Abstract
Titanium dioxide is a commonly used ingredient in cosmetics acting as a thickening agent and inorganic UV filter. However, TiO2 is difficult to disperse, which causes problems in spreading the formulations. The solution to this problem is to modify the titanium dioxide surface to change its properties by creation of the new type of hybrid inorganic–organic UV filter. Therefore, this study aimed to functionalize titanium dioxide with organosilicon compounds and determine how this modification will affect the dispersibility of TiO2 in the colloidal system and the stability of emulsions. First, the functionalized octaspherosilicates were obtained and characterized. Next, the synthesized compounds were applied as modifiers for titanium dioxide and were analyzed by FT-IR, UV-Vis, and laser diffraction. Furthermore, the hydrophilic–hydrophobic character was assessed by measuring the contact angle. The new materials were introduced into emulsions and the formulations were analyzed in terms of particle size distribution and stability by multiple light scattering. It was found that the modification of titanium dioxide with spherosilicates significantly improved both the stability of emulsion and the dispersibility of novel materials in the colloidal system compared to nonmodified TiO2. The covalent binding of the modifier with the titanium dioxide had an impact on the stability of the emulsion.
1. Introduction
Titanium dioxide (TiO2) is a commonly used ingredient in cosmetic products such as creams, powders, toothpaste, shampoos, soaps, nail polish, and eye shadows [1]. It acts as a thickening agent and a physical filter protecting against ultraviolet radiation [2,3,4]. Titanium dioxide naturally exists in different crystalline forms such as anatase, brookite, and rutile [5,6,7]. It was proved that rutile was more stable than anatase and brookite at room temperature [8]. Each form has different properties and applications. Titanium dioxide also exhibits high photocatalytic activity [9,10,11], which is associated with the generation of reactive oxygen species that may adversely affect the skin, accelerating the aging process [12,13]. Moreover, it is difficult to disperse TiO2 while preparing the formulations. Therefore, it is sometimes problematic to spread cosmetic products on the skin. Furthermore, it leaves white spots on its surface. The solution to this problem may be to modify the titanium dioxide, which enables to change its properties. So far, the surface of TiO2 has been functionalized with 3-aminopropyltriethoxysilane and N-2-(aminoethyl)-3-aminopropyltrimethoxysilane to be used in the adsorption processes of different dyes [14]. In other studies, the surface of titanium dioxide was treated with methacryloxypropylyrimethoxysilane to reduce the photocatalytic activity [15]. In turn, Yao et al. modified TiO2 with a silane coupling agent to change the surface properties from hydrophilic to hydrophobic [16]. The variation of surface nature was also observed when titanium dioxide was functionalized with sodium stearate and sodium oleate [17]. Recent studies have shown that the use of functionalized silsesquioxanes as modifiers of TiO2 increased its hydrophobicity and improved covering and dispersing properties [18].
Silsesquioxanes belong to the class of compounds that are hybrid systems, most often with a cubic structure and the general formula (RSiO1.5)n where R=H, alkyl, aryl, or their derivatives where n = 6, 8, 10, 12 [19]. An inorganic core and organic functional groups R attached to each of the eight corners of the cage can be distinguished in their structure. Silsesquioxanes can change the physicochemical properties of materials, such as thermal resistance [20], surface energy, and water contact angle [21] or they can improve dispersion [18] or even act as compatibilizers [22]. The factors influencing the dispersion capacity of particles in the system and the strength of their interaction with the modifier or matrix include particle size, specific surface area/porosity, chemical composition, and the presence/absence of surface functional groups in the case of fillers and pigments [23]. One of the most commonly used methods to improve dispersion is surface silanization, which reduces the ionic character of their surface and improves the wetting effect of the surface with the polymer. The modification of TiO2-SiO2 or SiO2 with the use of silsesquioxanes has been reported by Nowacka et al. [24] and Szwarc et al. [25], respectively.
The aim of this study was to functionalize titanium dioxide with organosilicon compounds (spherosilicates) and determine how this modification will affect the dispersibility of TiO2 in the colloidal system as well as the stability of emulsions. The trifunctional spherosilicates were selected because each introduced group exhibited specific functionality. Vinyl trimethoxysilane was used as a substrate to attach the anchoring group, derivative of cinnamate and eugenol were applied as the UV absorbing agent, whereas hexene and octene were used as the hydrophobic groups. Diethylene glycol monovinyl ether and allyl glycidyl ether were applied as substrates in the hydrosilylation reaction to introduce hydrophilic groups.
2. Materials and Methods
2.1. Synthesis of the Trifunctional Octasubstituted Spherosilicates
All spherosilicate derivatives were obtained by the hydrosilylation process, using commercially available olefins. The substrates applied to carry out the reactions are presented in Table 1. A precursor for the synthesis was obtained according to a literature procedure [26]. A total of 30 g of octaspherosilicate was placed in a three-neck, round-bottom flask (Glassco, Ambala, India), next, 250 mL of toluene and the mixture of:

Table 1.
Substrates used to perform reactions.
- (1)
- allyl cinnamate (22.20 g), hexene (7.45 g), and diethylene glycol monovinyl ether (3.90 g) in molar ratio 4:3:1
- (2)
- allyl cinnamate (16.65 g), hexene (9.93 g), and allyl glycidyl ether (3.37 g) in molar ratio 3:4:1
- (3)
- allyl cinnamate (22.20 g), octene (9.93 g), and vinyltrimethoxysilane (4.37 g) in molar ratio 4:3:1
- (4)
- eugenol (14.53 g), octene (9.93 g), and vinyltrimethoxysilane (8.74 g) in molar ratio 3:3:2 was added.
The reactions’ mixture was constantly stirred and heated to 60 °C (then 30 µL of Karstedt’s catalyst solution in xylene was added). The process was carried out for 24–48 h under reflux according to Scheme 1. The progress of the reaction was monitored by FT-IR-ATR analysis (Thermo Fisher Scientific, Waltham, MA, USA) until the Si-H bands’ disappearance (at 2141 cm−1 and 889 cm−1—stretching and bending vibrations, respectively).
