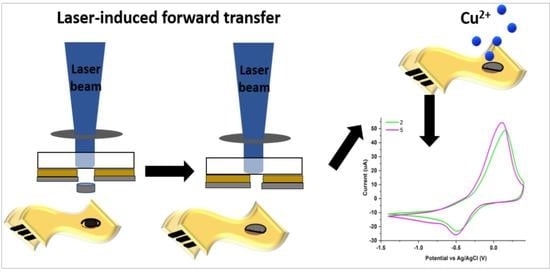
Fabrication of Hybrid Electrodes by Laser-Induced Forward Transfer for the Detection of Cu2+ Ions
Abstract
1. Introduction
2. Materials and Methods
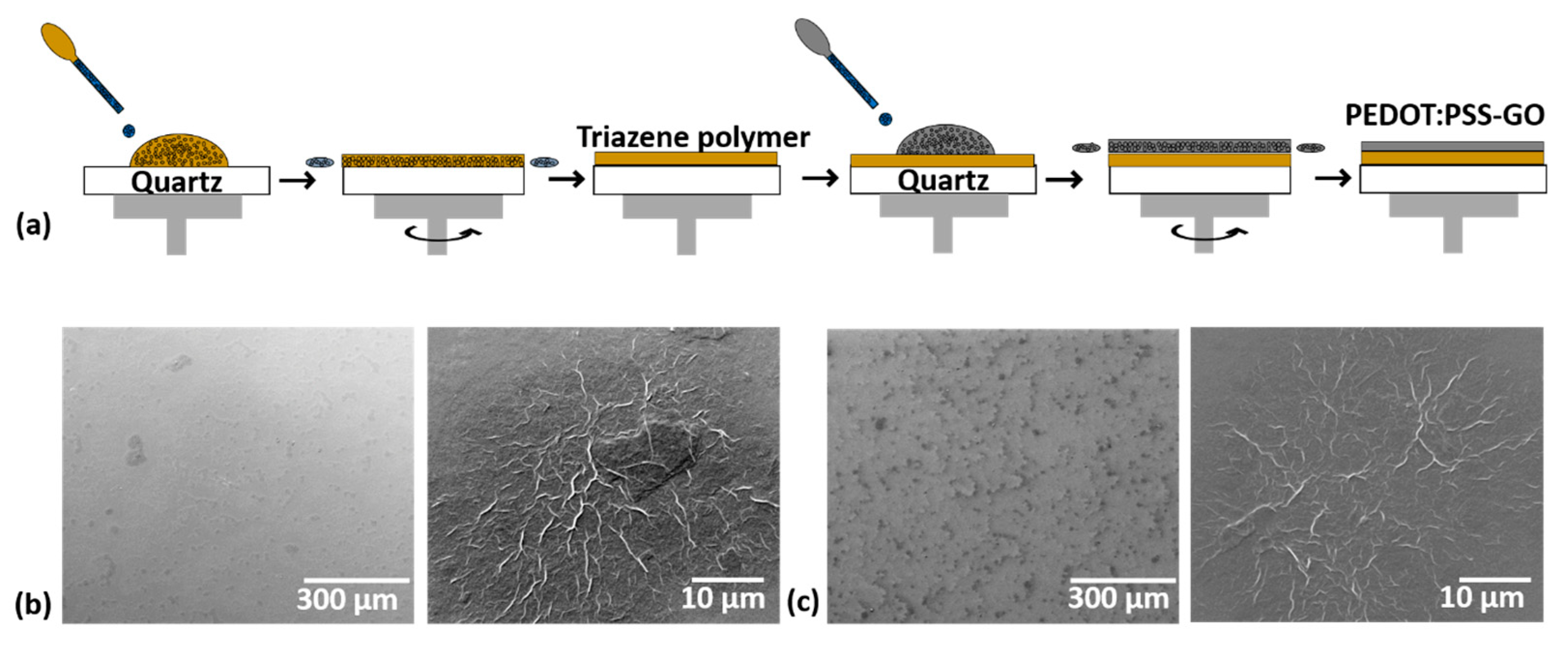
2.1. Preparation of Donor Films
2.2. Laser-Induced Forward Transfer (LIFT)
2.3. Characterization of the Laser-Transferred Materials and the As-Deposited Sensors
3. Results and Discussion
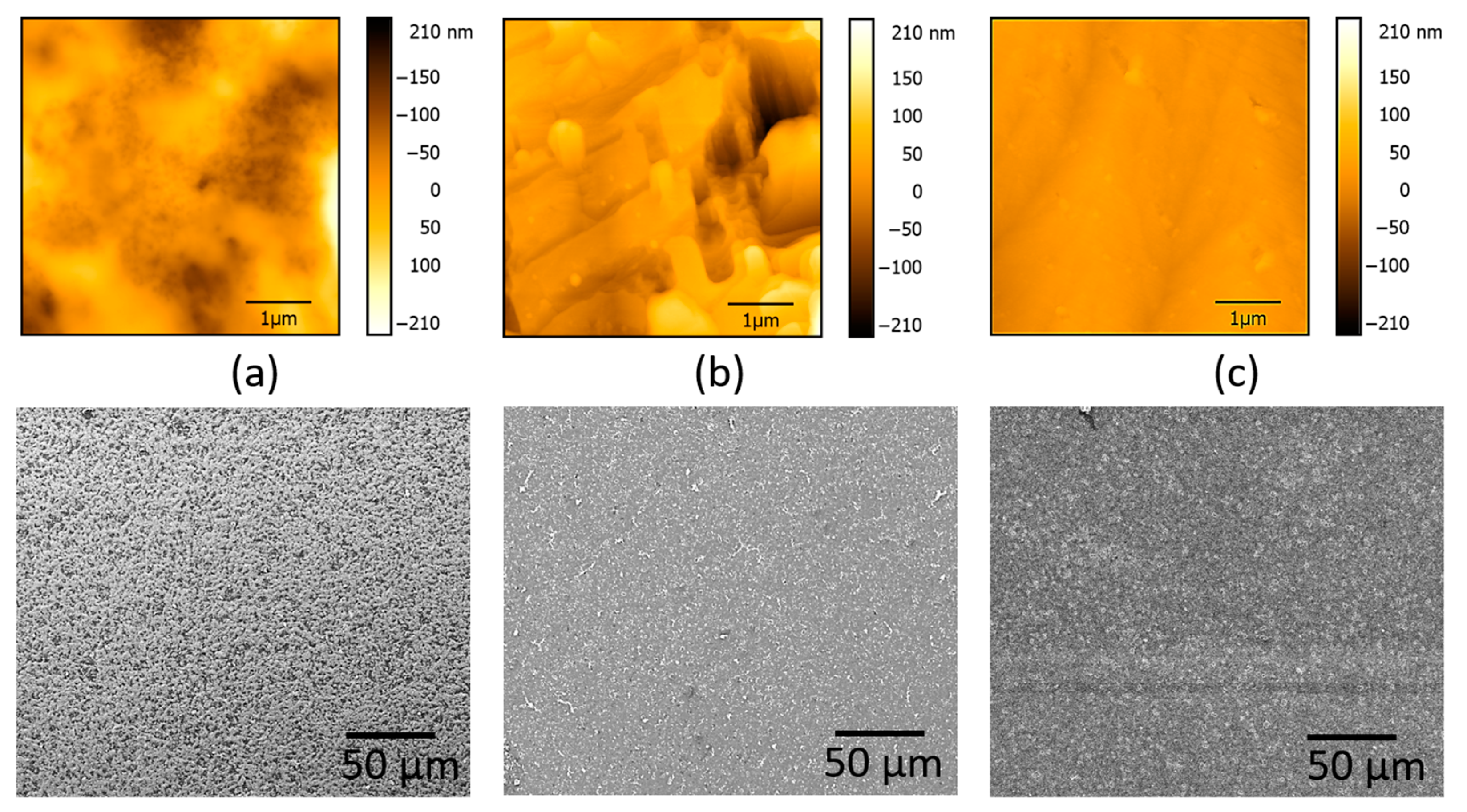
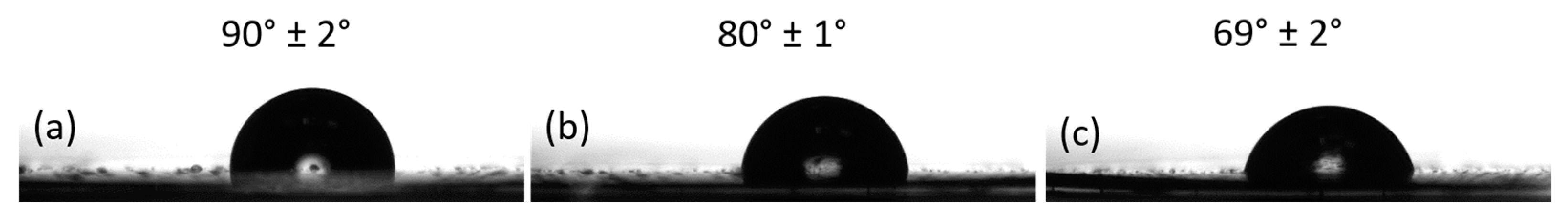
3.1. Donor Film Fabrication
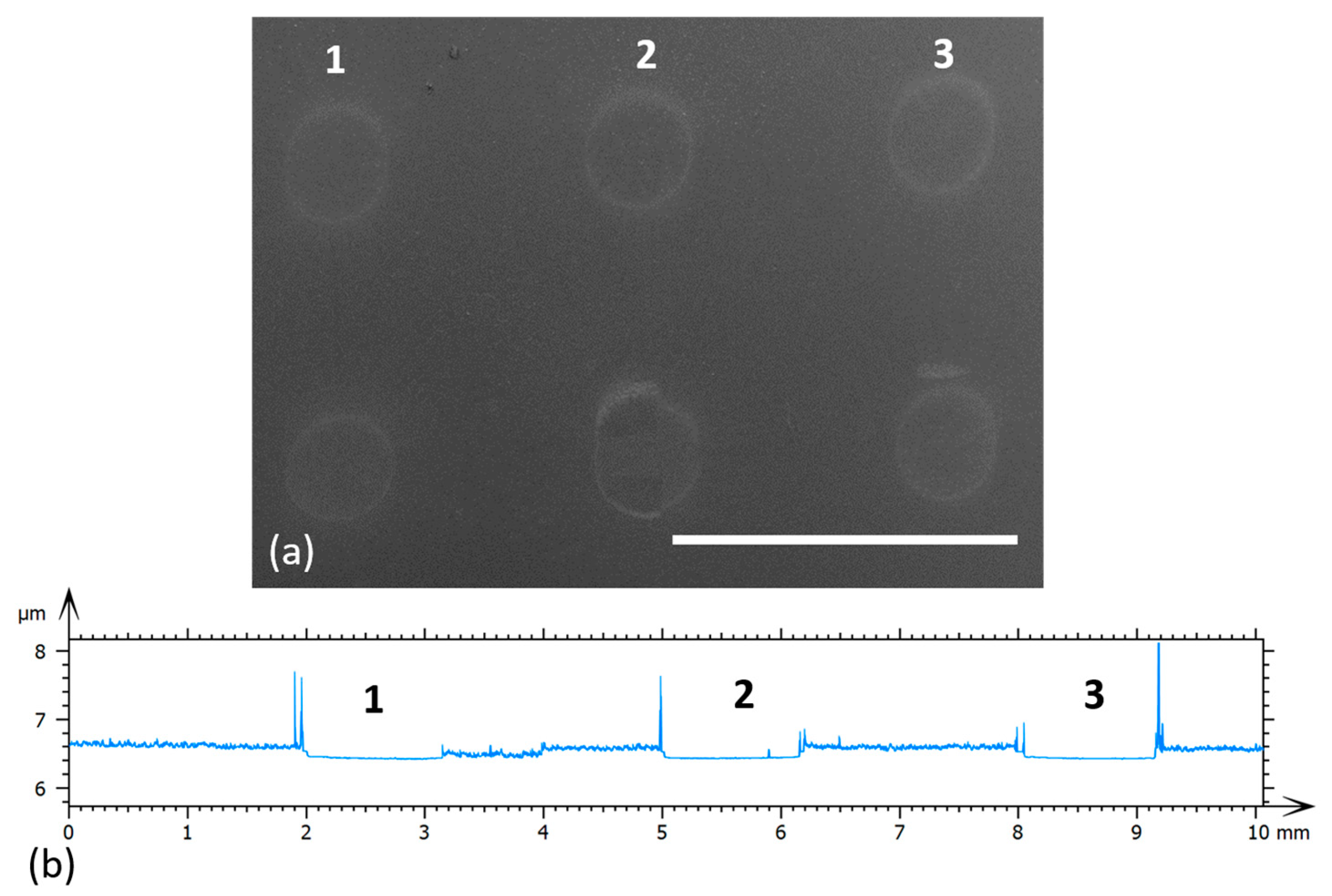
