Abnormal Eu3+ → Eu2+ Reduction in Ca9−xMnxEu(PO4)7 Phosphors: Structure and Luminescent Properties
Abstract
:1. Introduction
- (1)
- A broad excitation band which can be matched well with the light from the LED chip (usually, at 450–470 nm from the InGaN chip). A number of Eu3+-doped inorganic red phosphors have been developed with the narrow emission due to the electric dipole 5D0 → 7F2 transition located at 610–630 nm. This transition is dominant in most hosts due to the noncentrosymmetric environment. However, such luminescent materials mismatch the excitation wavelengths from the LED chip since the main excitation band of the Eu3+ ion is located obviously at 392–396 nm and has a narrow character;
- (2)
- (3)
2. Materials and Methods
3. Results
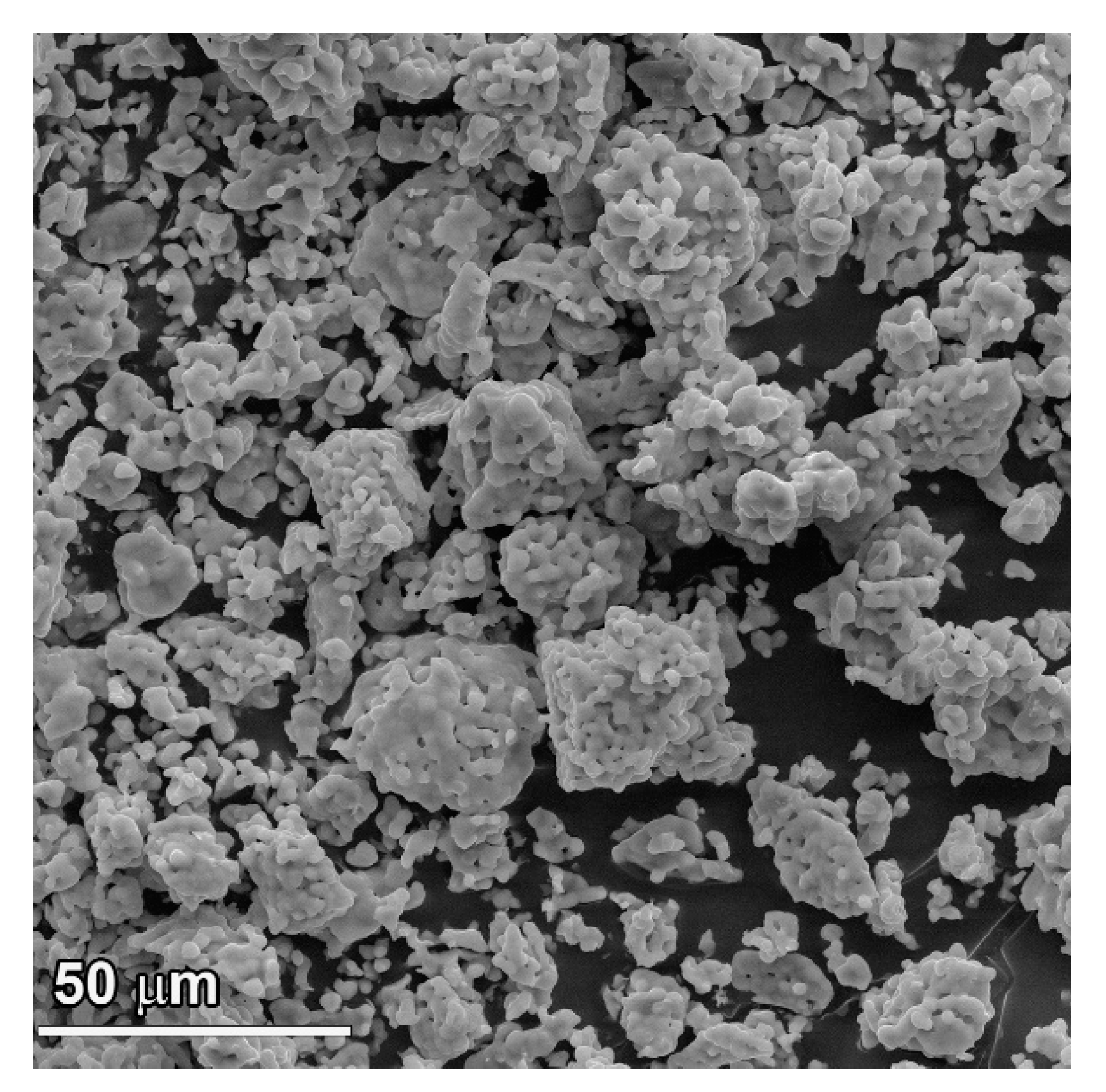
