Fabrication of 3D HierarchicalSphericalHoneycomb-Like Nd2O3/Co3O4/Graphene/Nickel Foam Composite Electrode Material for High-Performance Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
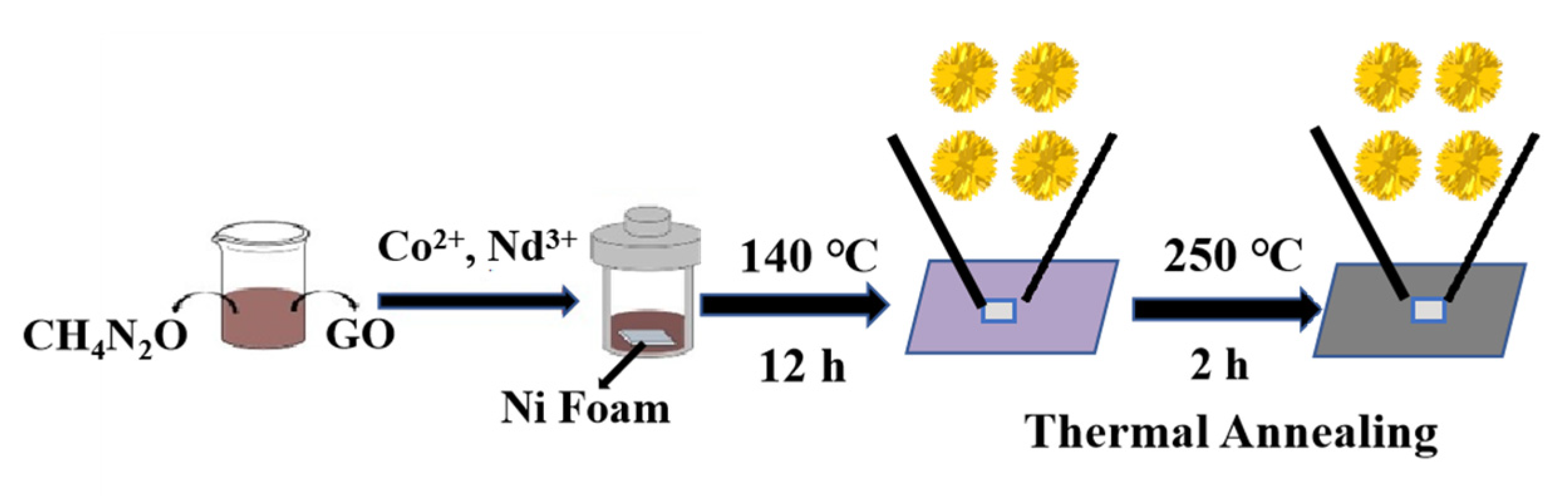
2.2. Preparation of Nd2O3/Co3O4/rGO/NF Composite Electrode
2.2.1. Pretreatment of Conductive Substrate NF
2.2.2. Synthesis of Nd2O3/Co3O4/rGO/NF Precursors
2.2.3. Synthesis of Nd2O3/Co3O4/rGO/NF
2.3. Structural Characterization
2.4. Electrochemical Characterization
2.4.1. Cyclic Voltammetry (CV)
2.4.2. Constant Current Charge and Discharge (GCD)
2.4.3. Electrochemical Impedance Spectroscopy (EIS)
2.4.4. Test of Cycling Stability
3. Results and Discussion
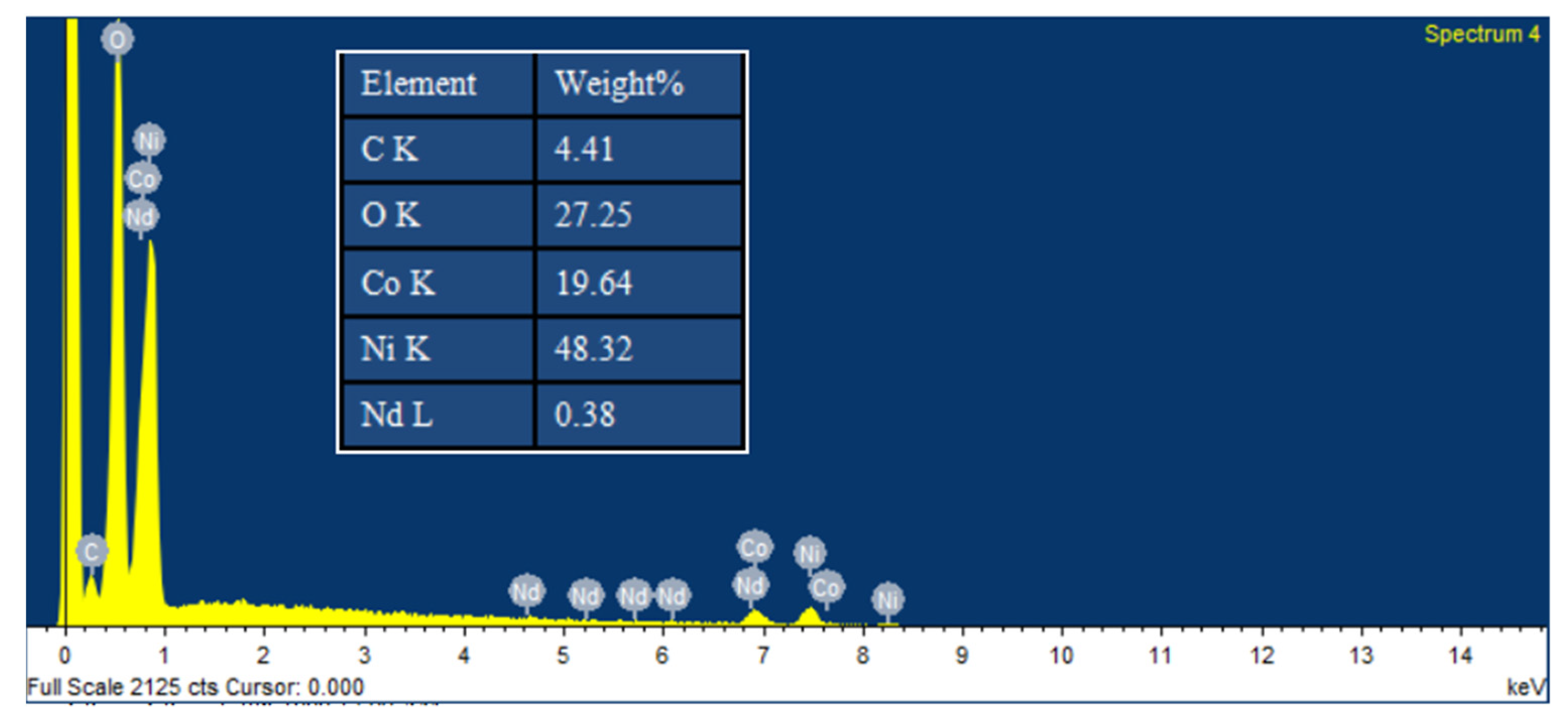
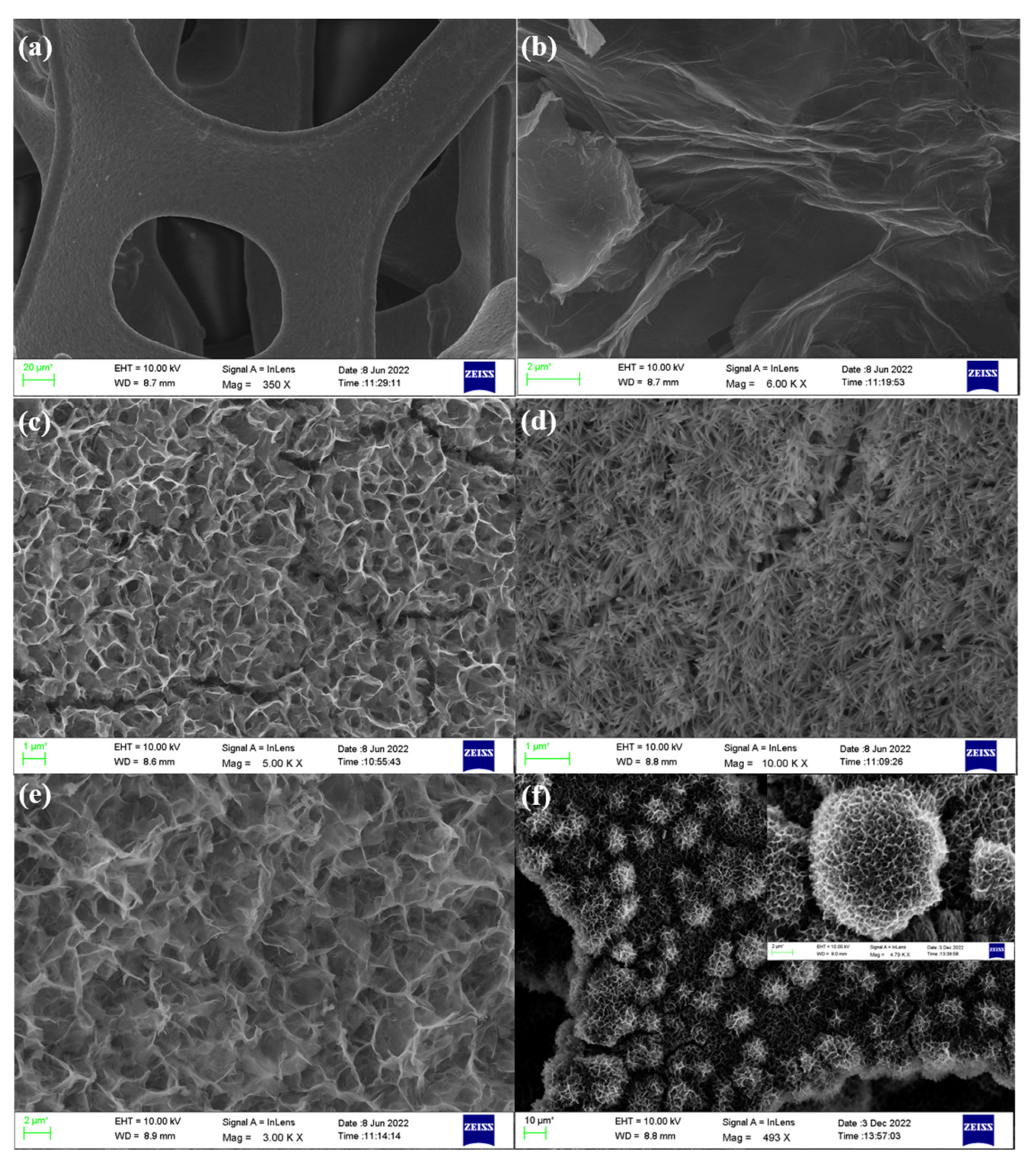
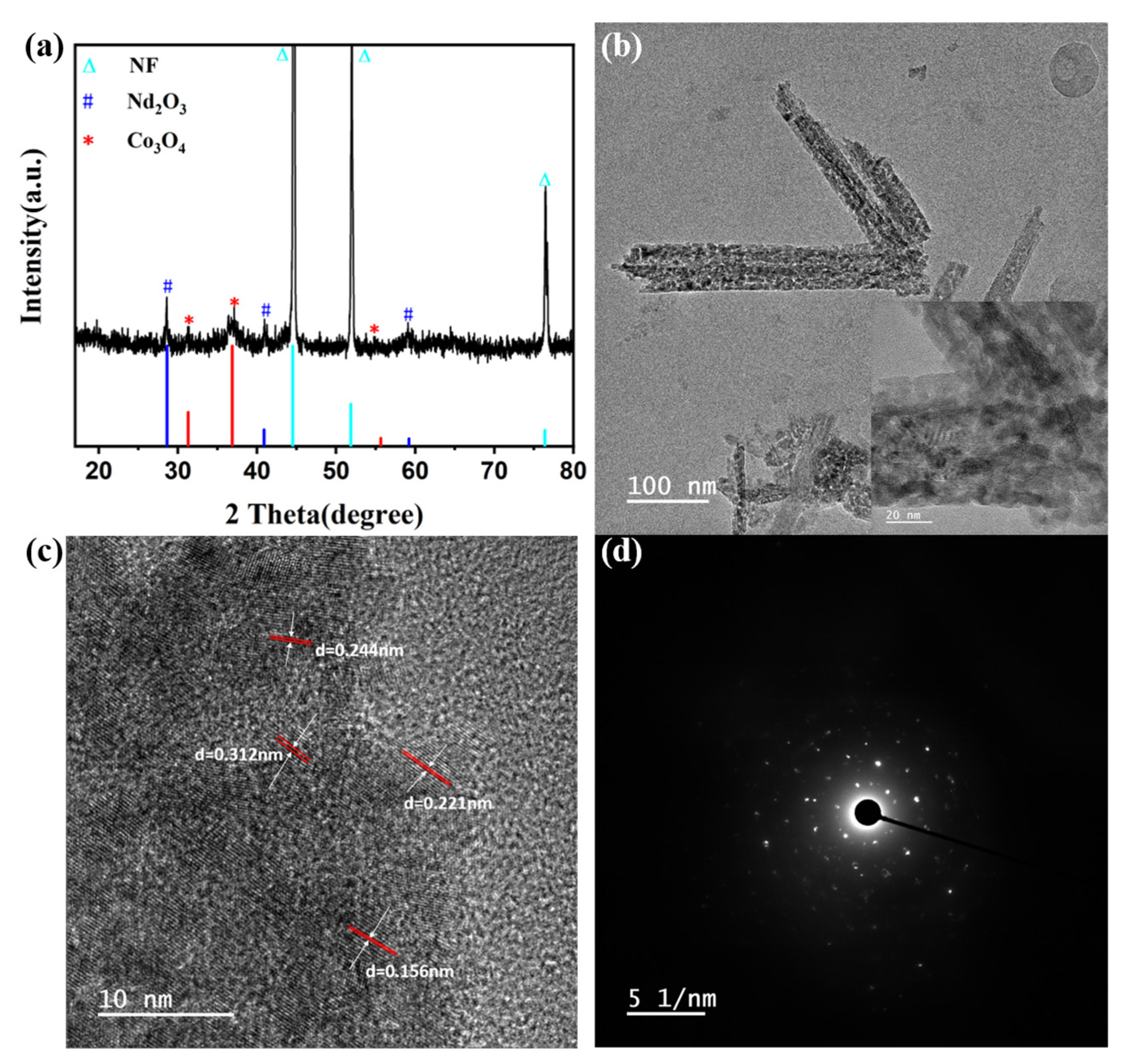
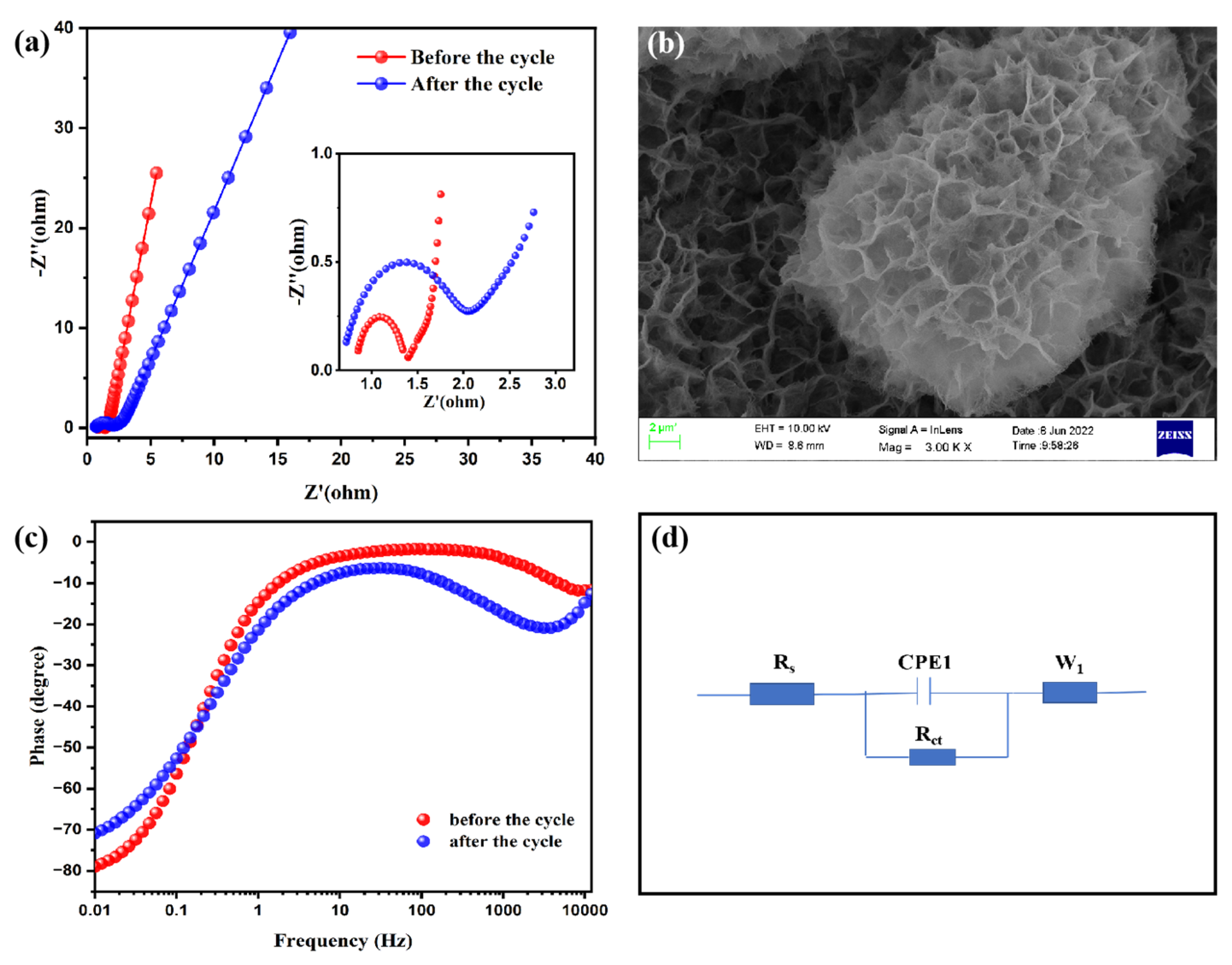
3.1. Analyses of Morphology and Structure
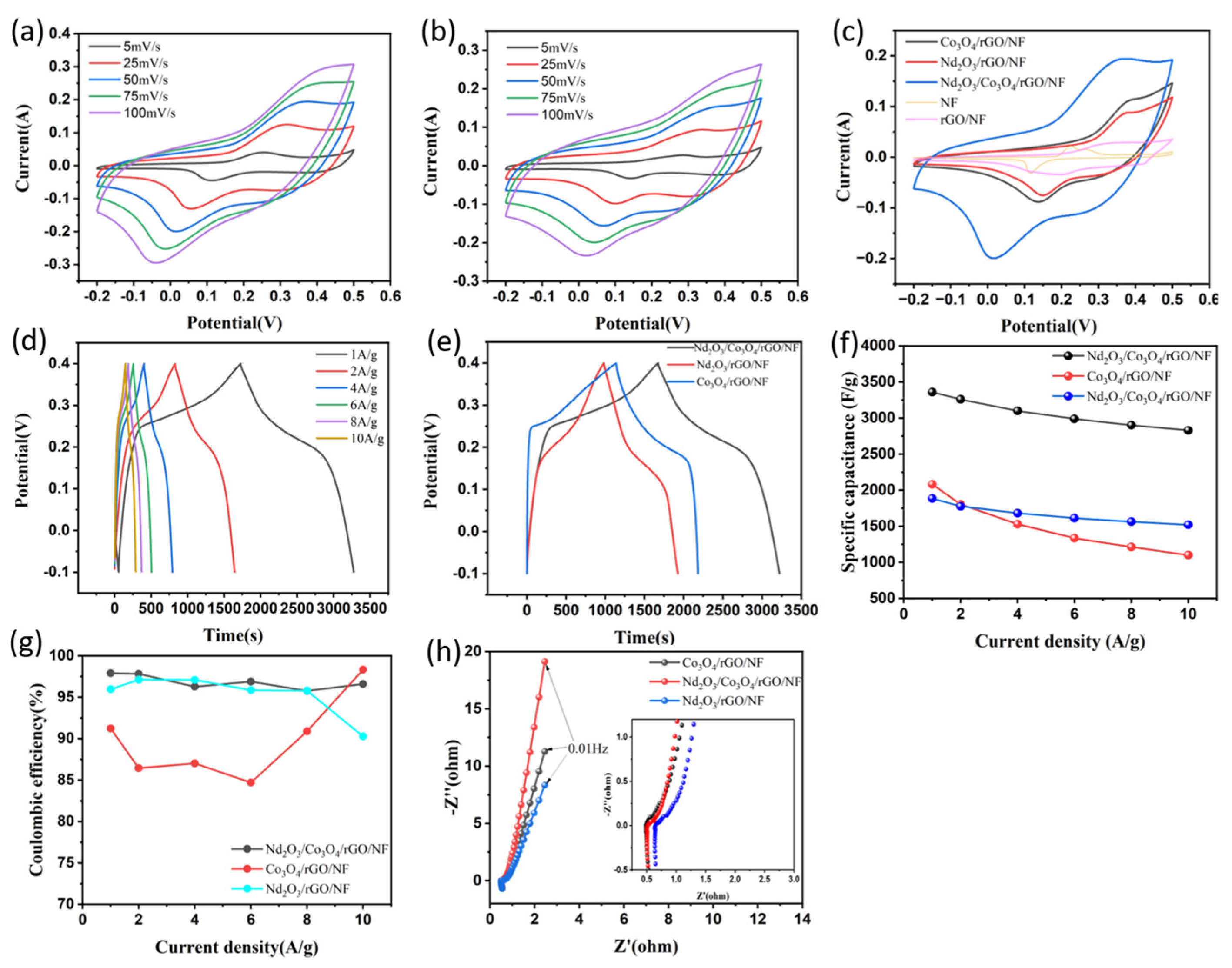
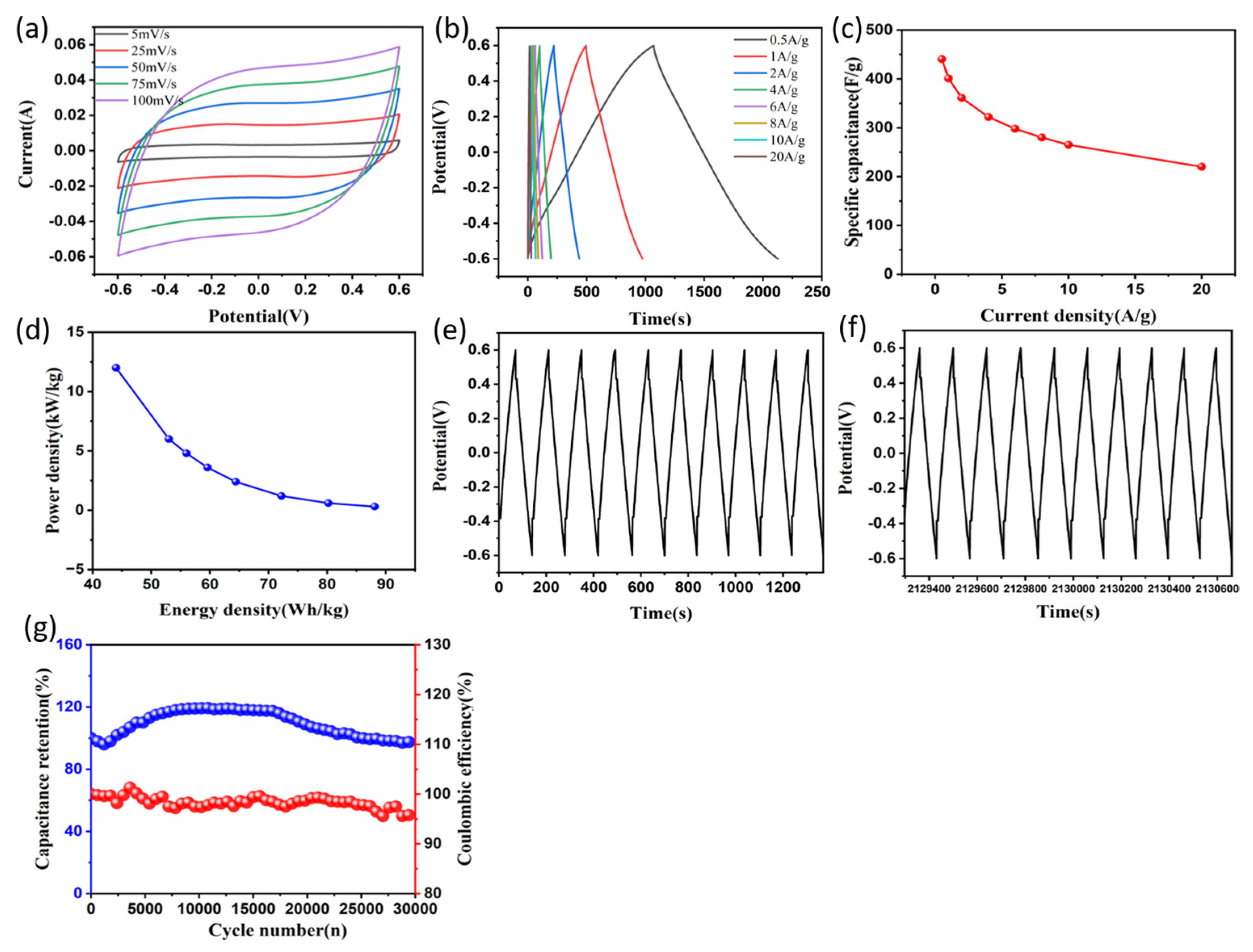
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, P.; Xu, L. Core-Shell Carbon Nanofibers@Ni(OH)2/NiO Composites for High-Performance Asymmetric Supercapacitors. Materials 2022, 15, 8377. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.K.; Jalak, M.B.; Karade, S.S.; Majumder, S.; Tamboli, M.S.; Truong, N.T.; Maile, N.C.; Kim, D.-Y.; Jagadale, A.D.; Yadav, H.M. A Novel Synthesized 1D Nanobelt-like Cobalt Phosphate Electrode Material for Excellent Supercapacitor Applications. Materials 2022, 15, 8235. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.E.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Zheng, J.P.; Huang, J.; Jow, T.R. The Limitations of Energy Density for Electrochemical Capacitors. J. Electrochem. Soc. 1997, 144, 2026. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Narenthiran, B.; Sivanantham, A.; Bhatlu, L.D.; Maridurai, T. Supercapacitor: Evolution and review. Mater. Today Proc. 2021, 46, 3984–3988. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Chen, S.; Xing, W.; Duan, J.; Hu, X.; Qiao, S.Z. Nanostructured morphology control for efficient supercapacitor electrodes. J. Mater. Chem. A 2013, 1, 2941–2954. [Google Scholar] [CrossRef]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Gu, W.; Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: Advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- Tan, Y.B.; Lee, J.-M. Graphene for supercapacitor applications. J. Mater. Chem. A 2013, 1, 14814–14843. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Wang, R.; Shen, G. Ternary oxide nanostructured materials for supercapacitors: A review. J. Mater. Chem. A 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Nandi, D.; Mohan, V.B.; Bhowmick, A.K.; Bhattacharyya, D. Metal/metal oxide decorated graphene synthesis and application as supercapacitor: A review. J. Mater. Sci. 2020, 55, 6375–6400. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, R.; Zheng, X.; Ru, Q.; Chen, Q.; Cui, C.; Li, G.; Zhang, D. Hierarchical porous NiCo2O4/CeO2 hybrid materials for high performance supercapacitors. Inorg. Chem. Front. 2018, 5, 3126–3134. [Google Scholar] [CrossRef]
- Liang, S.; Wang, H.; Li, Y.; Qin, H.; Luo, Z.; Huang, B.; Zhao, X.; Zhao, C.; Chen, L. Rare-earth based nanomaterials and their composites as electrode materials for high performance supercapacitors: A review. Sustainable Energy Fuels 2020, 4, 3825–3847. [Google Scholar] [CrossRef]
- Kai, T.; Ye, T. Magnetic characterization of rare-earth oxide nanoparticles. Appl. Phys. Lett. 2020, 117, 122410. [Google Scholar] [CrossRef]
- Arunachalam, S.; Kirubasankar, B.; Rajagounder Nagarajan, E.; Vellasamy, D.; Angaiah, S. A Facile Chemical Precipitation Method for the Synthesis of Nd(OH)3 and La(OH)3Nanopowders and their Supercapacitor Performances. ChemistrySelect 2018, 3, 12719–12724. [Google Scholar] [CrossRef]
