Synthesis and Crystal Structure of the Zintl Phases NaSrSb, NaBaSb and NaEuSb
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villars, P.; Calvert, L.D. (Eds.) Pearson’s Handbook of Crystallographic Data for Intermetallic Compounds, 2nd ed.; American Society for Metals: Materials Park, OH, USA, 1991. [Google Scholar]
- El Maslout, A.; Motte, J.P.; Gleitzer, C.; Aubry, J. Preparation et proprietes d’un nouveau compose dans la serie LiMP: Le phosphure de lithium-cadmium LiCdP. Comptes Rendus Seances L’academie Sci. Ser. C Sci. Chim. 1971, 273, 707–710. [Google Scholar]
- Tiburtius, C.; Schuster, H.U. LiBeSb und LiZnBi, ternaere Verbindungen mit Wurtzitgeruest. Z. Naturforsch. 1978, 33b, 35–38. [Google Scholar] [CrossRef]
- Krenkel, B.; Schuster, H.U. NaMgAs(Sb)—Ternaere Verbindungen mit modifizierter Cu2Sb-Struktur. Z. Naturforsch. 1978, 33b, 1080–1082. [Google Scholar]
- Hirt, H.; Deiseroth, H.J. Crystal structure of potassium calcium bismuthide, KCaBi. Z. Kristallogr. 2003, 218, 5. [Google Scholar]
- Tiburtius, C.; Schuster, H.U. NaBeAs(Sb)—Ternaere Phasen im ‘aufgefuellten’ NiAs (Ni2In)-Typ. Z. Naturforsch. 1978, 32b, 1133–1138. [Google Scholar]
- Dong, Y.K.; DiSalvo, F.J. Synthesis and single crystal structures of ternary phosphides Li4SrP2 and AAeP (A = Li, Na; Ae = Sr, Ba). J. Solid State Chem. 2007, 180, 432–439. [Google Scholar]
- Eisenmann, B.; Liebrich, O.; Schäfer, H.; Weiss, A. Darstellung und Kristallstruktur von CaLiSb (Ternaere E-Phasen von Hauptgruppenelementen II). Z. Naturforsch. 1969, 24b, 1344–1345. [Google Scholar] [CrossRef]
