Effects of Partial Manganese Substitution by Cobalt on the Physical Properties of Pr0.7Sr0.3Mn(1−x)CoxO3 (0 ≤ x ≤ 0.15) Manganites
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.2. Characterizations
3. Results and Discussion
3.1. Thermogravimetric Analysis
3.2. Structural and Morphological Characterization
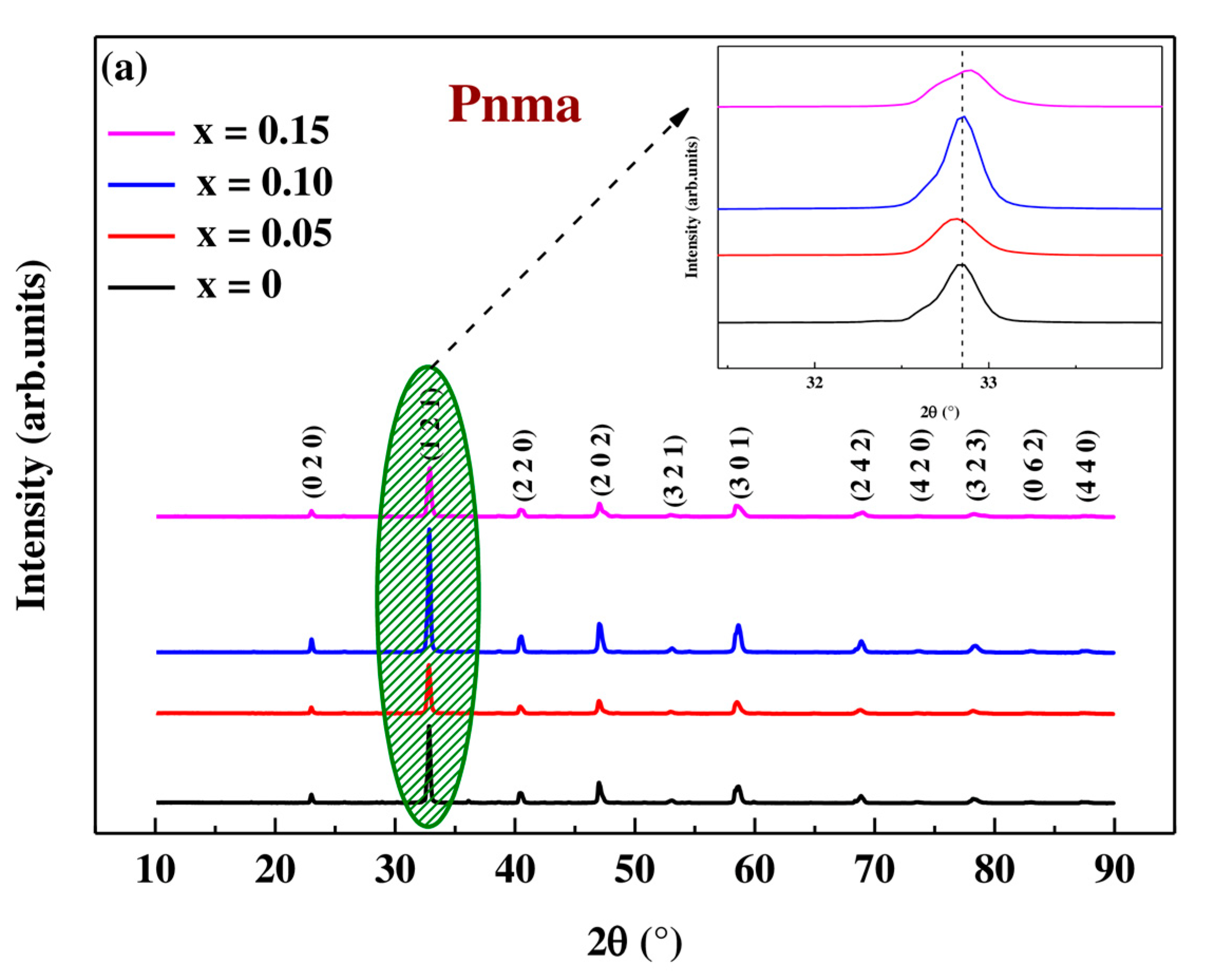
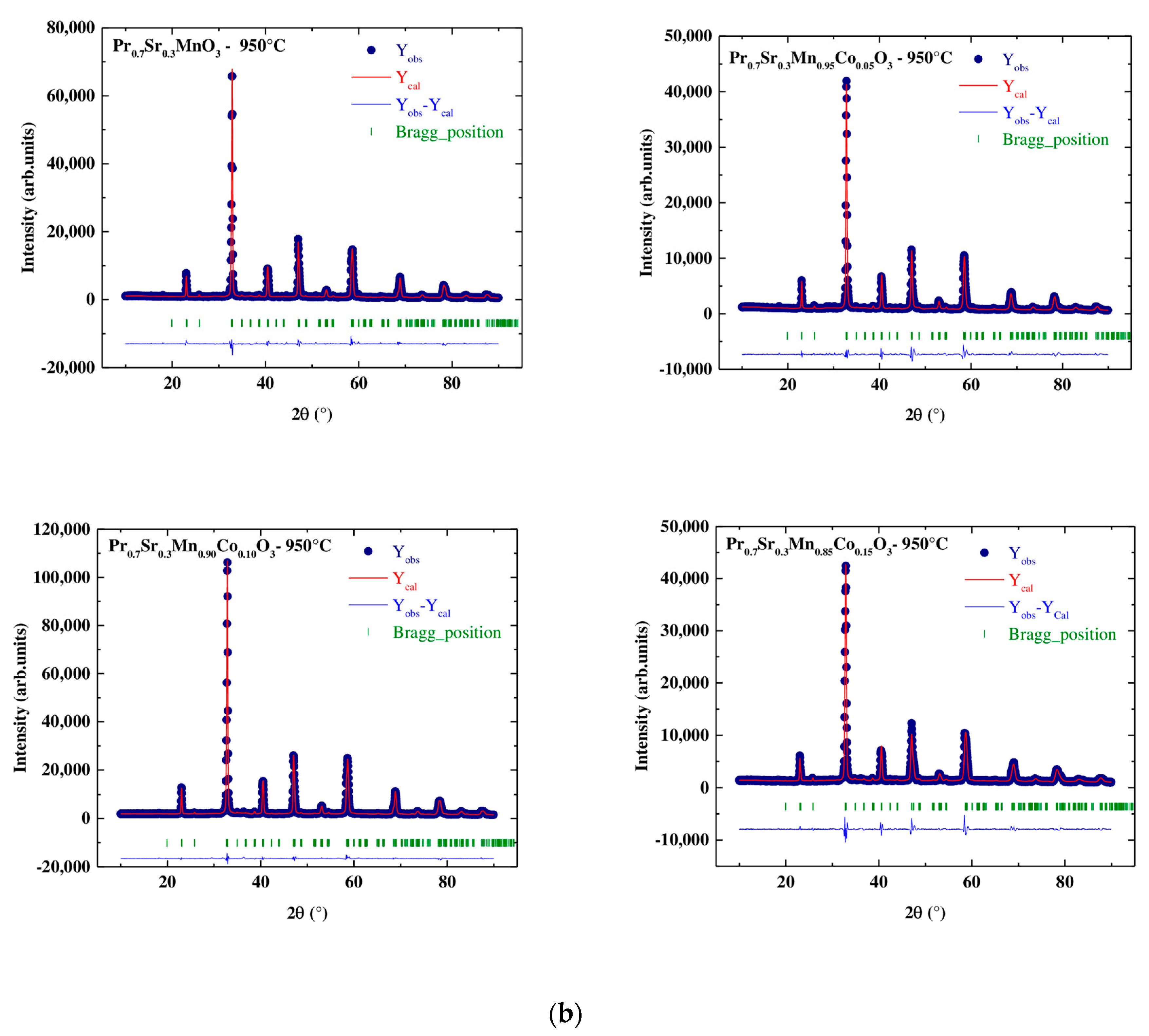
3.2.1. X-ray Diffraction Results
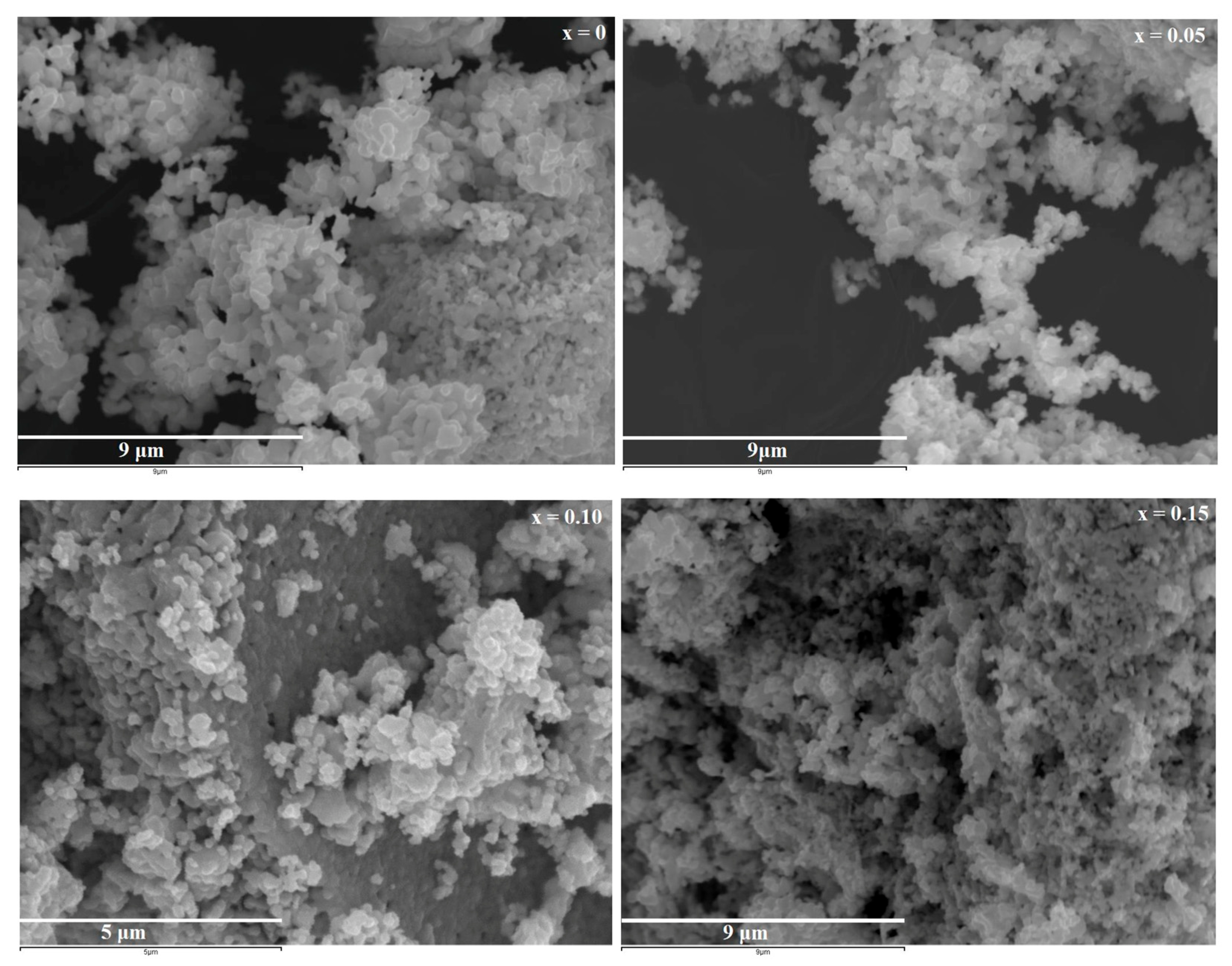
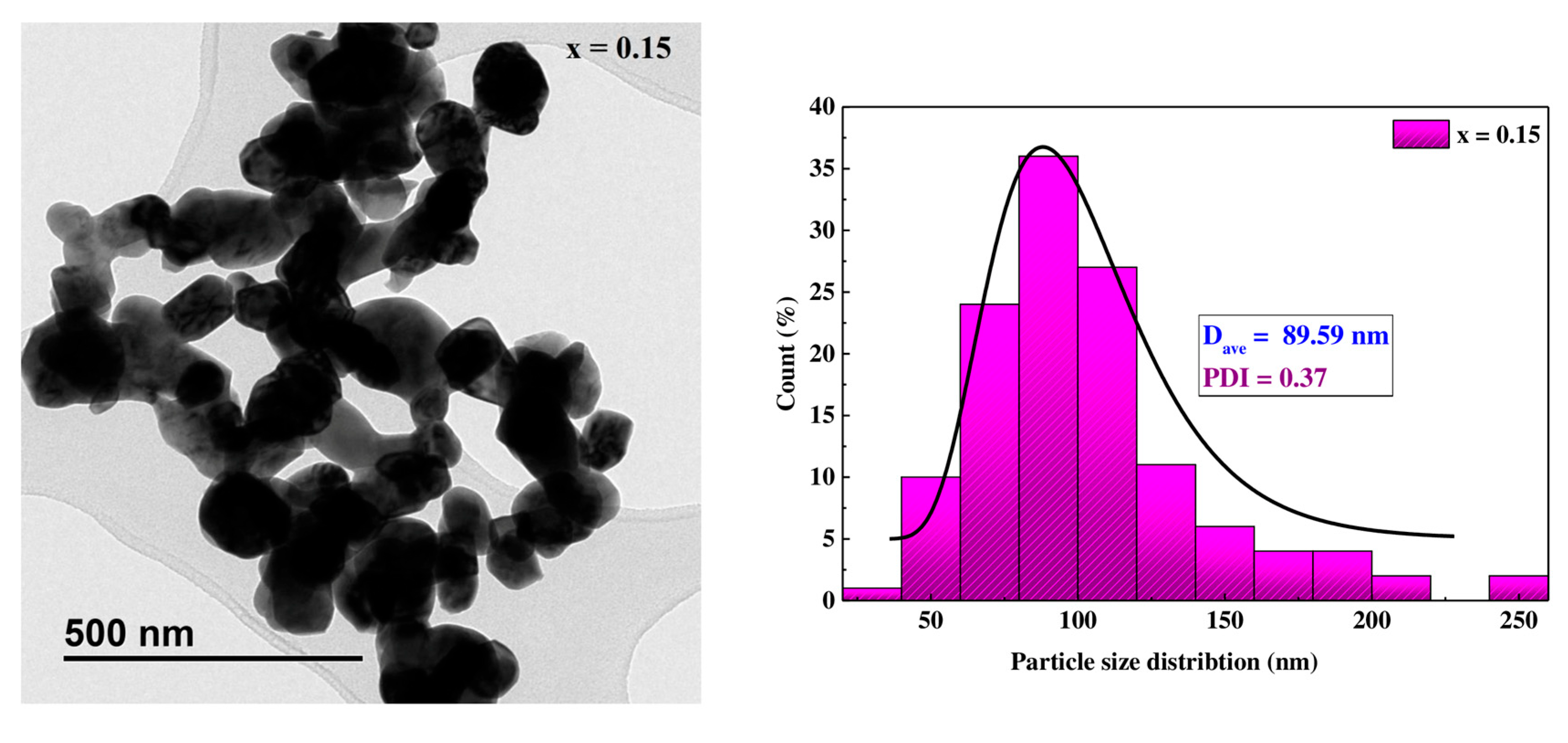
3.2.2. Morphological Characterization
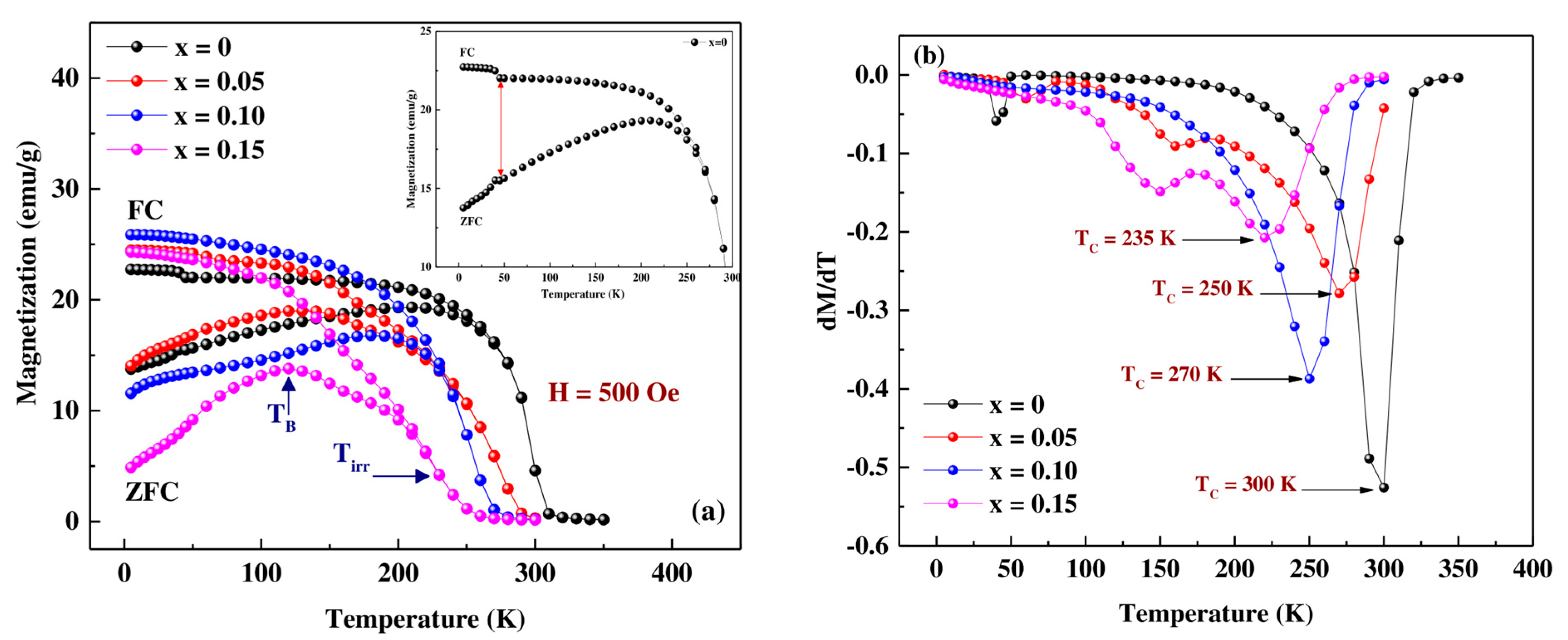
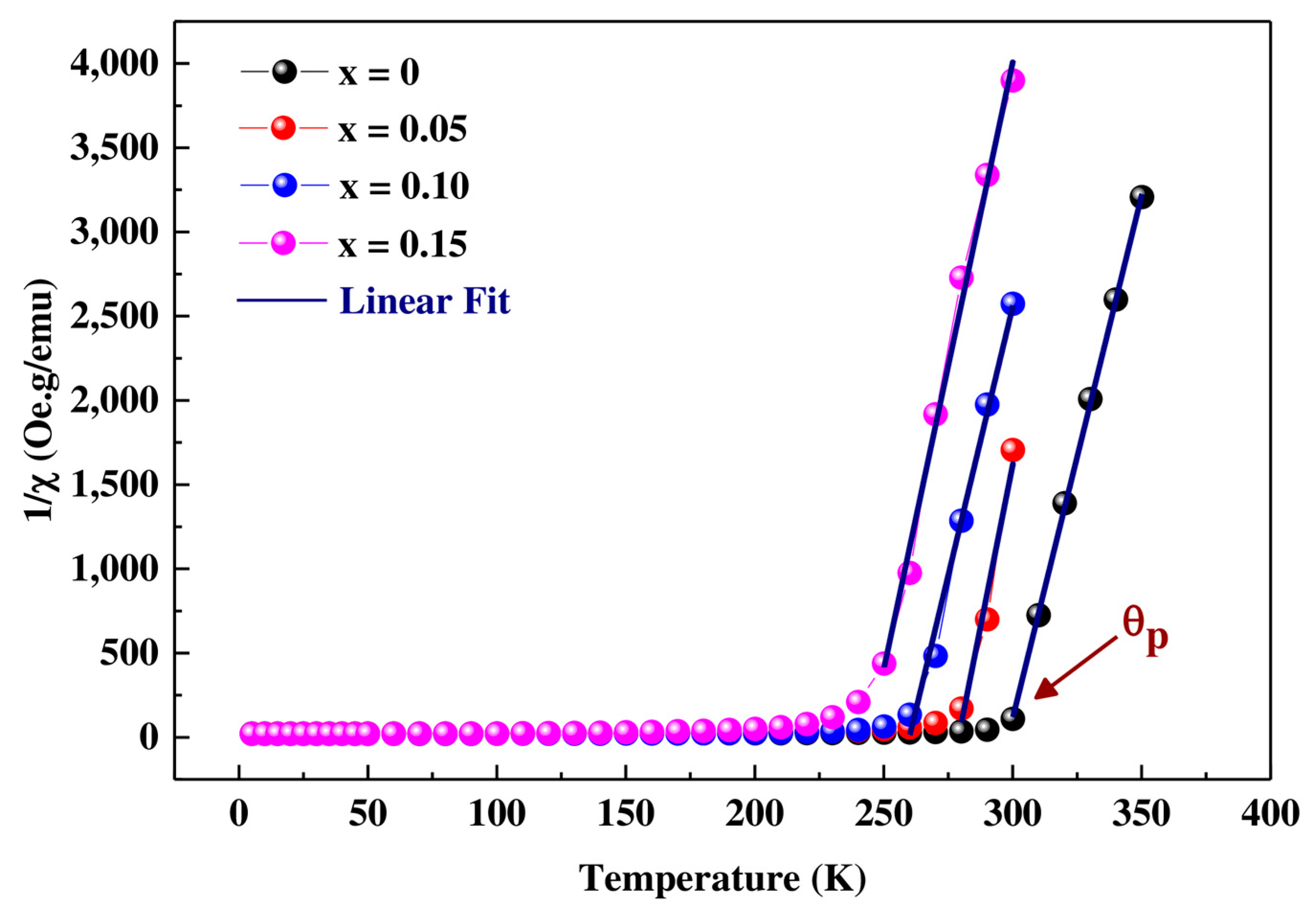
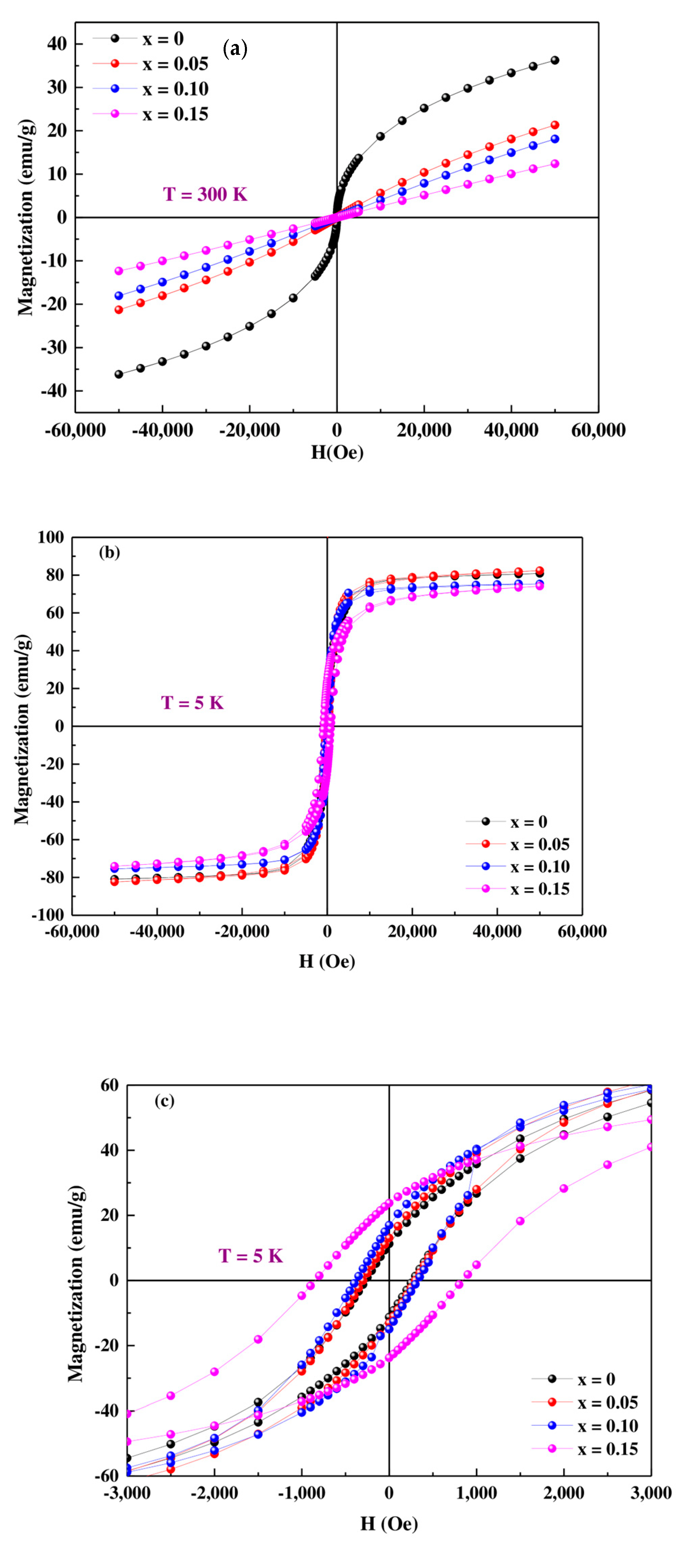
3.3. Magnetic Measurements
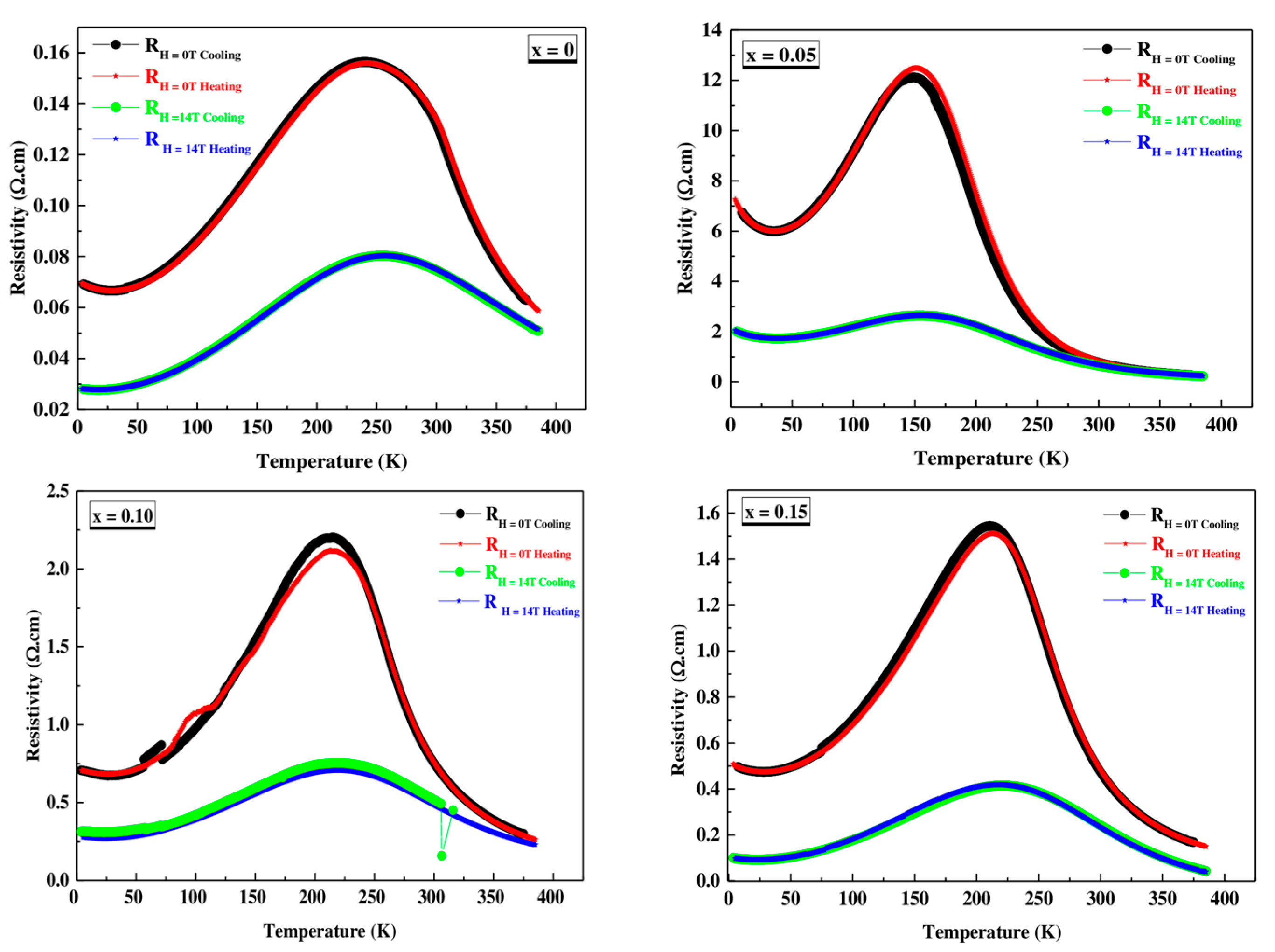
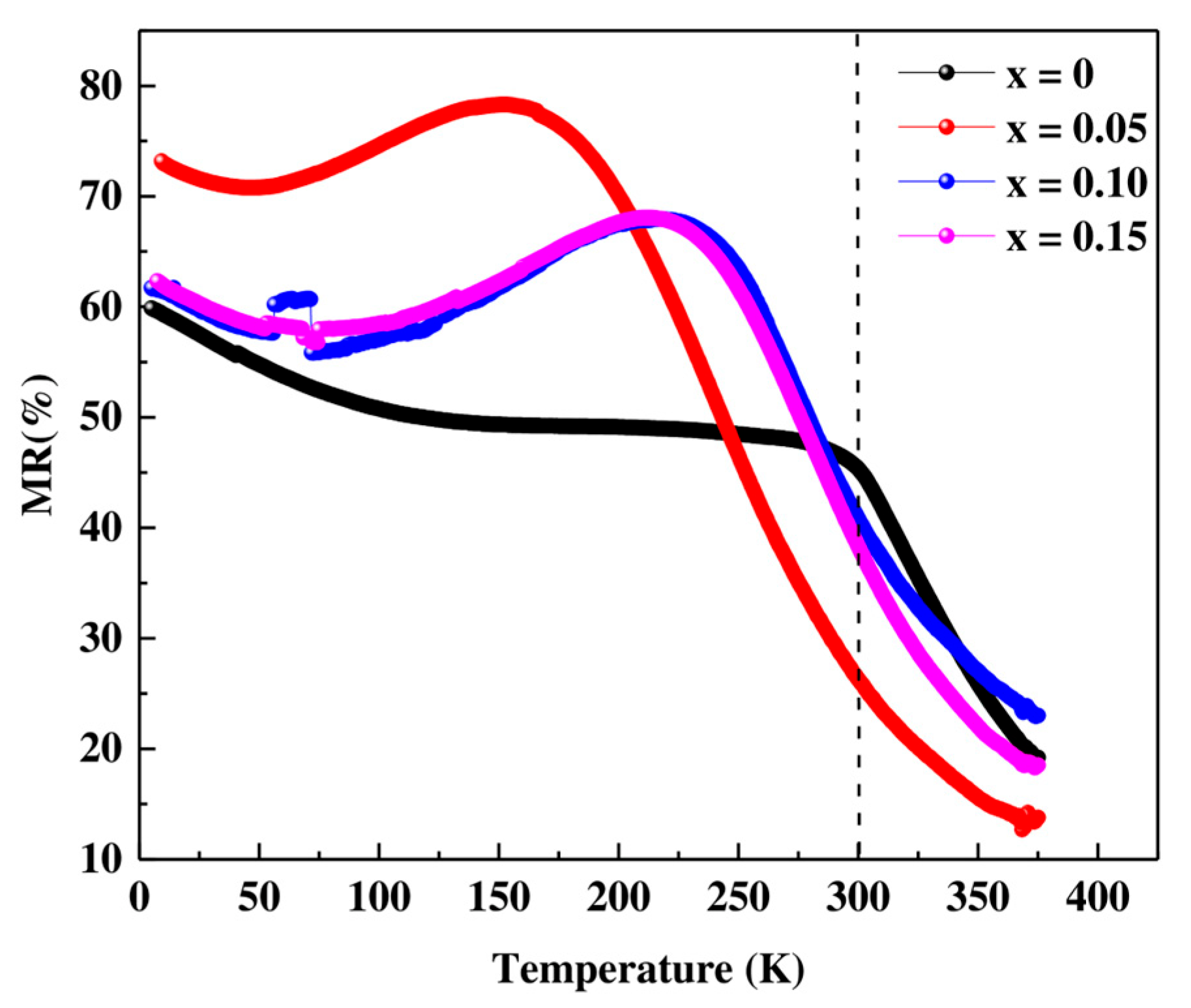
3.4. Electrical Transport Properties
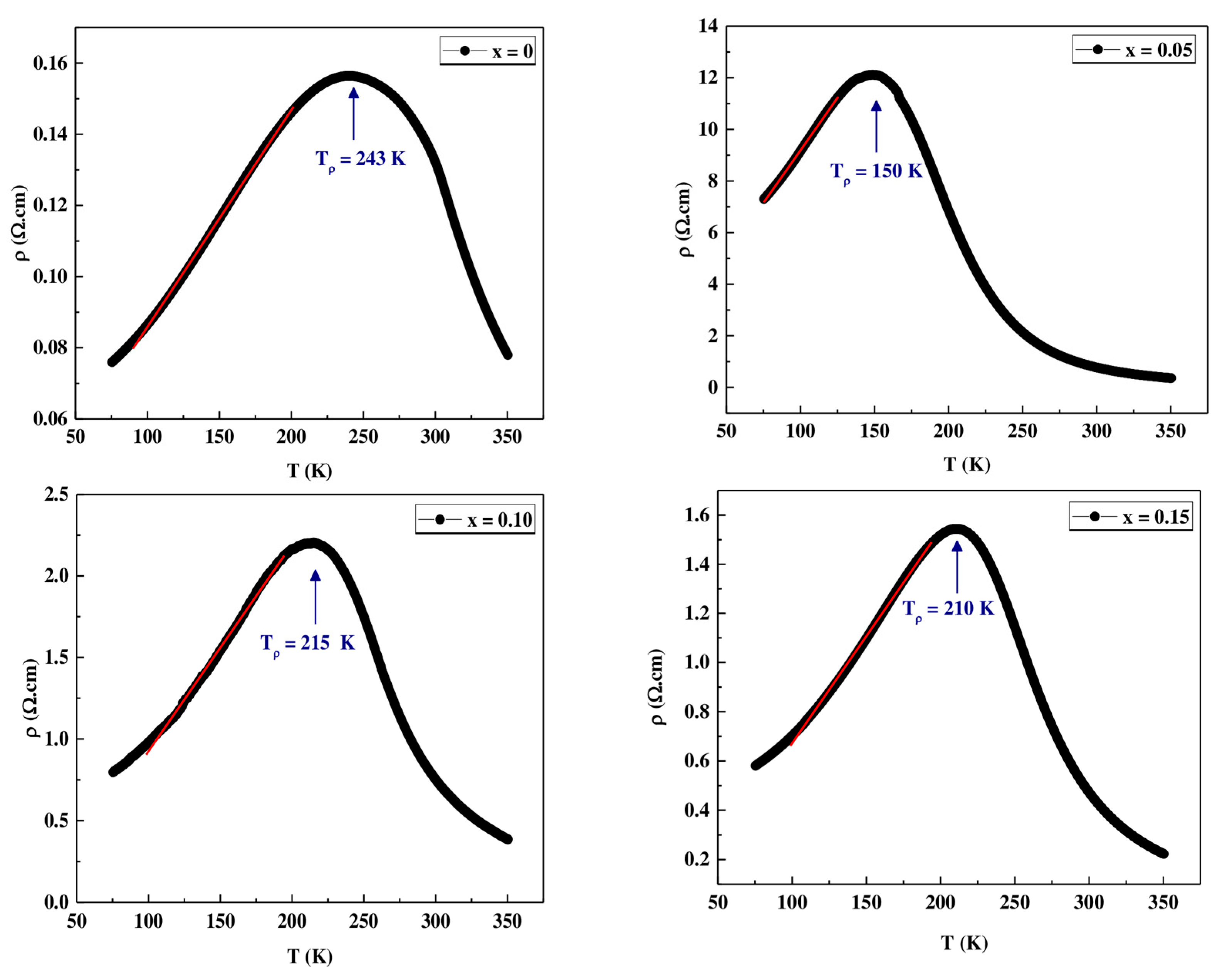
3.4.1. Electrical Behavior at Low Temperature (80 K < Tρ)
3.4.2. Electrical Behavior at High Temperature (T > Tρ)
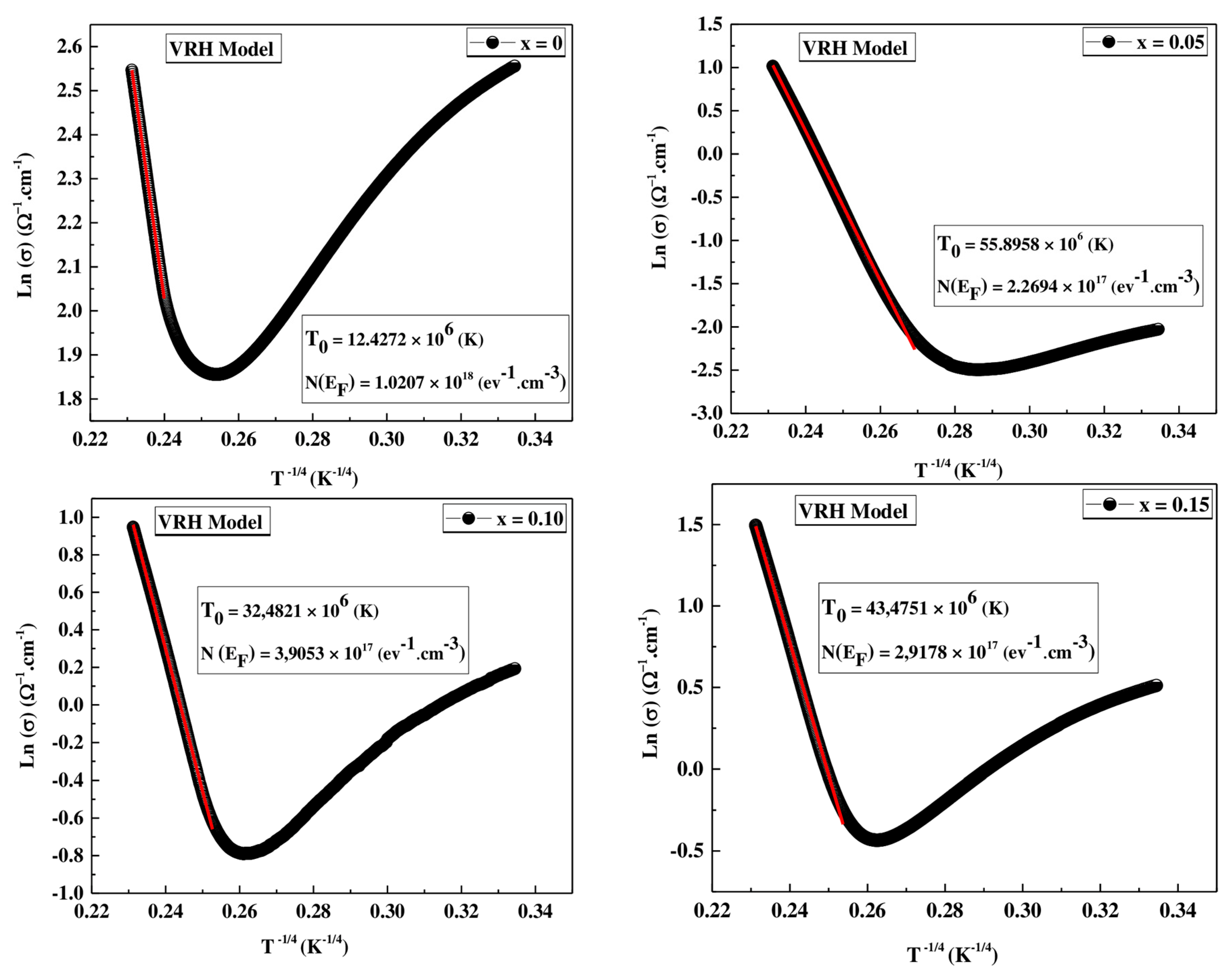
VRH Model
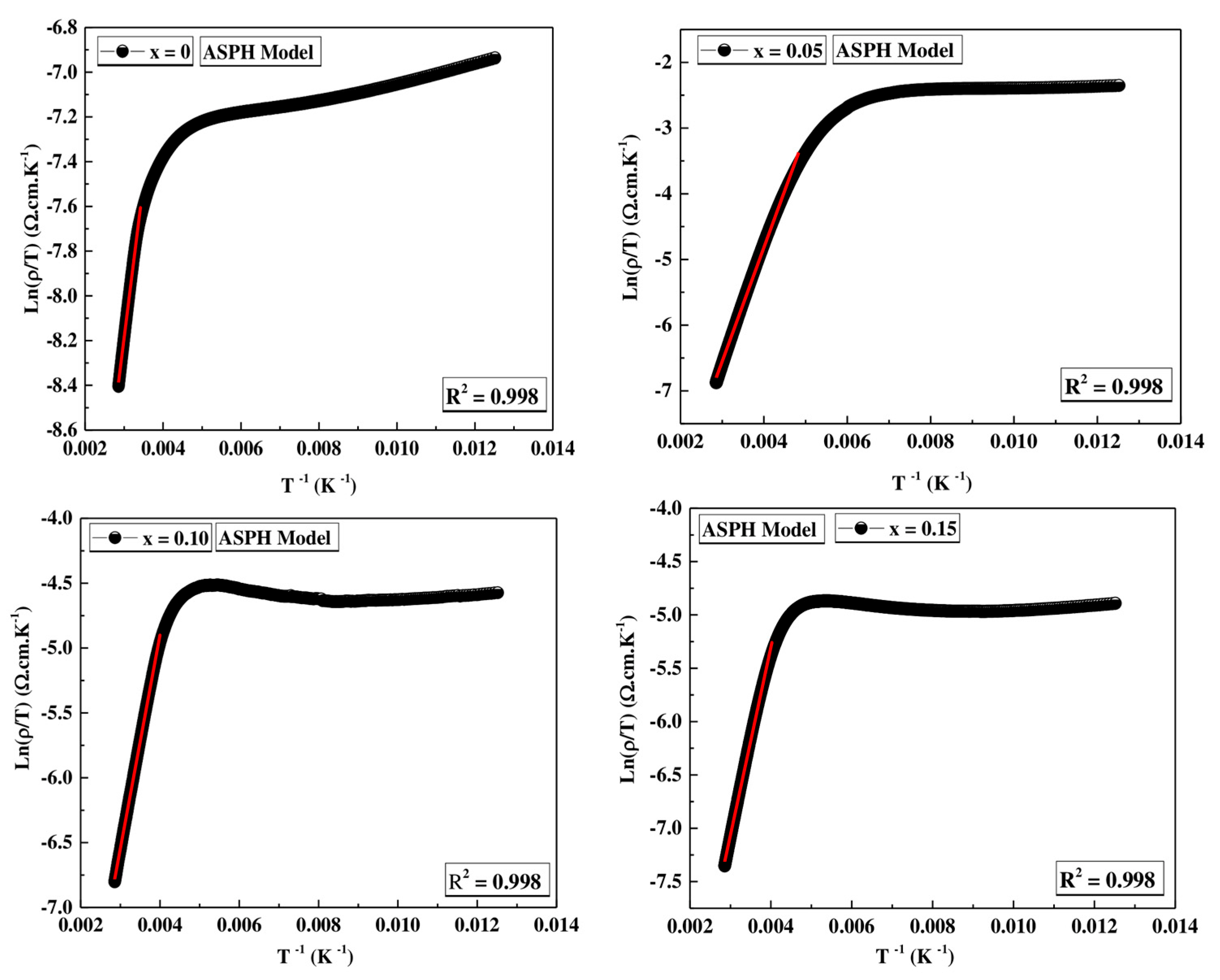
ASPH Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonker, G.H. Magnetic compounds with perovskite structure IV Conducting and non-conducting compounds. Physica 1950, 16, 337. [Google Scholar] [CrossRef]
- Raveau, B.; Rao, C.N.R. Colossal Magnetoresistance, Charge Ordering and Related Properties of Manganese Oxides; World Scientific: Singapore, 1998. [Google Scholar]
- von Helmolt, R.; Wecker, J.; Holzapfel, B.; Schultz, L.; Samwer, K. Giant negative magnetoresistance in perovskitelike La2/3 Ba1/3MnO3 ferromagnetic films. Phys. Rev. Lett. 1993, 71, 2331. [Google Scholar] [CrossRef] [PubMed]
- Zener, C. Interaction between the d -Shells in the Transition Metals. II. Ferromagnetic Compounds of Manganese with Perovskite Structure. Phys. Rev. 1951, 82, 403. [Google Scholar] [CrossRef]
- Töpfe, J.; Goodenough, J.B. Transport and Magnetic Properties of the Perovskites La1-yMnO3 and LaMn1-zO3. Chem. Mater. 1997, 9, 1467. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.R.; Perea, J.D.; Castillo, R.; Diosa, J.E.; Baca, E. Hybrid Superconducting-Ferromagnetic [Bi2Sr2(Ca,Y)2Cu3O10]0.99(La2/3Ba1/3MnO3)0.01 Composite Thick Films. Materials 2019, 12, 861. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Tan, W.; Huo, D. Magnetization reversal, critical behavior, and magnetocaloric effect in NdMnO3: The role of magnetic ordering of Nd and Mn moments. J. Appl. Phys. 2022, 132, 183907. [Google Scholar] [CrossRef]