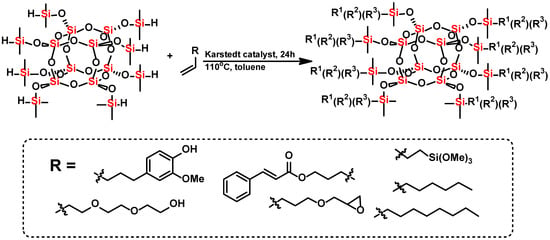
Scheme 1.
Hydrosilylation of olefins with octaspherosilicate.
The compound conversion as well as the purity of the products were determined by 1H, 13C, and 29Si NMR spectroscopy analysis.
2.2. Modification of Titanium Dioxide with Octaspherosilicate Derivatives
The procedure of mixing TiO2 with the selected organosilicon compound was carried out in accordance with our previous work [18]. In the modification process, all systems were obtained comprising of 1% w/w modifiers content in the dry TiO2.
2.3. Preparation of Emulsions with Modified Titanium Dioxide
The water phase and an oil phase were prepared separately with the compositions shown in the Table 2. Both phases were heated to 70 °C until all ingredients were dissolved. Modified TiO2 was added to the oil phase and mixed. Afterwards, both phases were combined and homogenized on a SilentCrusher M homogenizer (Heidolph, Schwabach, Germany) for 4 min. at 16,000 rpm. After cooling the emulsion to 30 °C, phenoxyethanol was added as preservative. Emulsions were prepared with each modified titanium dioxide and with the nonmodified one as the reference system.

Table 2.
Composition of emulsions.
2.4. Analytical Methods
1H, 13C, and 29Si Nuclear Magnetic Resonance (NMR) spectra were recorded at 25 °C on a Bruker Ascend 400 and Ultra Shield 300 spectrometers (Bruker, Billerica, MA, USA) using CDCl3 as a solvent. Chemical shifts were reported in ppm with reference to the residual solvent (CHCl3) peaks for 1H.
Density measurements of octaspherosilicate derivatives were performed three times on stationary densimeter KEM (Kyoto Electronics Manufacturing, Kyoto, Japan) at a temperature of 20 °C.
Water contact angle (WCA) analyses were performed five times by the sessile drop technique at room temperature and atmospheric pressure with a Krüss DSA100 goniometer (Krüss, Hamburg, Germany). Three independent measurements were taken for each sample, each with a 5 µL water drop.
UV spectra of octaspherosilicate derivatives were recorded using a UV spectrophotometer Cary 60 (Agilent Technologies, Santa Clara, CA, USA). Samples were dissolved in n-hexane. Solutions with the following concentrations were prepared: 1.0335 × 10−5 mol/L for SSQ1, SSQ2, SSQ3 and 2.1008 × 10−5 mol/L for SSQ4. Solid state UV-Vis spectra in reflectance mode were recorded by a UV-Vis spectrophotometer (Cary 60, Agilent) equipped with the solid sample holder.
The FT-IR spectra of modified and nonmodified titanium dioxide were recorded on a Nicolet iS50 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). For this purpose, KBr pellets were made (200 mg KBr + 2 mg of tested titanium dioxide). Pellets were put into spectrometer and transmission measurements were made.
The refractive index was measured three times using a refractometer InsMark IR120. Particle size distribution of modified and nonmodified titanium dioxide as well as emulsions was determined by a Mastersizer 3000 (Malvern Instruments Co. Ltd., Malvern, UK) using the laser diffraction method. The analyses were conducted in distilled water at room temperature. Five independent measurements were taken for each sample.
The zeta potential of titanium dioxide nonmodified and modified with SSQs was determined in distilled water by a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK).
A digital light microscope Keyence VHX 7000 (Keyence International, Mechelen, Belgium) with 100- x1000 VH-Z100T lens was used to examine the pigment dispersion in the emulsion. All images were recorded with a VHX 7020 camera.
The stability of the emulsion was measured using a Multiscan MS 20 (DataPhysics, Instruments GmbH, Filderstadt, Germany). The backscattering profiles were determined and presented as graphs versus sample height. All emulsions were analyzed for 31 days at a certain time at 25 °C.
3. Results and Discussion
3.1. Characterization of Organosilicon Modifiers
All organosilicon derivatives were obtained by the hydrosilylation reaction of octahydrospherosilicate and commercially available olefins with groups containing unsaturated bonds capable of absorbing UV radiation, as well as non-polar hydrophobic groups and polar groups such as oxirane group, trimethoxysilyl, or diethylene glycol ether. In addition, the trimethoxysilyl group was used to create weak chemical bonds between alkoxyl groups of SSQ and the hydroxyl groups located on the surface of titanium dioxide. Different derivatives containing three types of functional groups in the octaspherosilicate cage were synthesized. Their chemical structures are presented in Figure 1, Figure 2, Figure 3 and Figure 4. All compounds were obtained with high yield (>95%) and full conversion (>99%), which confirms the efficiency of the hydrosilylation reaction in obtaining functionalized organosilicon compounds. The conversion of the substrates and the purity of the products were determined by 1H NMR spectroscopy.
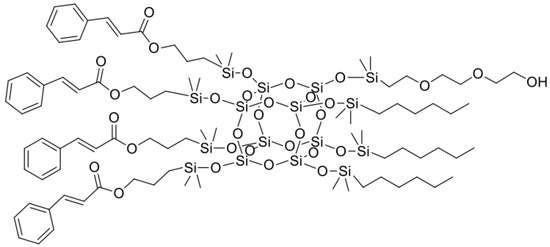
Figure 1.
The chemical structure of SSQ1.