- Rezanezhad, A.; Rezaie, E.; Ghadimi, L.S.; Hajalilou, A.; Abouzari-Lotf, E.; Arsalani, N. Outstanding supercapacitor performance of Nd–Mn co-doped perovskite LaFeO3@nitrogen-doped graphene oxide nanocomposites. Electrochim. Acta 2020, 335, 135699. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Durai, G.; Tatarchuk, T.; Sumathi, M.; Kuppusami, P.; Qin, J.; Choi, M.Y. Synthesis of hierarchical structured rare earth metal–doped Co3O4 by polymer combustion method for high performance electrochemical supercapacitor electrode materials. Ionics 2020, 26, 2051–2061. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-Layer Assembly of Ultrathin Composite Films from Micron-Sized Graphite Oxide Sheets and Polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Tyler, S.M.; Narendra, K.; Wang, X.; David, P.; Patrice, S.; Yury, G. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Su, D.; Zhou, L.; Wu, S.; Han, L.; Fang, S.; Cao, S. High-quality Porous Cobalt Monoxide Nanowires @ Ultrathin Manganese dioxide Sheets Core-Shell Nanowire Arrays on Ni Foam for High-Performance Supercapacitor. Electrochim. Acta 2016, 194, 376–384. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Huang, Z.; Lu, F.; He, T. NiCo2S4@Co(OH)2 core-shell nanotube arrays in situ grown on Ni foam for high performances asymmetric supercapcitors. J. Power Sources 2016, 312, 156–164. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Dubal, D.P.; Ji, S.-H.; Kim, D.-H. Superfast Electrodeposition of Newly Developed RuCo2O4 Nanobelts over Low-Cost Stainless Steel Mesh for High-Performance Aqueous Supercapacitor. Adv. Mater. Interfaces 2018, 5, 1800283. [Google Scholar] [CrossRef]
- Pan, T.-M.; Lu, C.-H. Response to “Comment on ‘Forming-free resistive switching in Nd2O3, Dy2O3, and Er2O3 films fabricated in full room temperature”. Appl. Phys. Lett. 2012, 100, 076102. [Google Scholar] [CrossRef]
- Murugalakshmi, M.; Karunamoorthy, S.; Min, P.C.; Velluchamy, M. Efficient photocatalytic degradation of sulfasalazine and reduction of hexavalent chromium over robust In2S3/Nd2O3 heterojunction under visible light. J. Water Process Eng. 2022, 45, 102492. [Google Scholar] [CrossRef]
