Enhanced Properties of Tailored Alumina–Magnesia-Based Dry Ramming Mixes by Calcium Magnesium Aluminate (CMA)
Abstract
1. Introduction
2. Materials and Methods
3. Results
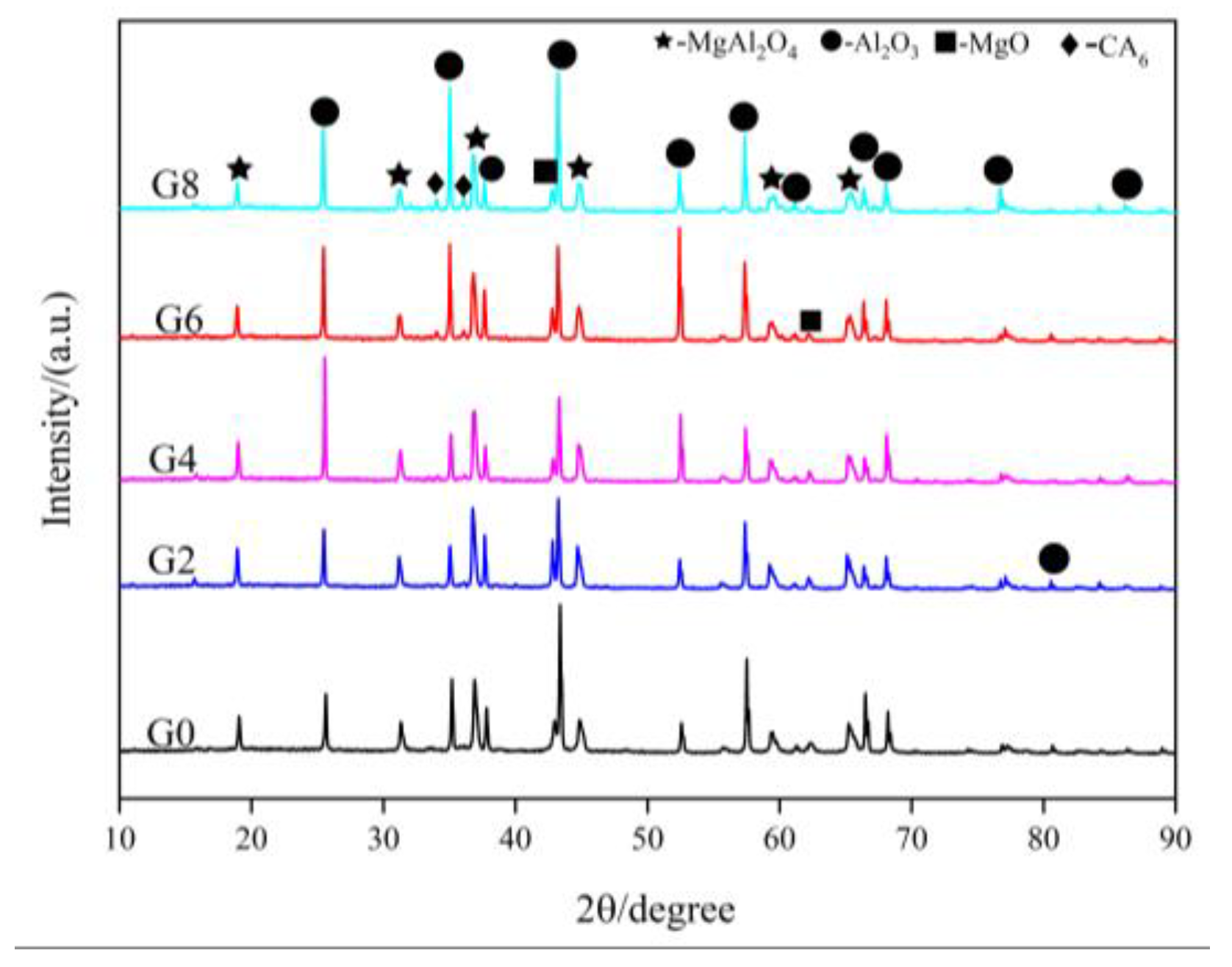