- Feng, X.J.; Prots, Y.; Schmidt, M.P.; Hoffmann, S.; Schnelle, W.; Burkhardt, U.; Zhao, J.-T.; Grin, Y. Synthesis, structure, and properties of two Zintl phases around the composition SrLiAs. Inorg. Chem. 2013, 52, 8971–8978. [Google Scholar] [CrossRef]
- Albering, J.H.; Ebel, T.; Jeitschko, W. Praeparation, Kristallstruktur und magnetische Eigenschaften der Verbindungen LiAX (A = Ca, Sr, Ba, Eu, Yb; X = P, As, Sb, Bi). Z. Kristallogr. Suppl. Issue 1997, 12, 242. [Google Scholar]
- Carrillo Cabrera, W.; Somer, M.; Peters, E.M.; Peters, K.; von Schnering, H.-G. Crystal structure of sodium strontium arsenide, NaSrAs. Z. Kristallogr. 1997, 212, 252. [Google Scholar] [CrossRef]
- Carrillo Cabrera, W.; Somer, M.; Peters, E.M.; Peters, K.; von Schnering, H.-G. Crystal structure of sodium barium phosphide, NaBaP. Z. Kristallogr. 1997, 212, 191. [Google Scholar] [CrossRef]
- Hirt, H.; Deiseroth, H.J. The new polar intermetallic compound NaBaBi. Z. Anorg. Allg. Chem. 2004, 630, 1357–1359. [Google Scholar] [CrossRef]
- Ovchinnikov, A.; Bobev, S. Zintl phases with group 15 elements and the transition metals: A brief overview of pnictides with diverse and complex structures. J. Solid State Chem. 2019, 270, 346. [Google Scholar]
- Baranets, S.; Ovchinnikov, A.; Bobev, S. Chapter 322: Structural diversity of the Zintl pnictides with rare-earth metals. In Handbook of Chemistry and Physics of the Rare Earths; Elsevier: Amsterdam, The Netherlands, 2021; Volume 60, pp. 227–324. [Google Scholar]
- Schäfer, M.C.; Suen, N.-T.; Bobev, S. Synthesis and crystal chemistry of new ternary pnictides containing lithium—Adding structural complexity one step at a time. Dalton Trans. 2014, 43, 1688. [Google Scholar]
