zhong, Y.; Shen, Z.J. Hardened austenite steel with columnar sub-grain structure formed by laser melting. Mater. Sci. Eng. A 2015, 625, 221–229. [Google Scholar] [CrossRef]
- Yang, H.J.; Yin, S.M.; Huang, C.X.; Zhang, Z.F.; Wu, S.D.; Li, S.X.; Liu, Y.D. EBSD Study on Deformation Twinning in AZ31 Magnesium Alloy during Quasi-in-Situ Compression. Adv. Eng. Mater. 2008, 10, 955–960. [Google Scholar] [CrossRef]
- Nagao, A.; Dadfarnia, M.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. Hydrogen-enhanced-plasticity mediated decohesion for hydrogen-induced intergranular and “quasi-cleavage” fracture of lath martensitic steels. J. Mech. Phys. Solids 2018, 112, 403–430. [Google Scholar] [CrossRef]
- Lu, K.; Lu, L.; Suresh, S. Strengthening Materials by Engineering Coherent Internal Boundaries at the Nanoscale. Science 2009, 324, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Hatano, M.; Fujinami, M.; Arai, K.; Fujii, H.; Nagumo, M. Hydrogen embrittlement of austenitic stainless steels revealed by deformation microstructures and strain-induced creation of vacancies. Acta Mater. 2014, 67, 342–353. [Google Scholar] [CrossRef]
- Eliezer, D.; Chakrapani, D.G.; Altstetter, C.J.; Pugh, E.N. The Influence of Austenite Stability on the Hydrogen Embrittlement and Stress-Corrosion Cracking of Stainless Steel. Metall. Trans. A 1979, 10, 935–941. [Google Scholar] [CrossRef]
- Hong, Y.J.; Zhou, C.S.; Zheng, Y.Y.; Zhang, L.; Zheng, J.Y.; Chen, X.Y.; An, B. Formation of strain-induced martensite in selective laser melting austenitic stainless steel. Mater. Sci. Eng. A 2019, 740–741, 420–426. [Google Scholar] [CrossRef]
- Cui, L.Q.; Jiang, F.Q.; Peng, R.L.; Mousavian, R.T.; Yang, Z.Q.; Moverare, J. Dependence of microstructures on fatigue performance of polycrystals: A comparative study of conventional and additively manufactured 316L stainless steel. Int. J. Plast. 2022, 149, 103172. [Google Scholar] [CrossRef]
- Trelewicz, J.R.; Halada, G.P.; Donaldson, O.K.; Manogharan, G. Microstructure and Corrosion Resistance of Laser Additively Manufactured 316L Stainless Steel. JOM 2016, 68, 850–859. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–71. [Google Scholar] [CrossRef]
- Lin, J.W.; Chen, F.D.; Liu, F.; Xu, D.X.; Gao, J.; Tang, X.B. Hydrogen permeation behavior and hydrogen-induced defects in 316L stainless steels manufactured by additive manufacturing. Mater. Chem. Phys. 2020, 250, 123038. [Google Scholar] [CrossRef]
- Izawa, C.; Wagner, S.; Deutges, M.; Martín, M.; Weber, S.; Pargeter, R.; Michler, T.; Uchida, H.-H.; Gemma, R.; Pundt, A. Role of surface oxide layers in the hydrogen embrittlement of austenitic stainless steels: A TOF-SIMS study. Acta Mater. 2019, 180, 329–340. [Google Scholar] [CrossRef]
- Dai, C.L.; Guo, F.; Wang, P.; Li, D.Z.; Zhang, L. Chemical states of ppm cerium in steel by ToF-SIMS analysis. Surf. Interface Anal. 2019, 52, 301–305. [Google Scholar] [CrossRef]


