Effect of Corrosive Aging Environments on the Flexural Properties of Silane-Coupling-Agent-Modified Basalt-Fiber-Reinforced Composites
Abstract
1. Introduction
2. Materials and Methods
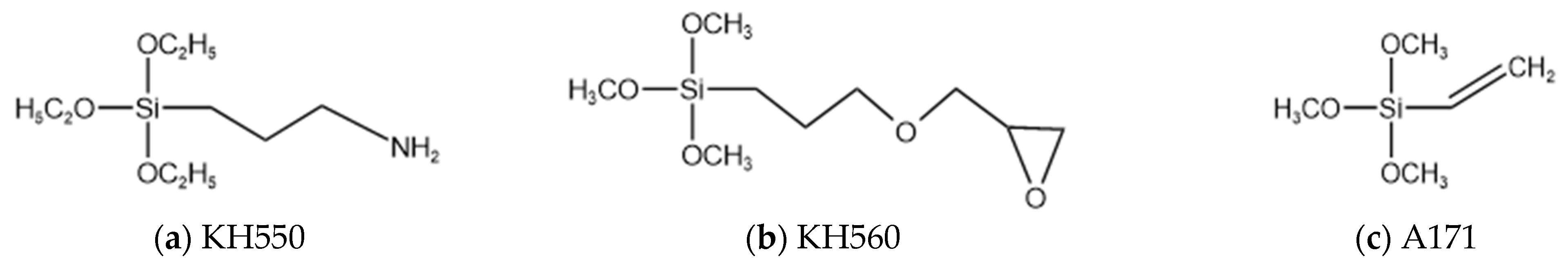
2.1. Materials
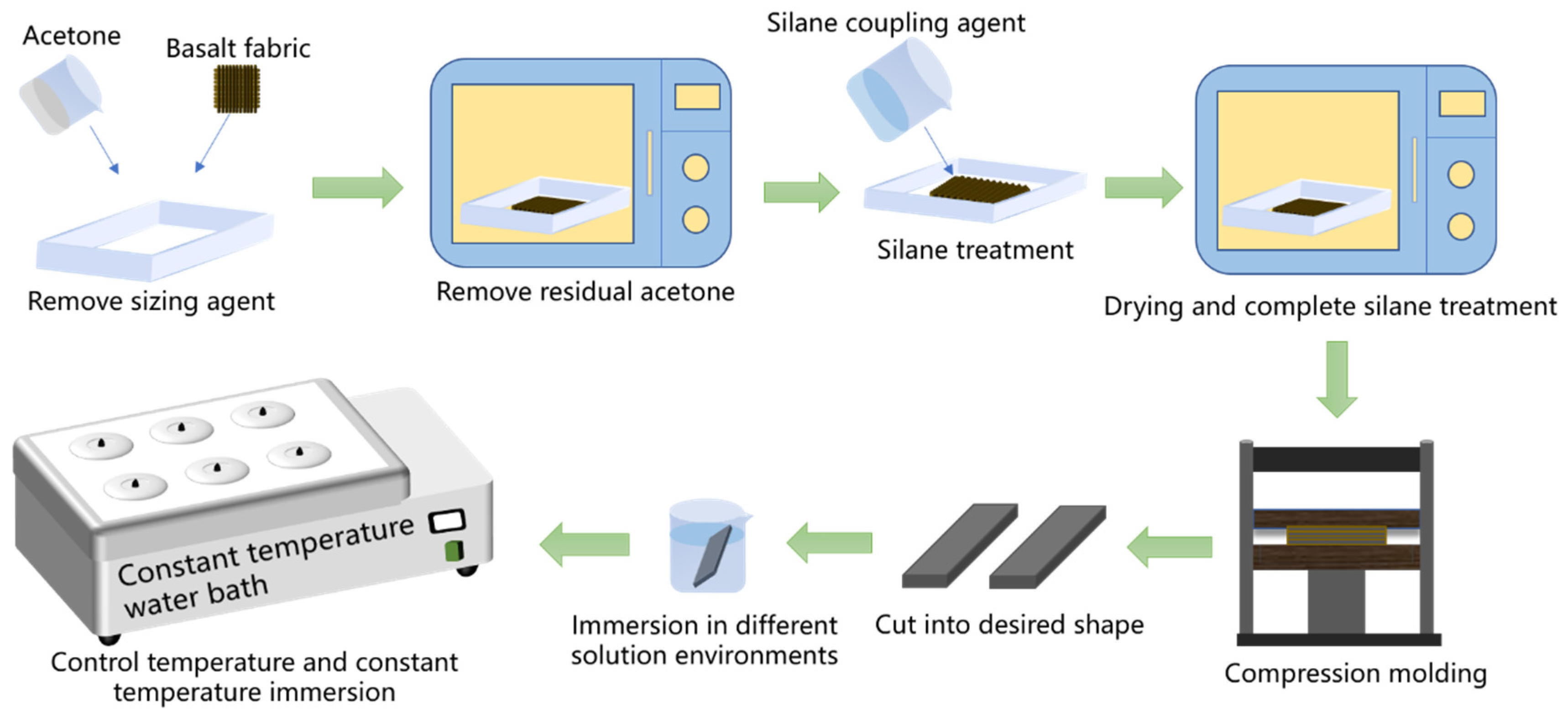
2.2. Preparation of BFRPs