3.1. SEM Observations
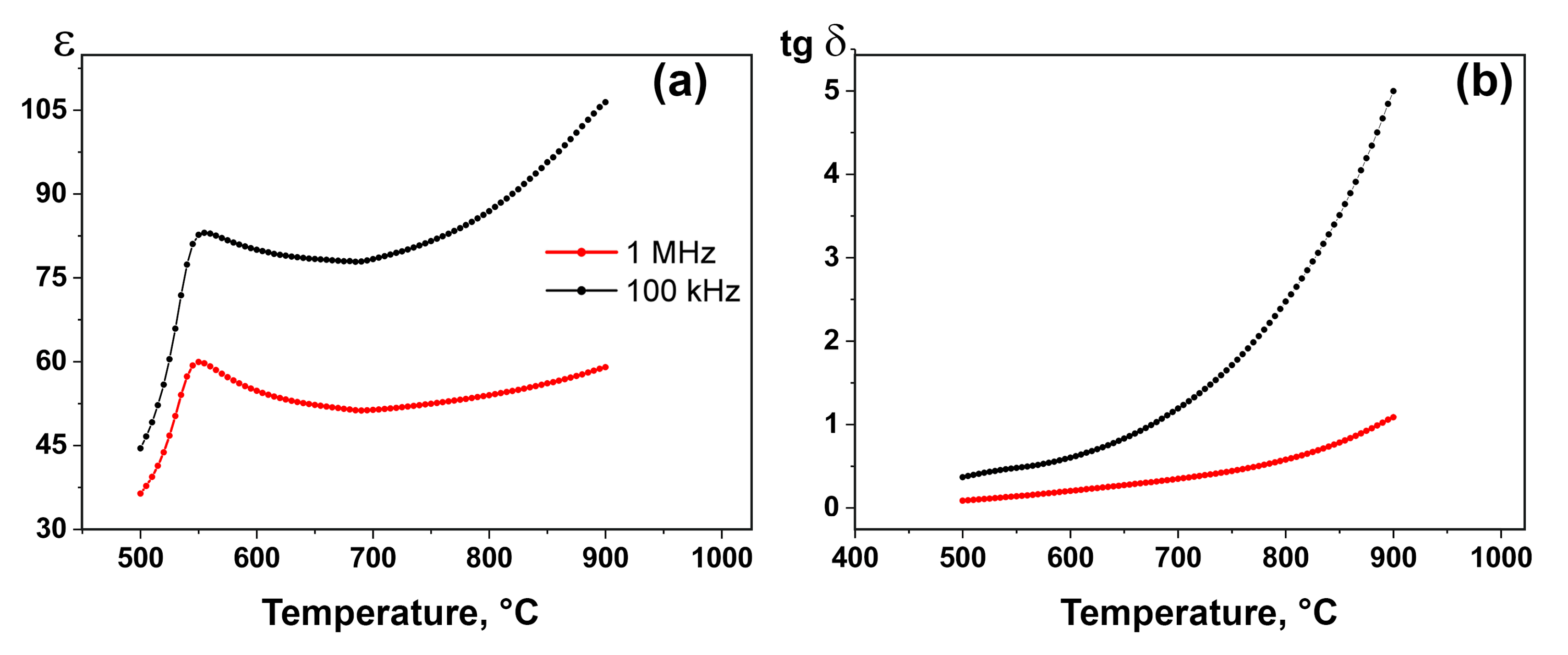
3.2. SHG, DSC and Dielectric Spectroscopy Measurements
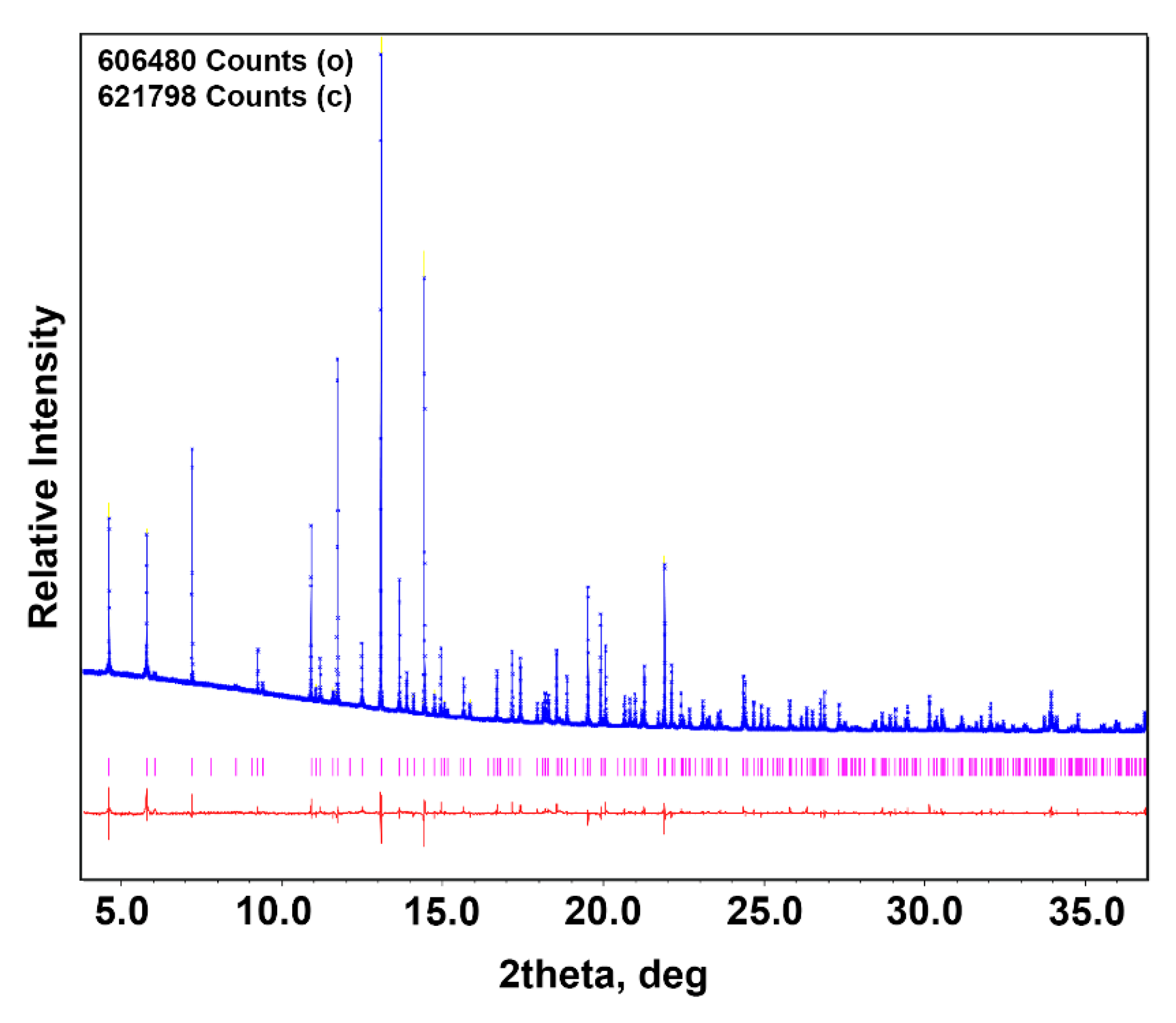
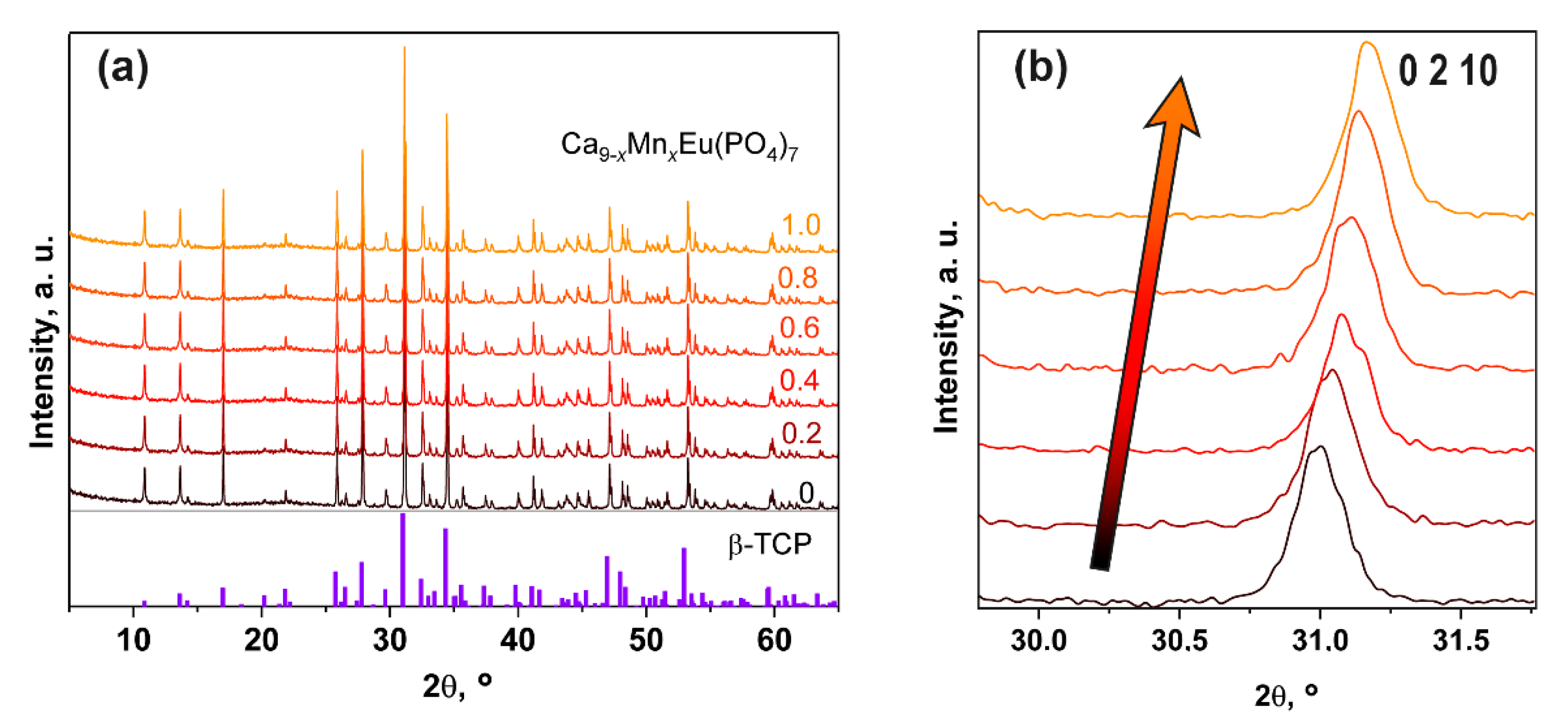
3.3. PXRD Study
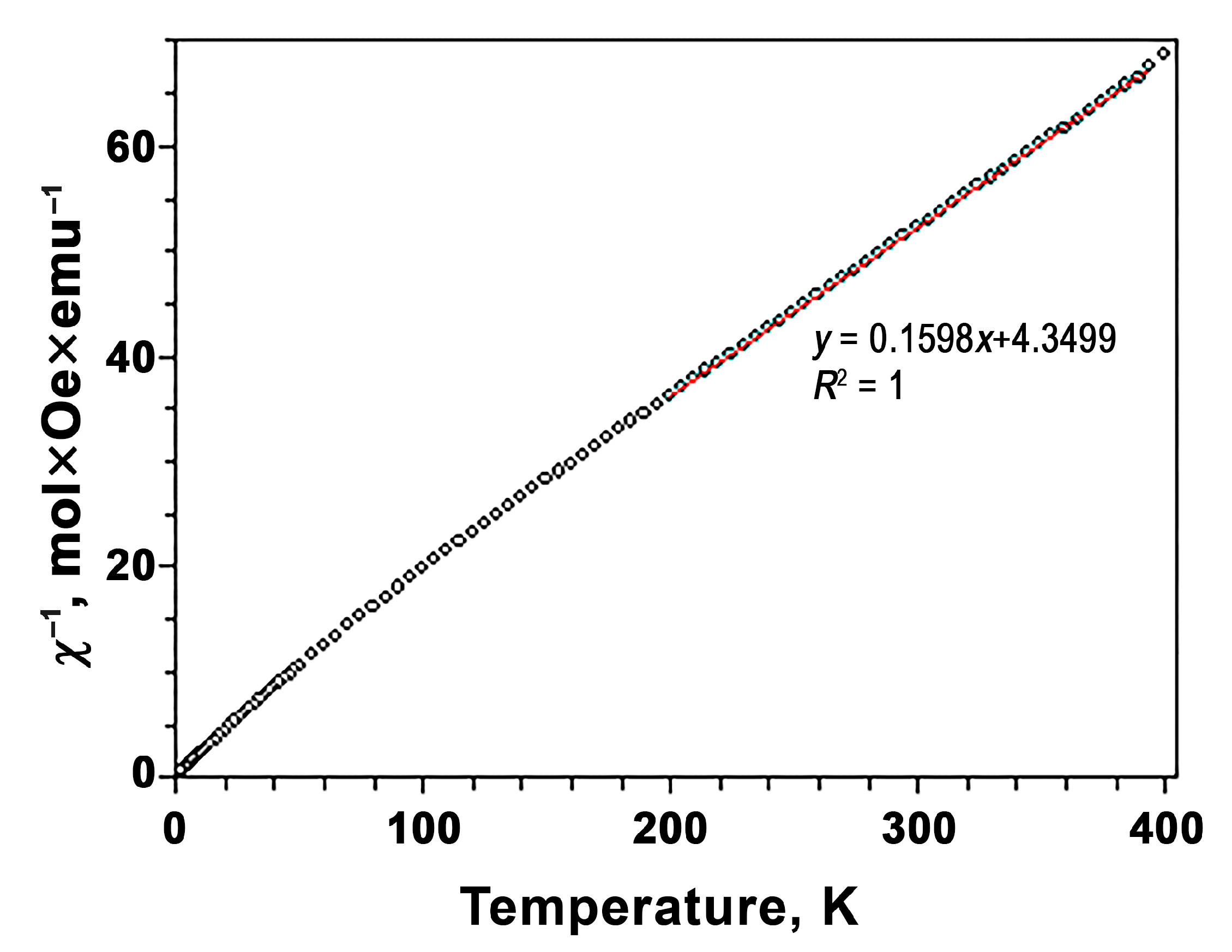
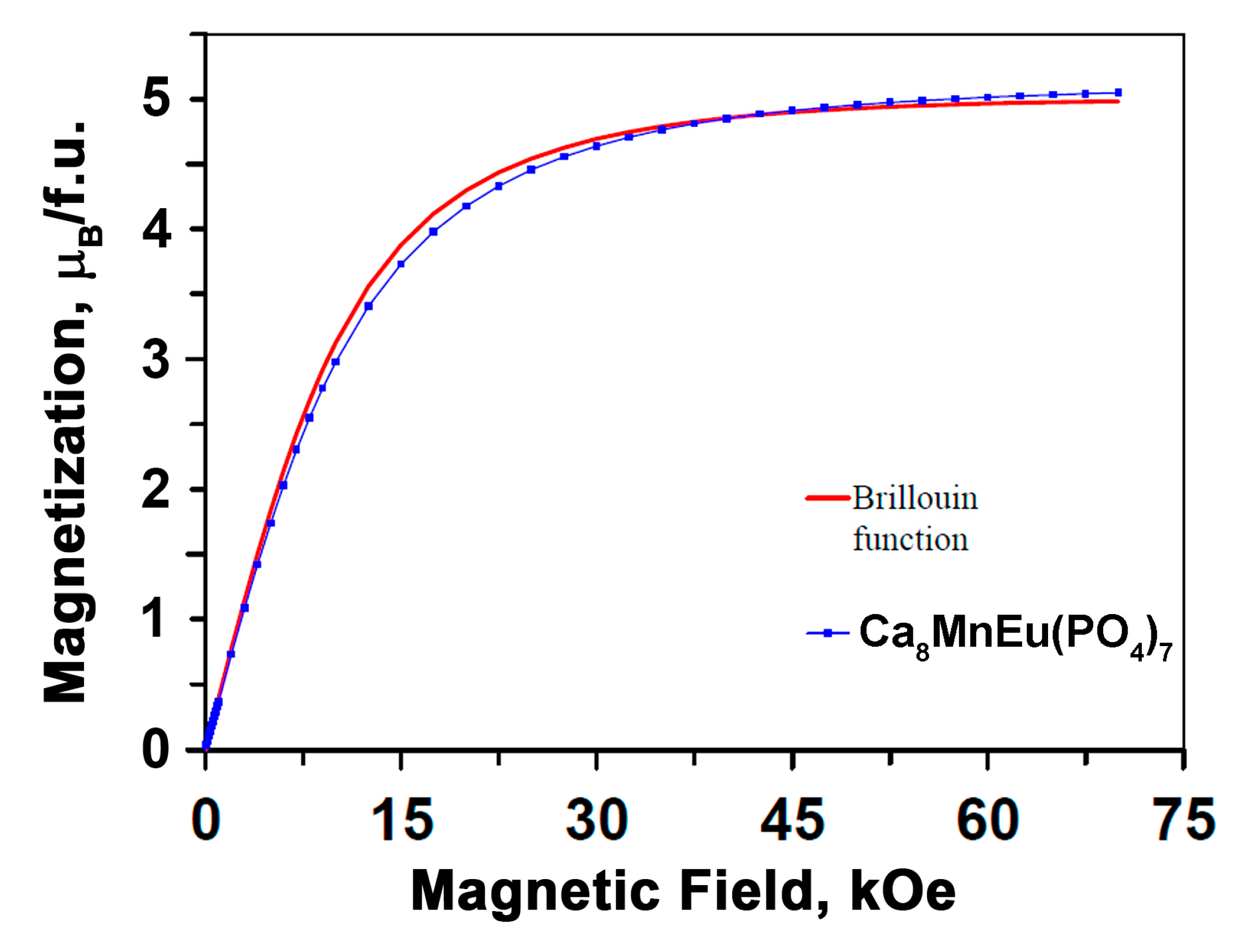
3.4. Magnetic Measurements
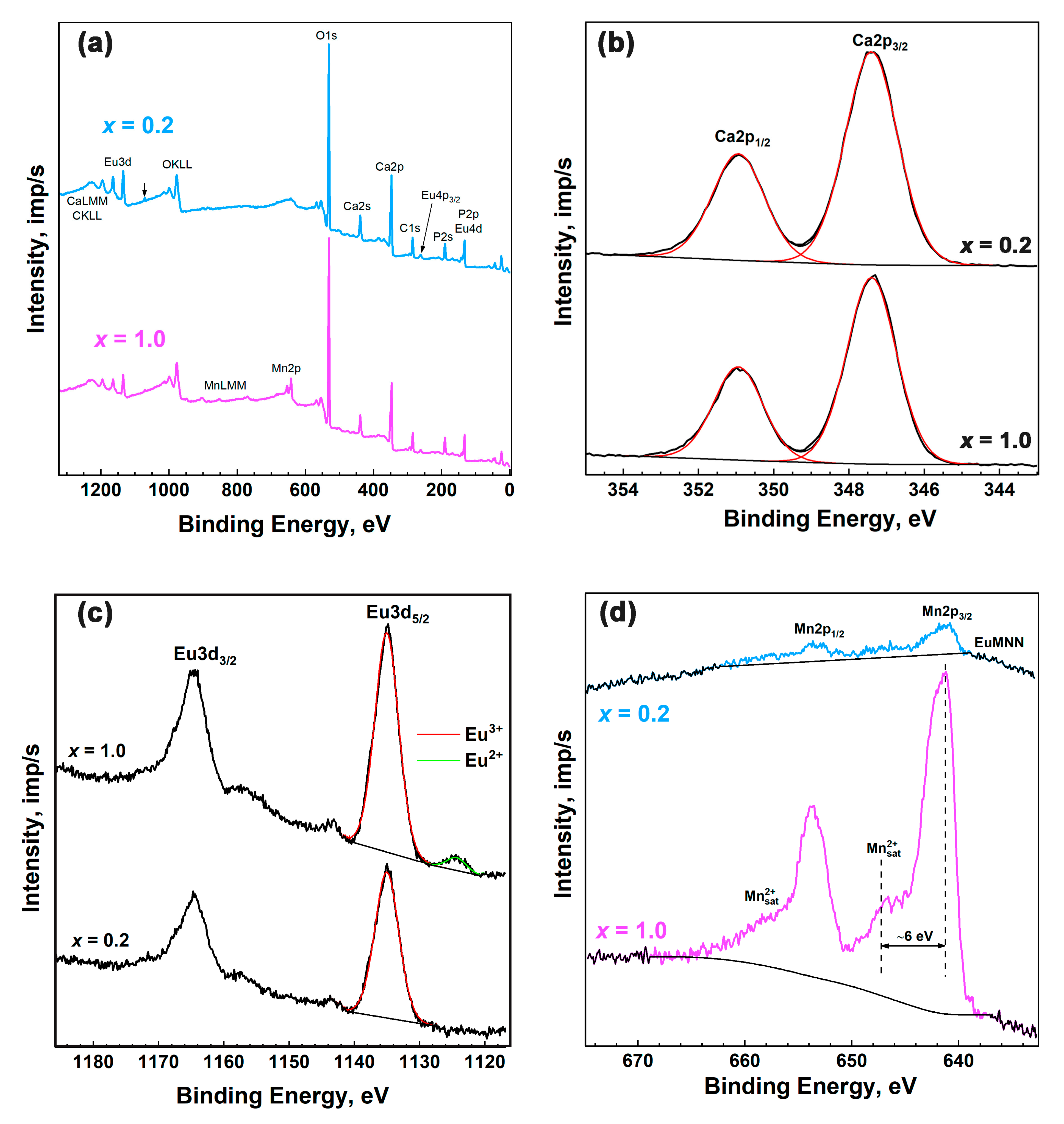
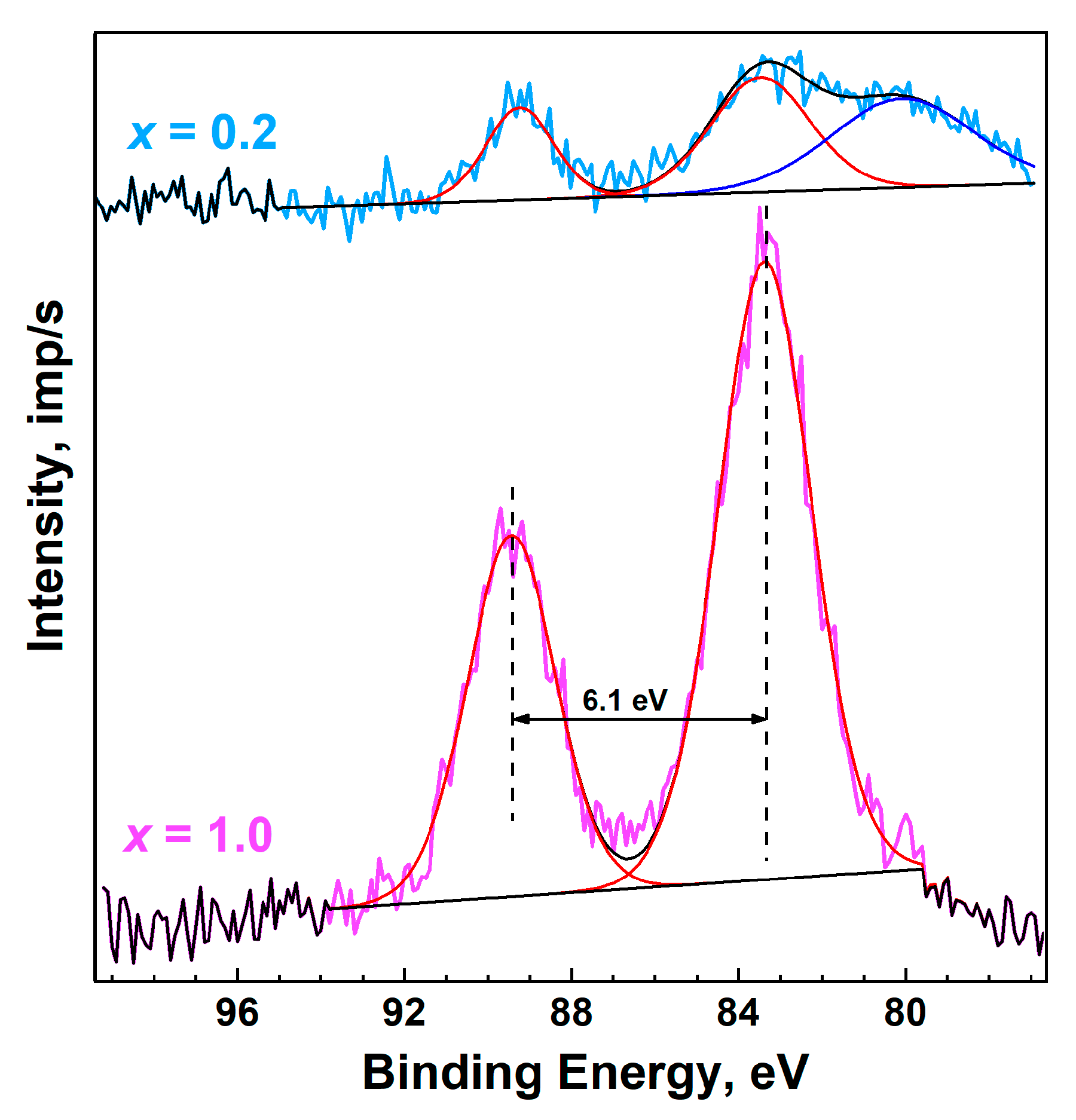