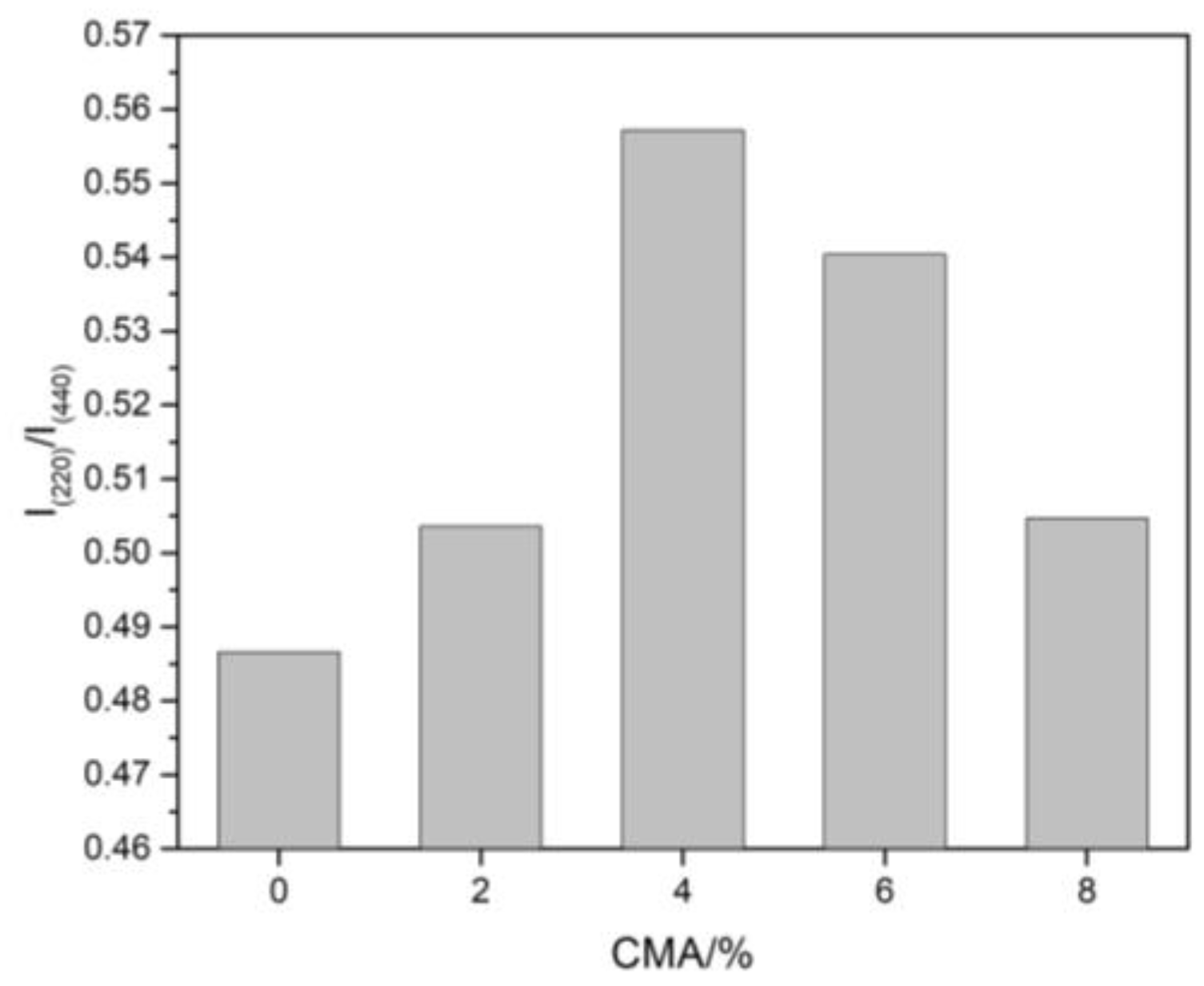
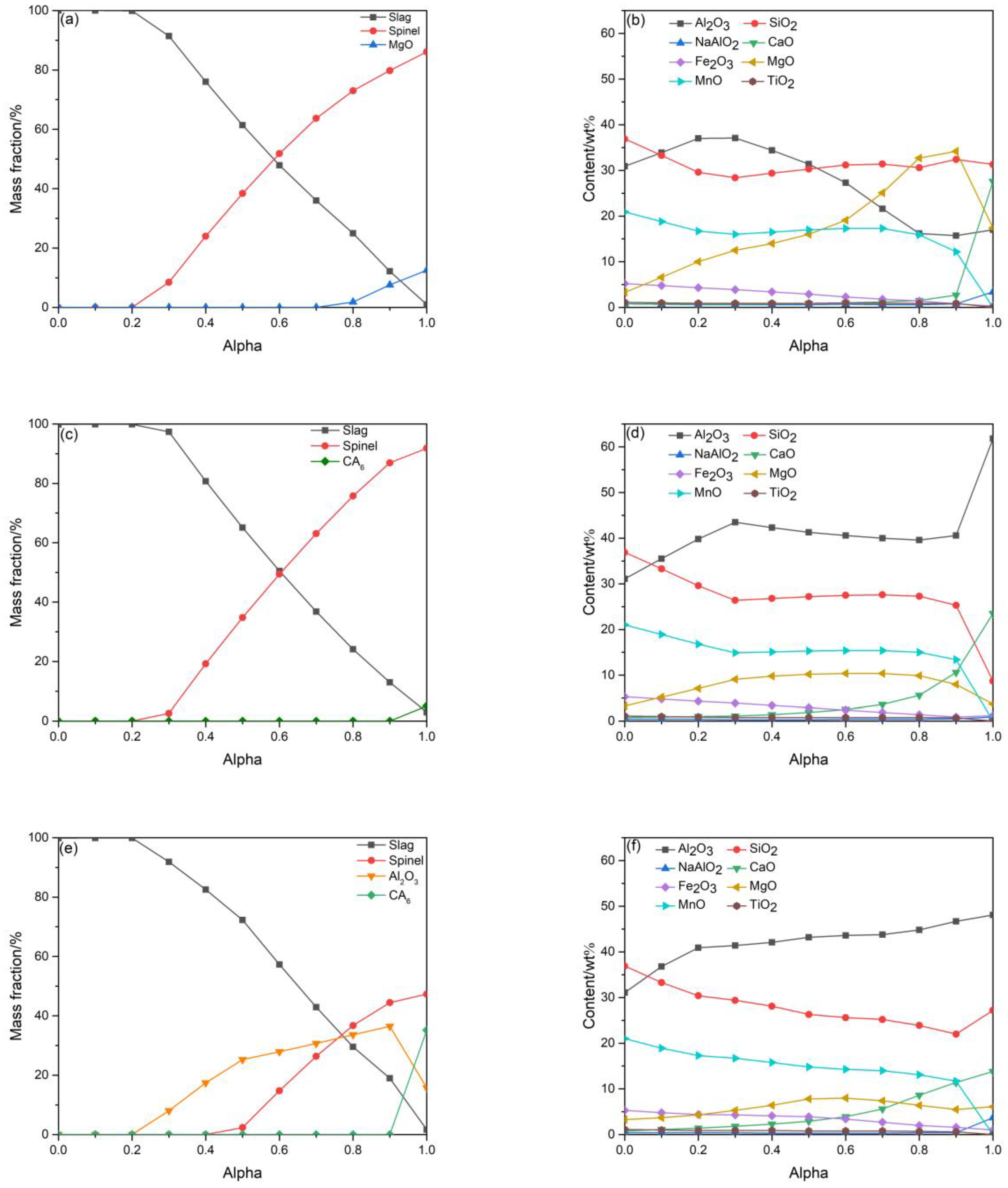
3.1. Phase Composition
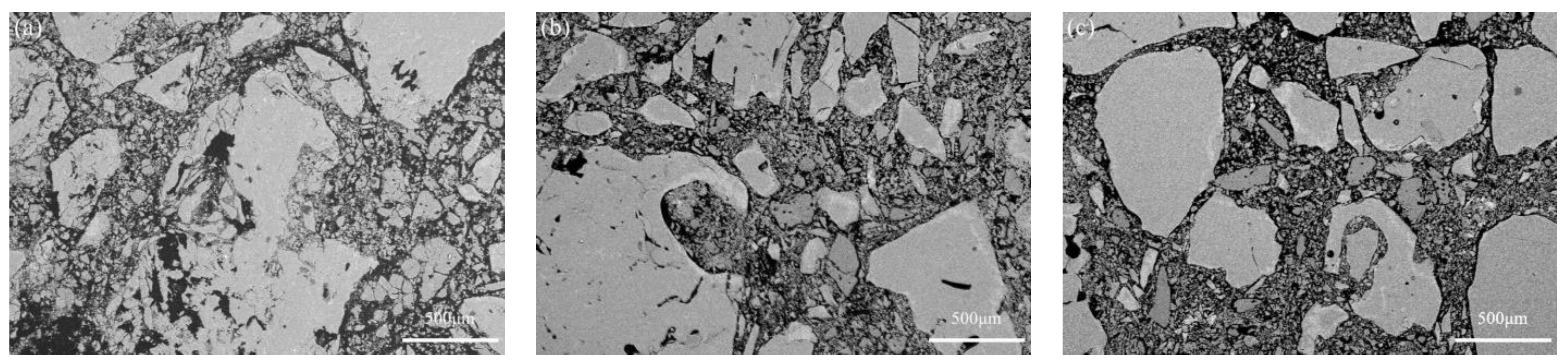
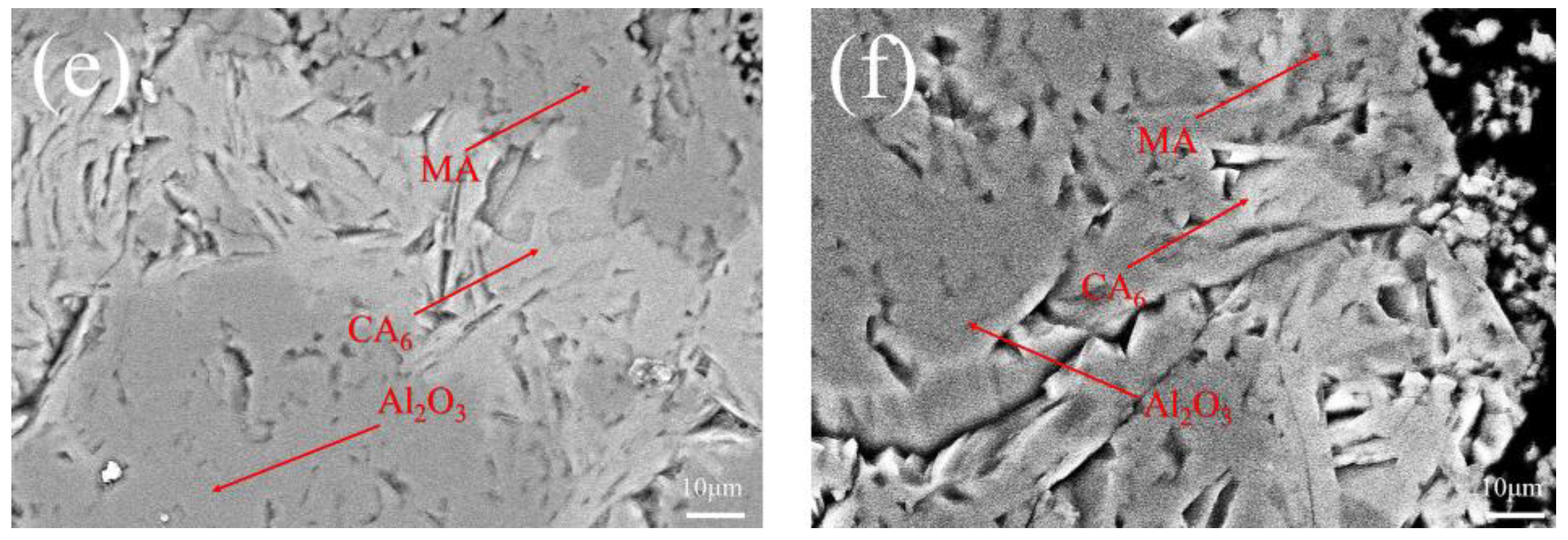
3.2. Uncorroded Microstructure