2.3. Immersion Experiment
2.4. SEM Imaging
2.5. Mass Change
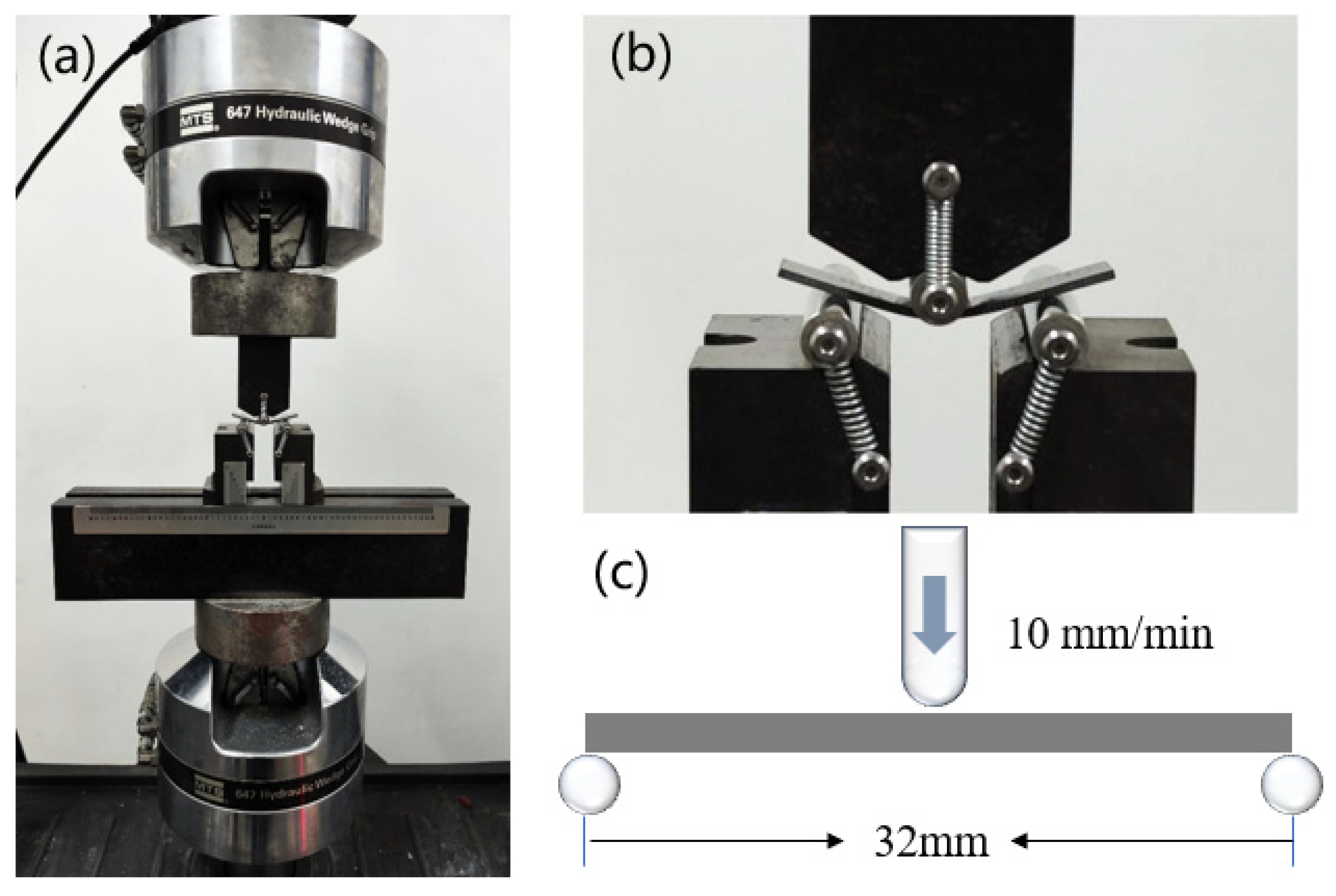
2.6. Three-Point Flexural Test
3. Results and Discussion
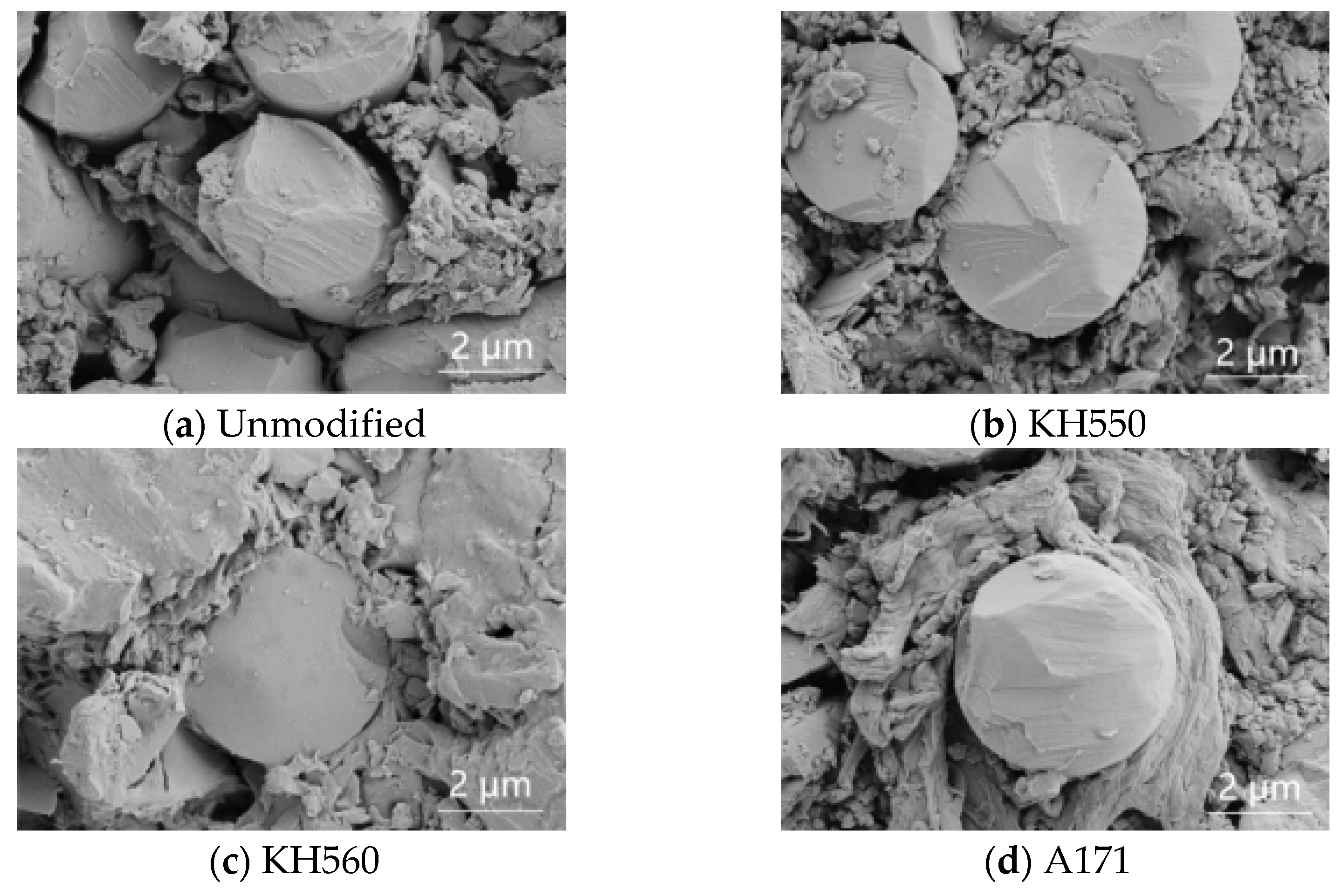
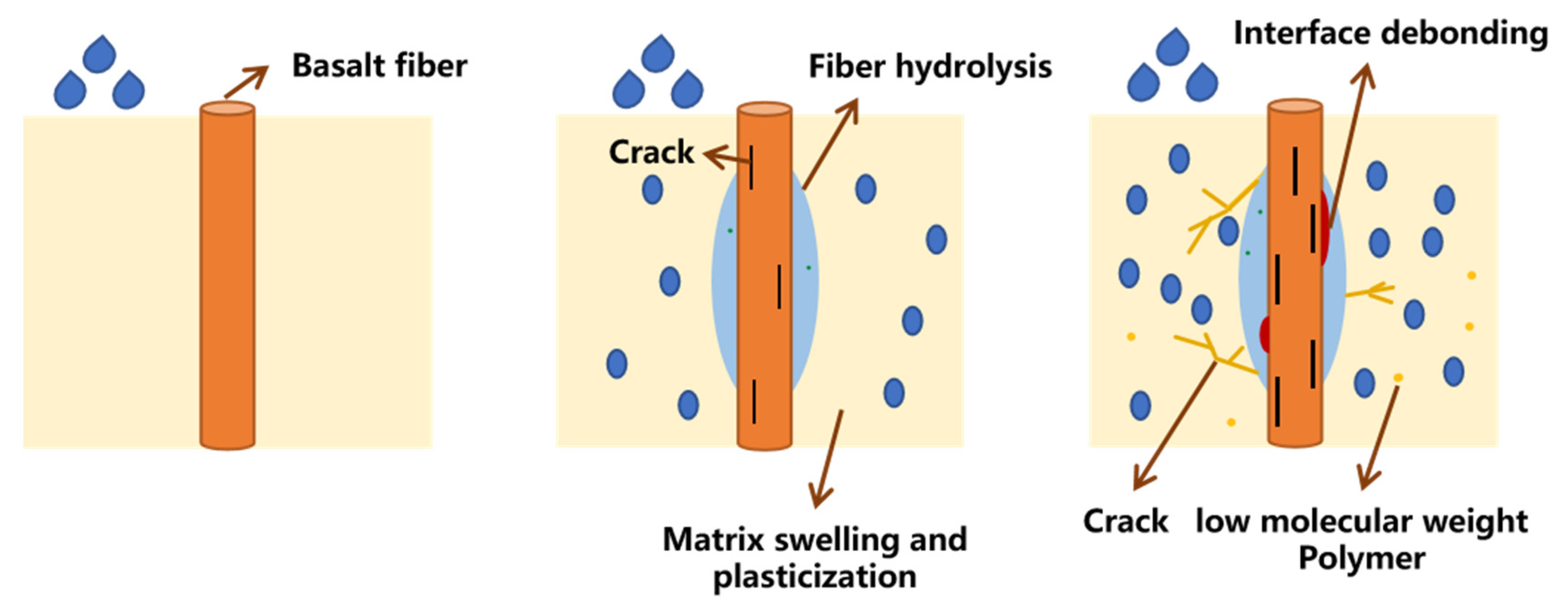
3.1. SEM Imaging
3.2. Change in Appearance
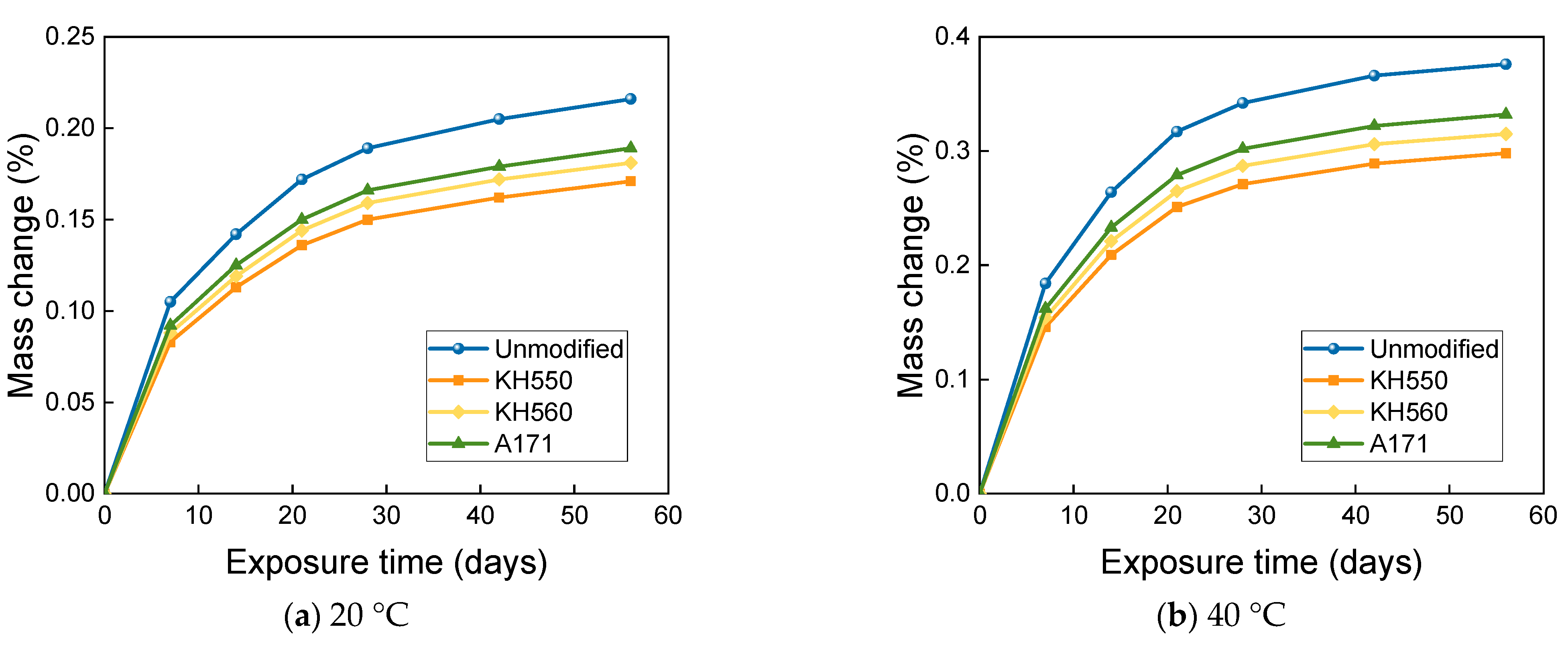