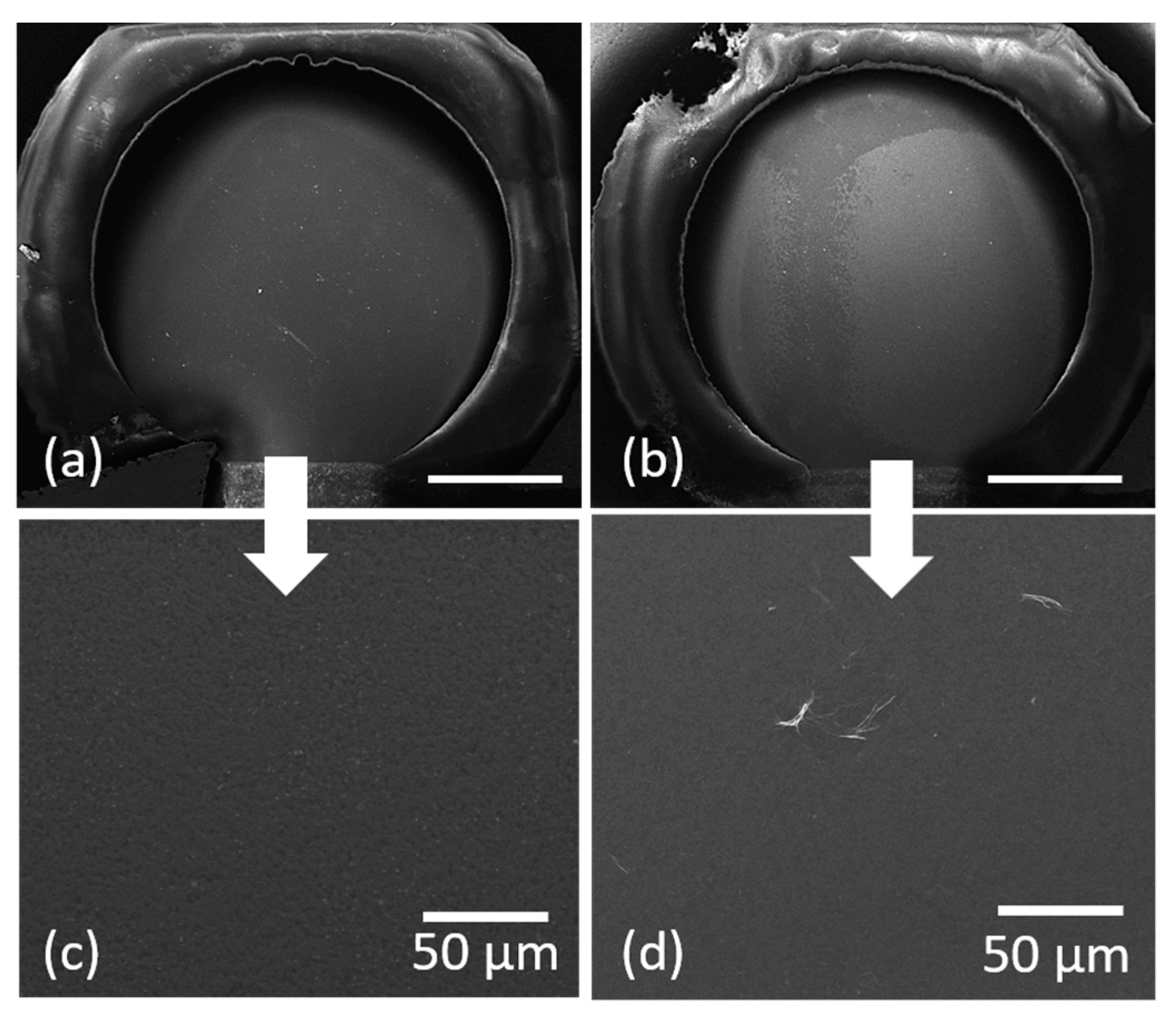
3.2. Laser Printing of PEDOT:PSS: GO Composites
3.3. Electrode Modification via LIFT—Influence of Laser Fluence
3.4. Electrode Modification via LIFT—Influence of Polymer: Graphene Oxide Ratio
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, T.; Liang, X.; Guo, X.; Yang, X.; Guo, J.; Zhou, X.; Huang, X.; Zhang, W.; Wang, Y.; Liu, Z.; et al. Smartphone-based colorimetric sensor array using gold nanoparticles for rapid distinguishment of multiple pesticides in real samples. Food Chem. 2023, 404, 134768. [Google Scholar] [CrossRef] [PubMed]
- Kovacova, Z.; Demcak, S.; Balintova, M.; Pla, C.; Zinicovscaia, I. Influence of Wooden Sawdust Treatments on Cu(II) and Zn(II) Removal from Water. Materials 2020, 13, 3575. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Musarrat, J.; Zaidi, A.; Khan, M.S.; Siddiqui, M.A.; Al-Khedhairy, A.A. Genotoxicity assessment of heavy metal-contaminated soils. Environ. Pollut. 2011, 20, 323–342. [Google Scholar] [CrossRef]
- Nan, Y.; Bai, Y. Sex-Based Differences in the Association between Serum Copper and Kidney Function: Evidence from NHANES 2011–2016. Int. J. Environ. Res. Public Health 2022, 19, 14086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shu, Z.; Sun, H.; Yan, L.; Peng, C.; Dai, Z.; Yang, L.; Fan, L.; Chu, Y. Cu(II)@ZIF-8 nanoparticles with dual enzyme-like activity bound to bacteria specifically for efficient and durable bacterial inhibition. Appl. Surf. Sci. 2023, 611, 155599. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Copper in Drinking Water. Copper in Drinking Water; National Academies Press: Washington, DC, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225402/ (accessed on 3 December 2022).