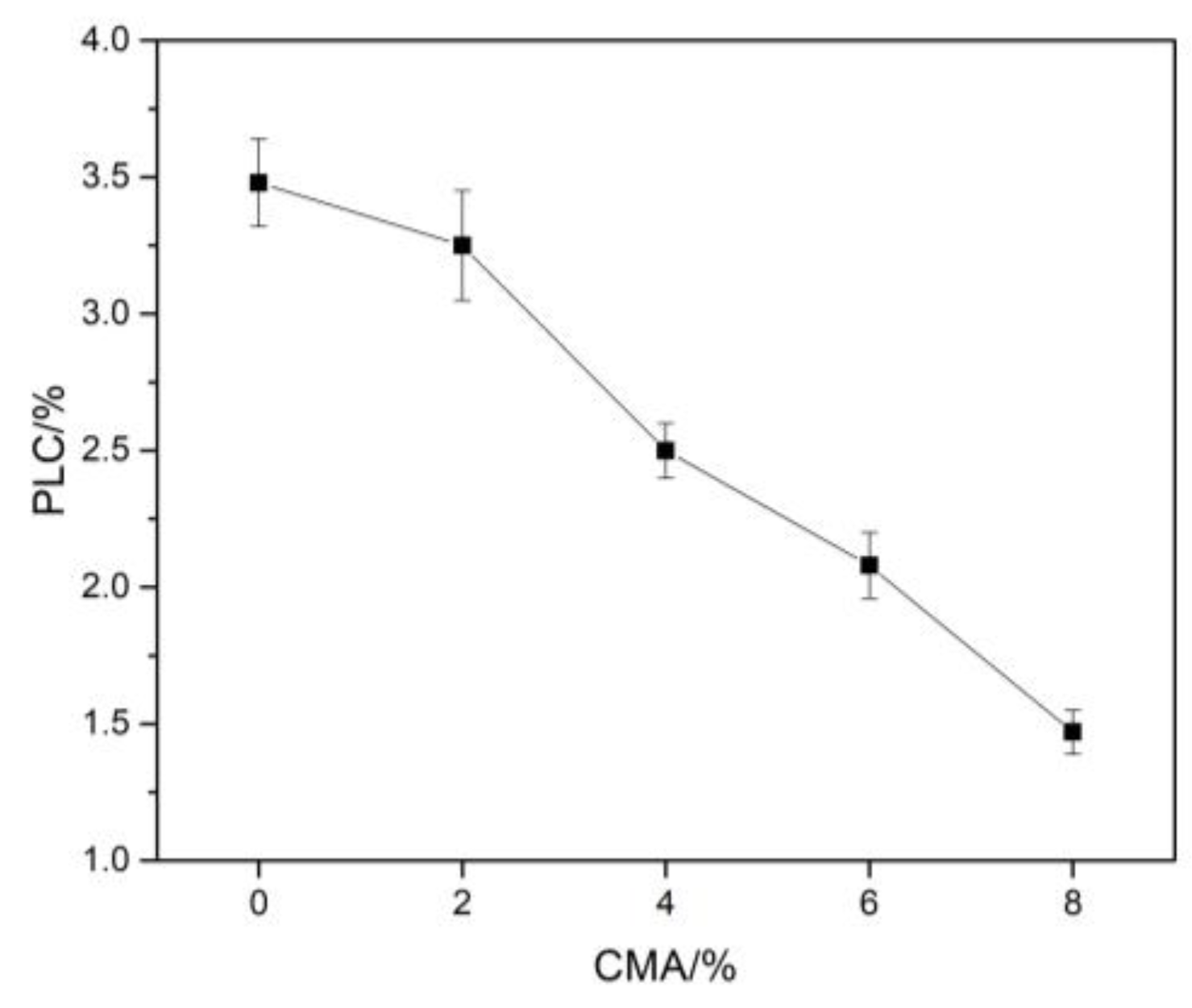
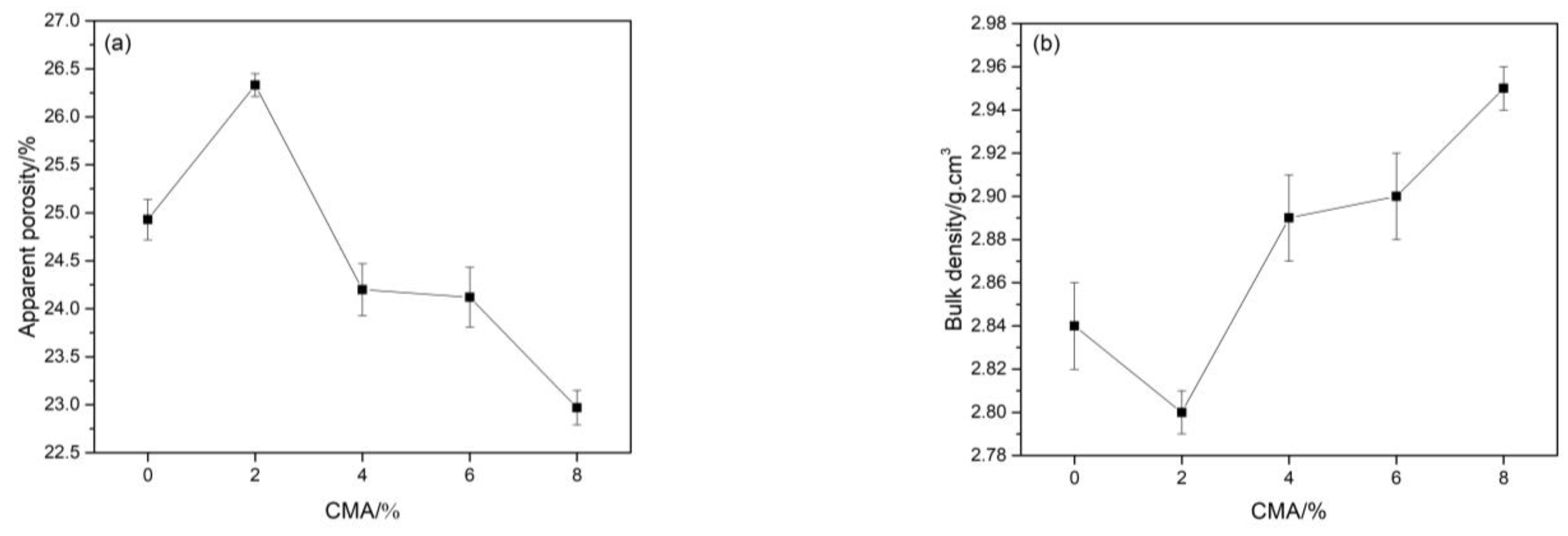
3.3. Physical and Mechanical Properties
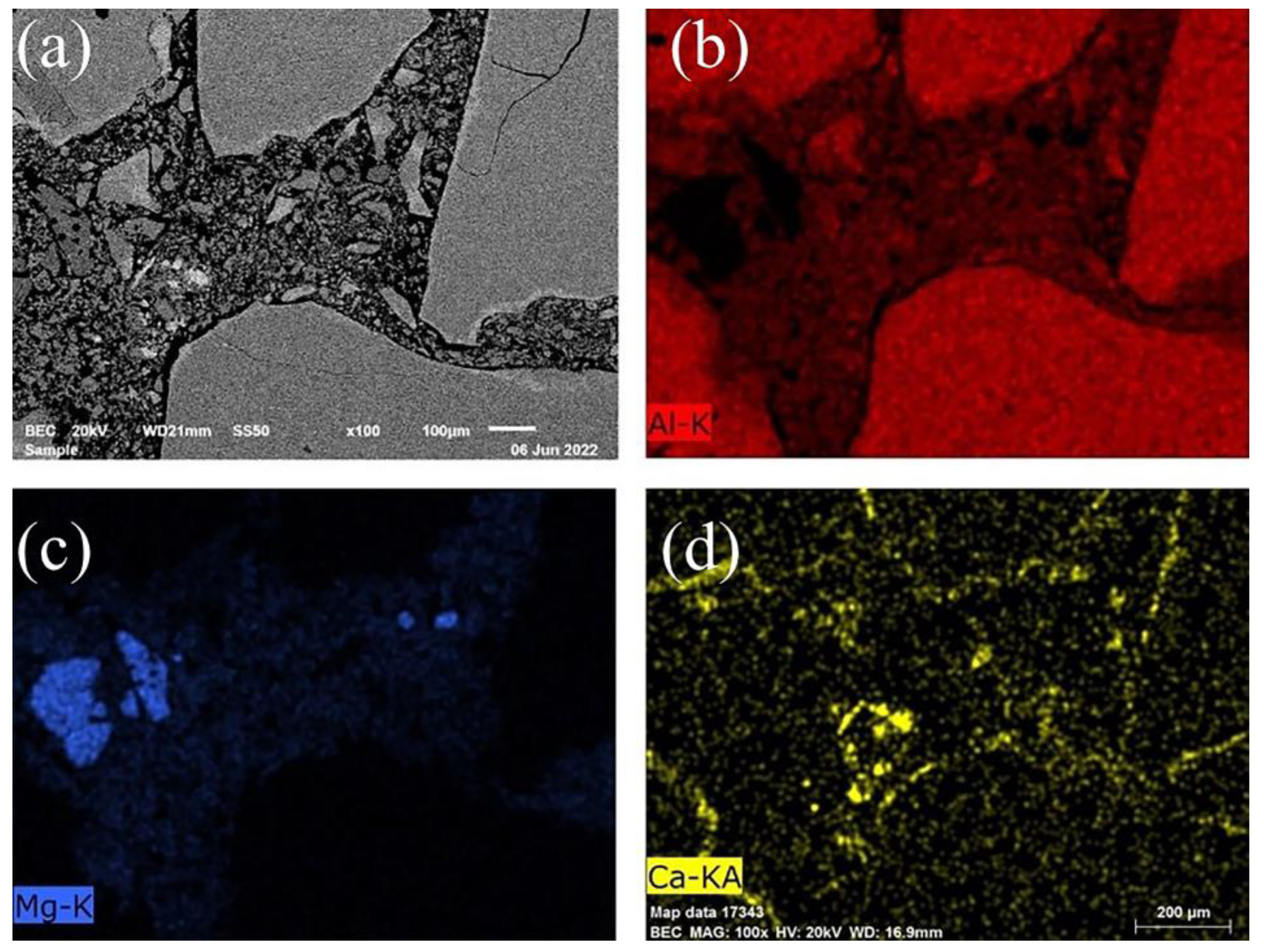
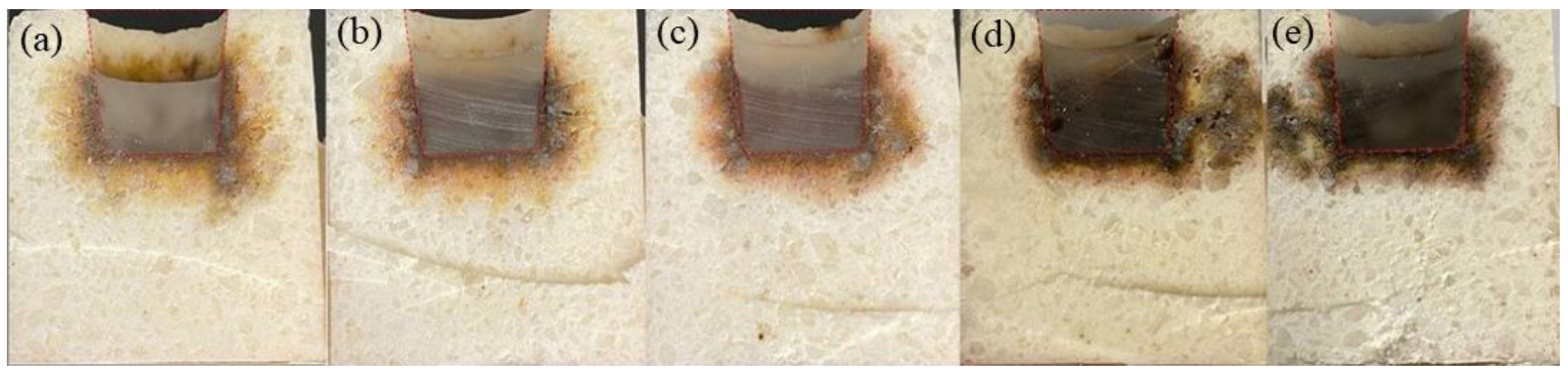
3.4. Slag Corrosion Resistance
4. Conclusions
- (1)
- The substitution of magnesia with CMA promoted the formation of the alumina-rich spinel and the bond structure of Al2O3-CA6 spinel in dry ramming mixes.
- (2)
- The incorporation of CMA powders can dramatically reduce linear changes after firing at 1600 °C for 3 h from 3.48% to 1.47% and greatly improve the cold crushing strength from 9.0 MPa to 19 MPa of alumina–magnesia-based dry ramming mixes.