- Makongo, J.P.A.; You, T.-S.; He, H.; Suen, N.-T.; Bobev, S. New lithium-containing pnictides with 1-D infinite chains of super-tetrahedral clusters. Synthesis, crystal and electronic structure of Ba4Li2Cd3Pn6 (Pn = P, As and Sb). Eur. J. Inorg. Chem. 2014, 2014, 5113. [Google Scholar] [CrossRef]
- Ojwang, D.O.; Bobev, S. Synthesis and structural characterization of Ba7Li11Bi10 and AE4(Li,Tr)7Pn6 (AE = Sr, Ba, Eu; Tr = Ga, In; Pn = Sb, Bi). Inorganics 2018, 6, 109. [Google Scholar]
- Wang, Y.; Suen, N.-T.; Kunene, T.; Stoyko, S.; Bobev, S. Synthesis and structural characterization of the Zintl phases Na3Ca3TrPn4, Na3Sr3TrPn4, and Na3Eu3TrPn4 (Tr = Al, Ga, In; Pn = P, As, Sb). J. Solid State Chem. 2017, 249, 160. [Google Scholar] [CrossRef]
- Wang, Y.; Stoyko, S.; Bobev, S. Quaternary pnictides with complex, non-centrosymmetric structures. Synthesis and structural characterization of the new Zintl phases Na11Ca2Al3Sb8, Na4CaGaSb3 and Na15Ca3In5Sb12. Inorg. Chem. 2015, 54, 1931. [Google Scholar]
- Saparov, B.; Bobev, S. Synthesis and crystal structure of the Zintl phases Na2CaCdSb2, Na2SrCdSb2 and Na2EuCdSb2. Inorganics 2022, 10, 265. [Google Scholar]
- Nesper, R. The Zintl-Klemm concept—A historical survey. Z. Anorg. Allg. Chem. 2014, 640, 2639–2648. [Google Scholar]
- Hoffmann, R.-D.; Pöttgen, R. AlB2-related intermetallic compounds—A comprehensive view based on group-subgroup relations. Z. Kristallogr. 2001, 216, 127–145. [Google Scholar]
- Oliynyk, A.O.; Adutwum, L.A.; Rudyk, B.W.; Pisavadia, H.; Lofti, S.; Hlukhyy, V.; Harynuk, J.J.; Mar, A.; Brgoch, J. Disentangling structural confusion through machine learning: Structure prediction and polymorphism of equiatomic ternary phases ABC. J. Am. Chem. Soc. 2017, 139, 17870–17881. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Effective ionic radii in oxides and fluorides. Acta Crystallogr. 1969, B25, 925–946. [Google Scholar]