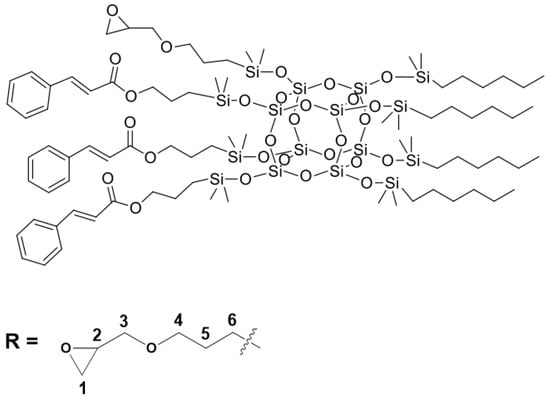
Figure 2.
The chemical structure of SSQ2.
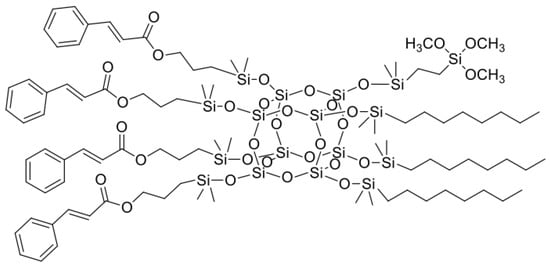
Figure 3.
The chemical structure of SSQ3.
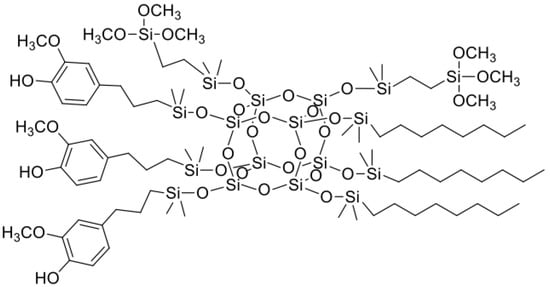
Figure 4.
The chemical structure of SSQ4.
1H NMR (600 MHz, CDCl3) δ (ppm): 7.70 (m, 4H, CH=CH-Ph), 7.54–7.39 (m, 20 H, Ph), 6.48 (m, 4 H, CH=CH-Ph), 4.20 (m, CH2- CH2- CH2-O), 4.02–3.63 (m, 10H, O-CH2- CH2-O) 2.96, 2.65,1.78 (m, CH2- CH2- CH2-O), 1.34–1.30 (m, 24 H, CH2-(CH2)4-CH3), 1.06–0.97 (m, 2H, Si-CH2-CH2-O) 0.90 (m, CH2-CH3), 0.68 (m, Si-CH2-CH2-CH2) 0.63 (m, CH2-(CH2)4-CH3), 0.47 (m, Si-CH2 backbiting product) 0.21–0.16 (m, 48H, SiMe2).
13C NMR (151 MHz, CDCl3) δ (ppm): 167.08 (C=O), 144.71 (Ph-CH=CH), 137.94, 134.58, 130.31, 129.14, 128.97, 128.33, 125.41, 118.36 (Ph-CH=CH), 67.02, 65.32 (O-CH2), 33.12, 31.70, 23.01, 22.72, 22.53, 21.57, 17.76, 14.27, 13.77, −0.20, −1.30 (SiMe2).
29Si NMR (119 MHz, CDCl3) δ (ppm): 13.15 (SiMe2), −109.01 (core).
1H NMR (600 MHz, CDCl3) δ (ppm): 7.70 (m, 3H, CH=CH-Ph), 7.53–7.39 (m, 15 H, Ph), 6.45 (m, 3 H, CH=CH-Ph) 4.19 (m, CH2- CH2- CH2-O), 3.68 (m, 1 H position 3), 3.45 (m, 2 H, position 4), 3.40 (m, 1 H, position 3), 3.15 (m, 1 H, position 2), 2.79 (m, 1 H, position 1), 2.61 (m, 1 H, position 1), 1.78 (m, CH2- CH2- CH2-O), 1.67 (m, 2 H, position 5), 1.34–1.30 (m, 32H, CH2-(CH2)4-CH3), 0.90 (m, 12H CH2-CH3), 0.69 (m, Si-CH2-CH2-CH2), 0.62(m, Si-CH2-CH2-), 0.46 (m, Si-CH2 backbiting product), 0.21–0.15 (m, 48H, SiMe2).
13C NMR (151 MHz, CDCl3) δ (ppm): 167.09(C=O), 145.97, 144.70 (Ph-CH=CH), 137.96, 134.60, 130.31, 129.15, 128.98, 128.34, 125.42, 118.37 (Ph-CH=CH), 74.21, 71.54, (glycidoxy group) 67.05 (O-CH2), 50.94 (OMe), 44.45, 33.14, 31.71, 23.03, 22.73, 22.54, 21.58, 17.76, 14.27, 13.79, −0.28, −0.37 (SiMe2).
29Si NMR (119 MHz, CDCl3) δ(ppm): 12.93 (SiMe2), −109.02 (core).
1H NMR (600 MHz, CDCl3) δ (ppm): 7.73 (m, 4 H, CH=CH-Ph), 7.53–7.39 (m, 20 H, Ph), 6.48 (m, 3 H, CH=CH-Ph), 4.19 (m, CH2- CH2- CH2-O), 3.59 (m, 9 H, Si-O-CH3), 1.78 (m, CH2- CH2- CH2-O), 1.28 (m, 36 H, CH2-(CH2)6-CH3), 0.90 (m, 9H CH2-CH3), 0.70 (m, Si-CH2-CH2-CH2-O) 0.62 (m, Si-CH2-CH2), 0.46 (m, Si-CH2 backbiting product), 0.21–0.16 (m, 48 H, SiMe2).