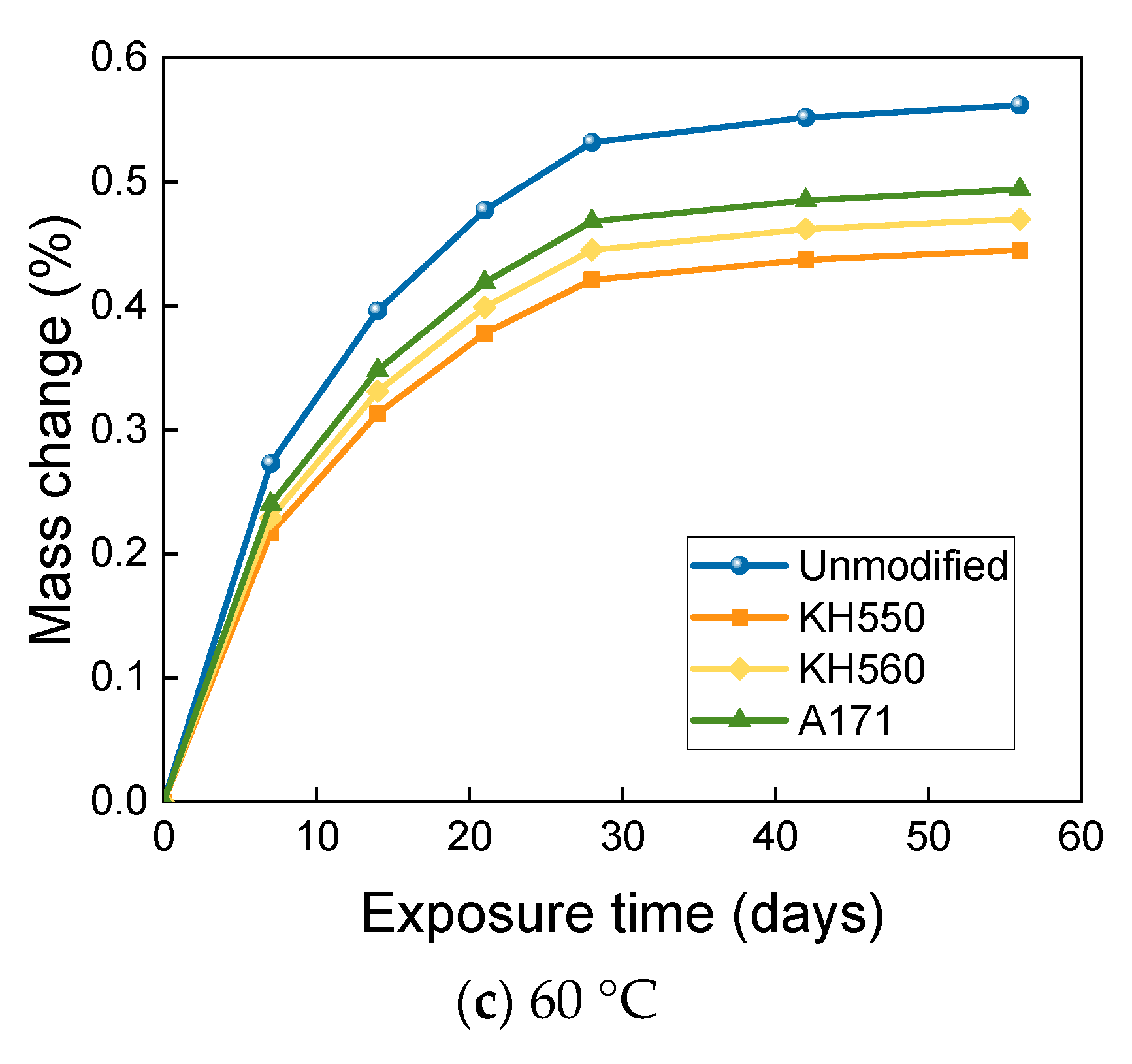
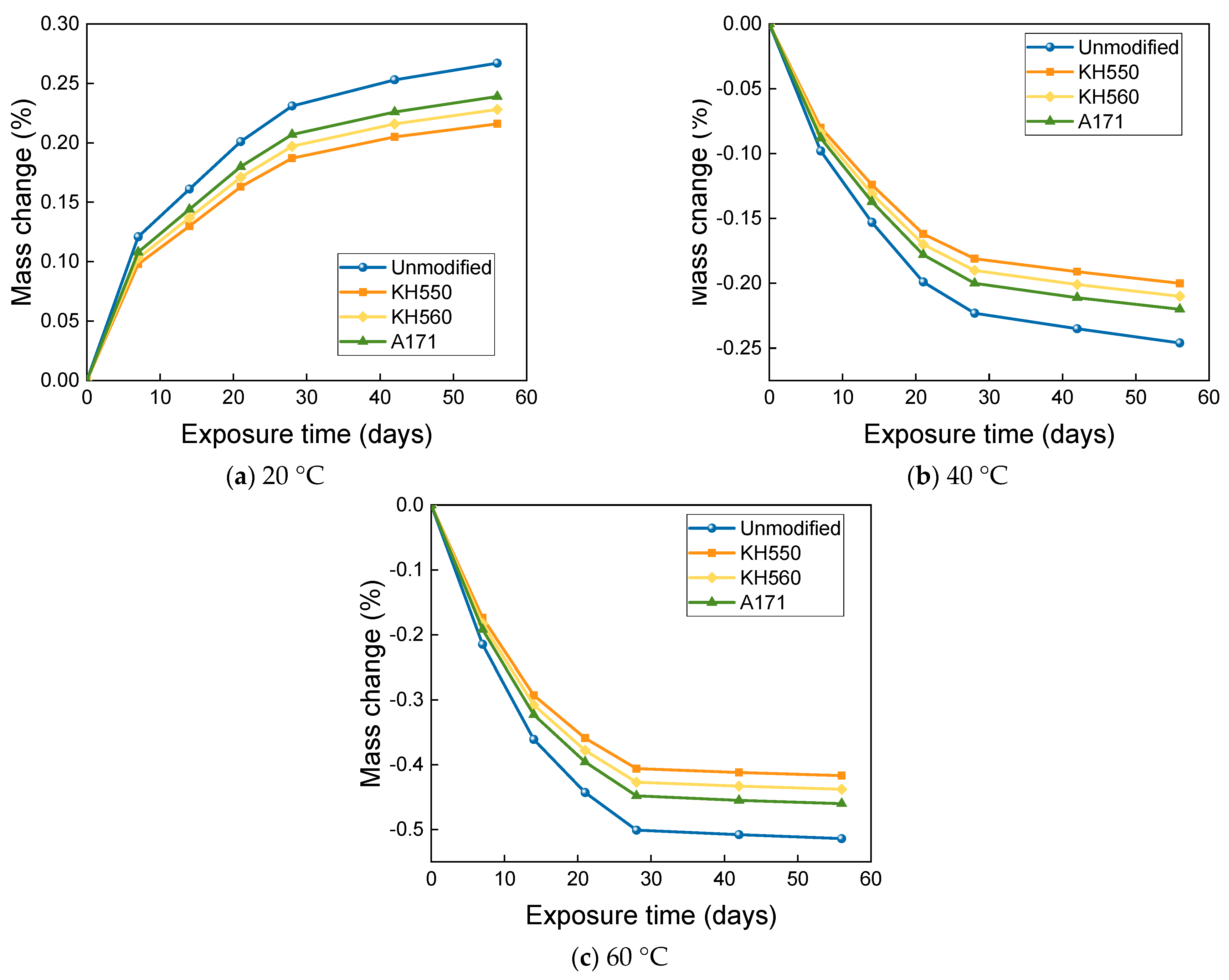
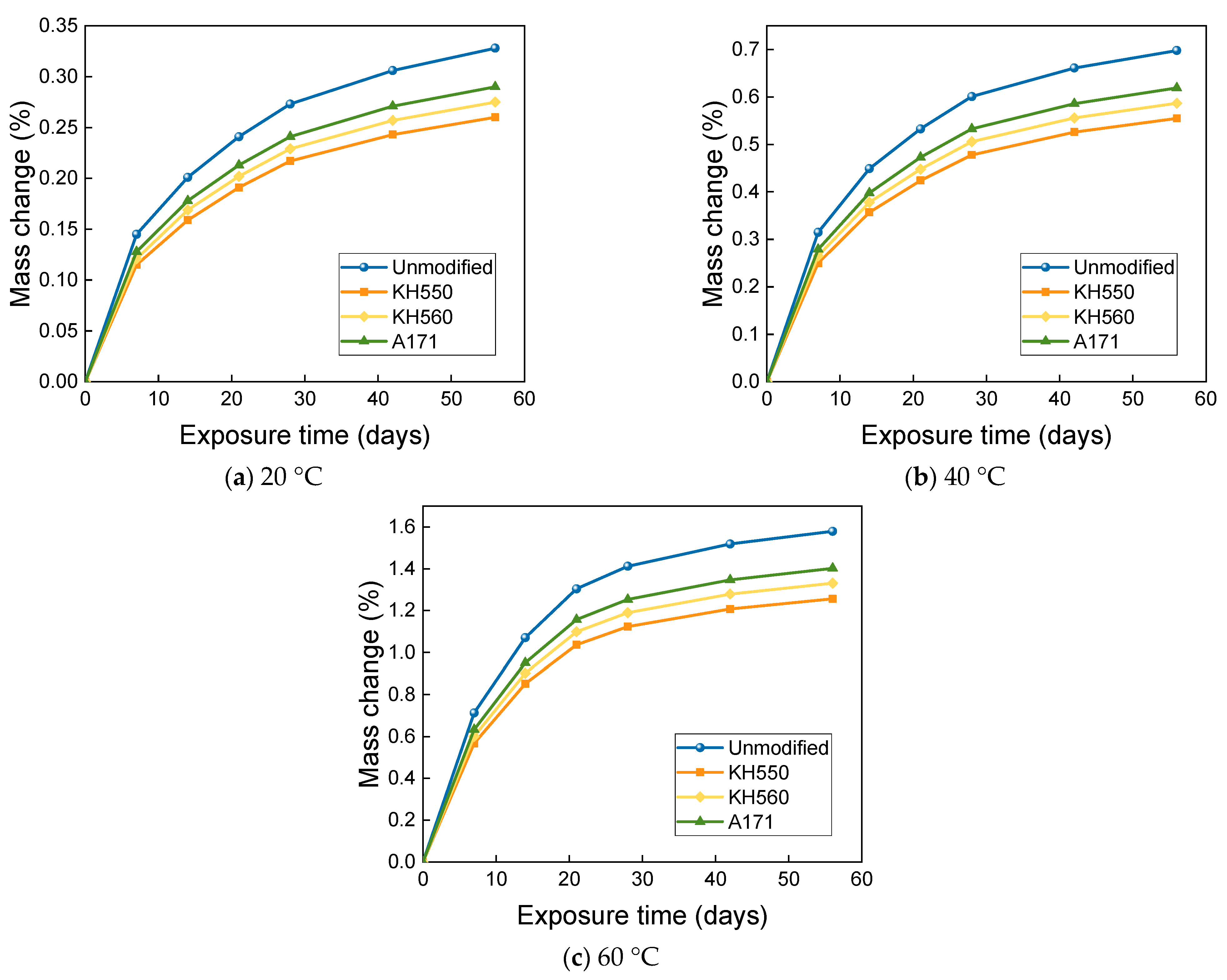
3.3. MASS Changes in Different Immersion Environments
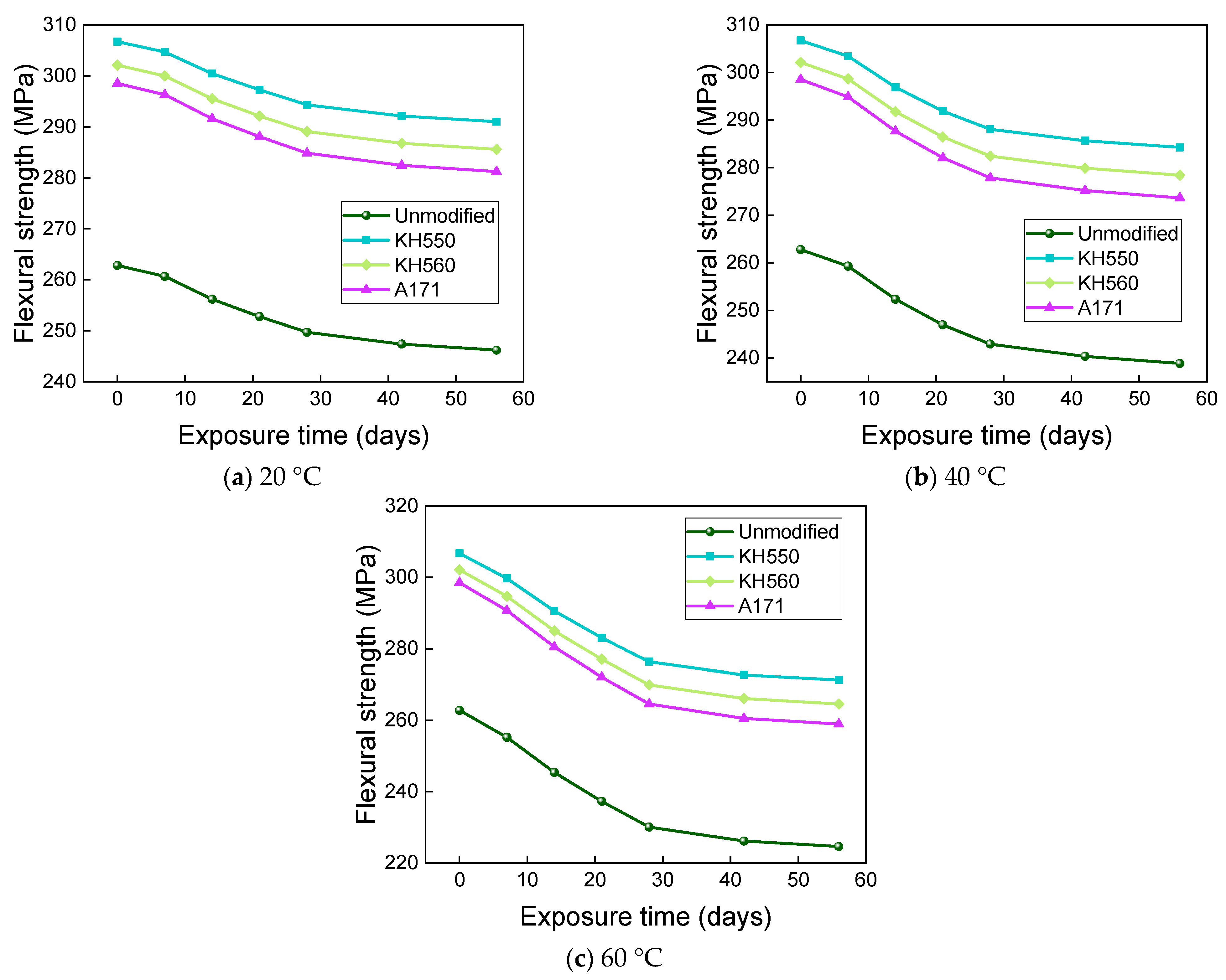
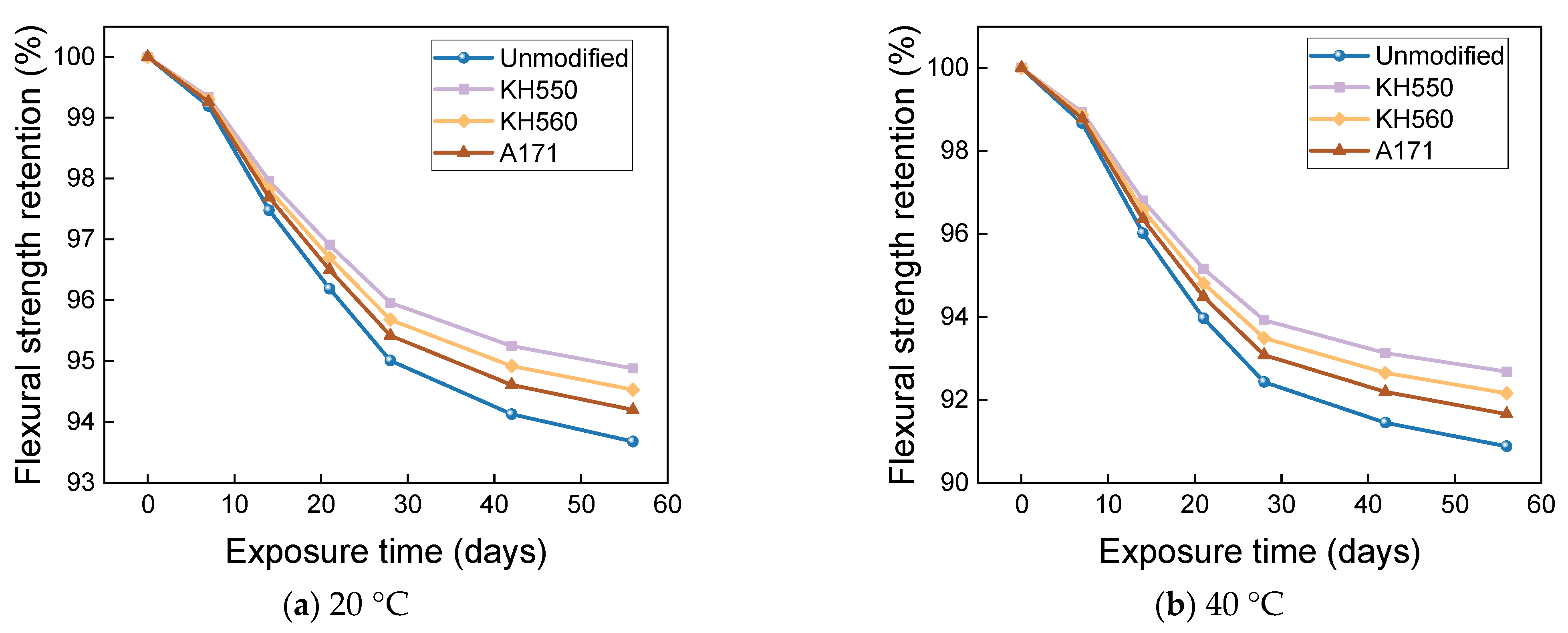
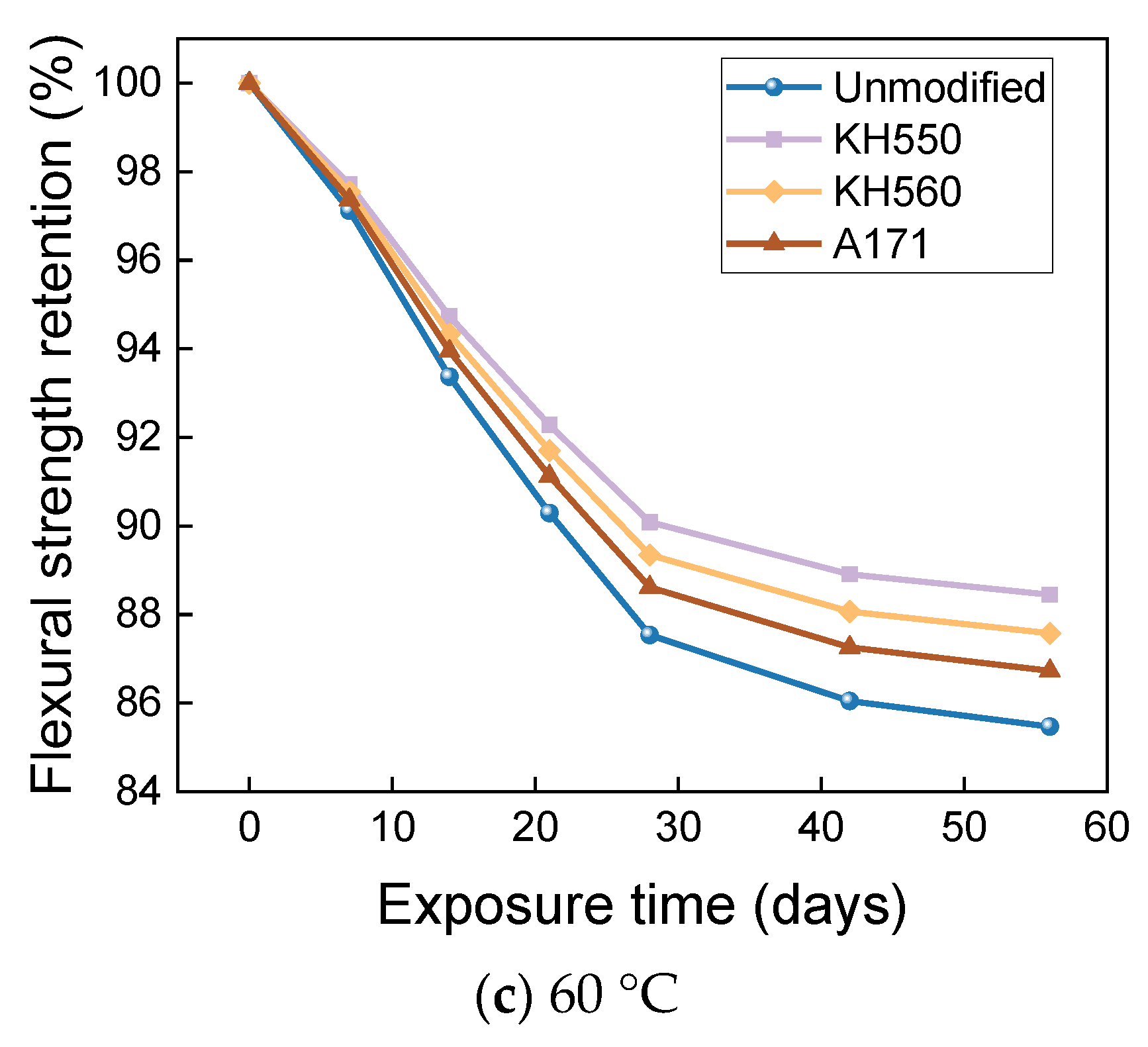