- Li, J.; Zou, P.-c.; Yao, W.-t.; Liu, P.; Kang, F.-y.; Yang, C. An asymmetric supercapacitor based on a NiO/Co3O4@NiCo cathode and an activated carbon anode. New Carbon Mater. 2020, 35, 112–120. [Google Scholar] [CrossRef]
- Tatsumi, K.; Tsutsui, M.; Beall, G.W.; Mullica, D.F.; Milligan, W.O. Satellite phenomena in the X-ray photoelectron spectra of lanthanide trihydroxides. J. Electron Spectrosc. Relat. Phenom. 1979, 16, 113–118. [Google Scholar] [CrossRef]
- Zhang, X.H.; Huang, J.M.; Tian, Y.B.; Ye, C.M.; Yang, S.H. Electrochemical Reduction Mechanism of Nd (III) in NaCl-KCl-NdCl3 Molten Salt on Tungsten. Electrode Chinese Rare Earths 2022, 43, 82–89. [Google Scholar] [CrossRef]
- Jeon, H.; Han, J.H.; Yu, D.M.; Lee, J.Y.; Kim, T.-H.; Hong, Y.T. Synthesis of mesoporous reduced graphene oxide by Zn particles for electrodes of supercapacitor in ionic liquid electrolyte. J. Ind. Eng. Chem. 2017, 45, 105–110. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Guo, W.; Chen, J.; He, W.; Peng, F. Synthesis of spherical PANI particles via chemical polymerization in ionic liquid for high-performance supercapacitors. Electrochim. Acta 2014, 135, 550–557. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Qian, Q.; Wang, F.; Xia, X. A green approach to the synthesis of graphene nanosheets. ACS nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhu, M.; Jiang, F.; Du, Y.; Wang, C.; Yang, P. Highly efficient electrocatalytic performance based on Pt nanoflowers modified reduced graphene oxide/carbon cloth electrode. J. Mater. Chem. 2012, 22, 13707. [Google Scholar] [CrossRef]
- Zhu, M.; Dong, Y.; Xiao, B.; Du, Y.; Wang, X. Enhanced photocatalytic hydrogen evolution performance based on Ru-trisdicarboxybipyridine-reduced graphene oxide hybrid. J. Mater. Chem. 2012, 22, 23773–23779. [Google Scholar] [CrossRef]