13C NMR (151 MHz, CDCl3) δ (ppm): 167.09(C=O), 145.98, 145.18, 144.70 (Ph-CH=CH), 137.97, 134.60, 132.42, 130.31, 128.98, 128.17, 125.42, 118.38 (Ph-CH=CH), 67.04, 65.33 (O-CH2), 50.67 (Si-(OMe)3), 33.56, 32.07, 29.45, 22.81, 22.54, 21.58, 17.78, 14.25, 13.77, 8.61, 0.43, −0.20, −0.39, −0.98 (SiMe2).
29Si NMR (119 MHz, CDCl3) δ (ppm):13.25 (SiMe2), −41.72 (Si(OMe)3, −108.99 (core).
1H NMR (600 MHz, CDCl3) δ (ppm): 6.82–6.67 (m, 9 H, CH2-Ph), 5.50 (s, 3 H, O-H), 3.87 (m, 9 H, Ph-O-CH3), 3.58 (m, 18 H, Si-O-CH3), 2.56 (m, 6 H, CH2-Ph), 1.64 (m, 6 H, CH2-CH2-Ph), 1.28 (m, 36 H, Si-CH2-(CH2)6-CH3) 0.89 (m, 9 H, Si-CH2-(CH2)6-CH3), 0.63 (m, 20 H, Si-CH2) 0.14 (m, 48 H, SiMe2)
13C NMR (151 MHz, CDCl3) δ (ppm): 146.20, 143.49, 137.77, 134.39, 128.95, 128.14, 125.22, 120.92, 114.05, 110.92, 55.73 (Ph-O-CH3), 50.48 (Si-(OMe)3), 39.12, 33.38, 31.89, 29.27, 25.17, 22.90, 22.62, 21.38, 17.60, 17.30, 14.05, 8.40, 0.22, −0.40, −0.42, −1.20 (SiMe2).
29Si NMR (119 MHz, CDCl3) δ (ppm): 12.84 (SiMe2), −41.59 (Si(OMe)3, −108.89 (core).
The UV spectra of spherosilicates were recorded and are depicted in Figure 5. SSQ1, SSQ2, and SSQ3 exhibit maximum absorbance at 271 nm, which can be attributed to π,π* transition with a molar absorption coefficient in the range of (6–8) × 104 M−1 cm−1. The absorption band corresponds to conjugated double bonds and the aromatic ring of allyl cinnamate [27].
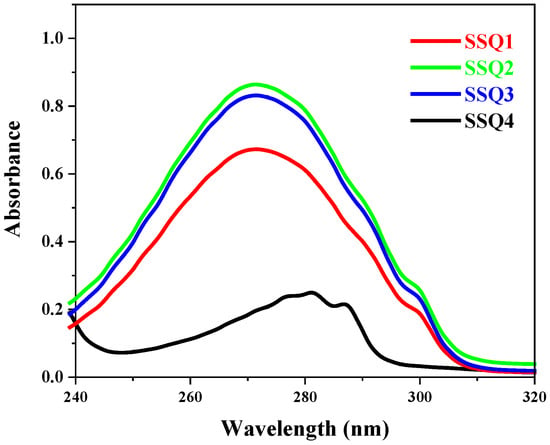
Figure 5.
UV spectra of synthesized silsesquioxanes.
In turn, the absorption spectrum of SSQ4 can be attributed to a single electronic transition with an oscillation structure band with λmax around 281 nm and molar absorption coefficient 1.1 × 104 M−1 cm−1 (π,π* transition). Similar results were obtained by Foudah et al. [28]. The absorption band at λmax at 281 nm corresponded to to the condensed benzene ring system of eugenol [29].
The density and refractive index were tested for the chemical compounds obtained (Table 3). The density value for all tested silsesquioxanes ranges from 1.093 to 1.112 g/mL, which is consistent with the data described by Joshi and Bhattacharyya [30]. Most of the volume is occupied by the functional organic groups, which are located outside the core. Typically, 5% of the volume is taken by the core [30].

Table 3.
Density, refractive index, and molecular weight of SSQs.
3.2. Characterization of Modified Titanium Dioxide
In the FT-IR spectra of the modified titanium dioxides, in addition to the bands characteristic of TiO2, new signals from the bonds present in the modifiers appeared (Figure 6). All modified systems showed additional bands in the range of 3000–2700 cm−1 (a) and 1550–1450 cm−1 (c) characteristic of unsaturated aliphatic chains, originating from the cinnamate and eugenol groups, as well as signals present in the range of 1100–1000 cm−1 (d) indicating presence of C-O-C ether bonds. In addition, in the range of 1750–1650 cm−1 (b), there is a signal coming from the stretching vibrations of ketone groups contained in allyl cinnamate used in modifiers SSQ1–SSQ3. The presence of additional signals in the FT-IR spectrum confirmed the modification of the TiO2 surface.
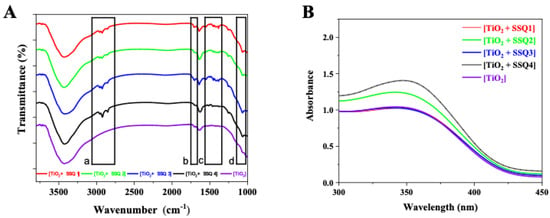
Figure 6.
FT-IR (A) and UV-Vis (B) spectra of modified titanium dioxide.
In Figure 6B, the UV-Vis spectra of nonmodified and modified titanium dioxide is presented. All materials absorb light at a wavelength below 400 nm. Similar spectra of pure TiO2 were recorded by Zhao et al. [31]. The results proved that all new materials exhibit strong absorption in the ultraviolet region. The modification of titanium dioxide did not shift the absorption maximum to higher wavelengths.
The influence of modifying titanium dioxide with spherosilicates on particle size distribution was also tested. A monomodal particle size distribution between 0.04 and 1.00 μm for titanium dioxide was observed (Figure 7). These results are in accordance with data recorded by Song et al. [32]. For all modified materials a monomodal particle size, distribution was also detected, and no significant changes compared to pure titanium dioxide were observed.