- M’nassri, R.; Cheikhrouhou-Koubaa, W.; Koubaa, M.; Boudjada, N.; Cheikhrouhou, A. Magnetic and magnetocaloric prop-erties of Pr0.6−xEuxSr0.4MnO3 manganese oxides. Solid State Commun. 2011, 151, 1579. [Google Scholar] [CrossRef]
- Wei, Z.; Liedienov, N.A.; Li, Q.; Pashchenko, A.V.; Xu, W.; Turchenko, V.A.; Yuan, M.; Fesych, I.V.; Levchenko, G.G. Influence of post-annealing, defect chemistry and high pressure on the magnetocaloric effect of non-stoichiometric La0.8-xK0.2Mn1+xO3 compounds. Ceram. Int. 2021, 47, 24553–24563. [Google Scholar] [CrossRef]
- M’nassri, R.; Cheikhrouhou, A. Evolution of Magnetocaloric Behavior in Oxygen Deficient La2/3Ba1/3MnO3−δ Manganites. J. Supercond. Nov. Magn. 2014, 27, 1463–1468. [Google Scholar] [CrossRef]
- Wali, M.; Skini, R.; Khlifi, M.; Dhahri, E.; Hlil, E. Effect of the oxygen deficiency on the physical properties of La0.8Na0.2MnO3− oxides (δ = 0 and 0.05). J. Magn. Magn. Mater. 2015, 394, 207–211. [Google Scholar] [CrossRef]
- Maignan, A.; Damay, F.; Barnab, A.; Martin, C.; Hervieu, M.; Raveau, B. The Effect of Mn-Site Doping on the Magneto-transport Properties of CMR Manganites. Phil. Trans. R. Soc. Lond. A 1998, 356, 1635–1659. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Li, S. Effect of particle size on magnetic and electric transport properties of La0.67Sr0.33MnO3 coatings. Phys. Chem. Chem. Phys. 2015, 17, 31161–31169. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.W.; Varshney, D. Structural and electrical properties of Pr1− xSrxMnO3 (x = 0.25, 0.3, 0.35 and 0.4) manganites. Mater. Sci. Semicond. Process. 2014, 27, 418–426. [Google Scholar] [CrossRef]
- Vadnala, S.; Rao, T.D.; Pal, P.; Asthana, S. Study of structural effect on Eu-substituted LSMO manganite for high temperature coefficient of resistance. Phys. B Condens. Matter 2014, 448, 277–280. [Google Scholar] [CrossRef]
- Hassen, A. A Comparative Study Between Pr1-xSrxMnO3 and Pr1-xCaxMnO3 at 0 < x < 0.30. J. Korean Phys. Soc. 2008, 52, 98–105. [Google Scholar]
- Selmi, A.; Bettaibi, A.; Rahmouni, H.; M’Nassri, R.; Boudjada, N.C.; Chiekhrouhou, A.; Khirouni, K. Physical properties of 20% Cr-doped Pr0.7Ca0.3MnO3 perovskite. Ceram. Int. 2015, 41, 11221–11227. [Google Scholar] [CrossRef]
- Boujelben, W.; Ellouze, M.; Cheikh-Rouhou, A.; Madar, R.; Fuess, H. Effect of Fe doping on the structural and magneto transport properties in Pr0.67Sr0.33MnO3 perovskite manganese. Phys. Status Solidi 2004, 201, 1410–1415. [Google Scholar] [CrossRef]