- Ai, Y.; Yan, L.; Zhang, S.; Ye, X.; Xuan, Y.; He, S.; Wang, X.; Sun, W. Ultra-sensitive simultaneous electrochemical detection of Zn(II), Cd(II) and Pb(II) based on the bismuth and graphdiyne film modified electrode. Microchem. J. 2023, 184, 108186. [Google Scholar] [CrossRef]
- Huang, R.; Lv, J.; Chen, J.; Zhu, Y.; Zhu, J.; Wågberg, T.; Hu, G. Three-dimensional porous high boron-nitrogen-doped carbon for the ultrasensitive electrochemical detection of trace heavy metals in food samples. J. Hazard. Mater. 2023, 442, 130020. [Google Scholar] [CrossRef]
- Al-Qasmi, N.; Al-Gethami, W.; Alhashmialameer, D.; Ismail, S.H.; Sadek, A.H. Evaluation of Green-Synthesized Cuprospinel Nanoparticles as a Nanosensor for Detection of Low-Concentration Cd(II) Ion in the Aqueous Solutions by the Quartz Crystal Microbalance Method. Materials 2022, 15, 6240. [Google Scholar] [CrossRef]
- Eddaif, L.; Shaban, A.; Telegdi, J.; Szendro, I. A piezogravimetric sensor platform for sensitive detection of lead (II) ions in water based on calyx [4] resorcinarene macrocycles: Synthesis, characterization, and detection. Arab. J. Chem. 2020, 13, 4448–4461. [Google Scholar] [CrossRef]
- Liu, G.; Xia, N.; Tian, L.; Sun, Z.; Liu, L. Progress in the Development of Biosensors Based on Peptide–Copper Coordination Interaction. Biosensors 2022, 12, 809. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Sharifuzzaman, M.; Sharma, S.; Xuan, X.; Zhang, S.; Ko, S.G.; Yoon, S.H.; Park, J.Y. High-Performance Flexible Electrochemical Heavy Metal Sensor Based on Layer-by-Layer Assembly of Ti3C2Tx/MWNTs Nanocomposites for Noninvasive Detection of Copper and Zinc Ions in Human Biofluids. ACS Appl. Mater. Interfaces 2020, 12, 48928–48937. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.C.; Wu, J.; Li, J.; Xie, C.G.; Liu, Y.S.; Zhong, Y.; Liu, J.H. Electrochemical determination of trace copper(II) with enhanced sensitivity and selectivity by gold nanoparticle/single-wall carbon nanotube hybrids containing three-dimensional l-cysteine molecular adapters. Sens. Actuators B Chem. 2013, 182, 382–389. [Google Scholar] [CrossRef]
- Oztekin, Y.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical copper (II) sensor based on self-assembled 4-amino-6-hydroxy-2-mercaptopyrimidine monohydrate. Sens. Actuators B Chem. 2011, 155, 612–617. [Google Scholar] [CrossRef]
- Cantalapiedra, A.; Gismera, M.J.; Procopio, J.R.; Sevilla, M.T. Electrochemical sensor based on polystyrene sulfonate–carbon nanopowders composite for Cu (II) determination. Talanta 2015, 139, 111–116. [Google Scholar] [CrossRef]
- Seenivasan, R.; Chang, W.-J.; Gunasekaran, S. Highly Sensitive Detection and Removal of Lead Ions in Water Using Cysteine-Functionalized Graphene Oxide/Polypyrrole Nanocomposite Film Electrode. ACS Appl. Mater. Interfaces 2015, 7, 15935–15943. [Google Scholar] [CrossRef]