3.5. XPS Study
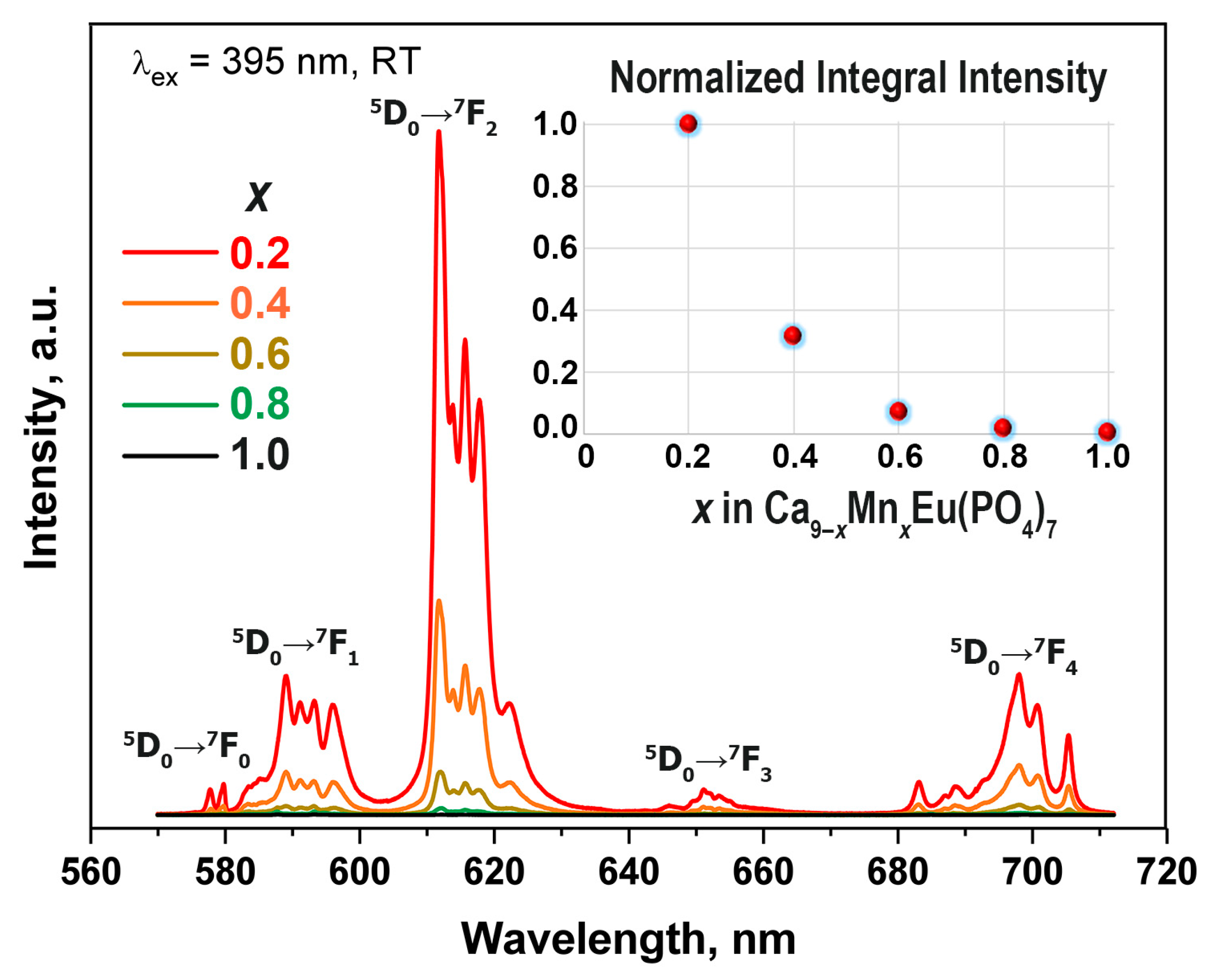
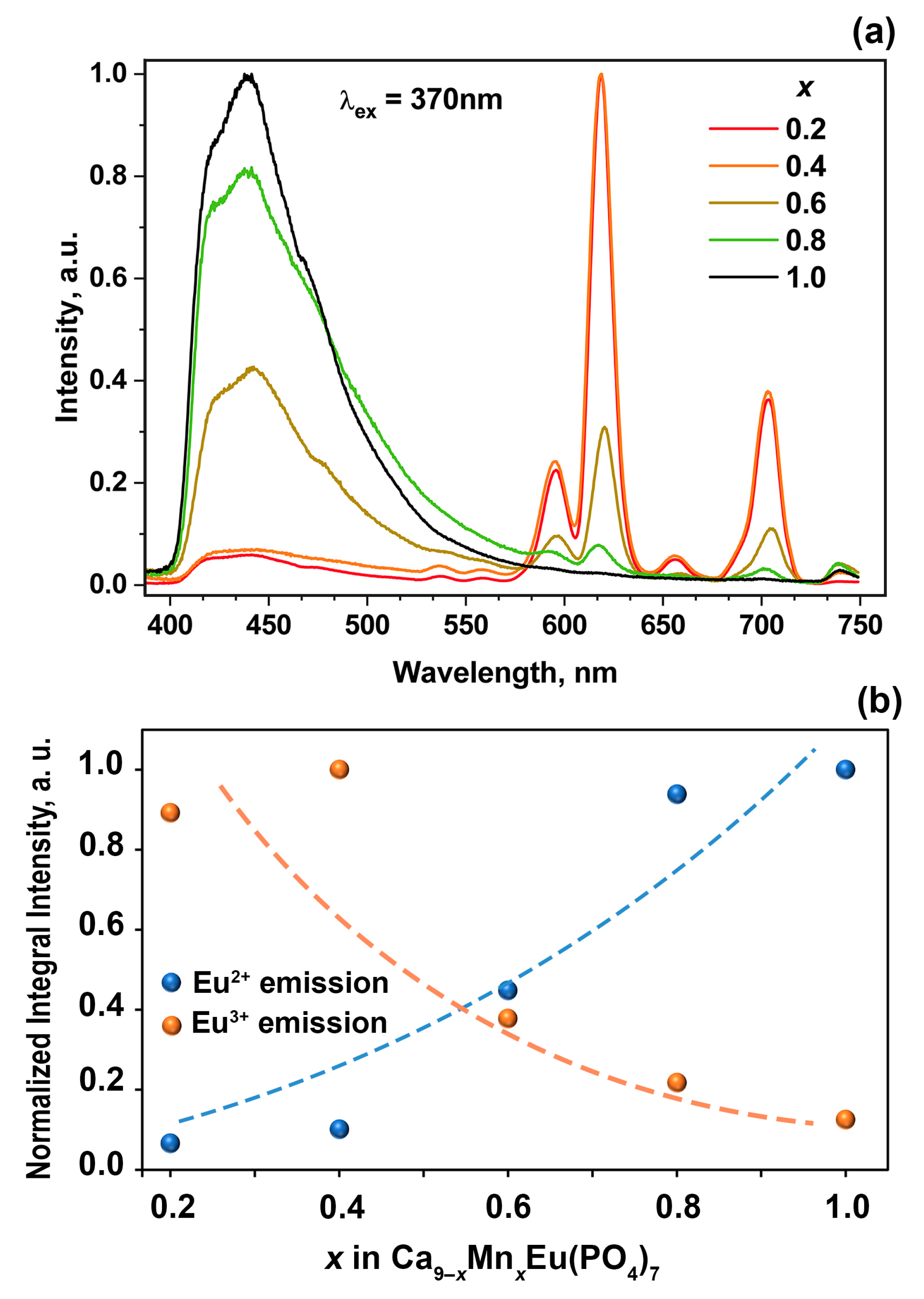
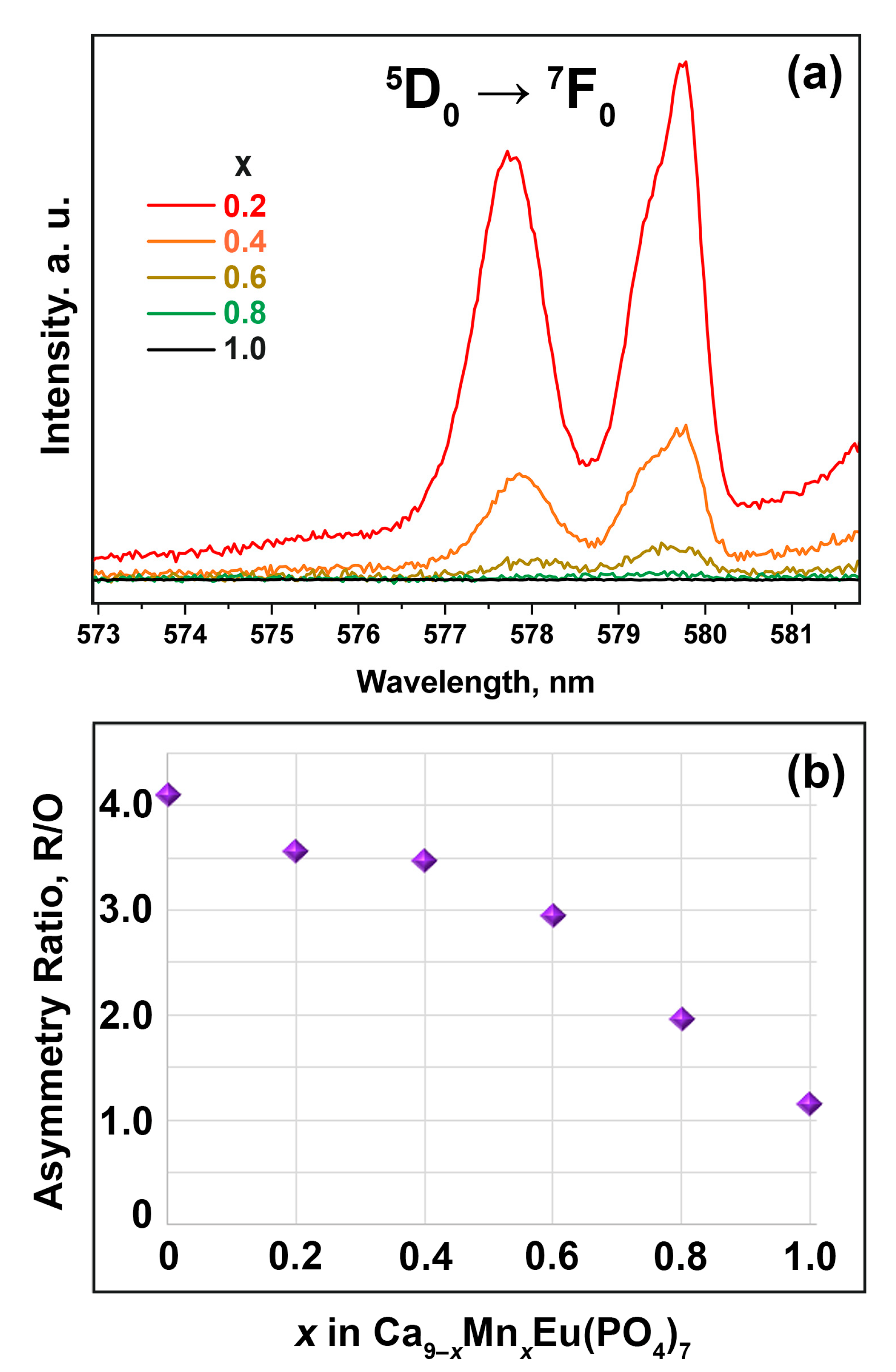
3.6. Photoluminescent Properties
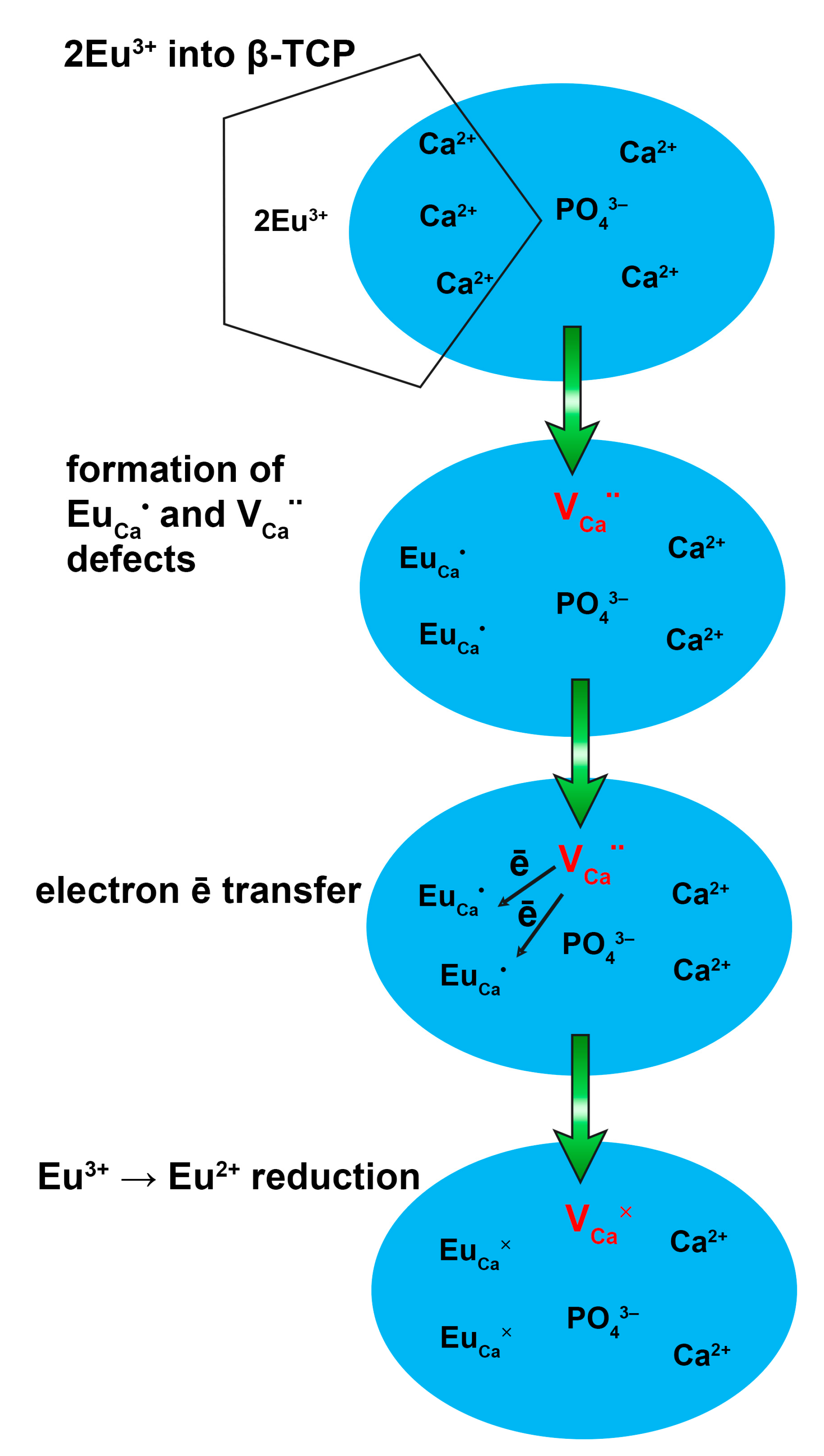
4. Discussion
- (1)
- There are no oxidizing ions in the structure;
- (2)
- The β-TCP-type host is based on tetrahedral anion groups (PO43−);
- (3)
- The doped ions (Eu3+ and Mn4+) substitute the ions with lower valences (Ca2+) in the host;
- (4)
- The substituted cation (Ca2+) has ionic radii close to Eu2+ (see Table 4 below).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US Department of Energy. Solid State Lighting Research and Development Plan. Available online: https://energy.gov/sites/prod/files/2016/06/f32/ssl_rd-plan_%25%0A20jun2016_2.pdf (accessed on 4 January 2023).