- Righi, L.; Gorria, P.; Insausti, M.; Gutiérrez, J.; Barandiarán, J.M. Influence of Fe in giant magnetoresistance ratio and magnetic properties of La0.7Ca0.3Mn1−xFexO3 perovskite type compounds. J. Appl. Phys. 1997, 81, 5767. [Google Scholar] [CrossRef]
- Dhahri, I.; Ellouze, M.; Mnasri, T.; Hlil, E.K.; Jotania, R. Structural, magnetic, magnetocaloric and critical exponents of oxide manganite La0.7Sr0.3Mn0.95Fe0.05O3. J. Mater. Sci. Mater. Electron. 2020, 31, 12493–12501. [Google Scholar] [CrossRef]
- Sotirova-Haralambeva, E.V.; Wang, X.L.; Liu, K.H.; Silver, T.; Konstantinov, K.; Horvat, J. Zinc doping effects on the structure, transport and magnetic properties of La0.7Sr0.3Mn1−xZnxO3 manganite oxide. Sci. Technol. Adv. Mater. 2003, 4, 149–152. [Google Scholar] [CrossRef]
- Dhahri, N.; Dhahri, A.; Cherif, K.; Dhahri, J.; Taibi, K.; Dhahri, E. Structural, magnetic and electrical properties of La0.67Pb0.33Mn1−xCoxO3. J. Alloy. Compd. 2010, 496, 69–74. [Google Scholar] [CrossRef]
- Ewas, A.M.; Hamad, M.A. Large magnetocaloric effect of La0.67Pb0.33Mn1−xCoxO3 in small magnetic field variation. Ceram. Int. 2017, 43, 7660–7662. [Google Scholar] [CrossRef]
- Shi, J.B.; Fan, Y.Y.; Tai, M.F.; Chen, H.Z.; Young, S.L. Magnetic behavior in the La0.7Pb0.3Mn1-xCoxO3 perovskite com-pounds. J. Magn. Magn. Mater. 2002, 239, 8–10. [Google Scholar] [CrossRef]
- Gdaiem, M.A.; Ghodhbane, S.; Dhahri, A.; Dhahri, J.; Hlil, E.K. Effect of cobalt on structural, magnetic and magnetocaloric properties of La0.8Ba0.1Ca0.1Mn1-xCoxO3 (x = 0.00, 0.05 and 0.10) manganites. J. Alloy. Compd. 2016, 681, 547–554. [Google Scholar] [CrossRef]
- Reshmi, C.P.; Pillai, S.S.; Vasundhara, M.; Raji, G.R.; Suresh, K.G.; Varma, M.R. Co-existence of magnetocaloric effect and magnetoresistance in Co substituted La0.67Sr0.33MnO3 at room temperature. J. Appl. Phys. 2013, 114, 033904. [Google Scholar] [CrossRef]
- Selmi, A.; M’Nassri, R.; Cheikhrouhou-Koubaa, W.; Boudjada, N.C.; Cheikhrouhou, A. The effect of Co doping on the magnetic and magnetocaloric properties of Pr0.7Ca0.3Mn1−xCoxO3 manganites. Ceram. Int. 2015, 41, 7723–7728. [Google Scholar] [CrossRef]
- Deac, I.G.; Vladescu, A.; Balasz, I.; Tunyagi, A.; Tetean, R. Electrical and magnetic behavior of transition metal oxides Ln0.7A0.3TMO3, Ln= La, Pr; A = Ca, Sr AND TM = Mn, Co. Int. J. Mod. Phys. B 2010, 24, 762–769. [Google Scholar] [CrossRef]