- Gong, X.; Bi, Y.; Zhao, Y.; Liu, G.; Teoh, W.Y. Graphene oxide-based electrochemical sensor: A platform for ultrasensitive detection of heavy metal ions. RSC Adv. 2014, 4, 24653–24657. [Google Scholar] [CrossRef]
- Andrei, F.; Boerasu, I.; Filipescu, M.; Palla-Papavlu, A. Facile Modification of Flexible Electrodes via Laser Transfer. Materials 2022, 15, 2488. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Sureshkumar, K.; Varley, T.S.; Nagaraju, D.H.; Ramakrishnappa, T. In situ reduction and functionalization of graphene oxide with l-cysteine for simultaneous electrochemical determination of cadmium(ii), lead(ii), copper(ii), and mercury(ii) ions. Anal. Methods 2014, 21, 8698–8705. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Chen, X.; Yang, Q.; Liu, J.H.; Huang, X.J. Selective adsorption toward toxic metal ions results in selective response: Electrochemical studies on a polypyrrole/reduced graphene oxide nanocomposite. Chem. Comm. 2012, 48, 2180–2182. [Google Scholar] [CrossRef]
- Revanappa, S.K.; Soni, I.; Siddalinganahalli, M.; Jayaprakash, G.K.; Flores-Moreno, R.; Bananakere Nanjegowda, C. A Fukui Analysis of an Arginine-Modified Carbon Surface for the Electrochemical Sensing of Dopamine. Materials 2022, 15, 6337. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, L.; Buscarino, A. Smart Materials. Materials 2022, 15, 6307. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, R.; Chen, R.; Wang, J.; Xiang, L. 3D Architectured Graphene/Metal Oxide Hybrids for Gas Sensors: A Review. Sensors 2018, 18, 1456. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, M.; Zhan, Y.; Zhang, Y.; Zhang, D. Electrodeposited poly(3,4-ethylene dioxythiophene) doped with graphene oxide for the simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Mikrochim. Acta 2020, 187, 94. [Google Scholar] [CrossRef]
- Li, T.J.; Lin, C.Y.; Balamurugan, A.; Kung, C.W.; Wang, J.Y.; Hu, C.W.; Wang, C.C.; Chen, P.Y.; Vittal, R.; Ho, K.C. Modification of glassy carbon electrode with a polymer/mediator composite and its application for the electrochemical detection of iodate. Anal. Chim. Acta 2012, 737, 55–63. [Google Scholar] [CrossRef]
- Jayaprakash, G.K. Pre-post redox electron transfer regioselectivity at the alanine modified nano graphene electrode interface. Chem. Phys. Lett. 2022, 789, 139295. [Google Scholar] [CrossRef]
- Jin, S.; Jun, G.H.; Jeon, S.; Hong, S.H. Design and application of carbon nanomaterials for photoactive and charge transport layers in organic solar cells. Nano Converg. 2016, 3, 8. [Google Scholar] [CrossRef]
- Khasim, S.; Pasha, A.; Badi, N.; Lakshmi, M.; Mishra, Y.M. High performance flexible supercapacitors based on secondary doped PEDOT–PSS–graphene nanocomposite films for large area solid state devices. RSC Adv. 2020, 10, 10526. [Google Scholar] [CrossRef]
- Sundramoorthy, A.K.; Premkumar, B.S.; Gunasekaran, S. Reduced Graphene Oxide-Poly(3,4-ethylenedioxythiophene) Polystyrenesulfonate Based Dual-Selective Sensor for Iron in Different Oxidation States. ACS Sens. 2016, 1, 151. [Google Scholar] [CrossRef]