| Laser Power/W | Scanning Speed/mm s−1 | Hatch Spacing/μm | Layer Thickness/μm |
|---|---|---|---|
| 370. | 1300 | 80 | 30 |
| Cr | Ni | Mo | Mn | Si | C | N | Fe |
|---|---|---|---|---|---|---|---|
| 17.0 | 11.3 | 2.63 | 0.86 | 0.49 | 0.013 | 0.09 | balance |
| Sample Details | Environment | H-Charging Condition | Ref. | |
|---|---|---|---|---|
| type-316L ODS-D5 | gaseous hydrogen | 10 MPa pressure | 37 | [21] |
| type-316L ODS-D150 | 0 | |||
| non-laser peened 316L | 1 mol/L of H2SO4 + 0.125 g/L Na4P2O7 · 10H2O | 50 mA/cm2 (24 h) | 21 | [22] |
| laser peened 316L | 7 | |||
| Not homogenized 316L | 0.5 M H2SO4 + 250 mg/L As2O3 | 200 A/m2 (50 min) | 11 | [23] |
| Homogenized 316L | 8 | |||
| non-laser peened 316L | 0.5 mol/L of H2SO4 + 1 g/L Na4P2O7 · 10H2O | 20 mA/cm2 (96 h) | 14 | [24] |
| laser peened 316L (6 GW/cm2) | 12 | |||
| laser peened 316L (8 GW/cm2) | 10 | |||
| laser peened 316L (10 GW/cm2) | 10 | |||
| hot-rolled 316L | electrochemical pre-charged | -- | 38 | [25] |
| hot-rolled 316L | gaseous hydrogen | 10 MPa pressure | 59 | |
| commercial type 316L | 0.5 M H2SO4 | 4.5 mA/cm2 (12 h) | 3 | [26] |
| 4.5 mA/cm2 (24 h) | 002012 | |||
| 4.5 mA/cm2 (36 h) | 9 | |||
| 4.5 mA/cm2 (48 h) | 14 | |||
| cold rolled 316L | 0.5 mol/L H2SO4 + 1 g/L CH4N2S | 20 mA/cm2 (18 h) | 26 | [27] |
| Types 316L (at −40 °C) | gaseous hydrogen | 102 MPa pressure | 72 | [28] |
| Types 316L (RT) | gaseous hydrogen | 83 MPa pressure | 39 | |
| DED 316L | gaseous hydrogen | 120 MPa pressure | 21 | [15] |
| AISI 316L | 0.5 M H2SO4 + 250 mg/L As2O3 | 400 A/m2 (50 min) | 14 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yang, H.; Meng, L.; Liu, D.; Xu, T.; Xu, D.; Shao, X.; Shao, C.; Li, S.; Zhang, P.; et al. Significance of Melt Pool Structure on the Hydrogen Embrittlement Behavior of a Selective Laser-Melted 316L Austenitic Stainless Steel. Materials 2023, 16, 1741. https://doi.org/10.3390/ma16041741
Liu J, Yang H, Meng L, Liu D, Xu T, Xu D, Shao X, Shao C, Li S, Zhang P, et al. Significance of Melt Pool Structure on the Hydrogen Embrittlement Behavior of a Selective Laser-Melted 316L Austenitic Stainless Steel. Materials. 2023; 16(4):1741. https://doi.org/10.3390/ma16041741
Chicago/Turabian StyleLiu, Jie, Huajie Yang, Lingxiao Meng, Di Liu, Tianqi Xu, Daokui Xu, Xiaohong Shao, Chenwei Shao, Shujun Li, Peng Zhang, and et al. 2023. "Significance of Melt Pool Structure on the Hydrogen Embrittlement Behavior of a Selective Laser-Melted 316L Austenitic Stainless Steel" Materials 16, no. 4: 1741. https://doi.org/10.3390/ma16041741
APA StyleLiu, J., Yang, H., Meng, L., Liu, D., Xu, T., Xu, D., Shao, X., Shao, C., Li, S., Zhang, P., & Zhang, Z. (2023). Significance of Melt Pool Structure on the Hydrogen Embrittlement Behavior of a Selective Laser-Melted 316L Austenitic Stainless Steel. Materials, 16(4), 1741. https://doi.org/10.3390/ma16041741








