Influence of Short-Pulse Microwave Radiation on Thermochemical Properties Aluminum Micropowder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
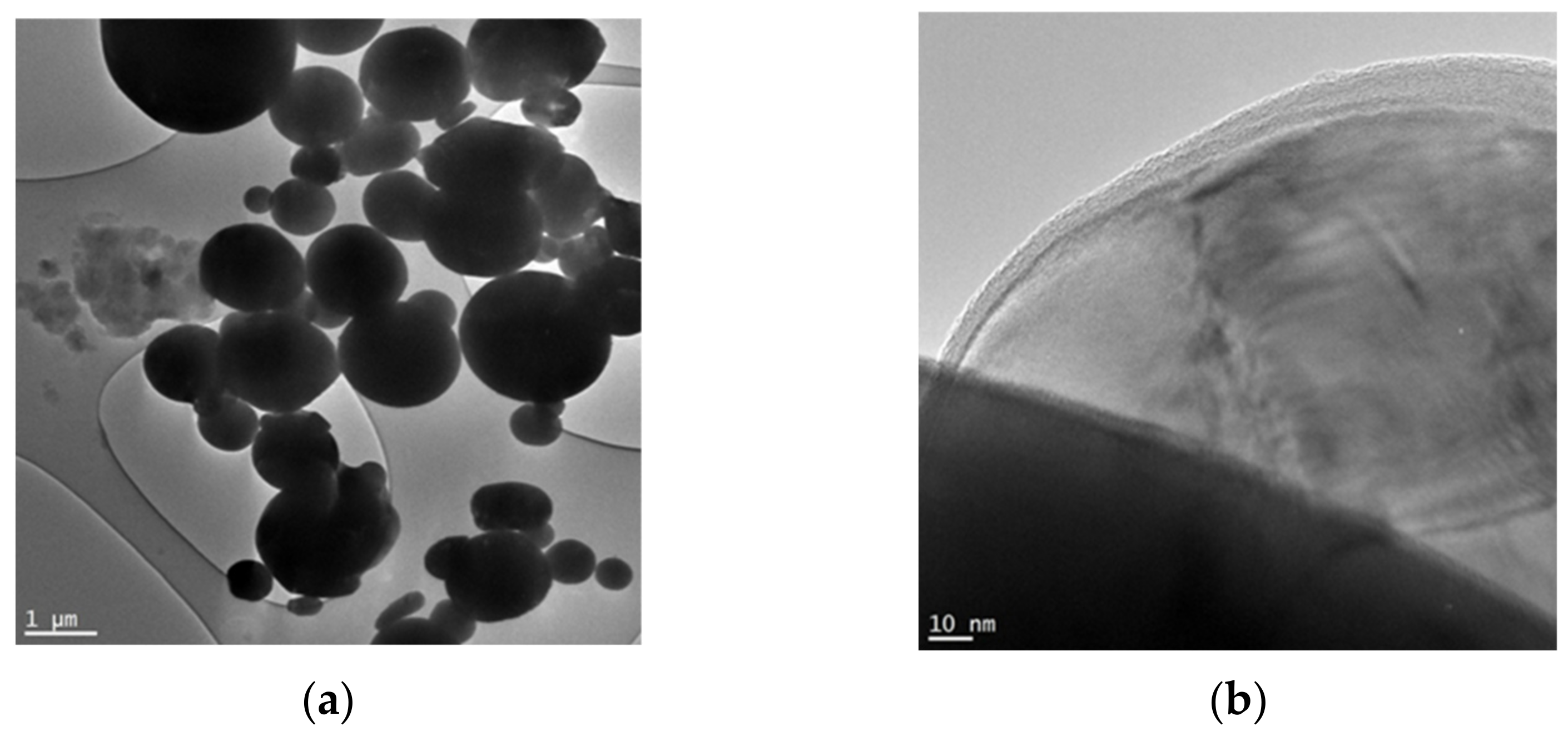
2.3. Characterization
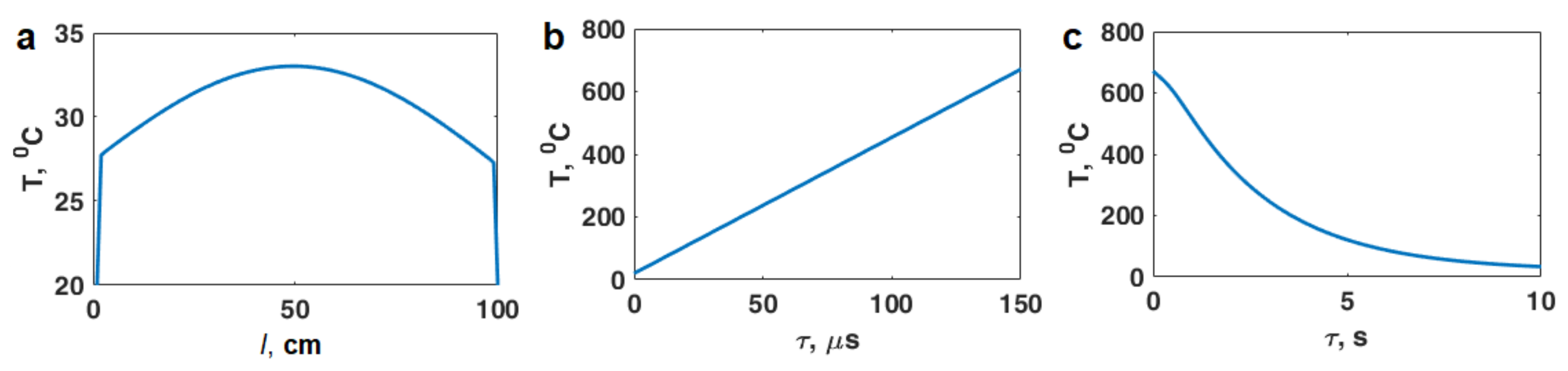
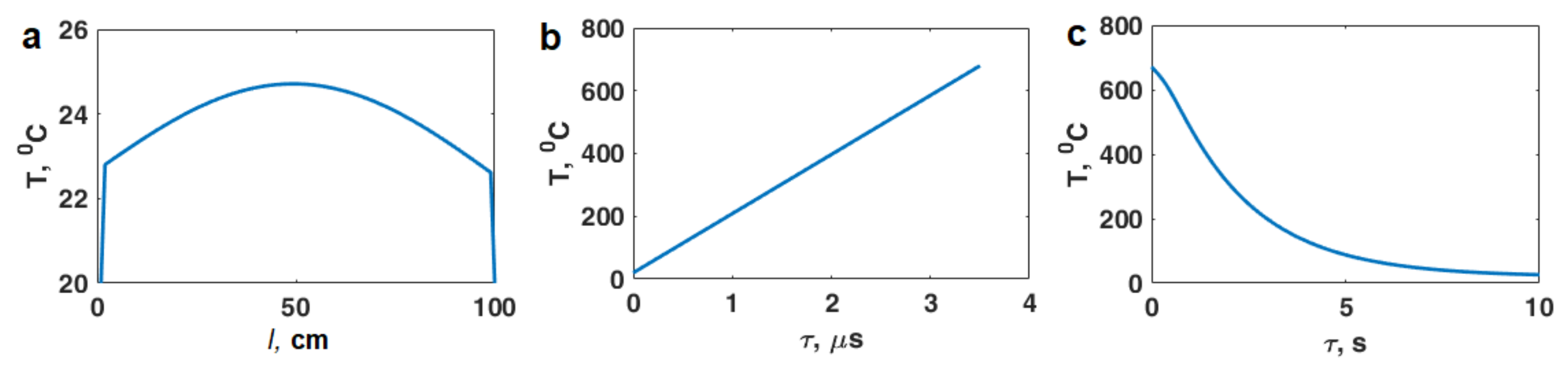
3. Estimation of Thermal Effects of Short-Pulse Microwave Radiation
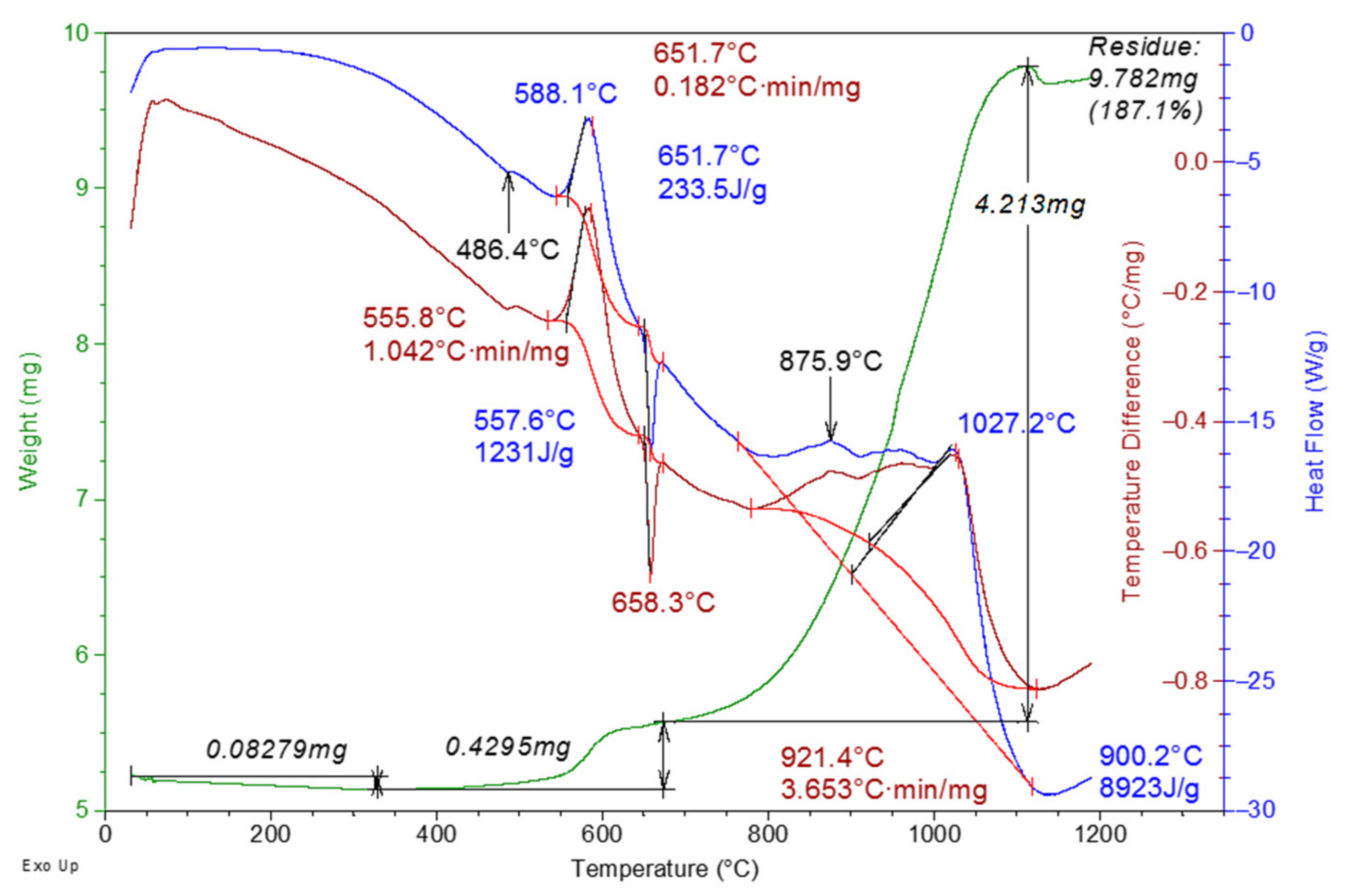
4. Thermal Analysis Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.; Hirayama, Y.; Takagi, K.; Kwon, H. Surface Cleaning Effect of Bare Aluminum Micro-Sized Powder by Low Oxygen Induction Thermal Plasma. Materials 2022, 15, 1553. [Google Scholar] [CrossRef]