- Das, P.T.; Panda, J.; Taraphder, A.; Nath, T.K. Electronic and magneto transport property of Pr0.7Sr0.3MnO3system in nanometric gran size modulation. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012142. [Google Scholar] [CrossRef]
- Dimesso, L. Pechini Processes: An Alternate Approach of the Sol-Gel Method, Preparation, Properties, and Applications. In Handbook of Sol-Gel Science and Technology; Springer: Cham, Switzerland, 2016; pp. 1067–1088. [Google Scholar]
- Rodriguez-Carvajal, J. FullProf, LLB Saclay, France. Physica B 1993, 192, 1995. [Google Scholar]
- Phokha, S.; Pinitsoontorn, S.; Maensiri, S.; Rujirawat, S. Structure, optical and magnetic properties of LaFeO3 nanoparticles prepared by polymerized complex method. J. Sol-Gel. Sci. Technol. 2014, 71, 333–341. [Google Scholar] [CrossRef]
- Majid, A.; Gollner, E.; Matam, M.; Tunney, J.; Post, M. Preparation of SrFeO3-X Thin Films by the Spin-Coating Method and its Gas Sensing Properties. MRS Proc. 2006, 972. [Google Scholar] [CrossRef]
- Goldschmit, V.M. Geochemische Verteilungsgesetz der Element. 7, (1927–1928), 8. Available online: https://searchworks.stanford.edu/view/9128152 (accessed on 14 December 2022).
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis; Materials Science Forum; Trans Tech Publications, Ltd.: Wollerau, Switzerland, 2000; Volume 378–383, pp. 118–123. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Radaelli, P.G.; Iannone, G.; Marezio, M.; Hwang, H.Y.; Cheong, S.-W.; Jorgensen, J.D.; Argyriou, D.N. Structural effects on the magnetic and transport properties of perovskite A1−xA′xMnO3 (x = 0.25, 0.30). Phys. Rev. B 1997, 56, 8265–8276. [Google Scholar] [CrossRef]
- Vazquez-Vazquez, C.; Blanco, M.C.; López-Quintela, M.A.; Sánchez, R.D.; Rivas, J.; Oseroff, S.B. Characterization of La0.67Ca0.33MnO3±δ particles prepared by the sol–gel route. J. Mater. Chem. 1998, 8, 991–1000. [Google Scholar] [CrossRef]
- Qianying, Y.; Jincang, Z.; Rongrong, J.; Chao, J.; Shixun, C. Double M–I transitions and low-temperature resistivity minimum of La2/3Ca1/3Mn1−xCoxO3(0 ≤ x ≤ 0.15) manganite. J. Magn. Magn. Mater. 2008, 320, 3313–3317. [Google Scholar]
- Park, J.-H.; Vescovo, E.; Kim, H.-J.; Kwon, C.; Ramesh, R.; En ligneVenkatesan, T. Propriétés magnétiques à la limite de surface d’un ferromagnétique semi-métallique. Lett. D’examen Phys. 1998, 81, 1953–1956. [Google Scholar] [CrossRef]