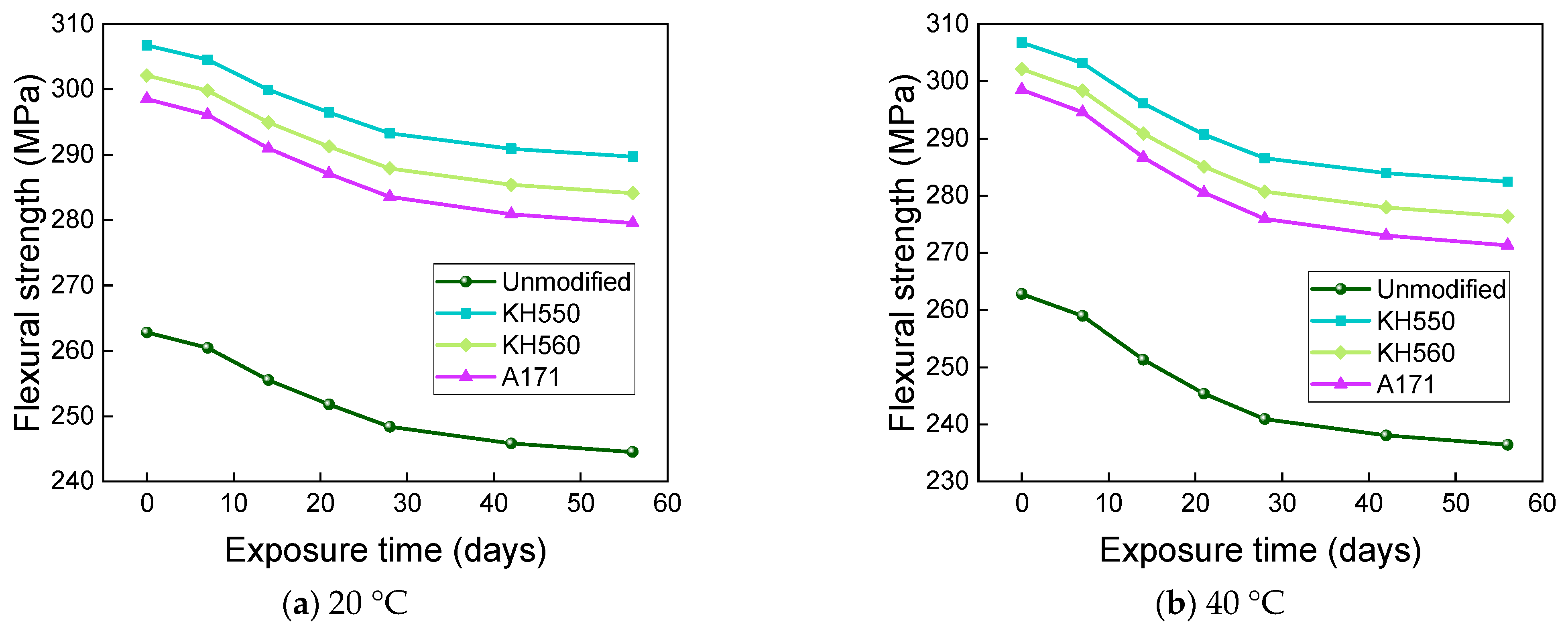
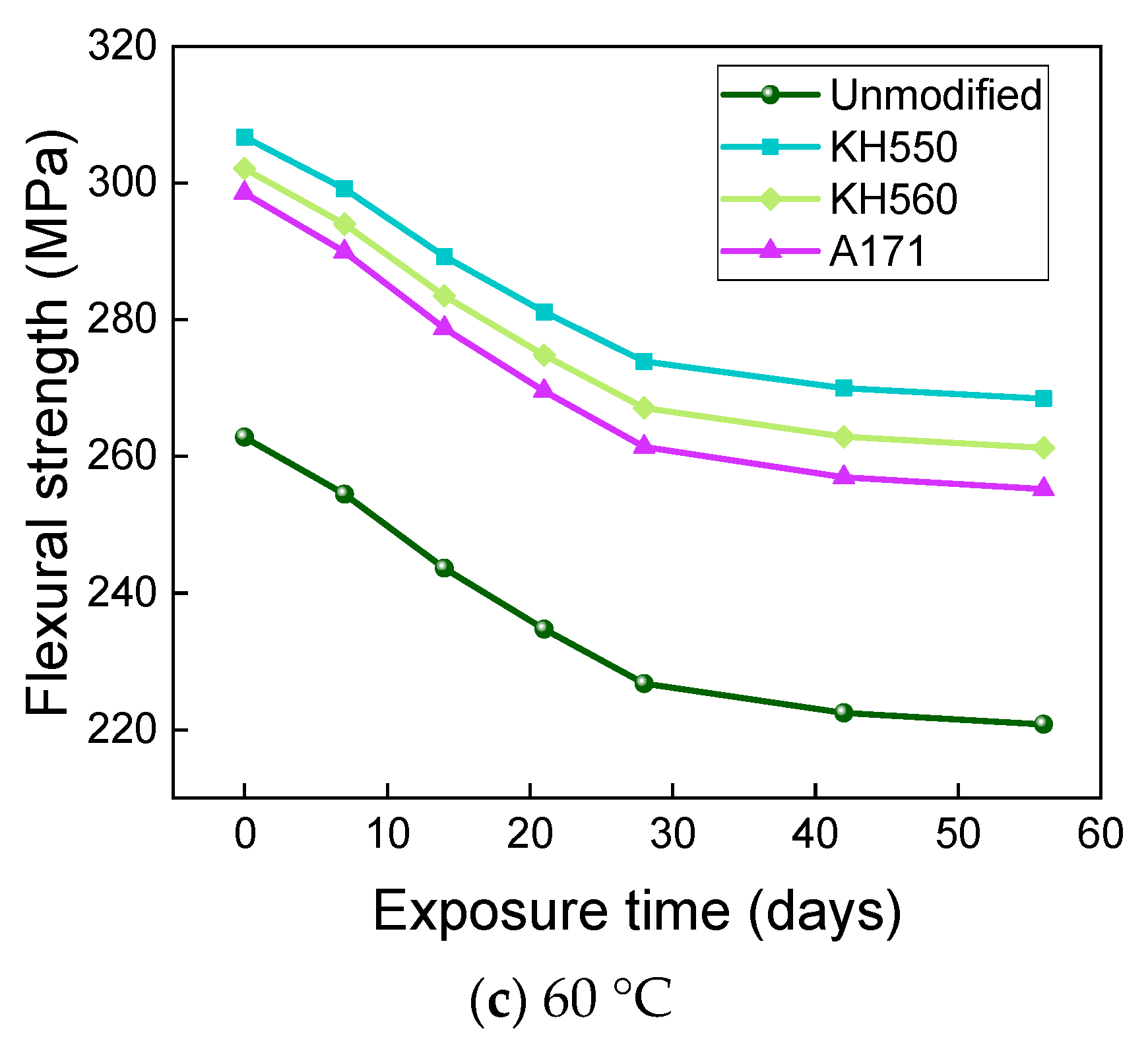
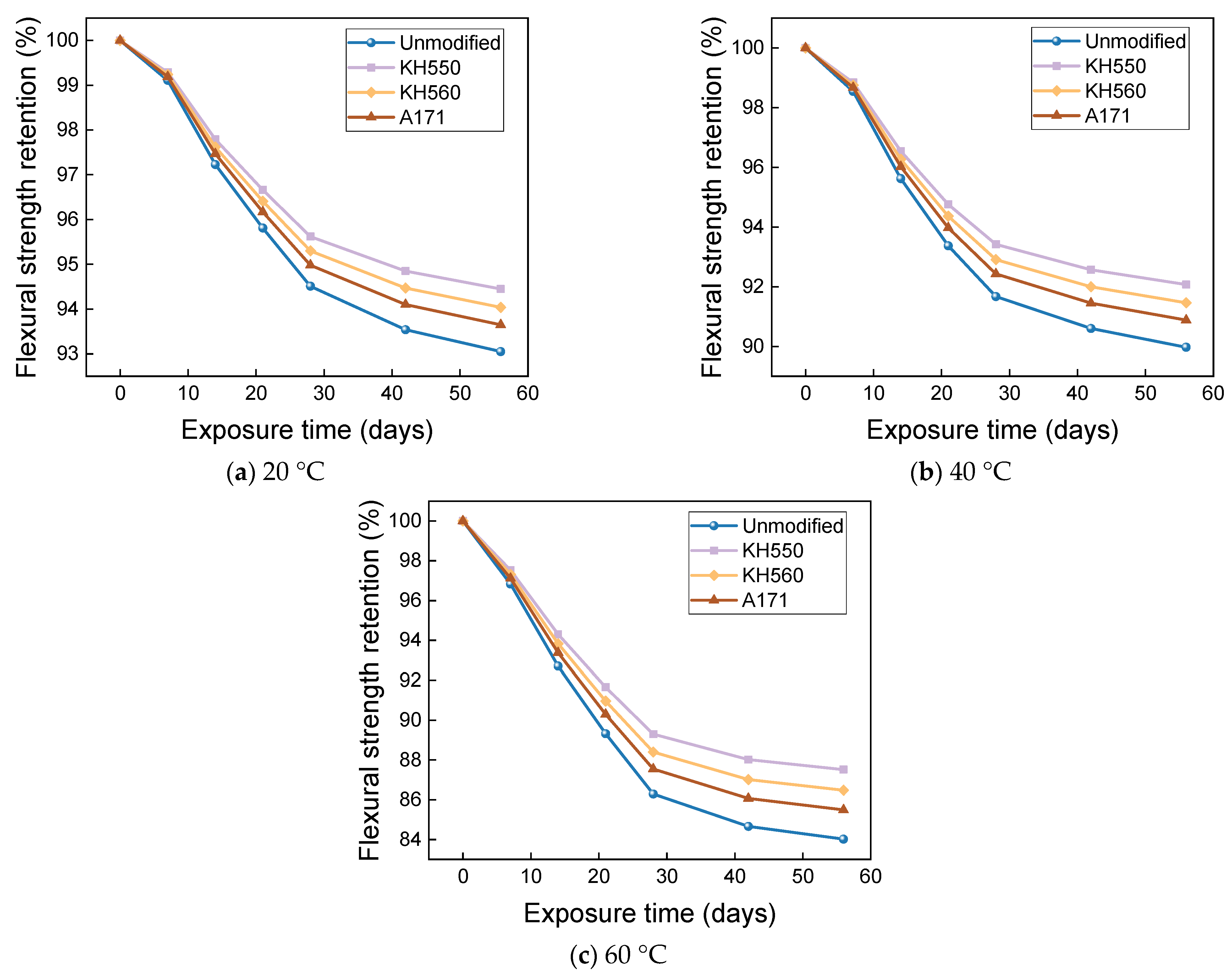
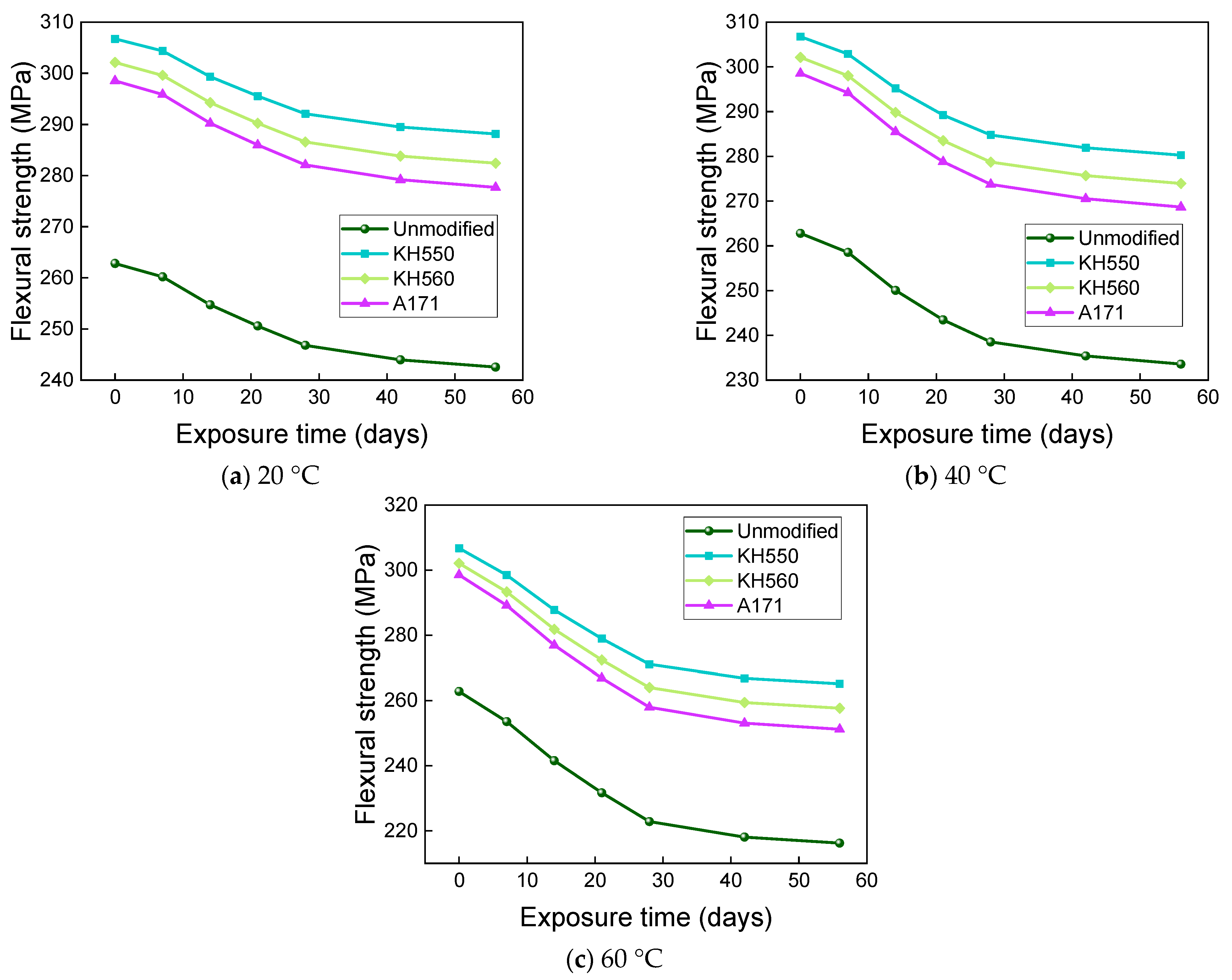
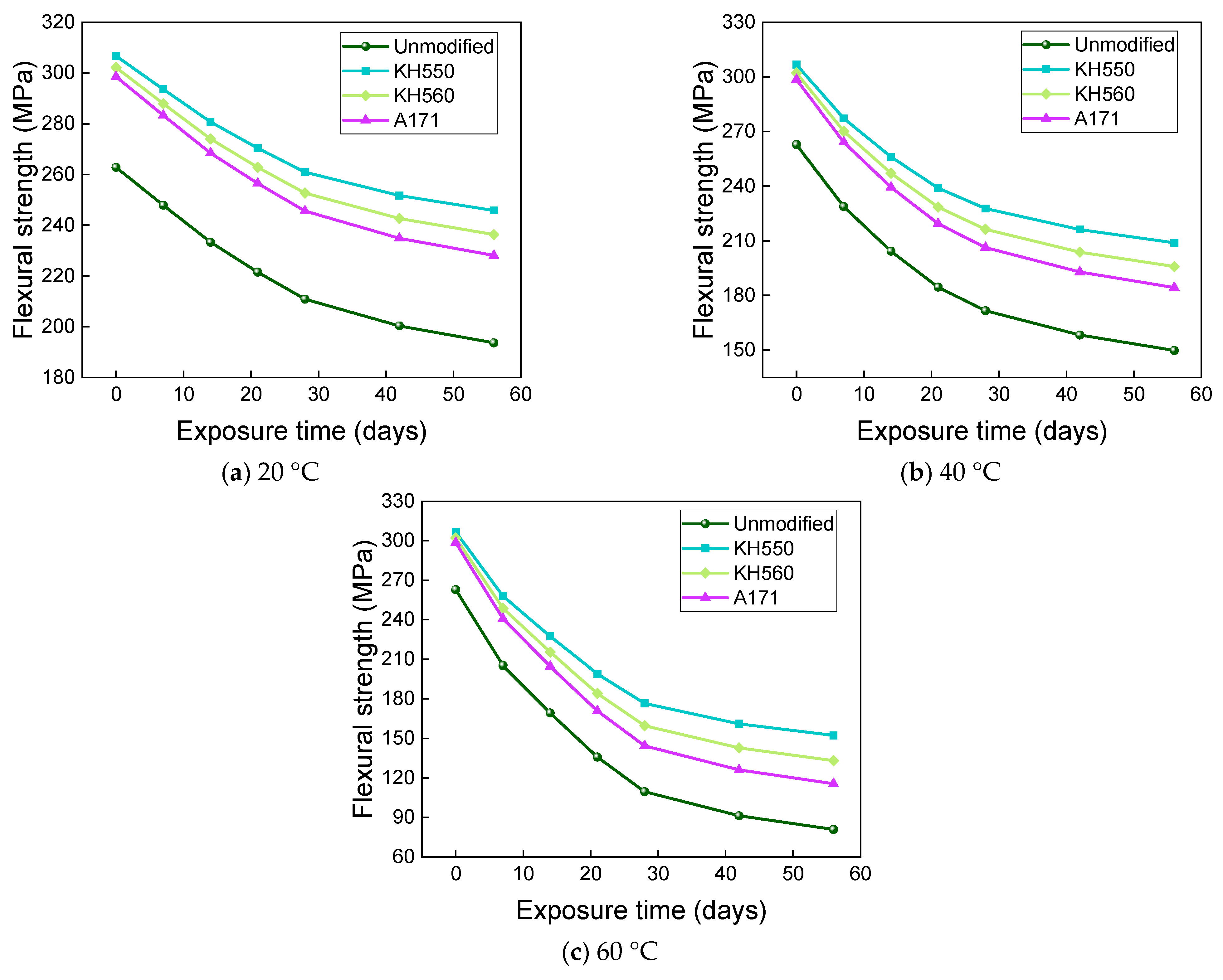
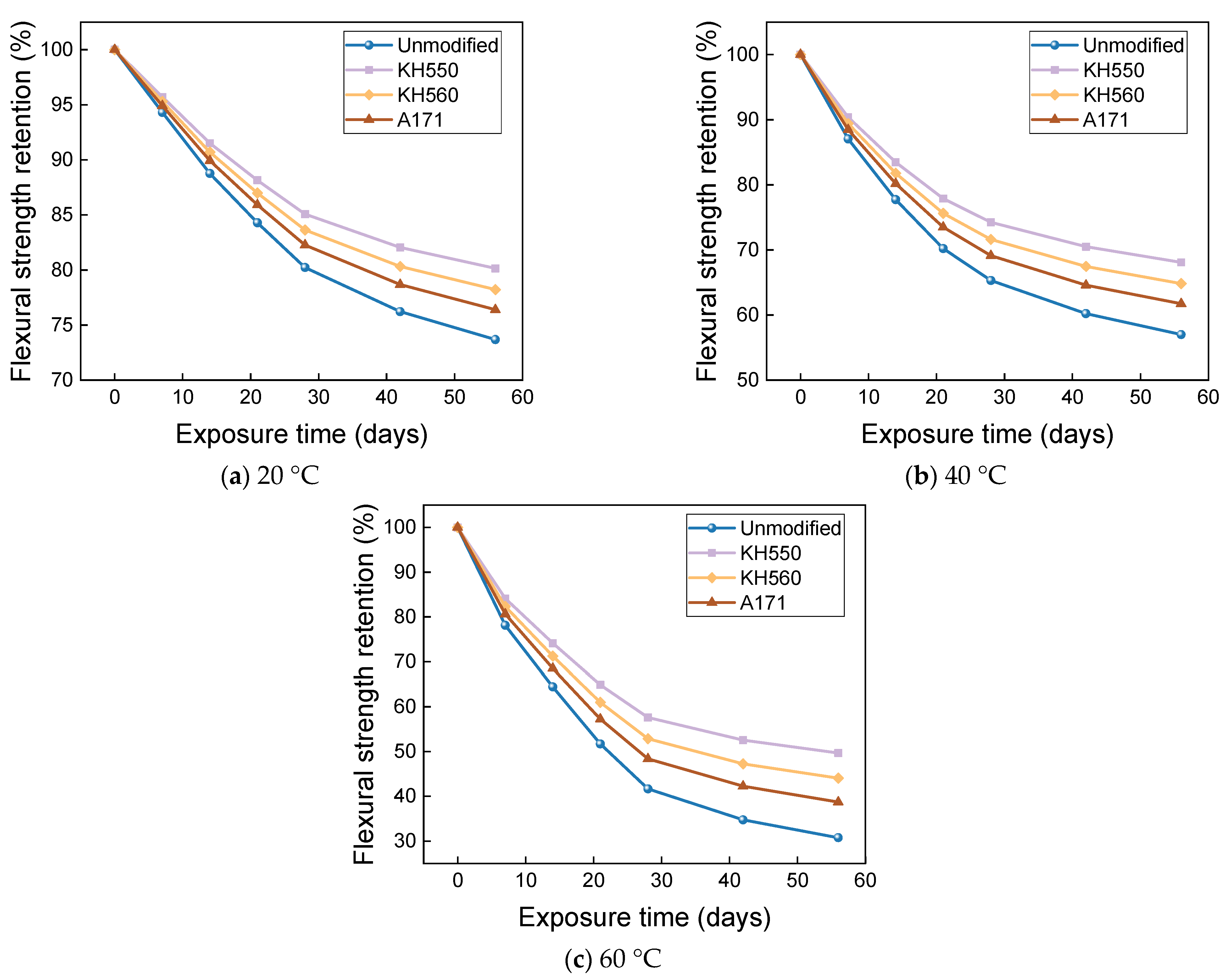
3.4. Flexural Properties in Different Immersion Environments
4. Conclusions
- (a)