- Xiong, J.; Kushwaha, S.K.; Liang, T.; Krizan, J.W.; Hirschberger, M.; Wang, W.; Cava, R.J.; Ong, N.P. Evidence for the chiral anomaly in the Dirac semimetal Na3Bi. Science 2015, 350, 413–416. [Google Scholar] [CrossRef]
- Narayan, A.; DiSante, D.; Picozzi, S.; Sanvito, S. Topological tuning in three-dimensional Dirac semimetals. Phys. Rev. Lett. 2014, 113, 256403. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, S.; Guo, S.; Qu, H.; Cai, B.; Chen, Z.; Zeng, H. High-performance monolayer Na3Sb shrinking transistors: A DFT-NEGF study. Nanoscale 2020, 12, 18931. [Google Scholar]
- Andersen, O.K. Linear Methods in Band Theory. Phys. Rev. B 1975, 12, 3060–3083. [Google Scholar]
- Andersen, O.K.; Jepsen, O. Explicit, First-Principles Tight-Binding Theory. Phys. Rev. Lett. 1984, 53, 2571. [Google Scholar] [CrossRef]
- Steinberg, S.; Dronskowski, R. The crystal orbital Hamilton population (COHP) method as a tool to visualize and analyze chemical bonding in intermetallic compounds. Crystals 2018, 8, 225. [Google Scholar]
- Sun, Y.; Wang, Q.-Z.; Wu, S.-C.; Felser, C.; Liu, C.-X.; Yan, B. Pressure-induced topological insulator in NaBaBi with right-handed surface spin texture. Phys. Rev. B 2016, 93, 205303. [Google Scholar] [CrossRef]
- Toberer, E.S.; May, A.F.; Snyder, G.J. Zintl chemistry for designing high efficiency thermoelectric materials. Chem. Mater. 2010, 22, 624. [Google Scholar]