- (3)
- The manganese-bearing slag penetration was attributed to the alumina-rich spinel and the interaction between slag and refractories. The accommodation of Mn and Fe ions in the slag by the alumina-rich spinel can depress slag penetration.
- (4)
- The optimal amount of 4% CMA was determined by comparing the comprehensive properties.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.W.; Li, X.C.; Zhu, B.Q. Influence of α-Al2O3 micropowder addition on properties of corundum dry ramming mix. China’s Refract. 2013, 22, 28–31. [Google Scholar] [CrossRef]
- Schacht, C.A. Refractories Handbook; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Pille, A.; Spiridigliozzi, H.; Amamra, M.; Billeton, T.; Zaghrioui, M.; Feldbach, E.; Kanaev, A.; Schoenstein, F. Morphology and luminescence of MgAl2O4 ceramics obtained via spark plasma sintering. Ceram. Int. 2019, 45, 8305–8312. [Google Scholar] [CrossRef]
- Lushchik, A.; Feldbach, E.; Kotomin, E.A.; Kudryavtseva, I.; Kuzovkov, V.N.; Popov, A.I.; Seeman, V.; Shablonin, E. Distinctive features of diffusion-controlled radiation defect recombination in stoichiometric magnesium aluminate spinel single crystals and transparent polycrystalline ceramics. Sci. Rep. 2020, 10, 7810. [Google Scholar] [CrossRef] [PubMed]
- Krause, O.; Kemnitz, H. Behaviour of spinel-forming DVM using crucible induction furnaces. A case study on material failure. Ceram. Int. 2016, 42, 10645–10654. [Google Scholar] [CrossRef]
- Routschka, G. Pocket Manual Refractory Materials; Vulkan-Verlag GmbH: Essen, Germany, 2004. [Google Scholar]
- Braulio, M.A.L.; Martinez, A.G.T.; Luz, A.P.L.; Liebske, C.; Pandolfelli, V.C. Basic slag attack of spinel-containing refractory castables. Ceram. Int. 2011, 37, 1935–1945. [Google Scholar] [CrossRef]
- Braulio, M.A.L.; Castro, J.F.R.; Pagliosa, C.; Bittencourt, L.R.M.; Pandolfelli, V.C. From macro to nanomagnesia: Designing the in situ spinel expansion. J. Am. Ceram. Soc. 2008, 91, 3090–3093. [Google Scholar] [CrossRef]
- Sako, E.Y.; Braulio, M.A.L.; Zinngrebe, E.; van der Laan, S.R.; Pandolfelli, V.C. Fundamentals and applications on in situ spinel formation mechanisms in Al2O3-MgO refractory castables. Ceram. Int. 2012, 38, 2243–2251. [Google Scholar] [CrossRef]
- Kang, R.; Wang, X.T.; Wang, Z.F.; Ma, Y.; Liu, H. Effect of pre-synthesized spinel addition on alumina-magnesia dry vibration mix. Refraction 2020, 54, 205–209. [Google Scholar] [CrossRef]
- Jiang, G.S.; Nie, J.H.; Li, H.B.; Liang, Y.H.; Cai, M.F. Influence of spinel micropowder on properties of alumina-magnesia dry ramming mixes. Refraction 2022, 56, 539–542,552. [Google Scholar] [CrossRef]
- Tripathi, H.S.; Mukherjee, B.; Das, S.; Haldar, M.K.; Das, S.K.; Ghosh, A. Synthesis and densification of magnesium aluminate spinel: Effect of MgO reactivity. Ceram. Int. 2003, 29, 915–918. [Google Scholar] [CrossRef]
- Sako, E.Y.; Braulio, M.A.L.; Brant, P.O.; Pandolfelli, V.C. The impact of pre-formed and in situ spinel formation on the physical properties of cement-bonded high alumina refractory castables. Ceram. Int. 2010, 36, 2079–2085. [Google Scholar] [CrossRef]
- Kumar, S.; Sarkar, R. Alumina-spinel castable for steel ladles: An overview. Int. J. Appl. Ceram. Technol. 2023, 20, 410–423. [Google Scholar] [CrossRef]
- De Aza, A.H.; Iglesias, J.E.; Pena, P.; De Aza, S. Ternary system Al2O3-MgO-CaO: Part II, phase relationships in the subsystem Al2O3-MgAl2O4-CaAl4O7. J. Am. Ceram. Soc. 2000, 83, 919–927. [Google Scholar] [CrossRef]
- Xu, L.; Chen, M.; Yin, X.L.; Wang, N.; Liu, L. Effect of TiO2 addition on the sintering densification and mechanical properties of MgAl2O4-CaAl4O7-CaAl12O19 composite. Ceram. Int. 2016, 42, 9844–9850. [Google Scholar] [CrossRef]
- Martinez, A.G.T.; Luz, A.P.; Braulio, M.A.L.; Pandolfelli, V.C. CA6 impact on the corrosion behavior of cement-bonded spinel-containing refractory castables: An analysis based on thermodynamic simulations. Ceram. Int. 2015, 41, 4714–4725. [Google Scholar] [CrossRef]
- Wöhrmeyer, C.; Gao, J.; Parr, C.; Szepizdyn, M.; Mineau, R.M.; Zhu, J.H. Corrosion mechanism of a density-reduced steel ladle lining containing porous spinel-calcium aluminate aggregates. Ceramics 2020, 3, 155–170. [Google Scholar] [CrossRef]
- Tang, H.; Li, C.; Gao, J.; Touzo, B.; Liu, C.; Yuan, W. Optimization of properties for alumina-spinel refractory castables by CMA (CaO-MgO-Al2O3) aggregates. Materials 2021, 14, 3050. [Google Scholar] [CrossRef]
- Aneziris, C.G.; Gehre, P. Effects of magnesium aluminate spinel raw materials on the material properties of MgO-C refractories. In Proceedings of the International Colloquium on Refractories, Aachen, Germany, 28–29 September 2016; pp. 242–245. [Google Scholar]
- Wöhrmeyer, C.; Gao, S.; Ping, Z.F.; Parr, C.; Aneziris, C.G.; Gehre, P. Corrosion mechanism of MgO-CMA-C ladle brick with high service life. Steel Res. Int. 2020, 91, 1900436. [Google Scholar] [CrossRef]
- Refractory Products-Determination of Permanent Change in Dimension on Heating. In Refractories Standard Compilation, 4th ed.; Standards Press of China: Beijing, China, 2010; Volume 2, pp. 81–90.