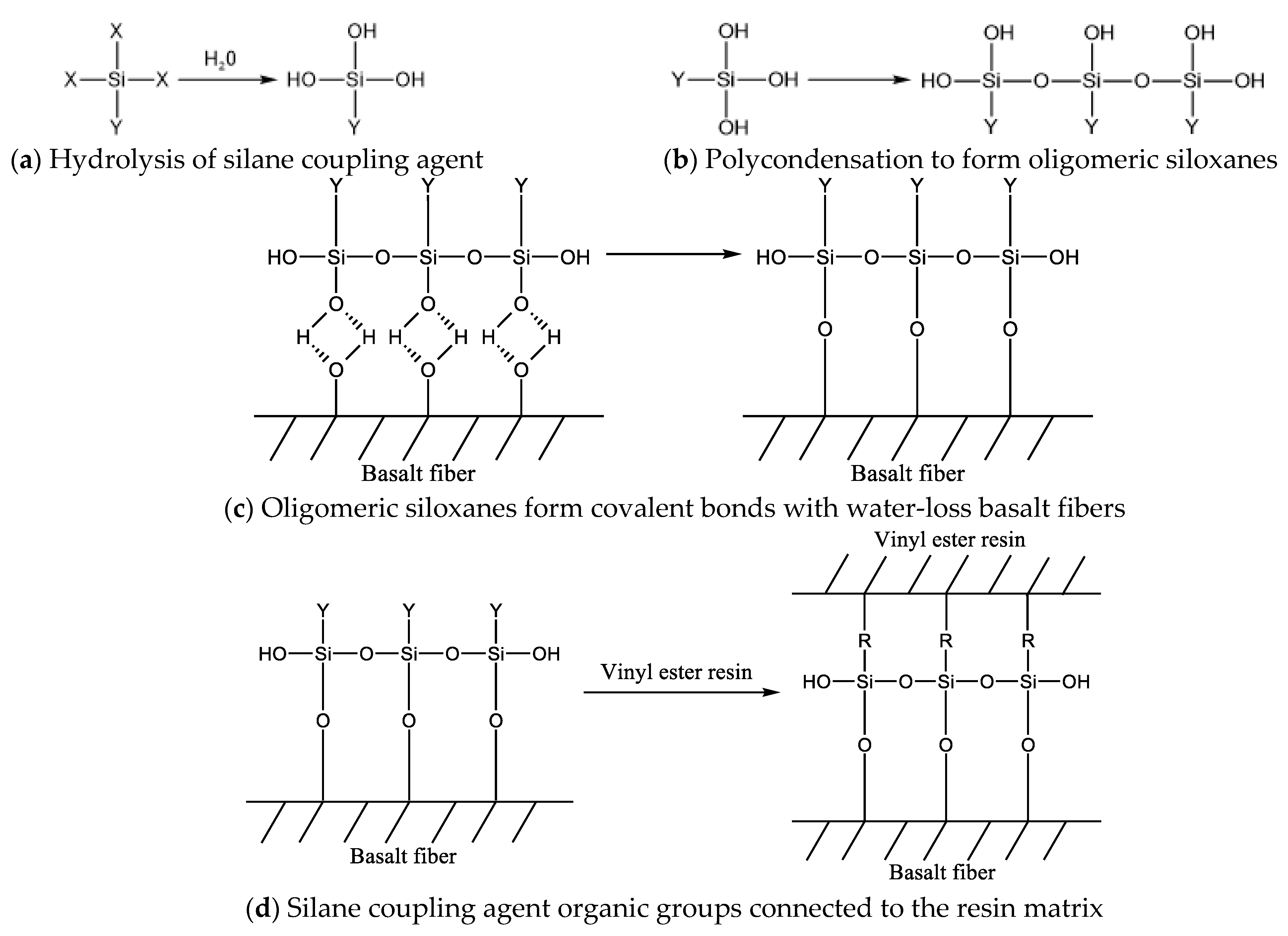
- The modification by silane coupling agents KH550, KH560, and A171 improved the interfacial bonding effect of BFRPs. Without the modification by silane coupling agents, there were large gaps between the fibers and the matrix, and the interfacial bonding effect was poor.