- Kauzlarich, S.M.; Brown, S.R.; Snyder, G.J. Zintl Phases for thermoelectric devices. Dalton Trans. 2007, 21, 2099. [Google Scholar]
- Liu, Z.K.; Zhou, B.; Zhang, Y.; Wang, Z.J.; Weng, H.M.; Prabhakaran, D.; Mo, S.-K.; Shen, Z.X.; Fang, Z.; Dai, X.; et al. Discovery of a Three-Dimensional Topological Dirac Semimetal, Na3Bi. Science 2014, 343, 864–867. [Google Scholar] [PubMed]
- Ogunbunmi, M.O.; Baranets, S.; Childs, A.B.; Bobev, S. The Zintl phases AIn2As2 (A = Ca, Sr, Ba): New topological insulators and thermoelectric material candidates. Dalton Trans. 2021, 50, 9173–9184. [Google Scholar] [PubMed]
- Guo, W.-T.; Huang, Z.; Zhang, J.-M. The Zintl phase compounds AEIn2As2 (AE = Ca, Sr, Ba): Topological phase transition under pressure. Phys. Chem. Chem. Phys. 2022, 24, 17337–17347. [Google Scholar] [CrossRef] [PubMed]
- Schindler, F.; Cook, A.M.; Vergniory, M.G.; Wang, Z.; Parkin, S.S.P.; Bernevig, B.A.; Neupert, T. Higher-order topological insulators. Sci. Adv. 2018, 4, eaat0346. [Google Scholar] [PubMed]
- Baranets, S.; He, H.; Bobev, S. Niobium-bearing arsenides and germanides from elemental mixtures not involving niobium: A new twist to an old problem in solid-state synthesis. Acta Crystallogr. C 2018, 74, 623. [Google Scholar]
- He, H.; Tyson, C.; Bobev, S. Synthesis and crystal structures of the quaternary Zintl phases RbNa8Ga3Pn6 (Pn = P, As) and Na10NbGaAs6. Crystals 2012, 2, 213–223. [Google Scholar] [CrossRef]
- SMART, version 2.10; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2003.
- SAINT, version 6.45; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2003.