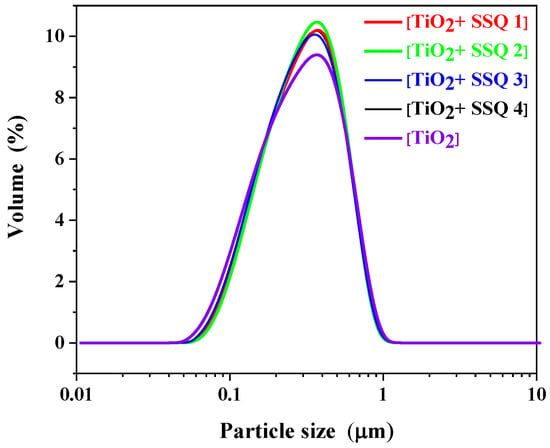
Figure 7.
Particle size distribution of nonmodified and modified titanium dioxide.
Table 4 presents the data in the form of d (0.1), d (0.5), and d (0.9). The obtained results indicated no meaningful effect of the applied modifiers on the variation in the particle size distribution compared to TiO2. Table 4 also shows the results of zeta potential of samples obtained. It was proved that the nonmodified TiO2 suspension had a positive zeta potential value. These results are in accordance with data recorded by Ridley et al. [33] and Halimi et al. [34]. The modification of titanium dioxide with SSQs changed the surface charge from positive to negative. It should be highlighted that all suspensions can be considered as stable. Based on the literature data, the dispersion with the ζ potential value higher than ±25 mV is less prone to change their particle size [35].

Table 4.
Particle size distribution and zeta potential of nonmodified and modified titanium dioxide.
In order to determine the hydrophilic–hydrophobic nature of the TiO2 pigment, water contact angle analysis (WCA) was performed. The study was carried out for unmodified TiO2 as a reference sample, as well as for TiO2 with the addition of SSQ1, SSQ2, SSQ3, and SSQ4 derivatives. For the laboratory test, samples in the form of pellets (load 120 MPa for 3 min.) were prepared. The TiO2 surface has a strongly polar, superhydrophilic character; therefore, the dosed drop was completely absorbed by the tested material (Figure 8). A similar effect was observed in our previous work [22]. Modification of the TiO2 surface with organosilicon compounds containing non-polar alkyl groups, i.e., hexyl and octyl groups, and polar groups, e.g., AGE, VTMOS, or DGME changed the character of the surface. Table 5 presents the values of contact angles for individual derivatives, and in Figure 8, the results are depicted as images of water droplets during sessile drop analysis. The smallest affinity to water molecules was reported for the SSQ2 sample in which the share of allyl groups is the highest. Other derivatives also showed a strongly hydrophobic character, and the WCA values are in the range of 130–142°.

Figure 8.
Images of water droplets during sessile drop analysis of TiO2+SSQ1, TiO2+SSQ2, TiO2+SSQ1, TiO2+SSQ1, and unmodified TiO2.

Table 5.
Contact angle.
The effect of modifiers on the dispersibility of titanium dioxide in emulsions was studied by an optical microscope. In Figure 9A–D, presenting the emulsions with modified TiO2, much smaller droplets were observed compared to formulations containing nonmodified titanium dioxide. In the image of the emulsion with TiO2 (Figure 5E), large clusters of undispersed titanium dioxide were detected. Based on the obtained images, it can be concluded that the addition of spherosilicates modifiers improved the dispersion of titanium(IV) oxide in emulsions.
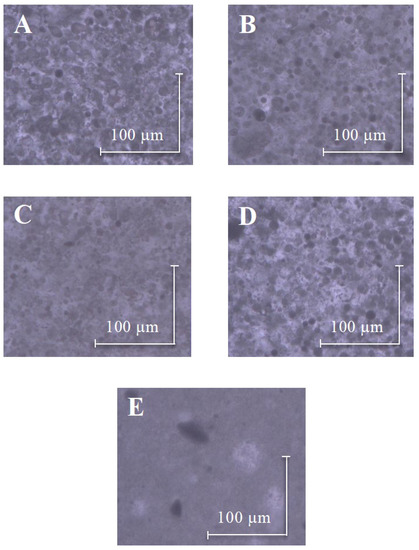
Figure 9.
Optical microscope images of E+[TiO2+SSQ1] (A), E+[TiO2+SSQ2] (B), E+[TiO2+SSQ3] (C), E+[TiO2+SSQ4] (D), E+[TiO2] (E).
It should be mentioned that particle size distribution might have an impact on the emulsions’ stability [36]. Therefore, all formulations were subjected to laser diffraction analyses. The results directly after the emulsions’ preparation are presented in Figure 10A. For all colloidal systems containing modified TiO2, the bimodal particle size distribution was detected. The predominant peak was identified in the range of 0.7 to 8 μm. In turn, the emulsion with pure titanium dioxide had trimodal particle size distribution. It was observed in Figure 10B that after 3 months of the emulsions’ storage, the volume fraction of predominant peak decreased, and the particle distribution shifted towards a larger size.
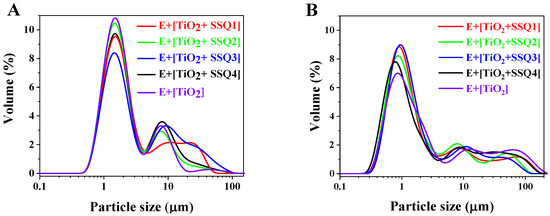
Figure 10.
Particle size distribution of emulsions after preparation (A) and after 3 months (B).
Based on the results presented in Table 6, it could be concluded that the highest changes in particle size distribution after 3 months were observed for emulsions containing nonmodified titanium dioxide.

Table 6.
Particle size distribution for emulsions.