- (b)
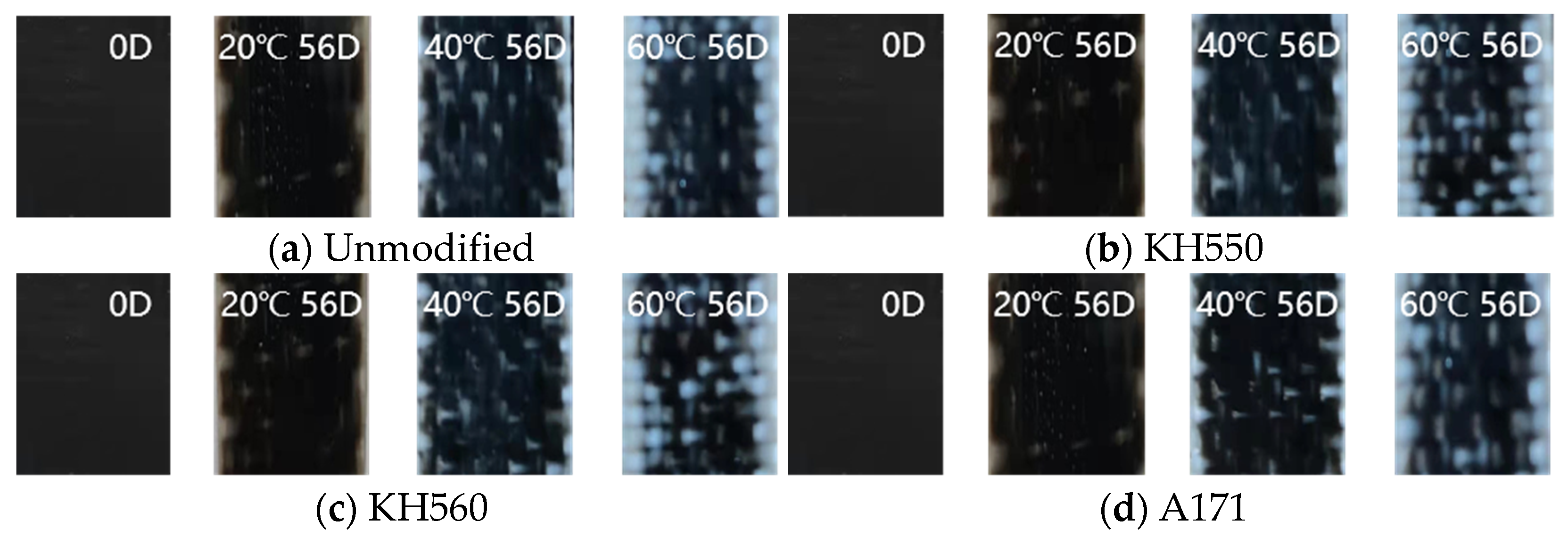
- The appearance of BFRPs varied greatly in different soaking environments. The samples soaked in water, 3.5% NaCl seawater, and 10% NaCl seawater environments did not show large changes, while the appearances of the samples soaked in acidic and alkaline environments showed great changes. Meanwhile, the appearance of BFRPs was greatly affected by the ambient temperature and soaking time, and a large number of white spots appeared on the surface of the samples after 56 days of soaking in the acidic and alkaline environments at 60 °C.
- (c)
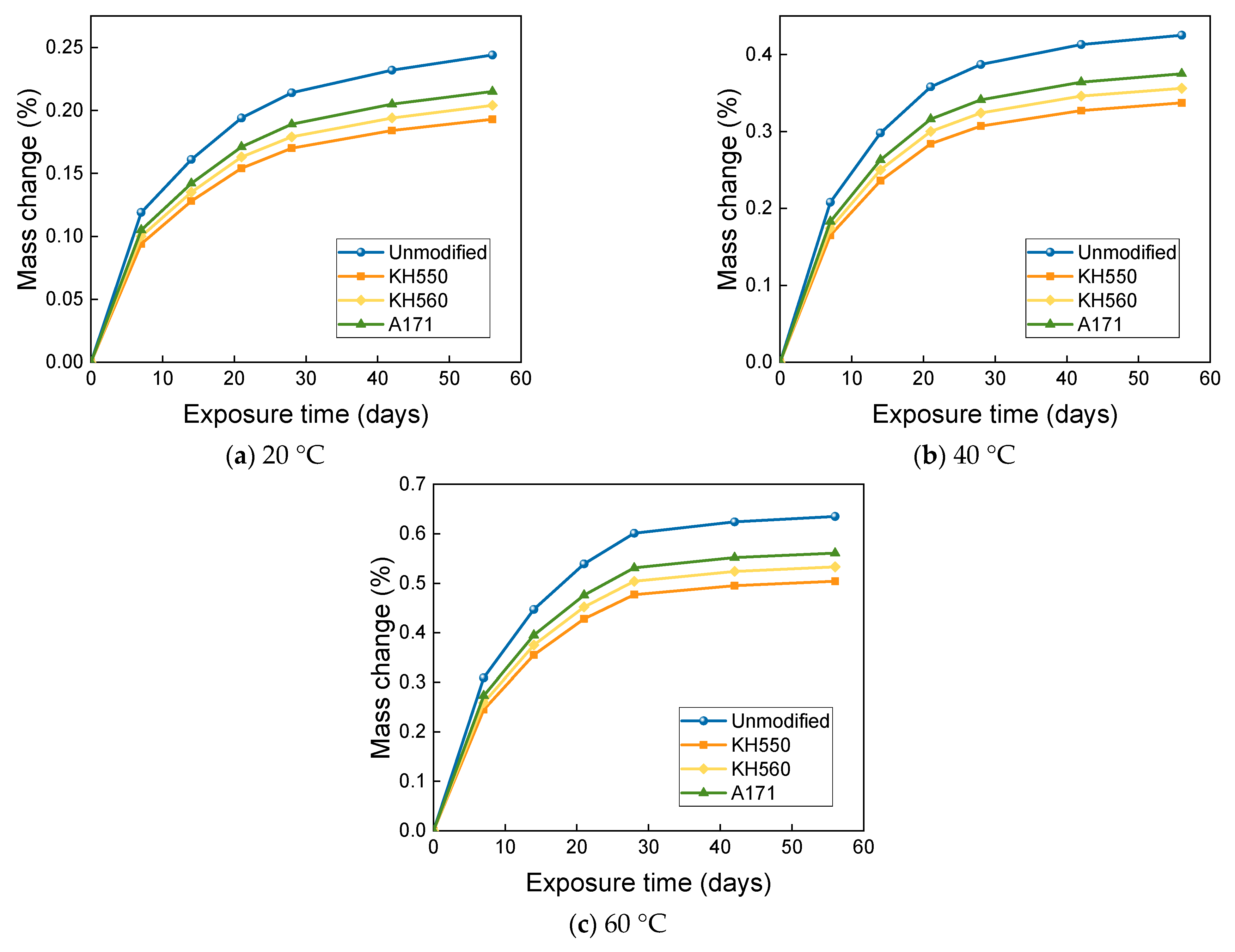
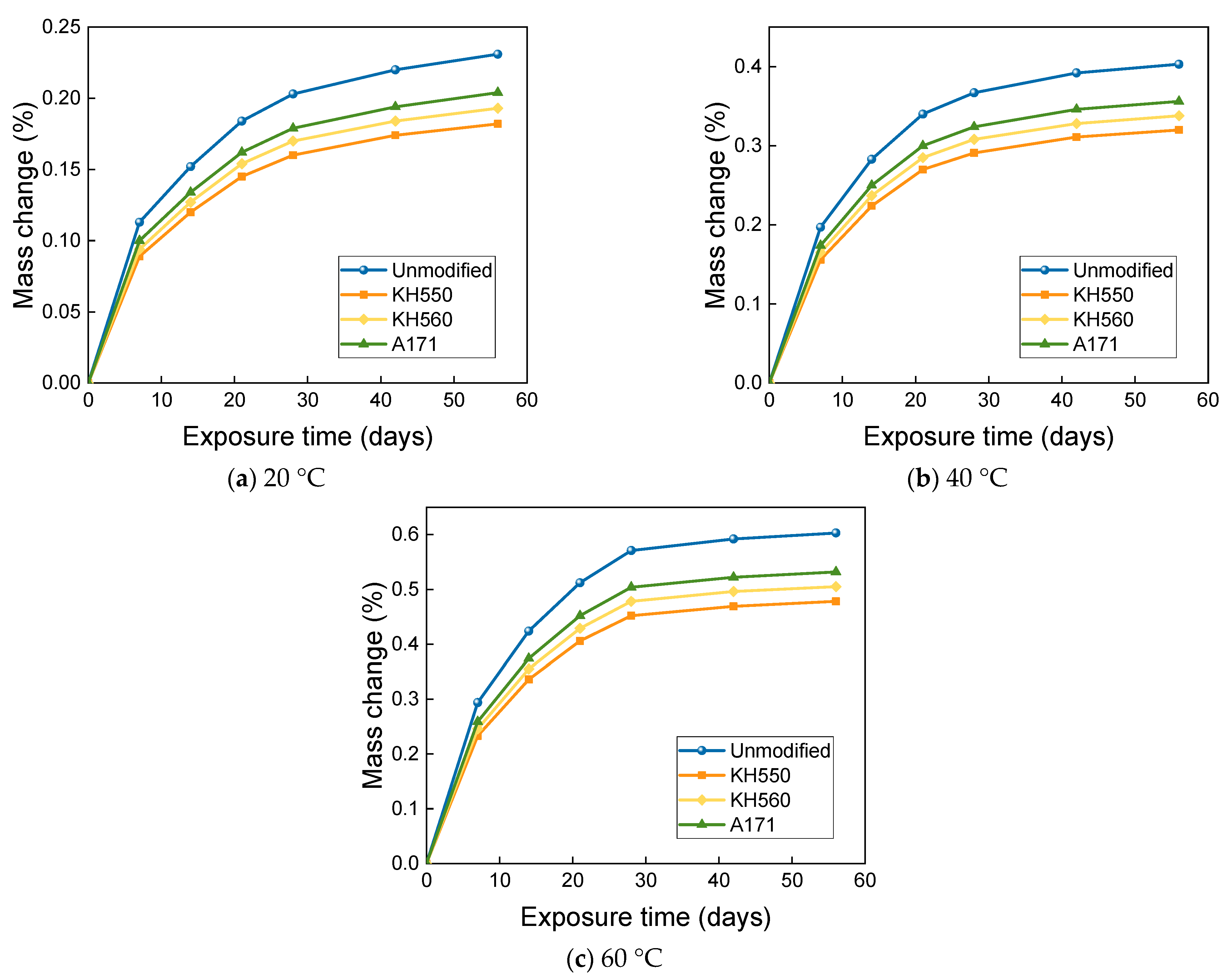
- The mass change of BFRPs accumulates gradually with soaking time, but the mass change rate decreases gradually with time. Temperature substantially enhances the mass change of BFRPs. The mass change of BFRPs in water at 20 °C, 40 °C, and 60 °C and 3.5% NaCl seawater, 10% NaCl seawater, and an alkaline environment mainly increased the mass. BFRPs exhibited mass gain in the acid environment at 20 °C, but a mass loss in the acid environments at 40 °C and 60 °C. The silane coupling agent modification reduced the mass change rate of BFRPs by forming a strong chemical bond connecting the fiber–resin matrix and enhancing the interfacial adhesion.