- Test Method for Bulk Density, Apparent Porosity and True Porosity of Dense Shaped Refractory Products. In Refractories Standard Compilation, 4th ed.; Standards Press of China: Beijing, China, 2010; Volume 2, pp. 3–8.
- Refractories-Determination of Cold Compressive Strength. In Refractories Standard Compilation, 4th ed.; Standards Press of China: Beijing, China, 2010; Volume 2, pp. 59–68.
- Refractories-Determination of Slag Resistance. In Refractories Standard Compilation, 4th ed.; Standards Press of China: Beijing, China, 2010; Volume 2, pp. 147–162.
- Braulio, M.A.L.; Rigaud, M.; Buhr, A.; Parr, C.; Pandolfelli, V.C. Spinel-containing alumina-based refractory castables. Ceram. Int. 2011, 37, 1705–1724. [Google Scholar] [CrossRef]
- Domínguez, C.; Chevalier, J.; Torrecillas, R.; Gremillard, L.; Fantozzi, G. Thermomechanical properties and fracture mechanisms of calcium hexaluminate. J. Eur. Ceram. Soc. 2001, 21, 907–917. [Google Scholar] [CrossRef]
- Braulio, M.A.L.; Pandolfelli, V.C. Tailoring the microstructure of cement-bonded alumina-magnesia refractory castables. J. Am. Ceram. Soc. 2010, 93, 2981–2985. [Google Scholar] [CrossRef]
- Yuan, W.J.; Deng, C.J.; Zhu, H.X. Effects of TiO2 addition on the expansion behavior of alumina-magnesia refractory castables. Mater. Chem. Phys. 2015, 162, 724–733. [Google Scholar] [CrossRef]
- Wu, H.D.; Liu, X.D.; Liu, X.X.; Zhang, J.H.; Yu, J.S. Effect of alumina content on the crystal structure, lattice thermal expansion and thermal conductivity of aluminium-rich spinel solid solutions. Mater. Chem. Phys. 2022, 288, 126366. [Google Scholar] [CrossRef]
- Sarpoolaky, H.; Zhang, S.; Lee, W.E. Corrosion of high alumina and near stoichiometric spinels in iron-containing silicate slags. J. Eur. Ceram. Soc. 2003, 23, 293–300. [Google Scholar] [CrossRef]
- Duan, K.; Xiang, W.; Yuan, G.; Huang, S.X.; Niu, J.J.; Jing, G.; Shan, Q. High-pressure synchrotron X-ray diffraction and Raman spectroscopic study of plumbogummite. Chin. Phys. B 2018, 27, 017402. [Google Scholar] [CrossRef]
- Akdogan, E.K.; Savkliyildiz, I.; Berke, B.; Zhong, Z.; Wang, L.; Weidner, D.; Croft, M.C.; Tsakalakos, T. Pressure effects on phase equilibria and solid solubility in MgO-Y2O3 nanocomposites. J. Appl. Phys. 2012, 111, 053506.1–053506.7. [Google Scholar] [CrossRef]
- Savkliyildiz, I.; Akdogan, E.K.; Zhong, Z.; Wang, L.; Weidner, D.; Vaughan, M.; Croft, M.C.; Tsakalakos, T. Phase transformations in hypereutectic MgO-Y2O3 nanocomposites at 5.5 GPa. J. Appl. Phys. 2013, 113, 203520.1–203520.6. [Google Scholar] [CrossRef]
- Podworny, J. Temperature dependence of the inversion degree in three-cation spinel solid solutions: Experimental evaluation by XRD. Powder Diffr. 2015, 30 (Suppl. 1), S104–S110. [Google Scholar] [CrossRef]
- Tkáčová, K.; Šepelák, V.; Števulová, N.; Boldyrev, V.V. Structure-reactivity study of mechanically activated zinc ferrite. J. Solid State Chem. 1996, 123, 100–108. [Google Scholar] [CrossRef]
- Datta, R.K.; Roy, R. Equilibrium order-disorder in spinels. J. Am. Ceram. Soc. 1967, 50, 578–583. [Google Scholar] [CrossRef]
- Erukhimovitch, V.; Mordekoviz, Y.; Hayun, S. Spectroscopic study of ordering in non-stoichiometric magnesium aluminate spinel. Am. Mineral. 2015, 100, 1744–1751. [Google Scholar] [CrossRef]
- Mordekovitz, Y.; Shoval, Y.; Froumin, N.; Hayun, S. Effect of structure and composition of non-stoichiometry magnesium aluminate spinel on water adsorption. Materials 2020, 13, 3195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, J.S.; Li, J.G.; Zeng, Y.N.; Hao, S.J.; Liu, P.Y.; Na, H. The properties characterization and strengthening-toughening mechanism of Al2O3-CA6-MA-Ni multi-phase composites prepared by adding calcined dolomite. Mater. Charact. 2022, 186, 111810. [Google Scholar] [CrossRef]
- Yi, S.; Huang, Z.; Huang, J.; Fang, M.; Liu, Y.; Zhang, S. Novel calcium hexaluminate/spinel-alumina composites with graded microstructures and mechanical properties. Sci. Rep. 2014, 4, 4333. [Google Scholar] [CrossRef]