- Patel, S.; Dhak, P.; Kim, M.K.; Lee, J.H.; Kim, S.K. Structural and magnetic properties of Co-doped Gd2O3 nanorods. J. Magn. Magn. Mater. 2015, 403, 155–160. [Google Scholar] [CrossRef]
- Gao, G.; Wu, H.B.; Dong, B.; Ding, S.; Lou, X.W. Growth of Ultrathin ZnCo2O4 Nanosheets on Reduced Graphene Oxide with Enhanced Lithium Storage Properties. Adv. Sci. 2015, 2, 1400014. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Wan, L.; Sun, S.; Chen, Q.; Sun, J.; Xia, Q.; Xia, H. Phosphate Ion Functionalized Co3O4 Ultrathin Nanosheets with Greatly Improved Surface Reactivity for High Performance Pseudocapacitors. Adv. Mater. 2017, 29, 1604167. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Luo, H.; He, M.; Wang, W.; Dai, Y. Controlled synthesis of NiCo2O4 nanowires and nanosheets on reduced graphene oxide nanosheets for supercapacitors. J. Solid State Electrochem. 2015, 19, 3309–3317. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, B.; Sun, Y.; Yang, H.; Zhang, X. Structure, morphology and electrochemical properties of LaxSr1−xCo0.1Mn0.9O3−δ perovskite nanofibers prepared by electrospinning method. J. Alloys Compd. 2015, 624, 31–39. [Google Scholar] [CrossRef]
- Babu, I.M.; William, J.J.; Muralidharan, G. Surfactant tuned morphology of mesoporous β-Co(OH)2/CMC nanoflakes: A prospective candidate for supercapacitors. J. Solid State Electrochem. 2019, 23, 1325–1338. [Google Scholar] [CrossRef]
- Bailmare, D.B.; Tripathi, P.; Deshmukh, A.D.; Gupta, B.K. Designing of two dimensional lanthanum cobalt hydroxide engineered high performance supercapacitor for longer stability under redox active electrolyte. Sci. Rep. 2022, 12, 3084. [Google Scholar] [CrossRef]
- Saleh Ghadimi, L.; Arsalani, N.; Ahadzadeh, I.; Hajalilou, A.; Abouzari-Lotf, E. Effect of synthesis route on the electrochemical performance of CoMnFeO4 nanoparticles as a novel supercapacitor electrode material. Appl. Surf. Sci. 2019, 494, 440–451. [Google Scholar] [CrossRef]