- (d)
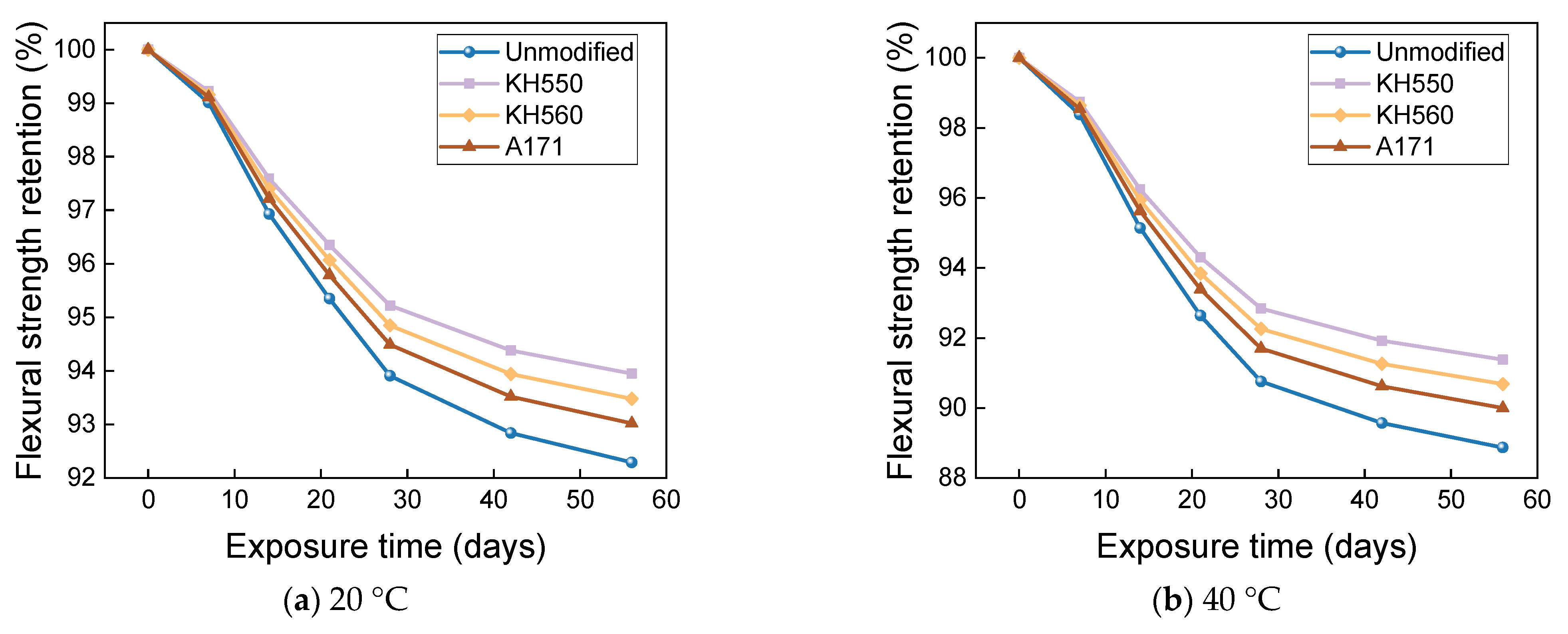
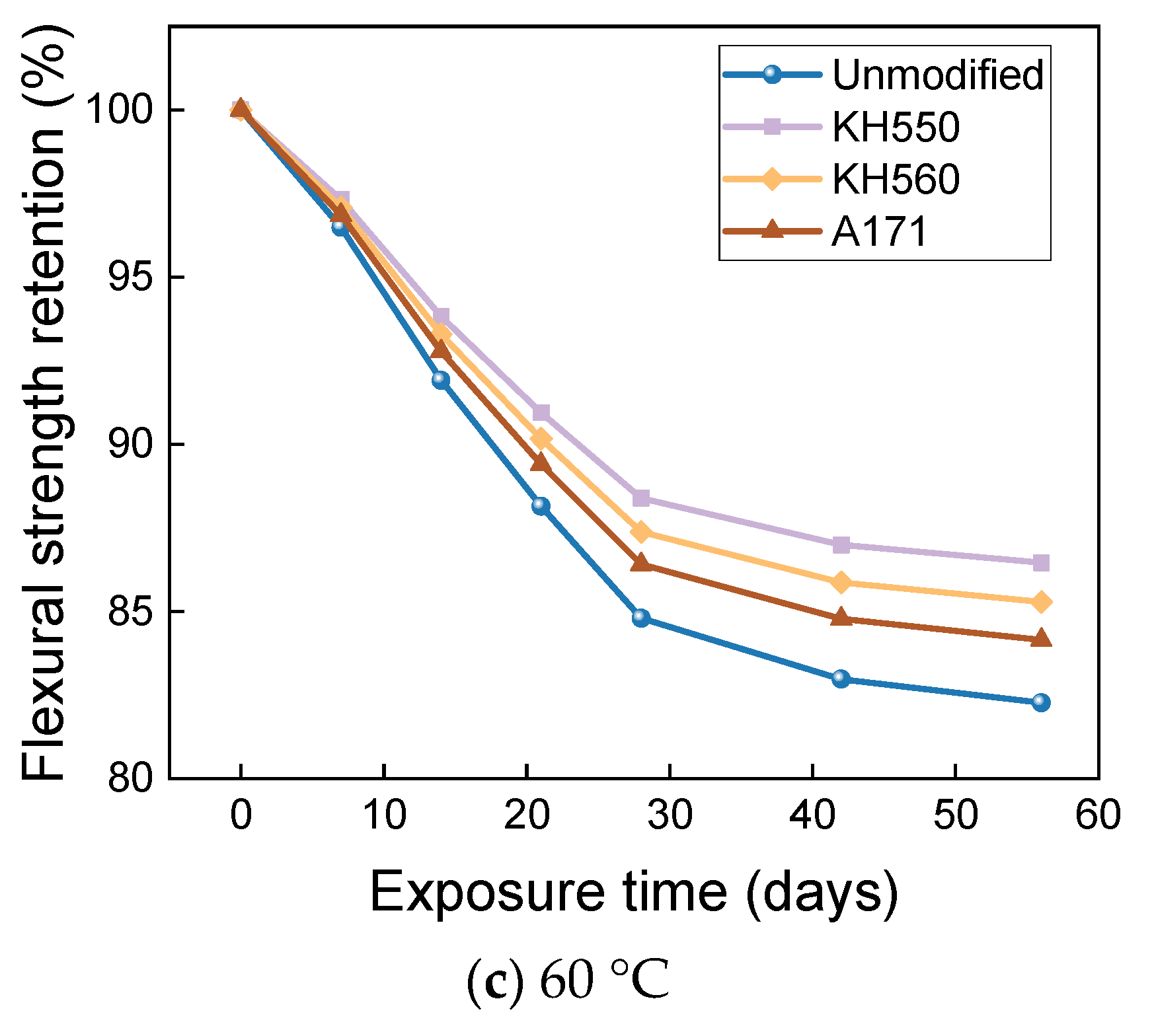
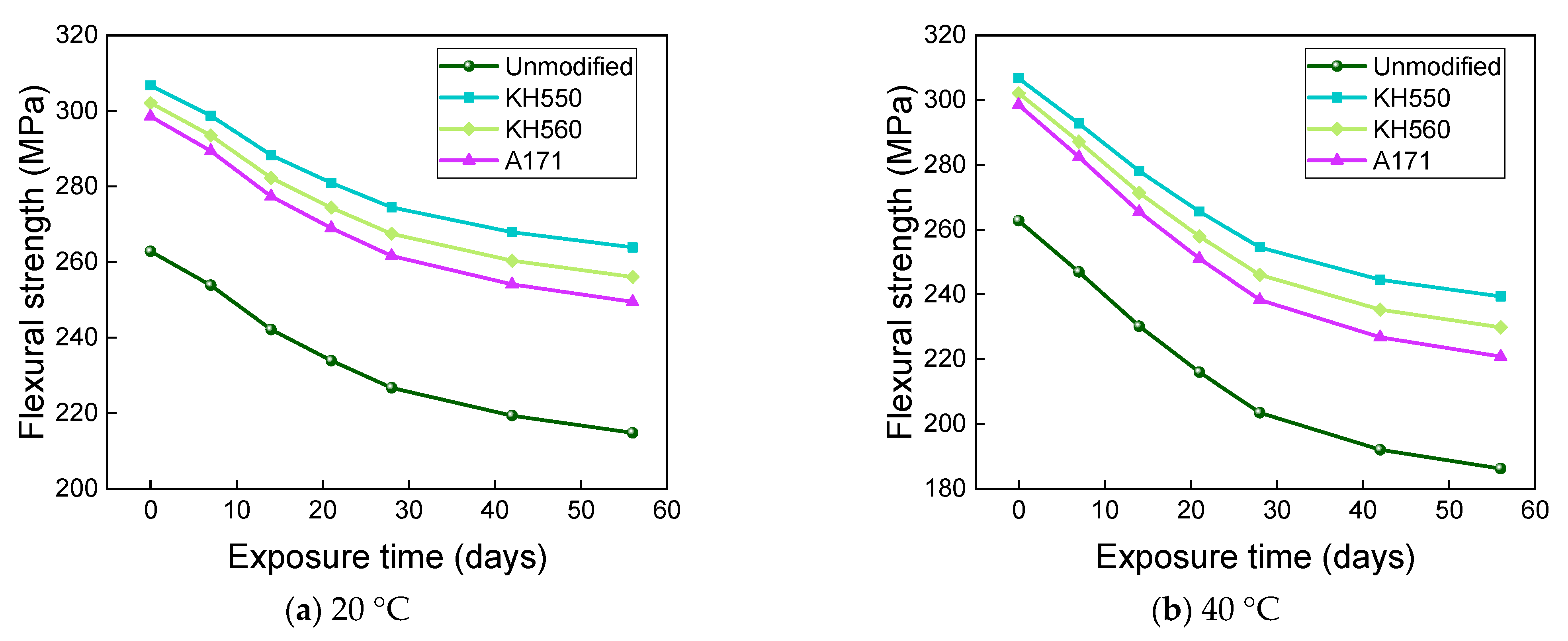
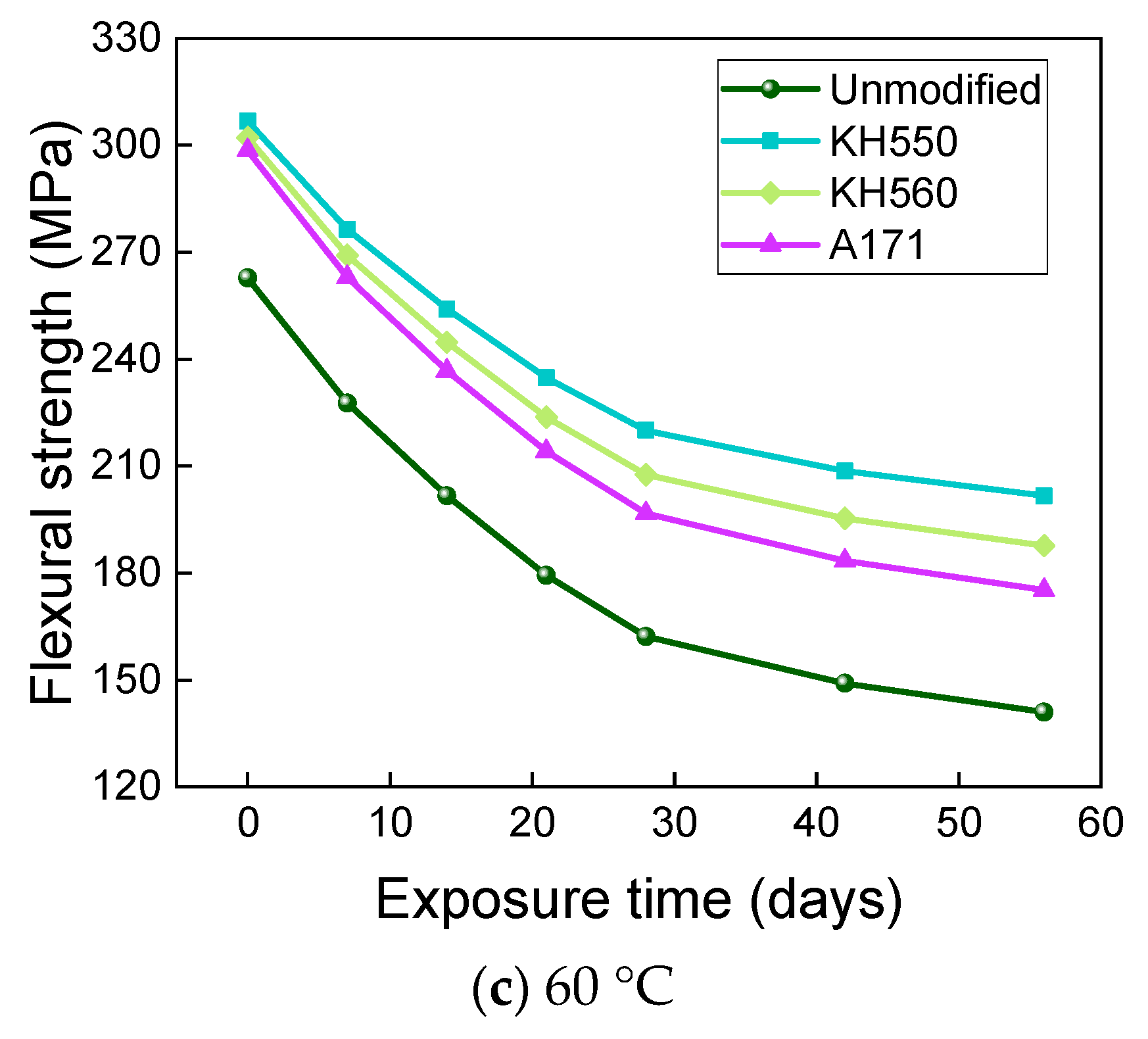
- The rate of the decrease in the flexural strength of BFRPs was positively correlated with the rate of mass change. The flexural strength gradually decreased with the increase in submergence time, but the decrease rate gradually slowed down with the increase in time. When the temperature increased, the flexural strength of the BFRPs decreased significantly. The alkaline environments had the greatest effect on the flexural properties of BFRPs compared to the water, seawater, and acid environments. The silane coupling agent modification improved the flexural properties of BFRPs by enhancing the interfacial bonding properties of the BFRPs and reduced the degradation of the flexural properties of BFRPs in corrosive aging environments. Considering the experimental results, the three silane coupling agents modified the corrosive aging performance of the composites in the order of KH550 > KH560 > A171.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mouritz, A.; Gellert, E.; Burchill, P.; Challis, K. Review of advanced composite structures for naval ships and submarines. Compos. Struct. 2001, 53, 21–42. [Google Scholar] [CrossRef]
- Tran, P.; Nguyen, Q.T.; Lau, K. Fire performance of polymer-based composites for maritime infrastructure. Compos. Part B Eng. 2018, 155, 31–48. [Google Scholar] [CrossRef]
- Van Den Einde, L.; Zhao, L.; Seible, F. Use of FRP composites in civil structural applications. Constr. Build. Mater. 2003, 17, 389–403. [Google Scholar] [CrossRef]
- Abdellatief, M.; Alanazi, H.; Radwan, M.K.H.; Tahwia, A.M. Multiscale Characterization at Early Ages of Ultra-High Performance Geopolymer Concrete. Polymers 2022, 14, 5504. [Google Scholar] [CrossRef] [PubMed]
- Tahwia, A.M.; Ellatief, M.A.; Heneigel, A.M.; Elrahman, M.A. Characteristics of eco-friendly ultra-high-performance geopolymer concrete incorporating waste materials. Ceram. Int. 2022, 48, 19662–19674. [Google Scholar] [CrossRef]
- Wang, H.; Hassan, E.A.; Memon, H.; Elagib, T.H.; Abad AllaIdris, F. Characterization of natural composites fabricated from Abutilon-fiber-reinforced Poly (Lactic Acid). Processes 2019, 7, 583. [Google Scholar] [CrossRef]
- Wang, H.; Memon, H.; Hassan, E.A.M.; Elagib, T.H.H.; Hassan, F.E.A.A.; Yu, M. Rheological and Dynamic Mechanical Properties of Abutilon Natural Straw and Polylactic Acid Biocomposites. Int. J. Polym. Sci. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Abdellatief, M.; Elemam, W.E.; Alanazi, H.; Tahwia, A.M. Production and optimization of sustainable cement brick incorporating clay brick wastes using response surface method. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Karbhari, V.M.; Xian, G. Hygrothermal effects on high VF pultruded unidirectional carbon/epoxy composites: Moisture uptake. Compos. Part B Eng. 2009, 40, 41–49. [Google Scholar] [CrossRef]
- Miah, S.; Yu, J.; Yang, Y.; Memon, H.; A Rashid, M. Durability and notch sensitivity analysis of environmental ageing induced glass fibre mat and kenaf fibre mat-reinforced composites. J. Ind. Text. 2019, 51, 24–47. [Google Scholar] [CrossRef]
- Yilmaz, T.; Sinmazcelik, T. Effects of hydrothermal aging on glass–fiber/polyetherimide (PEI) composites. J. Mater. Sci. 2010, 45, 399–404. [Google Scholar] [CrossRef]
- Zheng, Q.; Morgan, R.J. Synergistic Thermal-Moisture Damage Mechanisms of Epoxies and Their Carbon Fiber Composites. J. Compos. Mater. 1993, 27, 1465–1478. [Google Scholar] [CrossRef]
- Xiao, G.Z.; Delamar, M.; Shanahan, M.E.R. Irreversible interactions between water and DGEBA/DDA epoxy resin during hygrothermal aging. J. Appl. Polym. Sci. 1997, 65, 449–458. [Google Scholar] [CrossRef]
- Dhakal, H.; Zhang, Z.; Richardson, M. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Kaelble, D.H.; Dynes, P.J.; Crane, L.W.; Maus, L. Interfacial Mechanisms of Moisture Degradation in Graphite-Epoxy Composites. J. Adhes. 1975, 7, 25–54. [Google Scholar] [CrossRef]
- Hodzic, A.; Kim, J.-K.; Lowe, A.; Stachurski, Z. The effects of water aging on the interphase region and interlaminar fracture toughness in polymer–glass composites. Compos. Sci. Technol. 2004, 64, 2185–2195. [Google Scholar] [CrossRef]
- Bergeret, A.; Ferry, L.; Ienny, P. Influence of the fibre/matrix interface on ageing mechanisms of glass fibre reinforced thermoplastic composites (PA-6,6, PET, PBT) in a hygrothermal environment. Polym. Degrad. Stab. 2009, 94, 1315–1324. [Google Scholar] [CrossRef]
- Yan, L.; Chouw, N. Effect of water, seawater and alkaline solution ageing on mechanical properties of flax fabric/epoxy composites used for civil engineering applications. Constr. Build. Mater. 2015, 99, 118–127. [Google Scholar] [CrossRef]
- Bazli, M.; Ashrafi, H.; Oskouei, A.V. Effect of harsh environments on mechanical properties of GFRP pultruded profiles. Compos. Part B Eng. 2016, 99, 203–215. [Google Scholar] [CrossRef]
- Wang, H.; Memon, H.; Hassan, E.A.M.; Miah, S.; Ali, A. Effect of Jute Fiber Modification on Mechanical Properties of Jute Fiber Composite. Materials 2019, 12, 1226. [Google Scholar] [CrossRef]
- Feng, P.; Wang, J.; Wang, Y.; Loughery, D.; Niu, D. Effects of corrosive environments on properties of pultruded GFRP plates. Compos. Part B Eng. 2014, 67, 427–433. [Google Scholar] [CrossRef]
- Dilfi KF, A.; Balan, A.; Bin, H.; Xian, G.; Thomas, S. Effect of surface modification of jute fiber on the mechanical properties and durability of jute fiber-reinforced epoxy composites. Polym. Compos. 2018, 39, E2519–E2528. [Google Scholar] [CrossRef]
- Wang, X.; Petrů, M. Effect of hygrothermal aging and surface treatment on the dynamic mechanical behavior of flax fiber reinforced composites. Materials 2019, 12, 2376. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Abdul, H.P.S. Khalil the effect of environmental exposure upon the mechanical properties of coir or oil palm fiber reinforced composites. J. Appl. Polym. Sci. 2000, 77, 1322–1330. [Google Scholar] [CrossRef]
- Tual, N.; Carrere, N.; Davies, P.; Bonnemains, T.; Lolive, E. Characterization of sea water ageing effects on mechanical properties of carbon/epoxy composites for tidal turbine blades. Compos. Part A Appl. Sci. Manuf. 2015, 78, 380–389. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Kim, H.-J.; Jung, D.H. Investigation of seawater effects on the mechanical properties of untreated and treated MMT-based glass fiber/vinylester composites. Ocean Eng. 2015, 108, 393–401. [Google Scholar] [CrossRef]
- Monaldo, E.; Nerilli, F.; Vairo, G. Basalt-Based Fiber-Reinforced Materials and Structural Applications in Civil Engineering. Compos. Struct. 2019, 214, 246–263. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Di Bella, G.; Valenza, A. A review on basalt fibre and its composites. Compos. Part B Eng. 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Dhand, V.; Mittal, G.; Rhee, K.Y.; Park, S.-J.; Hui, D. A short review on basalt fiber reinforced polymer composites. Compos. Part B Eng. 2015, 73, 166–180. [Google Scholar] [CrossRef]
- Kim, M.T.; Kim, M.H.; Rhee, K.Y.; Park, S.J. Study on an oxygen plasma treatment of a basalt fiber and its effect on the interlaminar fracture property of basalt/epoxy woven composites. Compos. B Eng. 2011, 42, 499–504. [Google Scholar] [CrossRef]