Next, the stability of emulsions containing modified and nonmodified titanium dioxide was assessed by multiple light scattering. This method enables to detect any changes occurring in the sample without its dilution. Furthermore, it allows to shorten the aging and development processes of new formulations [37]. The changes in backscattering profiles were only considered because the samples were non-transparent and, therefore, the transmission signals were imperceptible. The sample is considered as stable when the curves overlap over time. In turn, for unstable emulsions, the changes in backscattering profiles will be detected. Two types of destabilization phenomena can be identified by multiple light scattering such as migration or particle size variation [38]. When the particles move from the bottom to the top, the creaming process occurs in the samples. In turn, when the movement of particles from the top to the bottom is observed, the sedimentation process takes place. On the other hand, when the variation in particle size occurred known as flocculation, the changes were visible in the backscattering profiles along the whole height of the samples [39]. Figure 11A–E presents the intensity of backscattered light versus sample height. The data were collected at a certain period for 31 days. The red color represents the first measurement and the blue one, the latest one. It was observed that the level of backscattering intensities for all emulsions obtained was similar and did not change significantly within 31 days. The backscattering profiles overlap over time. Only variations were observed at the top of the sample where the meniscus lowered down slightly, most likely due to an incomplete alignment. There were no significant changes in the stability over 31 days. According to Mengual et al., samples are considered as unstable when the variation of backscattering intensity exceeds 10%.
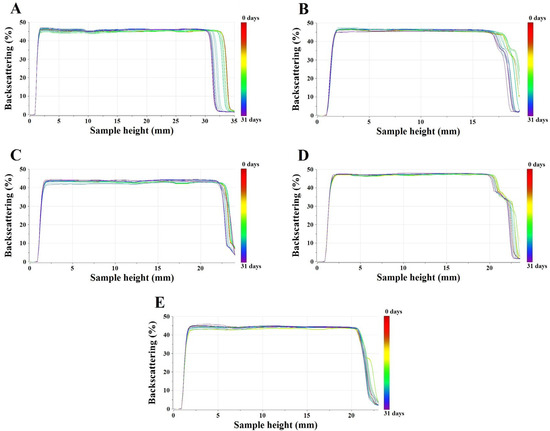
Figure 11.
Backscattering profiles of E+[TiO2+SSQ1] (A), E+[TiO2+SSQ2] (B), E+[TiO2+SSQ3] (C), E+[TiO2+SSQ4] (D), E+[TiO2] (E).
In order to better visualize the changes occurring in samples, the stability index was determined (Figure 12A). This parameter takes into consideration all the variations in size and concentration occurring in each sample [40]. It enables to compare the samples and select the most stable one. The lower value of the stability index is the more stable emulsion [41,42]. The value of stability index after 31 days of measurements decreased in the following order: E+[TiO2] (3.9%) > E+[TiO2+SSQ1] (3.1%) > E+[TiO2+SSQ2] (2.5%) > E+{TiO2+SSQ3] (2.4%) > E+[TiO2+SSQ4] (1%). Furthermore, based on the curves obtained, the rate of changes in the stability index was also determined (Figure 12B). It can be seen that the slowest variations in the stability index were observed for E+[TiO2+SSQ4]. Taking the above into consideration, it can be stated that emulsions containing TiO2 modified with the compound SSQ4 are the most stable. These results proved that the functionalization of titanium oxide increased the emulsion’s stability compared to pure titanium oxide.
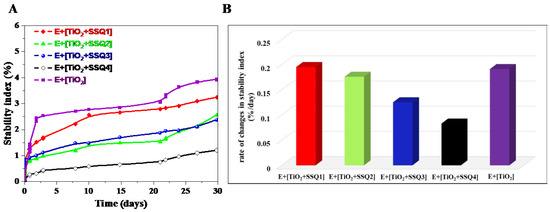
Figure 12.
Stability indexes (A) and rate of changes in stability index (B) for emulsions E+[TiO2+SSQ1], E+[TiO2+SSQ2], E+[TiO2+SSQ3], E+[TiO2+SSQ4], E+[TiO2].
This might be caused by the different nature of obtained materials that changed significantly after modification with spherosilicates derivatives (negative zeta potential) in relation with nonmodified TiO2 (positive zeta potential, Table 4). The results of zeta potential value may have influence on the stability of emulsions. It was observed that the highest value of zeta potential was observed for TiO2+SSQ4 and, thus, the emulsion containing this material was the most stable. As it was presented in Figure 9, the improvement of dispersibility of titanium dioxide by functionalization with selected spherosilicates had an influence on better stabilization of emulsions containing novel materials compared to sample E+[TiO2]
The demonstrated changes in the stability of the emulsion systems are related with two key factors such as the hydrophobic/hydrophilic properties of the modifiers and the covalent bond formed between the modifier and the hydroxyl surface of TiO2 via trimethoxysilyl groups in SSQ3 & SSQ4. The stability of emulsions containing modifiers that are not bound covalently to titanium dioxide such as SSQ1 and SSQ2 may be disturbed by the diffusion of the modifiers from the surface of TiO2 to the oil phase (Figure 13). SSQ1 and SSQ2 do not have in their structure the functional groups capable of forming covalent bonds. In turn, SSQ3 and SSQ4 are composed of a trimethoxysilyl group, which, during the deposition process, is hydrolyzed and condensed with hydroxyl groups located on the TiO2 surface [43].
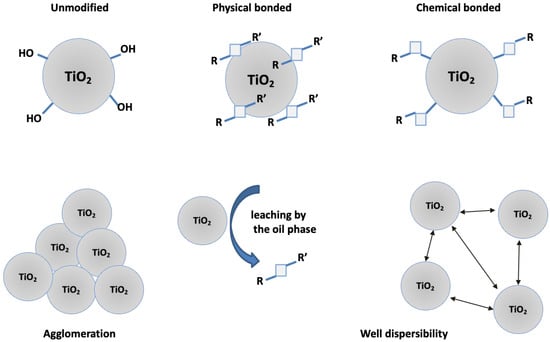
Figure 13.