- Lopez-Quintela, M.A.; Hueso, L.E.; Rivas, J.; Rivadulla, F. Magnétorésistance intergranulaire dans les na-nomanganites. Nanotechnologie 2003, 14, 212–219. [Google Scholar] [CrossRef]
- Curiale, J.; Granada, M.; Troiani, H.E.; Sánchez, R.D.; Leyva, A.G.; Levy, P.M.; Samwer, K. Magnetic dead layer in ferromagnetic manganite nanoparticles. Appl. Phys. Lett. 2009, 95, 043106. [Google Scholar] [CrossRef]
- Zhu, T.; Shen, B.G.; Sun, J.R.; Zhao, H.W.; Zhan, W.S. Surface spin-glass behavior in La2/3Sr1/3MnO3 nanoparticles. Appl. Phys. Lett. 2001, 78, 3863. [Google Scholar] [CrossRef]
- Mottaghi, N.; Trappen, R.B.; Kumari, S.; Huang, C.Y.; Yousefi, S.; Cabrera, G.B.; Aziziha, M.; Haertter, A.; Johnson, M.B.; Seehra, M.S.; et al. Observation and interpretation of negative remanent magnetization and inverted hysteresis loops in a thin film of La0.7Sr0.3MnO3. J. Phys. Condens. Matière 2018, 30, 405804. [Google Scholar] [CrossRef] [PubMed]
- Dwight, K.; Menyuk, N. Magnetic Properties of Mn3O4 and the Canted Spin Problem. Phys. Rev. 1960, 119, 1470–1479. [Google Scholar] [CrossRef]
- Hcini, S.; Zemni, S.; Triki, A.; Rahmouni, H.; Boudard, M. Size mismatch, grain boundary and bandwidth effects on structural, magnetic and electrical properties of Pr0.67Ba0.33MnO3 and Pr0.67Sr0.33MnO3 perovskites. J. Alloy. Compd. 2011, 509, 1394–1400. [Google Scholar] [CrossRef]
- Boujelben, W.; Cheikh-Rouhou, A.; Ellouze, M.; Joubert, J.C. Electrical Properties in Solid Solution Pr1-xSrxMnO3 (0 ≤ x ≤ 0.5). Phys. Status Solidi 2000, 177, 503–510. [Google Scholar] [CrossRef]
- Zemni, S.; Gasmi, A.; Boudard, M.; Oumezzine, M. Effect of nominal strontium deficiency on the structure and the magnetic properties of La0.6Sr0.4-delta MnO3 manganese perovskites. Mater. Sci. Eng. B 2007, 144, 117–122. [Google Scholar] [CrossRef]
- Martin, C.; Maignan, A.; Hervieu, M.; Raveau, B. Magnetic phase diagrams of L1-xAxMnO3 manganites (L = Pr, Sm; A = Ca, Sr…). Phys. Rev. B 1999, 60, 12191–12199. [Google Scholar] [CrossRef]
- Thanh, T.D.; Linh, D.C.; Yen, P.D.H.; Tuan, D.A.; Zhang, Y.D.; Phan, T.L.; Oh, S.K.; Yu, S.C. Electrical and magnetic properties of La0.7Ca0.3Mn0.9Co0.1O3 locating at a threshold of the first- and second-order phase transitions. J. Magn. Magn. Mater. 2019, 470, 59–63. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Wang, H.; Su, K.; Yang, D.; Huang, S.; Tan, W.; Liu, H.; Huo, D. The transport properties and large magnetoresistance effect in Pr0.7Sr0.3MnO3 film on SrTiO3. J. Mater. Sci. Mater. Electron. 2022, 33, 23834–23840. [Google Scholar] [CrossRef]