- Goljanian Tabrizi, A.; Arsalani, N.; Mohammadi, A.; Namazi, H.; Saleh Ghadimi, L.; Ahadzadeh, I. Facile synthesis of a MnFe2O4/rGO nanocomposite for an ultra-stable symmetric supercapacitor. New J. Chem. 2017, 41, 4974–4984. [Google Scholar] [CrossRef]
- Zhao, C.; Ju, P.; Wang, S.; Zhang, Y.; Min, S.; Qian, X. One-step hydrothermal preparation of TiO2/RGO/Ni(OH)2/NF electrode with high performance for supercapacitors. Electrochim. Acta 2016, 218, 216–227. [Google Scholar] [CrossRef]
- Mohammadi, A.; Arsalani, N.; Tabrizi, A.G.; Moosavifard, S.E.; Naqshbandi, Z.; Ghadimi, L.S. Engineering rGO-CNT wrapped Co3S4 nanocomposites for high-performance asymmetric supercapacitors. Chem. Eng. J. 2018, 334, 66–80. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X. Ultrathin Mesoporous NiCo2O4 Nanosheets Supported on Ni Foam as Advanced Electrodes for Supercapacitors. Adv. Funct. Mater. 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Arunachalam, S.; Kirubasankar, B.; Pan, D.; Liu, H.; Yan, C.; Guo, Z.; Angaiah, S. Research progress in rare earths and their composites based electrode materials for supercapacitors. Green Energy Environ. 2020, 5, 259–273. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, L.; Yin, X.; Huang, T.J.; Gong, H. A one step processed advanced interwoven architecture of Ni(OH)2 and Cu nanosheets with ultrahigh supercapacitor performance. J. Mater. Chem. A 2016, 4, 12144–12151. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Z.; Zhao, B.; Yong, C.; Zhang, L.; Wu, H.H.; Wang, M.S.; Dai, S.; Zhang, K.; Dong, D. Design and understanding of dendritic mixed-metal hydroxide [emailprotected] carbon nanotube array electrode for high-performance asymmetric supercapacitors. Energy Storage Mater. 2019, 16, 632–645. [Google Scholar] [CrossRef]
- Lv, A.; Lu, S.; Xu, W.; Wang, Z.; Shen, Y.; Liu, G. One-pot synthesis of NiCo2O4/rGO/NF hybrid electrode materials realizing ultrahigh capacitance and rapid charge/discharge at large current density. Appl. Surf. Sci. 2020, 511, 145538. [Google Scholar] [CrossRef]