- Kong, N.; Liu, J.; Kong, Q.; Wang, R.; Barrow, C.J.; Yang, W. Graphene modified gold electrode via π–π stacking interaction for analysis of Cu2+ and Pb2+. Sens. Actuators B 2013, 178, 426–433. [Google Scholar] [CrossRef]
- Gollavelli, G.; Chang, C.C.; Ling, Y.C. Facile Synthesis of Smart Magnetic Graphene for Safe Drinking Water: Heavy Metal Removal and Disinfection Control. ACS Sustain. Chem. Eng. 2013, 1, 462–472. [Google Scholar] [CrossRef]
- Yang, G.H.; Cao, J.T.; Li, L.L.; Rana, R.K.; Zhu, J.J. Carboxymethyl chitosan-functionalized graphene for label-free electrochemical cytosensing. Carbon 2013, 51, 124–133. [Google Scholar] [CrossRef]
- Coroş, M.; Pruneanu, S.; Stefan-van Staden, R.I. Review—Recent Progress in the Graphene-Based Electrochemical Sensors and Biosensors. J. Electrochem. Soc. 2020, 167, 037528. [Google Scholar] [CrossRef]
- Putra, B.R.; Nisa, U.; Heryanto, R.; Rohaeti, E.; Khalil, M.; Izzataddini, A.; Wahyuni, W.T. A facile electrochemical sensor based on a composite of electrochemically reduced graphene oxide and a PEDOT: PSS modified glassy carbon electrode for uric acid detection. Anal. Sci. 2022, 38, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Kim, S.W.; Lee, Y.J. Flexible sensor with electrophoretic polymerized graphene oxide/PEDOT: PSS composite for voltammetric determination of dopamine concentration. Sci. Rep. 2021, 11, 21101. [Google Scholar] [CrossRef]
- Arnold, C.B.; Serra, P.; Pique, A. Laser Direct-Write Techniques for Printing of Complex Materials. MRS Bull. 2007, 32, 23–31. [Google Scholar] [CrossRef]
- Delaporte, P.; Alloncle, A.-P. Laser-induced forward transfer: A high resolution additive manufacturing technology. Opt. Laser Technol. 2016, 78, 33–41. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Yang, F.; Wang, M.; Zhu, T. Fundamentals and Advances in Laser-Induced Transfer. Opt. Laser Technol. 2023, 160, 109065. [Google Scholar] [CrossRef]
- Rager, K.; Tang, B.; Schneemann, C.; Dworzak, A.; Oezaslan, M.; Dietzel, A. Ordered Porous Electrodes Obtained Using LIFT for Electrochemical Applications. Materials 2023, 16, 596. [Google Scholar] [CrossRef]
- Soulis, D.; Trigazi, M.; Tsekensis, G.; Chandrinou, C.; Klinakis, A.; Zergioti, I. Facile and Low-Cost SPE Modification Towards Ultra-Sensitive Organophosphorous and Carbamate Pesticide Detection in Olive Oil. Molecules 2020, 25, 4988. [Google Scholar] [CrossRef]
- Nagel, M.; Hany, R.; Lippert, T.; Molberg, M.; Nueesch, F.A.; Rentsch, D. Aryltriazene Photopolymers for UV-Laser Applications: Improved Synthesis and Photodecomposition Study. Macromol. Chem. Phys. 2007, 208, 277–286. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, Z.; Xiang, X. Graphene oxide composite hydrogels for wearable devices. Carbon Lett. 2022, 32, 1395–1410. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A review on the mechanical properties of polymer composites reinforced by carbon nanotubes and graphene. Carbon Lett. 2021, 31, 149–165. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry; Wiley-VCH, Inc.: New York, NY, USA, 1948; pp. 28–50. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications; John Wiley and Sons: New York, NY, USA, 1980; p. 228. [Google Scholar]