- Dunaevskii, S.M.; Malyshev, A.L.; Trunov, V.A.; Popov, V.V. Colossal magnetoresistance of the Sm1-xSrxMnO3system. Phys. Solid State 1997, 39, 1636–1637. [Google Scholar] [CrossRef]
- Kallel, N.; Fröhlich, K.; Oumezzine, M.; Ghedira, M.; Vincent, H.; Pignard, S. Magnetism and giant magnetoresistance in La0.7Sr0.3Mn1-xMxO3 (M = Cr, Ti) systems. Phys. Status Solidi 2004, 1, 1649–1654. [Google Scholar] [CrossRef]
- Urushibara, A.; Moritomo, Y.; Arima, T.; Asamitsu, A.; Kido, G.; Tokura, Y. Insulator-metal transition and giant magnetoresistance in La1-xSrxMnO3. Phys. Rev. B 1995, 51, 14103–14109. [Google Scholar] [CrossRef]
- Viret, M.; Ranno, L.; Coey, J.M.D. Magnetic localization in mixed-valence manganites. Phys. Rev. B 1997, 55, 8067–8070. [Google Scholar] [CrossRef]
- Sen, N.V.; Panwar, G.L.; Bhalla, S.K. Structural, magnetotransport and morphological studies of Sb-doped La2/3Ba1/3MnO3 ceramic perovskites. J. Phys. Chem. Solids 2007, 68, 1685. [Google Scholar] [CrossRef]
- Snyder, G.J.; Hiskes, R.; DiCarolis, S.; Beasley, M.R.; Geballe, T.H. MOCVD thin films and bulk material. Phys. Rev. B 1996, 53, 14434–14444. [Google Scholar] [CrossRef] [PubMed]
- Mott, N.F. Conduction dans les matériaux non cristallins: III. États localisés dans un pseudogap et près des extrémités des bandes de conduction et de valence. Philos. Mag. 1969, 19, 835–852. [Google Scholar] [CrossRef]
- Emin, D.; Holstein, T. Adiabatic Theory of an Electron in a Deformable Continuum. Phys. Rev. Lett. 1976, 36, 323–326. [Google Scholar] [CrossRef]
- Chen, X.G.; Fu, J.B.; Yun, C.; Zhao, H.; Yang, Y.B.; Du, H.L.; Han, J.Z.; Wang, C.S.; Liu, S.Q.; Zhang, Y.; et al. Magnetic and transport properties of cobalt doped La0.7Sr0.3MnO3. J. Appl. Phys. 2014, 116, 103907. [Google Scholar] [CrossRef]
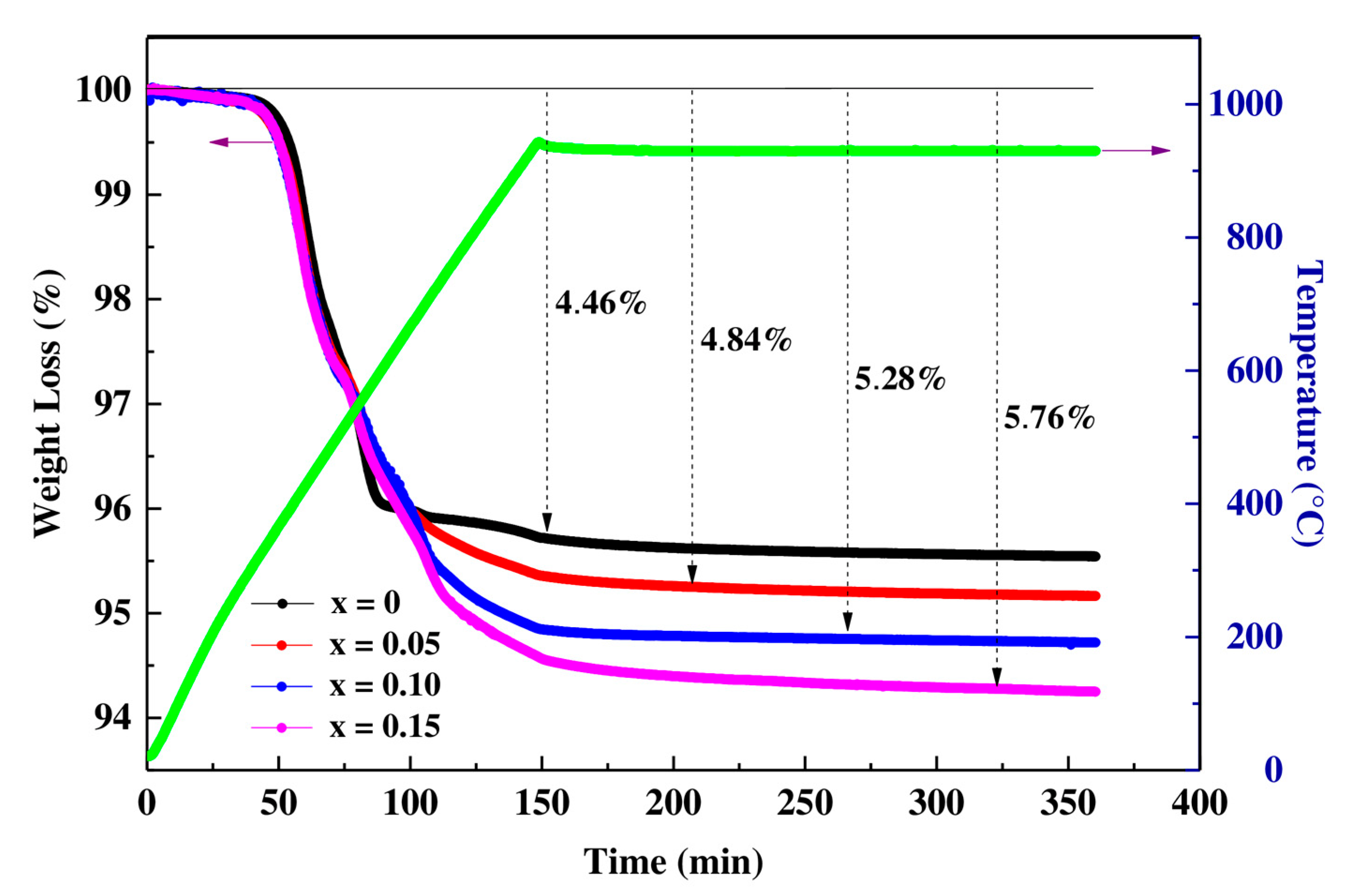


| Initial Mass (mg) | Final Mass (mg) | Oxygen Rate (y) | Compositions | |
|---|---|---|---|---|
| x = 0 | 47.67 (1) | 45.54 (1) | 2.98 (1) | |
| x = 0.05 | 72.64 (1) | 69.13 (1) | 2.99 (1) | |
| x = 0.10 | 58.31 (1) | 55.23 (1) | 3.00 (1) | |
| x = 0.15 | 55.59 (1) | 52.39 (1) | 3.01 (1) |
| x | 0 | 0.05 | 0.10 | 0.15 |
|---|---|---|---|---|
| Space group | Pnma | |||
| Lattice parameter | ||||
| a (Å) | 5.4494 (28) | 5.4426 (0) | 5.4465 (16) | 5.4590 (0) |
| b (Å) | 7.6913 (43) | 7.7355 (0) | 7.6921 (24) | 7.6651 (0) |