Interaction of SSQs (R/R’) with titanium dioxide in the colloidal system.
4. Conclusions
In this study, hybrid systems consisting of inorganic UV filter (TiO2) and organosilicon derivatives (containing cinnamate or eugenol groups) with ultraviolet absorbing properties were obtained. The modification of titanium dioxide with organofunctional spherosilicates changed the nature of the materials from hydrophilic to hydrophobic. It was proved that the functionalization of titanium dioxide with spherosilicates significantly improved the dispersibility of novel materials in the colloidal system compared to nonmodified TiO2. Therefore, the stability of emulsions containing novel materials was also enhanced. The research showed that the key factor that had an influence on the stability of emulsions was to create a covalent bond between the modifier and the surface of titanium dioxide. The new type of hybrid inorganic–organic UV filter might be a promising agent that improves dispersibility in colloidal systems.
Author Contributions
Conceptualization: B.S., R.E.P. and A.O. Formal analysis, B.S., R.E.P. and A.O. Funding acquisition: B.S. and A.O. Investigation: K.N., K.K., J.L. and M.F. Methodology: B.S. and A.O. Project administration: B.S. and A.O., Resources Supervision: B.S. and A.O. Visualization: K.N., M.F., K.K., J.L. and A.O. Writing: A.O., B.S. and M.F. Writing—review and editing: A.O., B.S. and R.E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Centre for Research and Development (Narodowe Centrum Badań i Rozwoju) grant number LIDER/5/0036/L-12/20/NCBR/2021 and LIDER/1/0001/L-10/18/NCBR/2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data were collected and provided in the main manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Korotcenkov, G. Titanium Dioxide (TiO2) and Its Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Bordes, C.; Bolzinger, M.; El Achak, M.; Pirot, F.; Arquier, D.; Agusti, G.; Chevalier, Y. Formulation of Pickering emulsions for the development of surfactant-free sunscreen creams. Int. J. Cosmet. Sci. 2021, 43, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Glówczyk-Zubek, J. Cosmetic application of micro-fine titanium dioxide. J. Appl. Cosmetol. 2004, 22, 143–153. [Google Scholar]
- Nunes, S.M.; Josende, M.E.; González-Durruthy, M.; Ruas, C.P.; Gelesky, M.A.; Romano, L.A.; Fattorini, D.; Regoli, F.; Monserrat, J.M.; Ventura-Lima, J. Different crystalline forms of titanium dioxide nanomaterial (rutile and anatase) can influence the toxicity of copper in golden mussel Limnoperna fortunei? Aquatic. Toxicol. 2018, 205, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Paola, A.D.; Palmisano, L.; Selli, E. Effect of titanium dioxide crystalline structure on the pho-tocatalytic production of hydrogen. Photochem. Photobiol. Sci. 2011, 10, 355–360. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.; Popov, A. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding polymorphic phase transformation behavior during growth of nano-crystalline aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Trestsova, M.A.; Utepova, I.A.; Chupakhin, O.N.; Semenov, M.V.; Pevtsov, D.N.; Nikolenko, L.M.; Tovstun, S.A.; Gadomska, A.; Shchepochkin, A.; Kim, G.A. Oxidative CH/CH Coupling of Dipyrromethanes with Azines by TiO2-Based Photocatalytic System. Synthesis of New BODIPY Dyes and Their Photophysical and Electro-chemical Properties. Molecules 2021, 26, 5549. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of Poly(Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Xue, C.; Wu, J.; Lan, F.; Liu, W.; Yang, X.; Zeng, F.; Xu, H. Nano Titanium Dioxide Induces the Generation of ROS and Potential Damage in HaCaT Cells Under UVA Irradiation. J. Nanosci. Nanotechnol. 2010, 10, 8500–8507. [Google Scholar] [CrossRef]
- Morsella, M.; D’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Krysztafkiewicz, A.; Jesionowski, T. Adsorption of organic dyes on the aminosilane modi-fied TiO2 surface. Dye. Pigment. 2004, 62, 121–130. [Google Scholar] [CrossRef]
- Siddiquey, I.A.; Ukaji, E.; Furusawa, T.; Sato, M.; Suzuki, N. The effects of organic surface treatment by meth-acryloxypropyltrimethoxysilane on the photostability of TiO2. Mater. Chem. Phys. 2007, 105, 162–168. [Google Scholar] [CrossRef]
- Yao, C.; Gao, G.S.; Lin, X.P.; Yang, X.J.; Lu, L.D.; Wang, X. Surface modification of nanosized TiO2 with silane coupling reagent. J. Inorg. Mater. 2006, 21, 315–321. [Google Scholar]
- Zhou, H.; Sun, S.; Ding, H. Surface Organic Modification of TiO2 Powder and Relevant Characterization. Adv. Mater. Sci. Eng. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Brząkalski, D.; Przekop, R.E.; Frydrych, M.; Pakuła, D.; Dobrosielska, M.; Sztorch, B.; Marciniec, B. Where ppm Quantities of Silsesquioxanes Make a Difference—Silanes and Cage Siloxanes as TiO2 Dispersants and Stabi-lizers for Pigmented Epoxy Resins. Materials 2022, 15, 494. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Xu, J. Polymer-POSS hybrid materials as fire retardants. In Polyhedral Oligomeric Silsesquioxane (POSS) Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 305–332. [Google Scholar]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Czech-Polak, J.; Oliwa, R.; Lenża, J.; Szołyga, M. Thermal Stability and Flame Retardancy of Polypropylene Composites Containing Siloxane-Silsesquioxane Resins. Polymers 2018, 10, 1019. [Google Scholar] [CrossRef]