- Lumileds Holding, B.V. Narrow Red Phosphor Technology. Available online: https://lumileds.com/company/blog/white-paper-narrow-red-phosphor-technology/ (accessed on 4 January 2023).
- Hu, T.; Ning, L.; Gao, Y.; Qiao, J.; Song, E.; Chen, Z.; Zhou, Y.; Wang, J.; Molokeev, M.S.; Ke, X.; et al. Glass crystallization making red phosphor for high-power warm white lighting. Light Sci. Appl. 2021, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.-J.; Li, Y.Q.; Hirosaki, N.; Yamamoto, H. Nitride Phosphors and Solid-State Lighting; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429103919. [Google Scholar]
- Luong, V.D.; Zhang, W.; Lee, H.-R. Preparation of Sr2Si5N8:Eu2+ for white light-emitting diodes by multi-step heat treatment. J. Alloys Compd. 2011, 509, 7525–7528. [Google Scholar] [CrossRef]
- Pust, P.; Weiler, V.; Hecht, C.; Tücks, A.; Wochnik, A.S.; Henß, A.-K.; Wiechert, D.; Scheu, C.; Schmidt, P.J.; Schnick, W. Narrow-band red-emitting Sr[LiAl3N4]:Eu2+ as a next-generation LED-phosphor material. Nat. Mater. 2014, 13, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Hoerder, G.J.; Seibald, M.; Baumann, D.; Schröder, T.; Peschke, S.; Schmid, P.C.; Tyborski, T.; Pust, P.; Stoll, I.; Bergler, M.; et al. Sr[Li2Al2O2N2]:Eu2+—A high performance red phosphor to brighten the future. Nat. Commun. 2019, 10, 1824. [Google Scholar] [CrossRef]
- Takeda, T.; Xie, R.-J.; Suehiro, T.; Hirosaki, N. Nitride and oxynitride phosphors for white LEDs: Synthesis, new phosphor discovery, crystal structure. Prog. Solid State Chem. 2018, 51, 41–51. [Google Scholar] [CrossRef]
- Osborne, R.A.; Cherepy, N.J.; Seeley, Z.M.; Payne, S.A.; Drobshoff, A.D.; Srivastava, A.M.; Beers, W.W.; Cohen, W.W.; Schlagel, D.L. New red phosphor ceramic K2SiF6:Mn4+. Opt. Mater. 2020, 107, 110140. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Rare Earths and the Balance Problem. J. Sustain. Metall. 2015, 1, 29–38. [Google Scholar] [CrossRef]
- Sinusaite, L.; Renner, A.M.; Schütz, M.B.; Antuzevics, A.; Rogulis, U.; Grigoraviciute-Puroniene, I.; Mathur, S.; Zarkov, A. Effect of Mn doping on the low-temperature synthesis of tricalcium phosphate (TCP) polymorphs. J. Eur. Ceram. Soc. 2019, 39, 3257–3263. [Google Scholar] [CrossRef]
- Lecointre, A.; Ait Benhamou, R.; Bessiére, A.; Wallez, G.; Elaatmani, M.; Viana, B. Red long-lasting phosphorescence (LLP) in β-TCP type Ca9.5Mn(PO4)7 compounds. Opt. Mater. 2011, 34, 376–380. [Google Scholar] [CrossRef]
- Wang, J.; Shang, M.; Cui, M.; Dang, P.; Liu, D.; Huang, D.; Lian, H.; Lin, J. Realizing an impressive red-emitting Ca9MnNa(PO4)7 phosphor through a dual function based on disturbing structural confinement and energy transfer. J. Mater. Chem. C 2020, 8, 285–295. [Google Scholar] [CrossRef]
- Liu, W.-R.; Chiu, Y.-C.; Yeh, Y.-T.; Jang, S.-M.; Chen, T.-M. Luminescence and Energy Transfer Mechanism in Ca10K(PO4)7:Eu2+,Mn2+ Phosphor. J. Electrochem. Soc. 2009, 156, J165. [Google Scholar] [CrossRef]
- Song, E.; Zhao, W.; Dou, X.; Zhu, Y.; Yi, S.; Min, H. Nonradiative energy transfer from Mn2+ to Eu3+ in K2CaP2O7:Mn2+,Eu3+ phosphor. J. Lumin. 2012, 132, 1462–1467. [Google Scholar] [CrossRef]
- Tao, P.; Liu, S.; Wong, W. Phosphorescent Manganese(II) Complexes and Their Emerging Applications. Adv. Opt. Mater. 2020, 8, 2000985. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, W.; Fan, J.; Sun, Z.; Zhang, X. Composition modification for tuning the luminescent property in Sr19(Mg,Mn)2(PO4)14: Eu2+ phosphors. J. Lumin. 2021, 239, 118369. [Google Scholar] [CrossRef]
- Cao, R.; Ran, Y.; Lv, X.; Xu, L.; Wan, H.; Hu, Q.; Chen, T.; Cao, C. Tunable multicolor luminescent properties of Ca8ZnLa(PO4)7:Ce3+, Mn2+ phosphor via efficient energy transfer. J. Lumin. 2019, 214, 116549. [Google Scholar] [CrossRef]
- Maciejewska, K.; Poźniak, B.; Tikhomirov, M.; Kobylińska, A.; Marciniak, Ł. Synthesis, Cytotoxicity Assessment and Optical Properties Characterization of Colloidal GdPO4:Mn2+, Eu3+ for High Sensitivity Luminescent Nanothermometers Operating in the Physiological Temperature Range. Nanomaterials 2020, 10, 421. [Google Scholar] [CrossRef]
- Félix-Quintero, H.; Falcony, C.; Hernández, A.J.; Camarillo, G.E.; Flores, J.C.; Murrieta, S.H. Mn2+ to Eu3+ energy transfer in zinc phosphate glass. J. Lumin. 2020, 225, 117337. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Wang, J.; Wu, M.; Wang, C. Color-Tunable Phosphor of Eu2+ and Mn2+ Codoped Ca2Sr(PO4)2 for UV Light-Emitting Diodes. J. Phys. Chem. C 2014, 118, 12494–12499. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, Z.; Hua, Z. Investigations on luminescence of Ca8MgGd(PO4)7:Eu2+, Mn2+, Yb3+, Er3+, Ho3+, Tm3+ phosphors. Mater. Res. Bull. 2016, 74, 34–40. [Google Scholar] [CrossRef]
- Belik, A.A.; Gutan, V.B.; Ivanov, L.N.; Lazoryak, B.I. Synthesis, structure, and luminescence properties of Ca9MnM(PO4)7 (M = Li, Na, K). Russ. J. Inorg. Chem. 2001, 46, 785–792. [Google Scholar]
- Kang, X.; Lü, W.; Wang, H.; Ling, D. Energy Transfer from Ce3+ to Tb3+/Dy3+/Mn2+ in Ca9Ga(PO4)7 Phosphors: Synthesis, Structure and Tunable Multicolor Luminescent Properties. ChemPhysChem 2019, 20, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Li, S.; Chen, J.; Li, J.; Zhao, Y.; Wang, L.; Jia, C.; Ouyang, R.; Lü, W. Photoluminescence properties of whitlockite-type Ca9MgK(PO4)7:Eu2+,Mn2+ phosphor. J. Lumin. 2016, 179, 328–333. [Google Scholar] [CrossRef]
- Ding, C.; Tang, W. Crystal structure, energy transfer and tunable luminescence properties of Ca8ZnCe(PO4)7:Eu2+,Mn2+ phosphor. Opt. Mater. 2018, 76, 56–62. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L.; Petrícek, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Aksenov, S.M.; Nikiforov, I.; Stefanovich, S.Y.; Lazoryak, B.I. Symmetry Inhomogeneity of Ca9–xZnxEu(PO4)7 Phosphor Determined by Second-Harmonic Generation and Dielectric and Photoluminescence Spectroscopy. Cryst. Growth Des. 2020, 20, 6461–6468. [Google Scholar] [CrossRef]
- Nikiforov, I.V.; Deyneko, D.V.; Spassky, D.A.; Baryshnikova, O.V.; Stefanovich, S.Y.; Lazoryak, B.I. Tunable luminescence and energy transfer in Eu3+ doped Ca8MTb(PO4)7 (M = Mg, Zn, Ca) phosphors. Mater. Res. Bull. 2020, 130, 110925. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Morozov, V.A.; Zhukovskaya, E.S.; Nikiforov, I.V.; Spassky, D.A.; Belik, A.A.; Lazoryak, B.I. The influence of second coordination-sphere interactions on the luminescent properties of β-Ca3(PO4)2-related compounds. J. Alloys Compd. 2020, 815, 152352. [Google Scholar] [CrossRef]