- SADABS, version 2.10; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2003.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Tank, R.; Jepsen, O.; Burkhardt, A.; Andersen, O. TB-LMTO-ASA Program; Max-Planck-Institut für Festkörperforschung: Stuttgart, Germany, 1994. [Google Scholar]
- von Barth, U.; Hedin, L. A local exchange-correlation potential for the spin polarized case. J. Phys. C Solid State Phys. 1972, 5, 1629. [Google Scholar]
- Yamada, T.; Matsuo, N.; Enoki, M.; Yamane, H. A novel ternary bismuthide, NaMgBi: Crystal amd electronic structure and electrical properties. Z. Naturforsch. 2021, 76b, 789–795. [Google Scholar]
- Vogel, R.; Schuster, H.U. Neue elektrovalente ternare Verbindungen des Kaliums mit Magnesium unde Elementen der 5 Hauptgruppe. Z. Naturforsch. 1979, 34b, 1719–1721. [Google Scholar] [CrossRef]
- Cardoso, G.; Caroca-Canalesr, N.; Hönle, W.; von Schnering, H.-G. Crystal structure of rubidium calcium arsenide, RbCaAs, and rubidium calcium antimonide, RbCaSb. Z. Kristallogr. NCS 2003, 218, 455–456. [Google Scholar] [CrossRef]
- Nowotny, H.M.; Holub, F. Untersuchungen an metalischen Systemen mit Flussspatphasen. Monatsh. Chem. 1960, 91, 877–887. [Google Scholar]
- Monconduit, L.; Belin, C. A new ternary antimonide phase, LiBaSb. Acta Cryst. E 2001, 57, 17–18. [Google Scholar]
- Gupta, S.; Ganguli, A.K. Synthesis, structure and properties of a new Zintl phase: SrLiSb. J. Soild State Chem. 2006, 179, 1318–1322. [Google Scholar] [CrossRef]
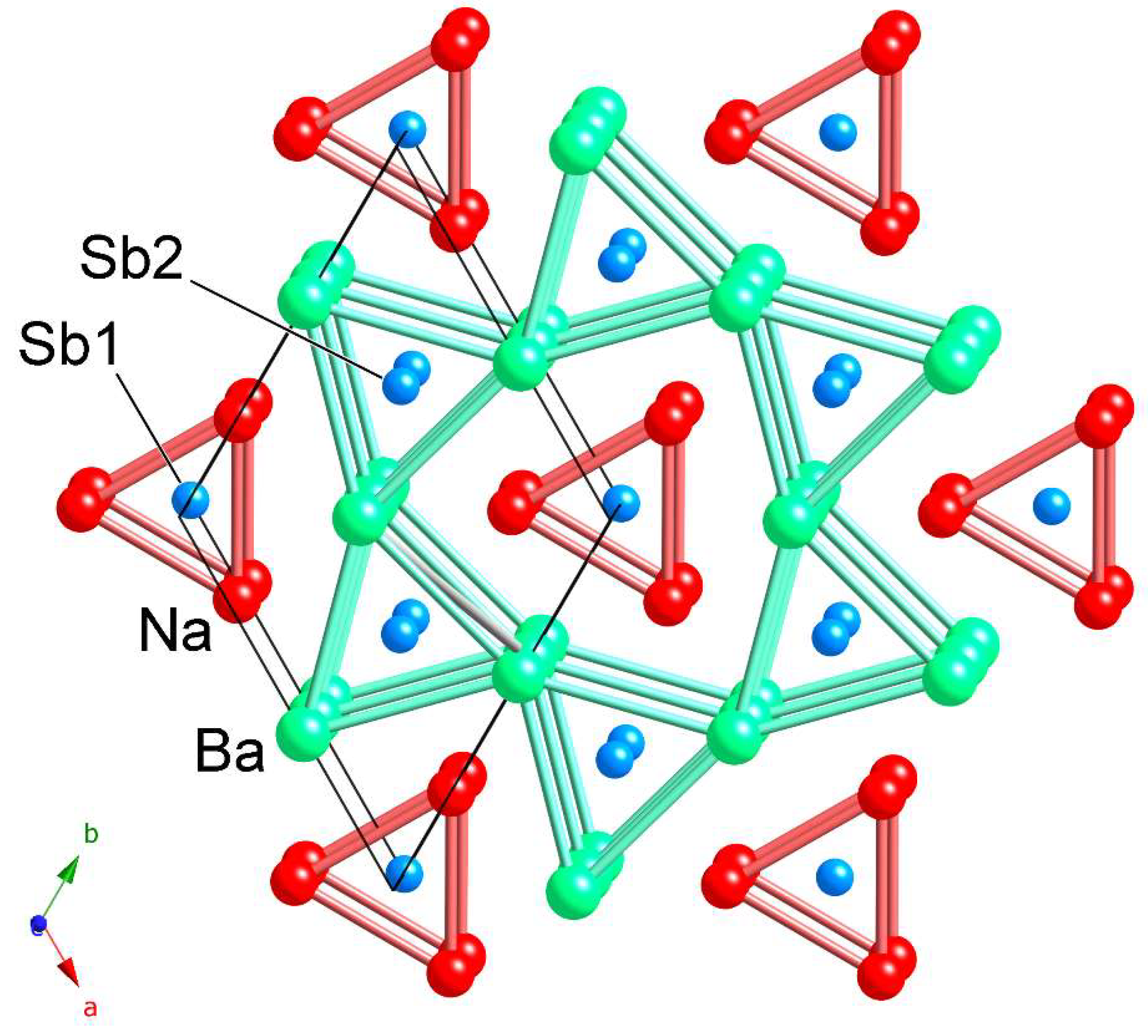
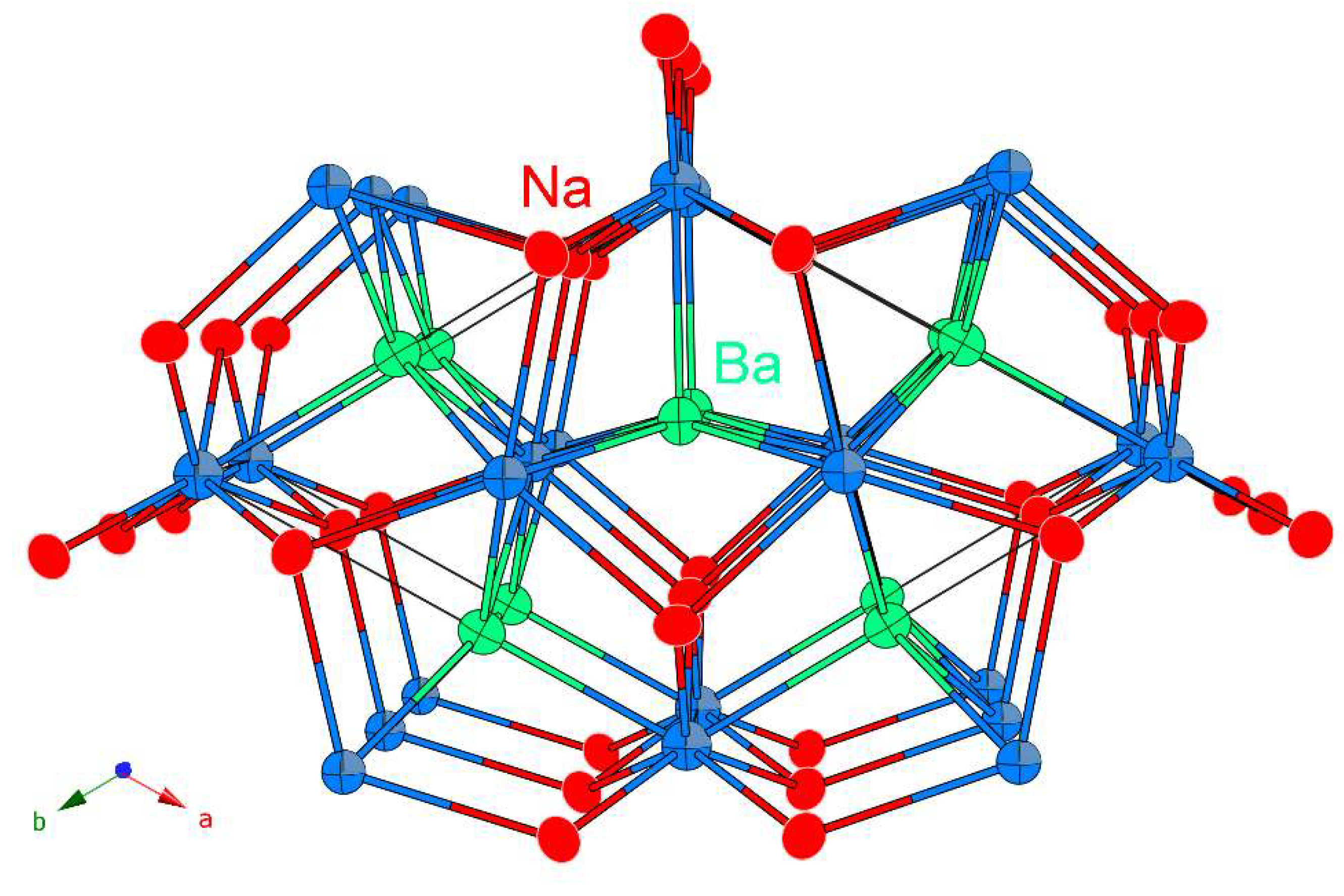
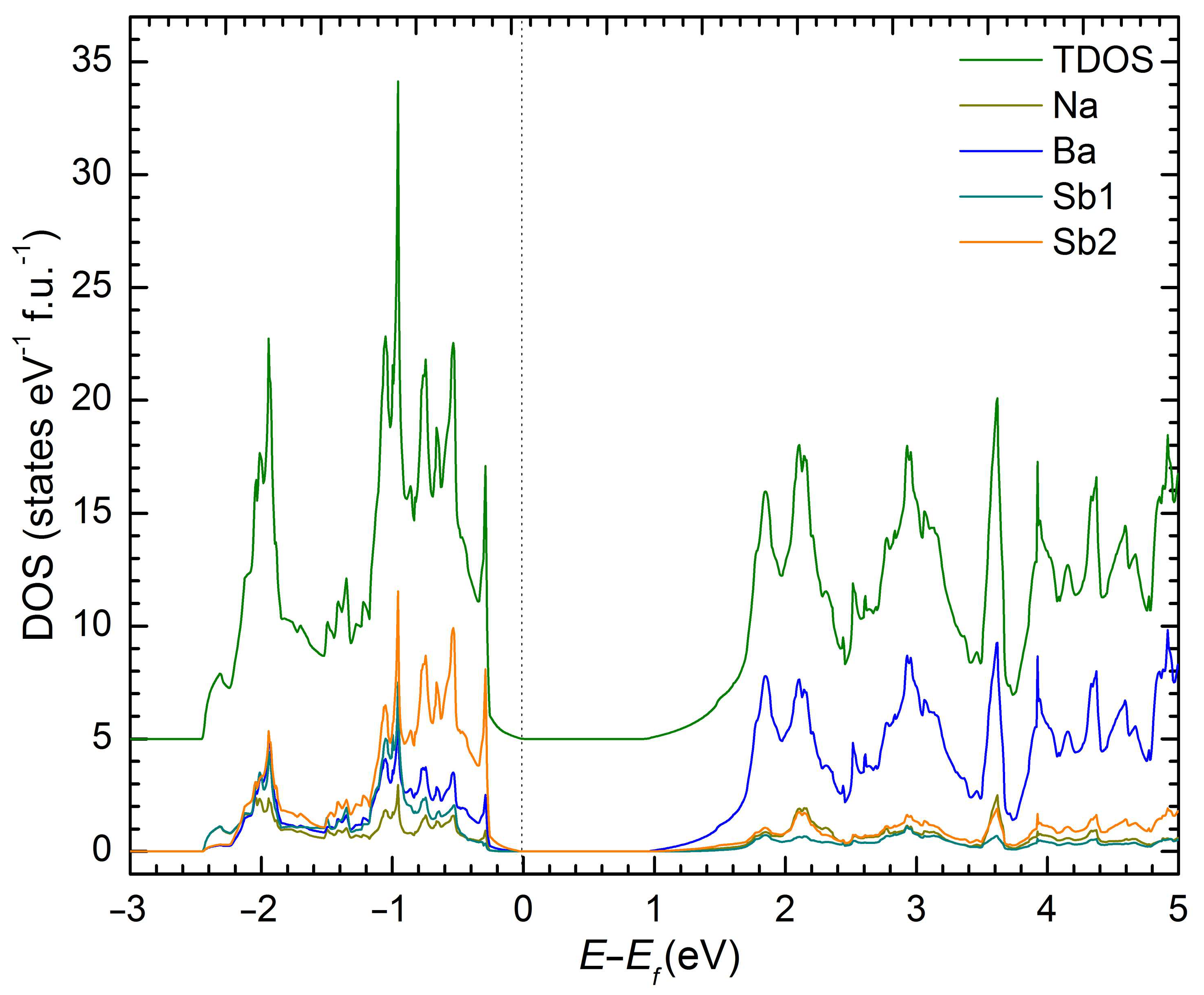

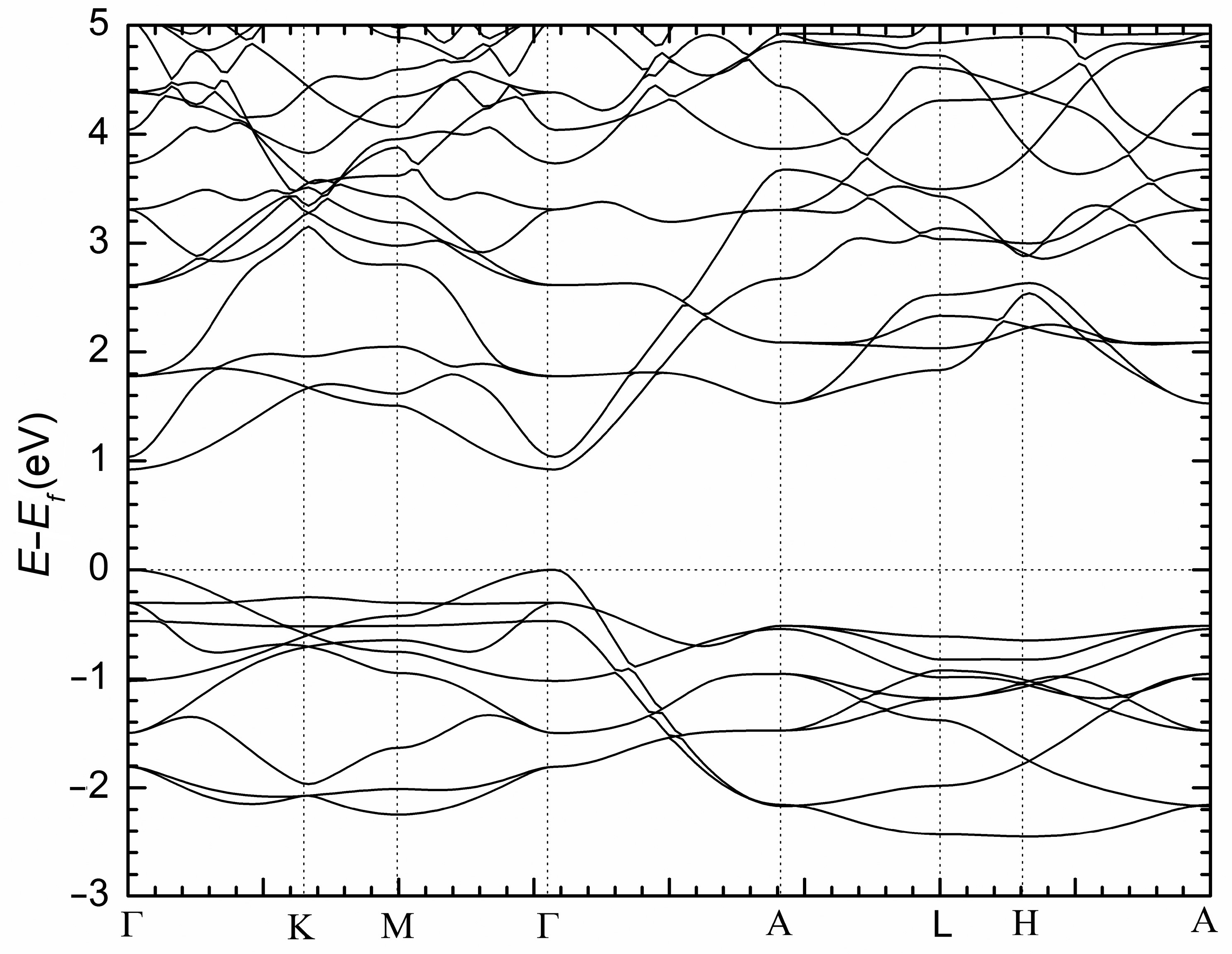
| Empirical Formula | NaSrSb | NaBaSb | NaEuSb |
|---|---|---|---|
| Formula weight | 232.36 | 282.08 | 296.70 |