- Asmi, D.; Low, I.M. Self-reinforced Ca-hexaluminate/alumina composites with graded microstructures. Ceram. Int. 2008, 34, 311–316. [Google Scholar] [CrossRef]
- Biswas, S.; Sarkar, D. Introduction to Refractories for Iron-and Steelmaking; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Sadatomi, Y.; Enomoto, N.; Hojo, J. Reaction behavior of slag and spinel in steel ladle castables. J. Ceram. Soc. Jpn. 2011, 119, 595–600. [Google Scholar] [CrossRef]
- Sako, E.Y.; Braulio, M.A.L.; Pandolfelli, V.C. The corrosion and microstructure relationship for cement-bonded spinel refractory castables. Ceram. Int. 2012, 38, 2177–2185. [Google Scholar] [CrossRef]
- De Bilbao, E.; Dombrowski, M.; Pilliere, H.; Poirier, J. Time-resolved high-temperature X-ray diffraction for studying the kinetics of corrosion of high-alumina refractory by molten oxides. Corros. Sci. 2018, 139, 346–354. [Google Scholar] [CrossRef]
- Zou, H.B.; Wang, C.; Xu, C.F.; Zhang, J.L.; Zhang, T. Effects of MnO on slag viscosity and wetting behaviour between slag and refractory. Ironmak. Steelmak. 2016, 43, 56–63. [Google Scholar] [CrossRef]
- Xu, L.; Chen, M.; Wang, N.; Yin, X.L. Corrosion mechanism of MgAl2O4-CaAl4O7-CaAl12O19 composite by steel ladle slag: Effect of additives. J. Eur. Ceram. Soc. 2017, 37, 2737–2746. [Google Scholar] [CrossRef]
- Yin, X.; Chen, M.; Wang, N.; Xu, L. Improvement of densification and mechanical properties of MgAl2O4-CaAl4O7-CaAl12O19 composite by addition of MnO. Ceram. Int. 2017, 43, 4706–4711. [Google Scholar] [CrossRef]



| Raw Materials | Content (wt%) | ||||
|---|---|---|---|---|---|
| G0 | G2 | G4 | G6 | G8 | |
| White fused alumina (5–3 mm) | 14 | 14 | 14 | 14 | 14 |
| White fused alumina (3–1 mm) | 35 | 35 | 35 | 35 | 35 |
| White fused alumina (1–0 mm) | 23 | 23 | 23 | 23 | 23 |
| White fused alumina (<0.075 mm) | 7 | 7 | 7 | 7 | 7 |
| Tabular alumina (<0.045 mm) | 3 | 3 | 3 | 3 | 3 |
| Calcium alumina (AC34B5) | 3 | 3 | 3 | 3 | 3 |
| Calcined magnesia (<0.3 mm) | 7 | 7 | 7 | 7 | 7 |
| Calcined magnesia (<0.075 mm) | 8 | 6 | 4 | 2 | 0 |
| MagArmour (<0.075 mm) | 0 | 2 | 4 | 6 | 8 |
| Compositions | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | TiO2 | Na2O |
|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 36.91 | 31.22 | 5.62 | 0.84 | 3.28 | 20.55 | 1.14 | 0.30 |
| Spinel | G0 | G2 | G4 | G6 | G8 |
|---|---|---|---|---|---|
| Content (wt%) | 48.7 | 57.4 | 57.5 | 45.5 | 36.8 |
| No. | G0 | G2 | G4 | G6 | G8 |
|---|---|---|---|---|---|
| Penetration index, P (%) | 25.96 | 20.35 | 19.53 | 20.24 | 20.62 |
| Samples | Zone | Al | Si | Mn | Mg | Fe | Ca | Na | O |
|---|---|---|---|---|---|---|---|---|---|
| G0 | 1 | 30.75 | — | 4.99 | 6.91 | 2.46 | — | — | 54.09 |
| 2 | 13.58 | 21.96 | 2.84 | 1.91 | 0.40 | — | 2.76 | 54.79 | |
| G2 | 3 | 29.91 | — | 4.18 | 5.23 | 3.55 | — | — | 56.07 |
| 4 | 32.04 | — | 3.59 | 9.31 | 1.21 | — | — | 52.90 | |
| G4 | 5 | 19.41 | 15.55 | 6.97 | 2.71 | 1.79 | 0.47 | 0.88 | 48.53 |
| 6 | 37.79 | — | 6.56 | 6.89 | 1.65 | 1.58 | — | 44.41 | |
| G8 | 7 | 34.67 | 3.34 | 2.73 | 7.58 | 1.02 | 0.75 | 0.52 | 47.91 |
| 8 | 33.03 | 5.65 | 3.03 | 9.10 | 1.00 | 0.40 | — | 46.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Jia, Z.; Li, B.; Chen, H.; Yuan, W. Enhanced Properties of Tailored Alumina–Magnesia-Based Dry Ramming Mixes by Calcium Magnesium Aluminate (CMA). Materials 2023, 16, 1707. https://doi.org/10.3390/ma16041707
Tang H, Jia Z, Li B, Chen H, Yuan W. Enhanced Properties of Tailored Alumina–Magnesia-Based Dry Ramming Mixes by Calcium Magnesium Aluminate (CMA). Materials. 2023; 16(4):1707. https://doi.org/10.3390/ma16041707
Chicago/Turabian StyleTang, Hu, Zhenggang Jia, Bing Li, Huazhong Chen, and Wenjie Yuan. 2023. "Enhanced Properties of Tailored Alumina–Magnesia-Based Dry Ramming Mixes by Calcium Magnesium Aluminate (CMA)" Materials 16, no. 4: 1707. https://doi.org/10.3390/ma16041707
APA StyleTang, H., Jia, Z., Li, B., Chen, H., & Yuan, W. (2023). Enhanced Properties of Tailored Alumina–Magnesia-Based Dry Ramming Mixes by Calcium Magnesium Aluminate (CMA). Materials, 16(4), 1707. https://doi.org/10.3390/ma16041707