- Szołyga, M.; Dutkiewicz, M.; Nowicki, M.; Sałasińska, K.; Celiński, M.; Marciniec, B. Phosphorus-Containing Silsesquioxane Derivatives as Additive or Reactive Components of Epoxy Resins. Materials 2020, 13, 5373. [Google Scholar] [CrossRef]
- Sztorch, B.; Pakuła, D.; Kustosz, M.; Romanczuk-Ruszuk, E.; Gabriel, E.; Przekop, R.E. The Influence of Orga-nofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF). Polymers 2022, 14, 5493. [Google Scholar] [CrossRef]
- Mittal, K.L. Silanes and Other Coupling Agents; CRC Press: Boka Raton, FL, USA, 2007; Volume 4. [Google Scholar]
- Nowacka, M.; Ambrożewicz, D.; Jesionowski, T. TiO2-SiO2/Ph-POSS functional hybrids: Preparation and characterisation. J. Nanomater. 2013, 2013, 19. [Google Scholar] [CrossRef]
- Szwarc, K.; Siwinska-Stefanska, K.; Marciniec, B.; Jesionowski, T. Synthesis and characterisation of SiO2/POSS hybrid systems obtained using the mechanical method. Physicochem. Probl. Miner. Process. 2012, 48, 181–192. [Google Scholar]
- Filho, N.L.D.; De Aquino, H.A.; Pires, G.; Caetano, L. Relationship between the dielectric and mechanical properties and the ratio of epoxy resin to hardener of the hybrid thermosetting polymers. J. Braz. Chem. Soc. 2006, 17, 533–541. [Google Scholar] [CrossRef]
- Madhurambal, G.; Ravindran, B.; Mariappan, M.; Mojumdar, S. Thermal, UV and FTIR spectral studies in al-kali metal cinnamates. J. Therm. Anal Calorim. 2010, 100, 811–815. [Google Scholar] [CrossRef]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Ross, S.A.; Salkini, M.A.; Alam, P. Simultaneous estimation of cin-namaldehyde and eugenol in essential oils and traditional and ultrasound-assisted extracts of different spe-cies of cinnamon using a sustainable/green HPTLC technique. Molecules 2021, 26, 2054. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eu-genol-containing essential oils loaded onto chitosan/polyvinyl alcohol blended films and their ability to eradicate Staphylococcus aureus or Pseudomonas aeruginosa from infected microenvironments. Pharmaceutics 2012, 13, 195. [Google Scholar] [CrossRef]
- Joshi, M.; Bhattacharyya, A. Nanotechnology—A new route to high-performance functional textiles. Text. Prog. 2011, 43, 155–233. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Wang, A.; Wu, L.; Wang, Q. Facile synthesis and photocatalytic activity of a novel titanium dioxide nanocomposite coupled with zinc porphyrin. Nanomater. Nanotechnol. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Song, J.; Qin, J.; Qu, J.; Song, Z.; Zhang, W.; Xue, X.; Shi, Y.; Zhang, T.; Ji, W.; Zhang, R.; et al. The effects of particle size distribution on the optical properties of titanium dioxide rutile pigments and their applications in cool non-white coatings. Sol. Energy Mater. Sol. Cells 2014, 130, 42–50. [Google Scholar] [CrossRef]
- Ridley, M.K.; Hackley, V.A.; Machesky, M.L. Characterization and surface-reactivity of nanocrystalline ana-tase in aqueous solutions. Langmuir 2006, 22, 10972–10982. [Google Scholar] [CrossRef]
- Halimi, S.U.; Abu Bakar, N.F.; Ismail, S.N.; Hashib, S.A.; Naim, M.N. Electrospray deposition of titanium dioxide (TiO2) nanoparticles. In Proceedings of the AIP Conference Proceedings, Surabaya, Indonesia, 23–25 October 2014; Volume 1586, pp. 57–62. [Google Scholar] [CrossRef]
- Olejnik, A.; Panek, R.; Madej, J.; Franus, W.; Goscianska, J. Low-cost zeolitic carriers for delivery of hy-droxychloroquine immunomodulatory agent with antiviral activity. Microporous Mesoporous Mater. 2022, 346, 112315. [Google Scholar] [CrossRef]
- Olejnik, A.; Glowka, A.; Nowak, I. Release studies of undecylenoyl phenylalanine from topical formulations. Saudi Pharm. J. 2018, 26, 709–718. [Google Scholar] [CrossRef]
- Santos, J.; Trujillo, L.A.; Calero, N.; Alfaro, M.C.; Munoz, J. Physical Characterization of a Commercial Suspo-emulsion as a Reference for the Development of Suspoemulsions. Chem. Eng. Technol. 2013, 36, 1883–1890. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayré, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Olejnik, A.; Kapuscinska, A.; Schroeder, G.; Nowak, I. Physico-chemical characterization of formulations containing endomorphin-2 derivatives. Amino Acids 2017, 49, 1719–1731. [Google Scholar] [CrossRef]
- Olejnik, A.; Schroeder, G.; Nowak, I. The tetrapeptide N-acetyl-Pro-Pro-Tyr-Leu in skin care formulations—Physicochemical and release studies. Int. J. Pharm. 2015, 492, 161–168. [Google Scholar] [CrossRef]
- Zalewska, A.; Kowalik, J.; Grubecki, I. Application of turbiscan lab to study the effect of emulsifier content on the stability of plant origin dispersion. Chem. Process Eng. 2019, 40, 399–409. [Google Scholar]
- Santos, J.; Calero, N.; Trujillo-Cayado, L.; Garcia, M.; Muñoz, J. Assessing differences between Ostwald ripening and coalescence by rheology, laser diffraction and multiple light scattering. Colloids Surf. B Biointerfaces 2017, 159, 405–411. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hossain, S.M.; Kang, H.-J.; Park, H.; Tijing, L.; Park, G.W.; Suzuki, N.; Fujishima, A.; Jun, Y.-S.; Shon, H.K.; et al. Hydrophilic/Hydrophobic Silane Grafting on TiO2 Nanoparticles: Photocatalytic Paint for Atmospheric Cleaning. Catalysts 2021, 11, 193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).