| Temperature (K) | 200(2) | 200(2) | 200(2) |
| Space group, Z | P2m, 3 | P2m, 3 | P2m, 3 |
| a (Å) | 8.2183(3) | 8.4779(4) | 8.1514(4) |
| c (Å) | 4.8475(4) | 5.0338(5) | 4.8154(5) |
| V (Å3) | 283.54(3) | 313.33(4) | 277.09(3) |
| c/a | 0.590 | 0.594 | 0.591 |
| ρcal (g/cm3) | 4.08 | 4.49 | 5.33 |
| μ (cm–1) | 210.9 | 156.9 | 239.7 |
| Goodness-of-fit on F2 | 1.11 | 1.12 | 1.02 |
| Unique reflections | 329 | 383 | 355 |
| Refined parameters | 15 | 15 | 15 |
| R1 (I > 2σI) a | 0.0153 | 0.0173 | 0.0187 |
| wR2 (I > 2σI) a | 0.0330 | 0.0368 | 0.0361 |
| R1 (all data) a | 0.0157 | 0.0179 | 0.0197 |
| wR2 (all data) a | 0.0331 | 0.0369 | 0.0364 |
| Largest peak and hole difference (e–/Å3) | 0.84 and –0.85 | 0.61 and –0.89 | 0.88 and –0.82 |
| CCDC deposition no. | 2237818 | 2237817 | 2237816 |
| Atom | Site | x | y | z | Ueq (Å2) |
|---|---|---|---|---|---|
| NaSrSb | |||||
| Na | 3g | 0.2424(3) | 0 | 1/2 | 0.0132(5) |
| Sr | 3f | 0.58090(8) | 0 | 0 | 0.0146(2) |
| Sb2 | 2d | 1/3 | 2/3 | 1/2 | 0.0119(1) |
| Sb1 | 1a | 0 | 0 | 0 | 0.0151(2) |
| NaBaSb | |||||
| Na | 3g | 0.2400(4) | 0 | 1/2 | 0.0173(8) |
| Ba | 3f | 0.58118(6) | 0 | 0 | 0.0122(1) |
| Sb2 | 2d | 1/3 | 2/3 | 1/2 | 0.0100(2) |
| Sb1 | 1a | 0 | 0 | 0 | 0.0114(2) |
| NaEuSb | |||||
| Na | 3g | 0.2416(5) | 0 | 1/2 | 0.0171(9) |
| Eu | 3f | 0.58180(7) | 0 | 0 | 0.0142(1) |
| Sb2 | 2d | 1/3 | 2/3 | 1/2 | 0.0125(2) |
| Sb1 | 1a | 0 | 0 | 0 | 0.0132(2) |
| Atom Pair | Distance (Å) | Atom Pair | Distance (Å) | Atom Pair | Distance (Å) |
|---|---|---|---|---|---|
| NaSrSb | NaBaSb | NaEuSb | |||
| Na–Sb1 (×2) | 3.138(2) | Na–Sb1 (×2) | 3.237(2) | Na–Sb1 (×2) | 3.111(3) |