- Sivakumar, P.; Jana, M.; Jung, M.G.; Gedanken, A.; Park, H.S. Hexagonal plate-like Ni–Co–Mn hydroxide nanostructures to achieve high energy density of hybrid supercapacitors. J. Mater. Chem. A 2019, 7, 11362–11369. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Chen, W.; Cha, D.; Alshareef, H.N. Substrate dependent self-organization of mesoporous cobalt oxide nanowires with remarkable pseudocapacitance. Nano Lett. 2012, 12, 2559–2567. [Google Scholar] [CrossRef]
- Cheng, S.; Shi, T.; Huang, Y.; Tao, X.; Li, J.; Cheng, C.; Liao, G.; Tang, Z. Rational design of nickel cobalt sulfide/oxide core-shell nanocolumn arrays for high-performance flexible all-solid-state asymmetric supercapacitors. Ceram. Int. 2017, 43, 2155–2164. [Google Scholar] [CrossRef]
- Hui, K.N.; Hui, K.S.; Tang, Z.; Jadhav, V.V.; Xia, Q.X. Hierarchical chestnut-like MnCo2O4 nanoneedles grown on nickel foam as binder-free electrode for high energy density asymmetric supercapacitors. J. Power Sources 2016, 330, 195–203. [Google Scholar] [CrossRef]
- Singh, A.; Akhtar, M.A.; Chandra, A. Trade-off between capacitance and cycling at elevated temperatures in redox additive aqueous electrolyte based high performance asymmetric supercapacitors. Electrochim.Acta 2017, 229, 291–298. [Google Scholar] [CrossRef]
- Zhu, S.J.; Jia, J.Q.; Wang, T.; Zhao, D.; Yang, J.; Dong, F.; Shang, Z.G.; Zhang, Y.X. Rational design of octahedron and nanowire CeO2@MnO2 core–shell heterostructures with outstanding rate capability for asymmetric supercapacitors. Chem. Commun. 2015, 51, 14840–14843. [Google Scholar] [CrossRef] [PubMed]
- Vivek, E.; Arulraj, A.; Krishnan, S.G.; Khalid, M.; Potheher, V. Novel Nanostructured Nd(OH)3/g-C3N4 Nanocomposites (Nanorolls Anchored on Nanosheets) as Reliable Electrode Material for Supercapacitors. Energy Fuels 2021, 35, 15205–15212. [Google Scholar] [CrossRef]