- Arslan, C.; Dogan, M. The effects of silane coupling agents on the mechanical properties of basalt fiber reinforced poly(butylene terephthalate) composites. Compos. Part B Eng. 2018, 146, 145–154. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Ji, J.; Yang, Y.; Shen, J.; Ye, M. The hygrothermal aging process and mechanism of the novolac epoxy resin. Compos. Part B Eng. 2016, 107, 1–8. [Google Scholar] [CrossRef]
- Wang, B.; Panigrahi, S.; Crerar, W.; Tabil, L. Application of pre-treated flax fibers in composites. CSAE/SCGR Paper 2003, 6, 03–367. [Google Scholar]
- Georgiopoulos, P.; Christopoulos, A.; Koutsoumpis, S.; Kontou, E. The effect of surface treatment on the performance of flax/biodegradable composites. Compos. Part B Eng. 2016, 106, 88–98. [Google Scholar] [CrossRef]
- Fergani, H.; Di Benedetti, M.; Oller, C.M.; Lynsdale, C.; Guadagnini, M. Durability and degradation mechanisms of GFRP reinforcement subjected to severe environments and sustained stress. Constr. Build. Mater. 2018, 170, 637–648. [Google Scholar] [CrossRef]
- Zafar, A.; Bertocco, F.; Schjødt-Thomsen, J.; Rauhe, J. Investigation of the long term effects of moisture on carbon fibre and epoxy matrix composites. Compos. Sci. Technol. 2012, 72, 656–666. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, Y.; Chen, P.; Wang, G.; Min, H.; Fang, W. Prediction of Long-Term Tensile Properties of Glass Fiber Reinforced Composites under Acid-Base and Salt Environments. Polymers 2022, 14, 3031. [Google Scholar] [CrossRef]
- Karbhari, V.M.; Chu, W. Degradation kinetics of pultruded E-glass/vinylester in alkaline media. ACI Mater. J. 2005, 102, 34. [Google Scholar]
- Xian, G.; Karbhari, V.M. Segmental relaxation of water-aged ambient cured epoxy. Polym. Degrad. Stab. 2007, 92, 1650–1659. [Google Scholar] [CrossRef]
- Regazzi, A.; Corn, S.; Ienny, P.; Bénézet, J.-C.; Bergeret, A. Reversible and irreversible changes in physical and mechanical properties of biocomposites during hydrothermal aging. Ind. Crop. Prod. 2016, 84, 358–365. [Google Scholar] [CrossRef]
- Nayak, R.K.; Mahato, K.K.; Ray, B.C. Water absorption behavior, mechanical and thermal properties of nano TiO2 enhanced glass fiber reinforced polymer composites. Compos. Part A: Appl. Sci. Manuf. 2016, 90, 736–747. [Google Scholar] [CrossRef]
- Huang, G.; Sun, H. Effect of water absorption on the mechanical properties of glass/polyester composites. Mater. Des. 2007, 28, 1647–1650. [Google Scholar] [CrossRef]
- Chen, Y.; Davalos, J.F.; Ray, I.; Kim, H.-Y. Accelerated aging tests for evaluations of durability performance of FRP reinforcing bars for concrete structures. Compos. Struct. 2007, 78, 101–111. [Google Scholar] [CrossRef]
- Ellyin, F.; Maser, R. Environmental effects on the mechanical properties of glass-fiber epoxy composite tubular specimens. Compos. Sci. Technol. 2004, 64, 1863–1874. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Feng, P. A comprehensive review on mechanical properties of pultruded FRP composites subjected to long-term environmental effects. Compos. Part B Eng. 2020, 191, 107958. [Google Scholar] [CrossRef]
- Li, H.; Zhang, K.; Fan, X.; Cheng, H.; Xu, G.; Suo, H. Effect of seawater ageing with different temperatures and concentrations on static/dynamic mechanical properties of carbon fiber reinforced polymer composites. Compos. Part B Eng. 2019, 173, 106910. [Google Scholar] [CrossRef]
- Mingchao, W.; Zuoguang, Z.; Yubin, L.; Min, L.; Zhijie, S. Chemical Durability and Mechanical Properties of Alkali-proof Basalt Fiber and its Reinforced Epoxy Composites. J. Reinf. Plast. Compos. 2008, 27, 393–407. [Google Scholar] [CrossRef]
- Isitman, N.A.; Aykol, M.; Kaynak, C. Interactions at fiber/matrix interface in short fiber reinforced amorphous thermoplastic composites modified with micro- and nano-fillers. J. Mater. Sci. 2011, 47, 702–710. [Google Scholar] [CrossRef]
- Takizawa, Y.; Chung, D.D.L. Through-thickness thermal conduction in glass fiber polymer–matrix composites and its enhancement by composite modification. J. Mater. Sci. 2015, 51, 3463–3480. [Google Scholar] [CrossRef]
- Zhao, D.; Hamada, H.; Yang, Y. Influence of Fiber Surface Treatment on Hydrothermal Aging Behavior of a Woven Thermoplastic Laminate. Fibers Polym. 2021, 22, 1633–1642. [Google Scholar] [CrossRef]
- Arslan, C.; Dogan, M. The effects of fiber silane modification on the mechanical performance of chopped basalt fiber/ABS composites. J. Thermoplast. Compos. Mater. 2019, 33, 1449–1465. [Google Scholar] [CrossRef]
- Tábi, T.; Tamas, P.; Kovacs, J.G. Chopped basalt fibres: A new perspective in reinforcing poly(lactic acid) to produce injection moulded engineering composites from renewable and natural resources. Express Polym. Lett. 2013, 7, 107–119. [Google Scholar] [CrossRef]
- Samper, M.; Petrucci, R.; Sánchez-Nacher, L.; Balart, R.; Kenny, J. Effect of silane coupling agents on basalt fiber-epoxidized vegetable oil matrix composite materials analyzed by the single fiber fragmentation technique. Polym. Compos. 2014, 36, 1205–1212. [Google Scholar] [CrossRef]
- Shao, L.; Huang, J.; Feng, X. Study on Preparation and Properties of Glass Fibre Fabric Reinforced Polyphenylene Sulphide Composites. Materials 2022, 15, 9036. [Google Scholar] [CrossRef] [PubMed]
- Ashbee, K.H.G.; Wyatt, R.C. Water damage in glass fibre/resin composites. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1969, 312, 553–564. [Google Scholar] [CrossRef]
- Lu, C.; Yang, Y.; He, L. Mechanical and durability properties of GFRP bars exposed to aggressive solution environments. Sci. Eng. Compos. Mater. 2021, 28, 11–23. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.; Song, S. Degradation of basalt fibre and glass fibre/epoxy resin composites in seawater. Corros. Sci. 2011, 53, 426–431. [Google Scholar] [CrossRef]
- Scheffler, C.; Förster, T.; Mäder, E.; Heinrich, G.; Hempel, S.; Mechtcherine, V. Aging of alkali-resistant glass and basalt fibers in alkaline solutions: Evaluation of the failure stress by Weibull distribution function. J. Non-Crystalline Solids 2009, 355, 2588–2595. [Google Scholar] [CrossRef]







| Group. | Immersion Environment | Temperature (°C) | Duration (Days) |
|---|---|---|---|
| 1 | Water | 20, 40, 60 | 7, 14, 21, 28, 42, 56 |
| 2 | 3.5%NaCI | 20, 40, 60 | 7, 14, 21, 28, 42, 56 |
| 3 | 10%NaCI | 20, 40, 60 | 7, 14, 21, 28, 42, 56 |
| 4 | 10%H2SO4 | 20, 40, 60 | 7, 14, 21, 28, 42, 56 |
| 5 | 10%NaOH | 20, 40, 60 | 7, 14, 21, 28, 42, 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wei, Y.; Ma, L.; Tian, W.; Zhu, C. Effect of Corrosive Aging Environments on the Flexural Properties of Silane-Coupling-Agent-Modified Basalt-Fiber-Reinforced Composites. Materials 2023, 16, 1543. https://doi.org/10.3390/ma16041543
Luo X, Wei Y, Ma L, Tian W, Zhu C. Effect of Corrosive Aging Environments on the Flexural Properties of Silane-Coupling-Agent-Modified Basalt-Fiber-Reinforced Composites. Materials. 2023; 16(4):1543. https://doi.org/10.3390/ma16041543
Chicago/Turabian StyleLuo, Xuanyao, Yuehai Wei, Leilei Ma, Wei Tian, and Chengyan Zhu. 2023. "Effect of Corrosive Aging Environments on the Flexural Properties of Silane-Coupling-Agent-Modified Basalt-Fiber-Reinforced Composites" Materials 16, no. 4: 1543. https://doi.org/10.3390/ma16041543
APA StyleLuo, X., Wei, Y., Ma, L., Tian, W., & Zhu, C. (2023). Effect of Corrosive Aging Environments on the Flexural Properties of Silane-Coupling-Agent-Modified Basalt-Fiber-Reinforced Composites. Materials, 16(4), 1543. https://doi.org/10.3390/ma16041543