- Nikiforov, I.V.; Deyneko, D.V.; Duskaev, I.F. Structural Features of Phosphates Ca9–xMxDy(PO4)7 (M = Zn2+ or Mg2+). Phys. Solid State 2020, 62, 860–864. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Nikiforov, I.V.; Spassky, D.A.; Dikhtyar, Y.Y.; Aksenov, S.M.; Stefanovich, S.Y.; Lazoryak, B.I. Luminescence of Eu3+ as a probe for the determination of the local site symmetry in β-Ca3(PO4)2 -related structures. CrystEngComm 2019, 21, 5235–5242. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Morozov, V.A.; Vasin, A.A.; Aksenov, S.M.; Dikhtyar, Y.Y.; Stefanovich, S.Y.; Lazoryak, B.I. The crystal site engineering and turning of cross-relaxation in green-emitting β-Ca3(PO4)2-related phosphors. J. Lumin. 2020, 223, 117196. [Google Scholar] [CrossRef]
- Dikhtyar, Y.Y.; Deyneko, D.V.; Boldyrev, K.N.; Baryshnikova, O.V.; Belik, A.A.; Morozov, V.A.; Lazoryak, B.I. Crystal structure, dielectric and optical properties of β-Ca3(PO4)2-type phosphates Ca9-xZnxLa(PO4)7:Ho3+. J. Lumin. 2021, 236, 118083. [Google Scholar] [CrossRef]
- Dikhtyar, Y.Y.; Deyneko, D.V.; Spassk, D.A.; Lazoryak, B.I.; Stefanovich, S.Y. A novel high color purity blue-emitting Tm3+-doped β-Ca3(PO4)2-type phosphor for WLED application. Optik 2020, 227, 166027. [Google Scholar] [CrossRef]
- Belik, A.A.; Morozov, V.A.; Deyneko, D.V.; Savon, A.E.; Baryshnikova, O.V.; Zhukovskaya, E.S.; Dorbakov, N.G.; Katsuya, Y.; Tanaka, M.; Stefanovich, S.Y.; et al. Antiferroelectric properties and site occupations of R3+ cations in Ca8MgR(PO4)7 luminescent host materials. J. Alloys Compd. 2017, 699, 928–937. [Google Scholar] [CrossRef]
- Lazoryak, B.I.; Zhukovskaya, E.S.; Baryshnikova, O.V.; Belik, A.A.; Leonidova, O.N.; Deyneko, D.V.; Savon, A.E.; Dorbakov, N.G.; Morozov, V.A. Luminescence, structure and antiferroelectric-type phase transition in Ca8ZnEu(PO4)7. Mater. Res. Bull. 2018, 104, 20–26. [Google Scholar] [CrossRef]
- Dikhtyar, Y.Y.; Spassky, D.A.; Morozov, V.A.; Deyneko, D.V.; Belik, A.A.; Baryshnikova, O.V.; Nikiforov, I.V.; Lazoryak, B.I. Site occupancy, luminescence and dielectric properties of β-Ca3(PO4)2-type Ca8ZnLn(PO4)7 host materials. J. Alloys Compd. 2022, 908, 164521. [Google Scholar] [CrossRef]
- Stefanovich, S.Y.; Petrova, D.A.; Morozov, V.A.; Fortalnova, E.A.; Belov, D.A.; Deyneko, D.V.; Lazoryak, B.I. Enhanced nonlinear optical activity and Ca2+-conductivity in Ca10.5-xPbx(VO4)7 ferroelectrics. J. Alloys Compd. 2018, 735, 1826–1837. [Google Scholar]
- Altomare, A.; Rizzi, R.; Rossi, M.; El Khouri, A.; Elaatmani, M.; Paterlini, V.; Della Ventura, G.; Capitelli, F. New Ca2.90(Me2+)0.10(PO4)2 β-tricalcium Phosphates with Me2+ = Mn, Ni, Cu: Synthesis, Crystal-Chemistry, and Luminescence Properties. Crystals 2019, 9, 288. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Nikiforov, I.V.; Lazoryak, B.I.; Aksenov, S.M. The role of anionic heterovalent [PO4]3− → [GeO4]4− substitution on the luminescence properties of inorganic phosphors with the β-Ca3(PO4)2-type structure: New data based on accurate crystal structure refinement. Dalt. Trans. 2022, 51, 655–663. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Titkov, V.V.; Fedyunin, F.D.; Spassky, D.A.; Volkov, S.N.; Borovikova, E.Y.; Lazoryak, B.I.; Aksenov, S.M. «Ellestadite»-type anionic [PO4]3− → [SO4]2− substitutions in β-Ca3(PO4)2 type compounds: A new route to design the inorganic phosphors. Ceram. Int. 2022, 48, 24012–24020. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley and Sons: New York, NY, USA, 2005. [Google Scholar]
- Baltrus, J.P.; Keller, M.J. Rare earth oxides Eu2O3 and Nd2O3 analyzed by XPS. Surf. Sci. Spectra 2019, 26, 014001. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Choudhary, R.J.; Phase, D.M. Structural, XPS and magnetic studies of pulsed laser deposited Fe doped Eu2O3 thin film. Mater. Res. Bull. 2015, 70, 392–396. [Google Scholar] [CrossRef]
- Shin, Y.C.; Leem, S.J.; Kim, C.M.; Kim, S.J.; Sung, Y.M.; Hahn, C.K.; Baek, J.H.; Kim, T.G. Deposition of Europium Oxide on Si and its optical properties depending on thermal annealing conditions. J. Electroceramics 2009, 23, 326–330. [Google Scholar] [CrossRef]
- Vercaemst, R.; Poelman, D.; Fiermans, L.; Van Meirhaeghe, R.L.; Laflère, W.H.; Cardon, F. A detailed XPS study of the rare earth compounds EuS and EuF3. J. Electron Spectros. Relat. Phenom. 1995, 74, 45–56. [Google Scholar] [CrossRef]
- Schneider, W.-D.; Laubschat, C.; Nowik, I.; Kaindl, G. Shake-up excitations and core-hole screening in Eu systems. Phys. Rev. B 1981, 24, 5422–5425. [Google Scholar] [CrossRef]
- Kang, J.-G.; Jung, Y.; Min, B.-K.; Sohn, Y. Full characterization of Eu(OH)3 and Eu2O3 nanorods. Appl. Surf. Sci. 2014, 314, 158–165. [Google Scholar] [CrossRef]
- Mercier, F.; Alliot, C.; Bion, L.; Thromat, N.; Toulhoat, P. XPS study of Eu(III) coordination compounds: Core levels binding energies in solid mixed-oxo-compounds EumXxOy. J. Electron Spectros. Relat. Phenom. 2006, 150, 21–26. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Langell, M.; Hutchings, C.W.; Carson, G.A.; Nassir, M.H. High resolution electron energy loss spectroscopy of MnO(100) and oxidized MnO(100). J. Vac. Sci. Technol. A 1996, 14, 1656–1661. [Google Scholar] [CrossRef]
- Soares, E.A.; Paniago, R.; de Carvalho, V.E.; Lopes, E.L.; Abreu, G.J.P.; Pfannes, H.-D. Quantitative low-energy electron diffraction analysis of MnO(00) films grown on Ag(100). Phys. Rev. B 2006, 73, 035419. [Google Scholar] [CrossRef]
- Stranick, M.A. Mn2O3 by XPS. Surf. Sci. Spectra 1999, 6, 39–46. [Google Scholar] [CrossRef]
- Stranick, M.A. MnO2 by XPS. Surf. Sci. Spectra 1999, 6, 31–38. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Q.; Xia, Z. Structural Engineering of Eu2+ -Doped Silicates Phosphors for LED Applications. Acc. Mater. Res. 2020, 1, 137–145. [Google Scholar] [CrossRef]
- Zhang, H.; Lü, M.; Xiu, Z.; Wang, S.; Zhou, G.; Zhou, Y.; Wang, S.; Qiu, Z.; Zhang, A. Synthesis and photoluminescence properties of a new red emitting phosphor: Ca3(VO4)2:Eu3+; Mn2+. Mater. Res. Bull. 2007, 42, 1145–1152. [Google Scholar] [CrossRef]
- Dai, W.B. Mechanism of the reduction and energy transfer between Eu2+ and Eu3+ in Eu-doped CaAl2Si2O8 materials prepared in air. J. Mater. Chem. C 2014, 2, 3951–3959. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Y.; Zhu, Y.; Liu, S.; Li, H.; Lei, W. Abnormal reduction of Eu3+ to Eu2+ in Sr5(PO4)3Cl:Eu phosphor and its enhanced red emission by the charge compensation. J. Lumin. 2019, 214, 116569. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y. Effect of Oxygen Vacancies on the Reduction of Eu3+ in Mg3Ca3(PO4)4 in Air Atmosphere. Inorg. Chem. 2017, 56, 10396–10403. [Google Scholar] [CrossRef]