- Lan, J.; Hou, H.; Liu, X.; Yu, X.; Rong, J.; Chen, F. Hierarchical dumbbell-like Fe3O4/C electrode forthe supercapacitor. Mater. Lett. 2023, 331, 133302. [Google Scholar] [CrossRef]


| Electrode Materials | Specific Capacitance | Cycling Performance | Energy Density at Power Density | Ref. |
|---|---|---|---|---|
| Co3O4/rGO composites | 1152 F/g at 1 A/g | 89.1 % (5000 cycles) | 57.26 Wh/kg at 2240.68 W/kg | [54] |
| CeO2/Co3O4 composites | 255 F/g at 0.25 A/g | 90.1 % (3000 cycles) | 27.5 Wh/kg at 500 W/kg | [55] |
| Nd(OH)3/g-C3N4composites | 426.40 F/g at 1A/g | 88.3% (3000 cycles) | no data | [56] |
| Fe3O4/C composites | 490.2 F/g at 1A/g | 90.7 % (1000 cycles) | 34.3 Wh/kg at 1003.9 W/kg | [57] |
| Nd2O3/Co3O4/rGO/NF | 3359.6 F/g at 1A/g | 95.7% (30,000 cycles) | 88.1 Wh/kg at 300 W/kg | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Wang, S.; Lu, S.; Xu, W.; Zhou, M. Fabrication of 3D HierarchicalSphericalHoneycomb-Like Nd2O3/Co3O4/Graphene/Nickel Foam Composite Electrode Material for High-Performance Supercapacitors. Materials 2023, 16, 1694. https://doi.org/10.3390/ma16041694
Liang H, Wang S, Lu S, Xu W, Zhou M. Fabrication of 3D HierarchicalSphericalHoneycomb-Like Nd2O3/Co3O4/Graphene/Nickel Foam Composite Electrode Material for High-Performance Supercapacitors. Materials. 2023; 16(4):1694. https://doi.org/10.3390/ma16041694
Chicago/Turabian StyleLiang, Huihui, Shasha Wang, Shixiang Lu, Wenguo Xu, and Min Zhou. 2023. "Fabrication of 3D HierarchicalSphericalHoneycomb-Like Nd2O3/Co3O4/Graphene/Nickel Foam Composite Electrode Material for High-Performance Supercapacitors" Materials 16, no. 4: 1694. https://doi.org/10.3390/ma16041694
APA StyleLiang, H., Wang, S., Lu, S., Xu, W., & Zhou, M. (2023). Fabrication of 3D HierarchicalSphericalHoneycomb-Like Nd2O3/Co3O4/Graphene/Nickel Foam Composite Electrode Material for High-Performance Supercapacitors. Materials, 16(4), 1694. https://doi.org/10.3390/ma16041694