| c (Å) | 5.4811 (27) | 5.4715 (0) | 5.4787 (16) | 5.4697 (0) |
| V (Å3) | 229.738 (0) | 230.359 (0) | 229.533 (0) | 228.878 (0) |
| W (u.a) | 0.1092 | 0.0960 | 0.0941 | 0.0779 |
| tG | 0.9198 | 0.9206 | 0.9214 | 0.9222 |
| <rB> (Å) | 0.6105 | 0.6087 | 0.6070 | 0.6052 |
| dMn,Co-O1 (Å) | 1.956 (4) | 1.94685 (0) | 1.9540 (17) | 1.94540 (0) |
| θMn,Co-O1-Mn,Co (°) | 159 (10) | 166.7649 (0) | 159.6 (6) | 160.1462(0) |
| dMn,Co-O2 (Å) | 1.80 (4) | 1.94644 (0) | 1.959 (14) | 2.1907 (0) |
| θMn,Co-O2-Mn,Co (°) | 170.9 (15) | 158.9012 (0) | 162.6 (7) | 170.8186 (0) |
| <dMn,Co-O> (Å) | 1.878 | 1.9466 | 1.9565 | 2.068 |
| <θMn,Co-O-Mn,Co> (°) | 164.95 | 162.833 | 161.1 | 165.4824 |
| Discrepancy factors (%) | ||||
| Rp (%) | 12.7 | 17.5 | 7.46 | 16.5 |
| Rwp (%) | 13.6 | 17 | 6.68 | 15 |
| RF (%) | 4.03 | 4.27 | 3.37 | 4.01 |
| χ2 (%) | 9.57 | 10.2 | 3.5 | 8.75 |
| x | 0 | 0.05 | 0.10 | 0.15 |
|---|---|---|---|---|
| DSCH (nm) | 33.78 | 29.00 | 33.61 | 24.27 |
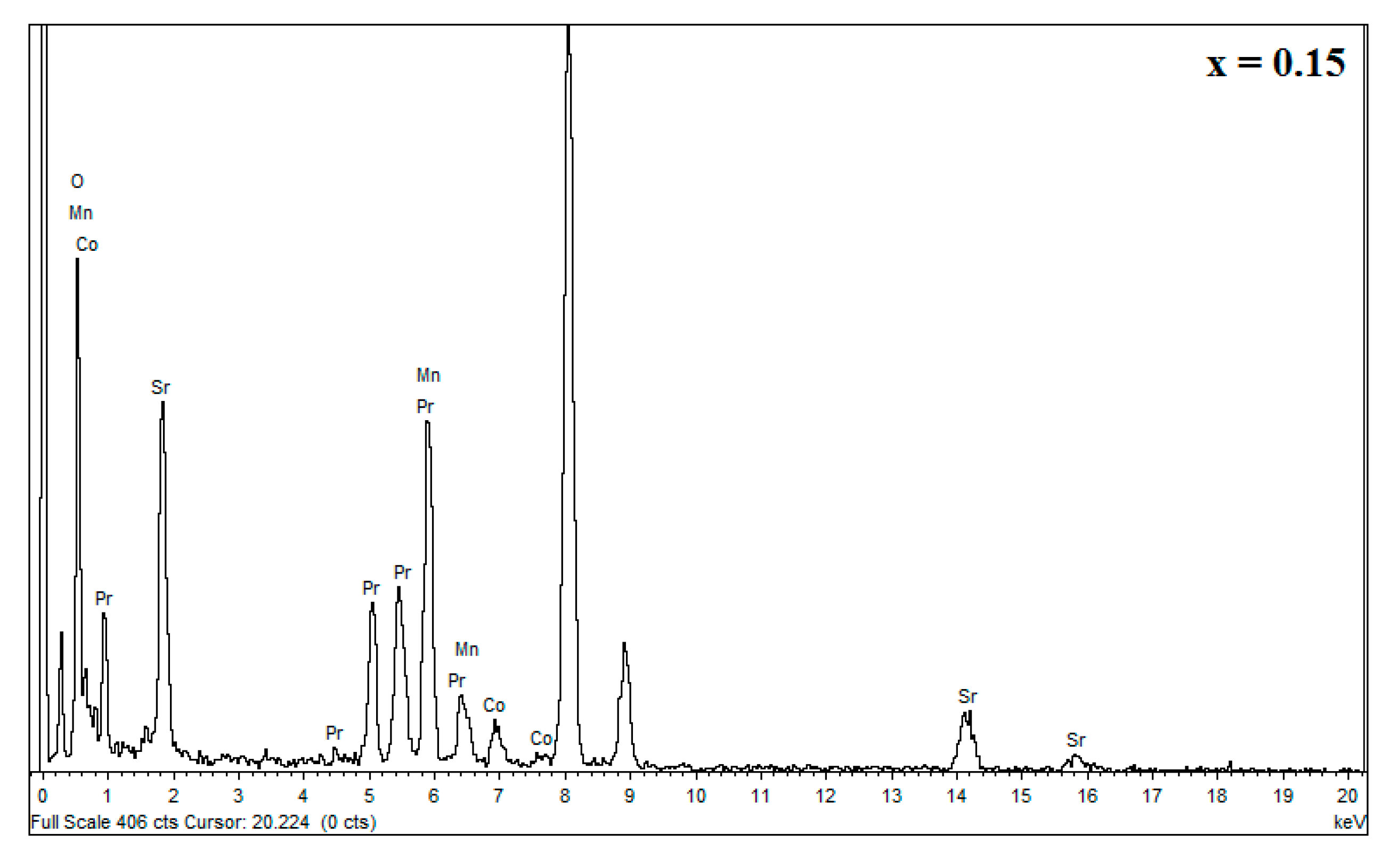
| Sample | Element | Weight (%) | Nominal Chemical Composition | Experimental Chemical Composition |
|---|---|---|---|---|
| x = 0.15 | Mn | 27.98 | 0.85 | 0.861 |
| Co | 4.49 | 0.15 | 0.138 | |
| Sr | 24.90 | 0.30 | 0.368 | |
| Pr | 42.63 | 0.70 | 0.630 |
| Composition Pr0.7Sr0.3Mn(1−x)CoxO3 | TB (K) | Tirr (K) | TC (K) | θp (K) | C (emu mol−1 Oe−1 K) | ||
|---|---|---|---|---|---|---|---|
| x = 0 | 220 | 229 | 300 | 295 | 0.0161 | 5.5019 | 3.5899 |
| x = 0.05 | 139 | 190 | 270 | 275 | 0.0129 | 5.5015 | 3.2239 |
| x = 0.10 | 190 | 210 | 250 | 255 | 0.0156 | 5.5010 | 3.5417 |
| x = 0.15 | 119 | 180 | 235 | 238 | 0.0138 | 5.5006 | 3.3310 |
| Composition Pr0.7Sr0.3Mn(1−x)CoxO3 | Hc (Oe) | Mr (emu/g) | Ms (emu/g) | Hc (Oe) | Mr (emu/g) | Ms (emu/g) |
|---|---|---|---|---|---|---|
| 5 K | 300 K | |||||
| x = 0 | 272.13 | 11.14 | 80.92 | 8.76 | 0.157 | 36.24 |
| x = 0.05 | 299.01 | 13.06 | 82.40 | 12.89 | 0.007 | 21.32 |
| x = 0.10 | 377.28 | 16.86 | 75.10 | 12.64 | 0.005 | 18.00 |
| x = 0.15 | 852.36 | 23.70 | 74.13 | 12.42 | 0.003 | 12.17 |
| Compositions | TC (K) | Tρ (K) | MR (%) | Ref | ||
|---|---|---|---|---|---|---|
| T (K) | H (kOe) | % | ||||
| Pr0.7Sr0.3MnO3 | 300 | 243 | 320 | 10 | 35.9 | This work |
| Pr0.7Sr0.3Mn0.95Co0.05O3 | 270 | 150 | 300 | 10 | 20.5 | This work |
| Pr0.7Sr0.3Mn0.90Co0.10O3 | 250 | 215 | 300 | 10 | 34.5 | This work |
| Pr0.7Sr0.3Mn0.85Co0.15O3 | 235 | 210 | 320 | 10 | 25.3 | This work |