- Shaikh, A.A.; Firdaws, J.; Badrunnessa, S.S.; Rahman, M.S.; Bakshi, P.K. Electrochemical Studies of the pH Dependence of Cu(II) Reduction in Aqueous Britton-Robinson Buffer Solution. Int. J. Electrochem. Sci. 2011, 6, 2333–2343. [Google Scholar]
- Haque, F.; Rahman, M.; Ahmed, E.; Bakshi, P.; Shaikh, A. A Cyclic Voltammetric Study of the Redox Reaction of Cu(II) in Presence of Ascorbic Acid in Different pH Media. Dhaka Univ. J. Sci. 2013, 61, 161–166. [Google Scholar] [CrossRef]
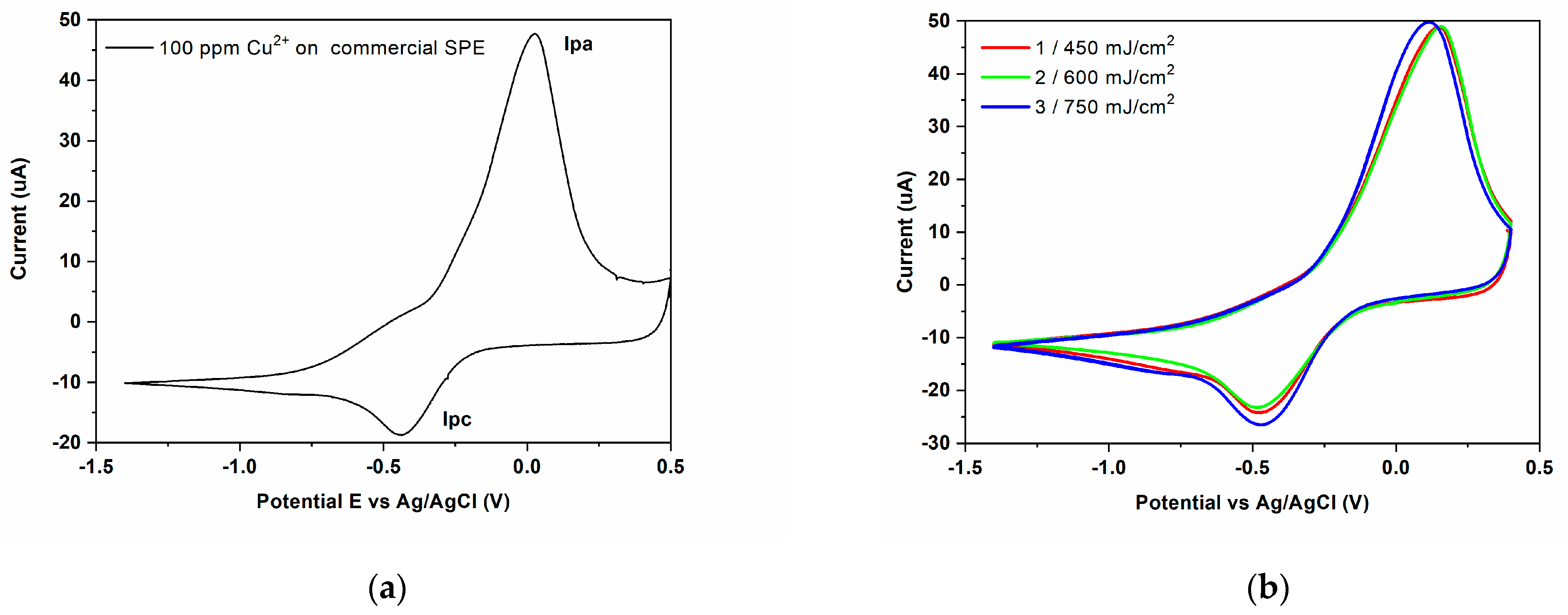


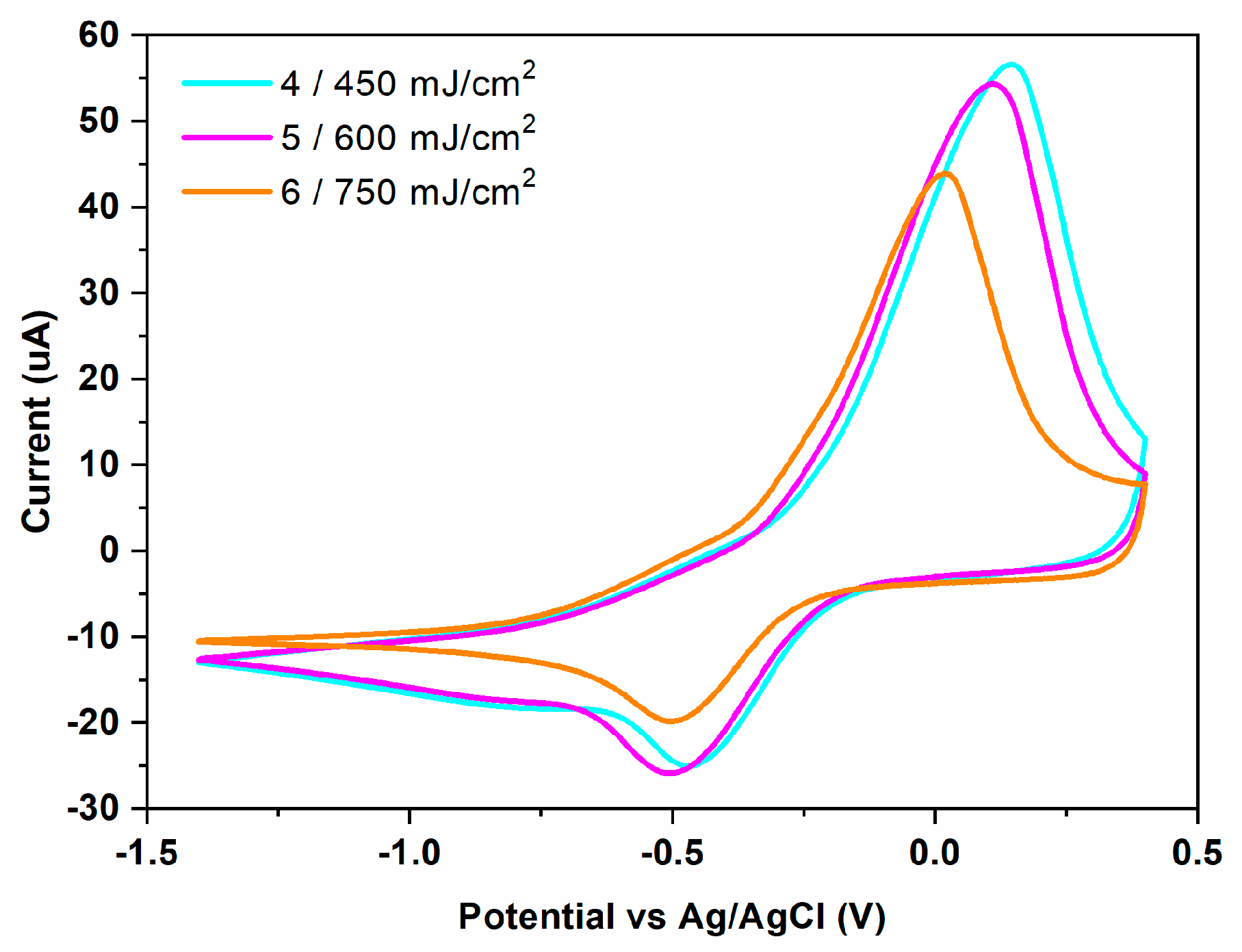
| Sensor | Experimental Conditions | Peak Current (μA) | Peak Potential (mV) | ∆Ep = Epa − Epc | ipa/ipc | ||
|---|---|---|---|---|---|---|---|
| (−) ipc | ipa | (−) Epc | Epa | ||||
| Commercial sensor | 11.41 | 42.9 | 430 | 26 | 450 | 3.76 | |
| 1 * | 25:75 wt.% PEDOT:PSS: GO Donor without TP 450 mJ/cm2 | 17.09 | 35.32 | 470 | 140 | 610 | 1.97 |
| 2 * | 25:75 wt.% PEDOT:PSS: GO Donor without TP 600 mJ/cm2 | 14.59 | 40.66 | 480 | 150 | 630 | 2.79 |
| 3 * | 25:75 wt.% PEDOT:PSS: GO Donor without TP 750 mJ/cm2 | 17. 9 | 42.98 | 470 | 110 | 580 | 2.51 |
| 4 * | 25:75 wt.% PEDOT:PSS: GO Donor with TP 450 mJ/cm2 | 17.34 | 47.94 | 460 | 150 | 610 | 2.76 |
| 5 * | 25:75 wt.% PEDOT:PSS: GO Donor with TP 600 mJ/cm2 | 16.14 | 48.84 | 500 | 110 | 610 | 3.02 |
| 6 * | 25:75 wt.% PEDOT:PSS: GO Donor with TP 750 mJ/cm2 | 11.84 | 39.52 | 500 | 10 | 510 | 3.33 |
| 7 * | 50:50 wt.% PEDOT:PSS: GO Donor without TP 600 mJ/cm2 | 7.72 | 38.68 | 460 | 22 | 480 | 5.01 |
| SnO2 | Ref. [19] | 10.01 | 38.03 | 460 | −31 | 429 | 2.90 |
| Pd-SnO2 | Ref. [19] | 15.18 | 44.06 | 340 | 110 | 450 | 3.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonciu, A.F.; Andrei, F.; Palla-Papavlu, A. Fabrication of Hybrid Electrodes by Laser-Induced Forward Transfer for the Detection of Cu2+ Ions. Materials 2023, 16, 1744. https://doi.org/10.3390/ma16041744
Bonciu AF, Andrei F, Palla-Papavlu A. Fabrication of Hybrid Electrodes by Laser-Induced Forward Transfer for the Detection of Cu2+ Ions. Materials. 2023; 16(4):1744. https://doi.org/10.3390/ma16041744
Chicago/Turabian StyleBonciu, Anca Florina, Florin Andrei, and Alexandra Palla-Papavlu. 2023. "Fabrication of Hybrid Electrodes by Laser-Induced Forward Transfer for the Detection of Cu2+ Ions" Materials 16, no. 4: 1744. https://doi.org/10.3390/ma16041744
APA StyleBonciu, A. F., Andrei, F., & Palla-Papavlu, A. (2023). Fabrication of Hybrid Electrodes by Laser-Induced Forward Transfer for the Detection of Cu2+ Ions. Materials, 16(4), 1744. https://doi.org/10.3390/ma16041744