- Dang, P.; Liu, D.; Li, G.; Liang, S.; Lian, H.; Shang, M.; Lin, J. Mixing the valence control of Eu2+/Eu3+ and energy transfer construction of Eu2+/Mn2+ in the solid solution (1 − x)Ca3(PO4)2 − x Ca9Y(PO4)7 for multichannel photoluminescence tuning. Inorg. Chem. Front. 2019, 6, 2837–2849. [Google Scholar] [CrossRef]
- Peng, M.; Pei, Z.; Hong, G.; Su, Q. The reduction of Eu3+ to Eu2+ in BaMgSiO4:Eu prepared in air and the luminescence of BaMgSiO4:Eu2+ phosphor. J. Mater. Chem. 2003, 13, 1202–1205. [Google Scholar] [CrossRef]
- Xu, W.; Chen, D.; Yuan, S.; Zhou, Y.; Li, S. Tuning excitation and emission of Mn4+ emitting center in Y3Al5O12 by cation substitution. Chem. Eng. J. 2017, 317, 854–861. [Google Scholar] [CrossRef]
- Yu, H.; Ruan, F.; Chen, L.; Deng, D. Dual-emitting Eu2+/Eu3+ co-doped Ca9Zn1.5(PO4)7 phosphor for self-calibrated optical thermometry. Opt. Mater. 2020, 100, 109678. [Google Scholar] [CrossRef]
- Lu, J.; Pan, Y.; Wang, J.; Chen, X.; Huang, S.; Liu, G. Reduction of Mn4+ to Mn2+ in CaAl12O 19 by co-doping charge compensators to obtain tunable photoluminescence. RSC Adv. 2013, 3, 4510–4513. [Google Scholar] [CrossRef]
- Hu, J.Q.; Song, E.H.; Ye, S.; Zhou, B.; Zhang, Q.Y. Anomalous spontaneous-reduction of Mn7+/Mn4+ to Mn2+ and luminescence properties in Zn2GeO4:Mn. J. Mater. Chem. C 2017, 5, 3343–3351. [Google Scholar] [CrossRef]




| Host | RE/Mn2+ Combination | PL Spectra (Band Location/Peak of the Band) | Features | Ref. |
|---|---|---|---|---|
| Ca9Mn1−xNa(PO4)7:xM2+, M = Zn, Mn | Mn2+ and Eu2+/Mn2+ | 600–750 nm/655 nm | The co-doping of Zn2+/Mg2+ and Mn2+ broke the intrinsic structural confinement of Mn2+ and improved its red emission. | [13] |
| Sr19(Mg1−xMnx)2(PO4)14: yEu2+ | Eu2+/Mn2+ | 550–750 nm/610 nm | The emission bands were attributed to Eu2+ and Mn2+ in different sites. | [17] |
| Ca8MgGd(PO4)7:Eu2+/Mn2+ | Eu2+/Mn2+ | 600–750 nm/650 nm λex = 365 nm | Efficient Eu2+ → Mn2+ energy transfer was observed, Eu2+ emission intensity decreased. | [22] |
| Ca9MMn(PO4)7 (M = Li, Na, K) | Mn2+ | 580–720 nm/645 nm | The excitation by β-source 90Sr-90Y. Mn2+ occupies M5 site, M+ ions (Li, Na, K) are located in M4 site. | [23] |
| Ca8.82−zGa(PO4)7:0.18Ce3+, zMn2+ | Ce3+/Mn2+ | Two broad emission bands: 350–450 nm/380 nm (Ce3+ emission) 600–700 nm/625 nm (Mn2+ emission) | The concentration quenching was observed above 9 mol. % Mn2+; the decreasing of quantum yield from 62.3% to 67 % with increasing Mn2+ concentration was explained by energy loss during Ce3+ → Mn2+ energy transfer process. | [24] |
| Ca10K(PO4)7:Eu2+, Mn2+ | Eu2+/Mn2+ | Two broad emission bands (λex = 347 nm): 425–500 nm/467 nm (Eu2+ emission) 600–700 nm/634 nm (Mn2+ emission) | Decreasing of PL intensity with increasing of Mn2+ concentration. The concentration quenching was observed above 7 mol. % Mn2+; resonant type of Eu2+ → Mn2+ energy transfer process via a dipole–quadrupole mechanism. | [14] |
| Ca9MgK(PO4)7:Eu2+, Mn2+ | Eu2+/Mn2+ | Two broad emission bands (λex = 347 nm) 425–500 nm/467 nm (Eu2+ emission) 600–700 nm/634 nm (Mn2+ emission) | Resonant type of Eu2+ → Mn2+ energy transfer with mechanism via a dipole–quadrupole interaction. | [25] |
| Ca8ZnCe(PO4)7:Eu2+, Mn2+ | Ce3+/Eu2+/Mn2+ | Three broad bands peaked (λex = 285 nm) 320–420 nm/375 nm (Ce3+ emission) 450–575 nm/500 nm (Eu2+ emission) 580–700 nm/645 nm (Mn2+ emission) | The energy transfers of Ce3+→Eu2+/Mn2+ and Eu2+ → Mn2+ ions were investigated. The emitting color can be adjusted from violet-blue to green/red-orange/white by doping/co-doping. | [26] |
| Sample Composition | Ca8MnEu(PO4)7 |
|---|---|
| Lattice parameters: a, Å | 10.39826(1) |
| c, Å | 37.17350(5) |
| Unit cell volume V, Å3 | 3480.851(7) |
| Calculated density, g/cm3 | 3.413 |
| Data Collection: | |
| Diffractometer | BL15XU beamline of SPring-8 |
| Radiation/Wavelength (λ, Å) | Synchrotron/0.65298 |
| Absorption coefficient, μ (mm−1) | 4.381 |
| F(000) | 3462 |
| 2θ range (°) | 2.040–60.237 |
| Step scan (2θ) | 0.003 |
| Imax | 606,480 |
| Number of points | 19,391 |
| Refinement: | |
| Refinement | Rietveld |
| Background function | Legendre polynoms, 15 terms |
| No. of reflections (all/observed) | 945/922 |
| No. of refined parameters/refined atomic parameters | 43/34 |
| R and Rw (%) for Bragg reflections (Rall/Robs) | 5.29/5.95 and 5.05/4.81 |
| RP and RwP; Rexp | 2.06, 3.31, 0.63 |
| Goodness of fit (ChiQ) | 5.25 |
| Max./min. residual density(e) (Å3) | 0.65/−0.88 |
| Sample | ΔMn2psat | ΔMn3s | Reference |
|---|---|---|---|
| Ca8MnEu(PO4)7 | ~6.0 | 6.1 | This work |
| Ca8.8Mn0.2Eu(PO4)7 | ~6.0 | 5.8 | This work |
| MnO | ~6.0 | 6.1 | [53,54] |
| Mn2O3 | 10.1 | 5.4 | [55] |
| MnO2 | 11.8 | 4.4 | [56] |
| Site | Dr Value, %, for Doped Ion | |||
|---|---|---|---|---|
| Mn2+ | Mn4+ | Eu2+ | Eu3+ | |
| Ca1–Ca3 CN8 (r =1.12 Å) | 14 (r = 0.96 Å) | - | 11 (r = 1.25 Å) | 4 (r = 1.07 Å) |
| Ca5 CN6 (r =1.00 Å) | 17 (r = 0.83 Å) | 47 (r = 0.53 Å) | 17 (r = 1.17 Å) | 5 (r = 0.95 Å) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipina, E.V.; Spassky, D.A.; Krutyak, N.R.; Morozov, V.A.; Zhukovskaya, E.S.; Belik, A.A.; Manylov, M.S.; Lazoryak, B.I.; Deyneko, D.V. Abnormal Eu3+ → Eu2+ Reduction in Ca9−xMnxEu(PO4)7 Phosphors: Structure and Luminescent Properties. Materials 2023, 16, 1383. https://doi.org/10.3390/ma16041383
Sipina EV, Spassky DA, Krutyak NR, Morozov VA, Zhukovskaya ES, Belik AA, Manylov MS, Lazoryak BI, Deyneko DV. Abnormal Eu3+ → Eu2+ Reduction in Ca9−xMnxEu(PO4)7 Phosphors: Structure and Luminescent Properties. Materials. 2023; 16(4):1383. https://doi.org/10.3390/ma16041383
Chicago/Turabian StyleSipina, Elena V., Dmitry A. Spassky, Nataliya R. Krutyak, Vladimir A. Morozov, Evgenia S. Zhukovskaya, Alexei A. Belik, Mikhail S. Manylov, Bogdan I. Lazoryak, and Dina V. Deyneko. 2023. "Abnormal Eu3+ → Eu2+ Reduction in Ca9−xMnxEu(PO4)7 Phosphors: Structure and Luminescent Properties" Materials 16, no. 4: 1383. https://doi.org/10.3390/ma16041383
APA StyleSipina, E. V., Spassky, D. A., Krutyak, N. R., Morozov, V. A., Zhukovskaya, E. S., Belik, A. A., Manylov, M. S., Lazoryak, B. I., & Deyneko, D. V. (2023). Abnormal Eu3+ → Eu2+ Reduction in Ca9−xMnxEu(PO4)7 Phosphors: Structure and Luminescent Properties. Materials, 16(4), 1383. https://doi.org/10.3390/ma16041383