| La0.7Ca0.3Mn0.9Co0.1O3 | 188 | 155 | 170 | 4 | 14.3 | [51] |
| Pr0.7Sr0.3MnO3 | - | - | 175 | 10 | 47.1 | [52] |
| La0.67Sr0.33Mn0.90Co0.10O3 | 294 | 250 | 300 | 50 | 21 | [26] |
| Sm0.7Sr0.3MnO3 | - | - | 77 | 30 | 90 | [53] |
| La0.7Sr0.3MnO3 | 369 | >360 | 78 | 5 | 15 | [54] |
| ρ0 (Ω.cm) | ρ2 (Ω.cm.K−2) | ρ4.5 (Ω.cm.K−4.5) | ρmax (Ω.cm) | R2 | Tρ (K) | ρ0 (Ω.cm) | |
|---|---|---|---|---|---|---|---|
| x = 0 | 0.0061 | 9.1812 × 10−8 | 6.1581 × 10−12 | 0.1560 | 0.996 | 243 | 0.0061 |
| x = 0.05 | 0.0785 | 7.5708 × 10−6 | 5.6774 × 10−10 | 12.0645 | 0.997 | 150 | 0.0785 |
| x = 0.10 | 0.0124 | 3.0995 × 10−7 | 6.4824 × 10−11 | 2.2014 | 0.992 | 215 | 0.0124 |
| x = 0.15 | 0.0085 | 2.2599 × 10−7 | 5.0214 × 10−11 | 1.5447 | 0.996 | 210 | 0.0085 |
| Models | VRH Model | ASPH Model | |||
|---|---|---|---|---|---|
| T0 (106) (K) | N(EF) (ev−1.cm−3) | R2 | Ea (meV) | R2 | |
| x = 0 | 12.4272 | 1.0207 × 1018 | 0.999 | 107.51 | 0.998 |
| x = 0.05 | 55.8958 | 2.2694 × 1017 | 0.998 | 153.84 | 0.998 |
| x = 0.10 | 32.4821 | 3.9053 × 1017 | 0.999 | 140.57 | 0.998 |
| x = 0.15 | 43.4751 | 2.9178 × 1017 | 0.998 | 153.87 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdiri, F.; Alonso, J.M.; Mnasri, T.; de la Presa, P.; Morales, I.; Martínez, J.L.; Ben Younes, R.; Marin, P. Effects of Partial Manganese Substitution by Cobalt on the Physical Properties of Pr0.7Sr0.3Mn(1−x)CoxO3 (0 ≤ x ≤ 0.15) Manganites. Materials 2023, 16, 1573. https://doi.org/10.3390/ma16041573
Zdiri F, Alonso JM, Mnasri T, de la Presa P, Morales I, Martínez JL, Ben Younes R, Marin P. Effects of Partial Manganese Substitution by Cobalt on the Physical Properties of Pr0.7Sr0.3Mn(1−x)CoxO3 (0 ≤ x ≤ 0.15) Manganites. Materials. 2023; 16(4):1573. https://doi.org/10.3390/ma16041573
Chicago/Turabian StyleZdiri, Feriel, José María Alonso, Taoufik Mnasri, Patricia de la Presa, Irene Morales, José Luis Martínez, Rached Ben Younes, and Pilar Marin. 2023. "Effects of Partial Manganese Substitution by Cobalt on the Physical Properties of Pr0.7Sr0.3Mn(1−x)CoxO3 (0 ≤ x ≤ 0.15) Manganites" Materials 16, no. 4: 1573. https://doi.org/10.3390/ma16041573
APA StyleZdiri, F., Alonso, J. M., Mnasri, T., de la Presa, P., Morales, I., Martínez, J. L., Ben Younes, R., & Marin, P. (2023). Effects of Partial Manganese Substitution by Cobalt on the Physical Properties of Pr0.7Sr0.3Mn(1−x)CoxO3 (0 ≤ x ≤ 0.15) Manganites. Materials, 16(4), 1573. https://doi.org/10.3390/ma16041573