| Na–Sb2 (×2) | 3.180(2) | Na–Sb2 (×2) | 3.293(2) | Na–Sb2 (×2) | 3.158(3) |
| Na–Sr (×2) | 3.690(2) | Na–Ba (×2) | 3.834(3) | Na–Eu (×2) | 3.672(3) |
| Na–Sr (×4) | 3.8529(5) | Na–Ba (×4) | 3.9822(5) | Na–Eu (×4) | 3.8187(5) |
| Na–Na (×2) | 3.451(4) | Na–Na (×2) | 3.525(6) | Na–Na (×2) | 3.412(7) |
| Sr–Sb1 | 3.4443(6) | Ba–Sb1 | 3.5508(6) | Eu–Sb1 | 3.4089(6) |
| Sr–Sb2 (×4) | 3.4562(2) | Ba–Sb2 (×4) | 3.5774(2) | Eu–Sb2 (×4) | 3.4320(2) |
| Sr–Na (×2) | 3.690(2) | Ba–Na (×2) | 3.834(3) | Eu–Na (×2) | 3.672(3) |
| Sr–Na (×4) | 3.8529(5) | Ba–Na (×4) | 3.9822(5) | Eu–Na (×4) | 3.8187(5) |
| Sb1–Na (×6) | 3.138(2) | Sb1–Na (×6) | 3.237(2) | Sb1–Na (×6) | 3.111(3) |
| Sb1–Sr (×3) | 3.4443(6) | Sb1–Ba (×3) | 3.5508(6) | Sb1–Eu (×3) | 3.4089(6) |
| Sb2–Na (×3) | 3.180(2) | Sb2–Na (×3) | 3.293(2) | Sb2–Na (×3) | 3.158(3) |
| Sb2–Sr (×6) | 3.4562(2) | Sb2–Ba (×6) | 3.5774(2) | Sb2–Eu (×6) | 3.4320(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Bobev, S. Synthesis and Crystal Structure of the Zintl Phases NaSrSb, NaBaSb and NaEuSb. Materials 2023, 16, 1428. https://doi.org/10.3390/ma16041428
Wang Y, Bobev S. Synthesis and Crystal Structure of the Zintl Phases NaSrSb, NaBaSb and NaEuSb. Materials. 2023; 16(4):1428. https://doi.org/10.3390/ma16041428
Chicago/Turabian StyleWang, Yi, and Svilen Bobev. 2023. "Synthesis and Crystal Structure of the Zintl Phases NaSrSb, NaBaSb and NaEuSb" Materials 16, no. 4: 1428. https://doi.org/10.3390/ma16041428
APA StyleWang, Y., & Bobev, S. (2023). Synthesis and Crystal Structure of the Zintl Phases NaSrSb, NaBaSb and NaEuSb. Materials, 16(4), 1428. https://doi.org/10.3390/ma16041428