- Cañadilla, A.; Romero, A.; Rodríguez, G.P.; Caminero, M.Á.; Dura, Ó.J. Mechanical, Electrical, and Thermal Characterization of Pure Copper Parts Manufactured via Material Extrusion Additive Manufacturing. Materials 2022, 15, 4644. [Google Scholar] [CrossRef]
- Shinkaryov, A.S.; Cherkasova, M.V.; Pelevin, I.A.; Ozherelkov, D.Y.; Chernyshikhin, S.V.; Kharitonova, N.A.; Gromov, A.A.; Nalivaiko, A.Y. Aluminum Powder Preparation for Additive Manufacturing Using Electrostatic Classification. Coatings 2021, 11, 629. [Google Scholar] [CrossRef]
- De Luca, L.T.; Galfetti, L.; Severini, F.; Meda, L.; Marra, G.; Vorozhtsov, A.B.; Sedoi, V.S.; Babuk, V.A. Burning of Nano-Aluminized Composite Rocket Propellants. Combust. Explos. Shock Waves 2005, 41, 680–692. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-saving pathways for thermoelectric nanomaterial synthesis: Hydrothermal/solvothermal, microwave-assisted, solution-based, and powder processing. Adv. Sci. 2022, 9, 2106052. [Google Scholar] [CrossRef]
- Gromov, A.A.; Strokova, Y.I.; Teipel, U. Stabilization of Metal Nanoparticles—A Chemical Approach. Chem. Eng. Technol. 2009, 32, 1049–1060. [Google Scholar] [CrossRef]
- Ju, Z.; An, J.; Guo, C.; Li, T.-R.; Jia, Z.-Y.; Wu, R.-F. The oxidation reaction and sensitivity of aluminum nanopowders coated by hydroxyl-terminated polybutadiene. J. Energ. Mater. 2020, 39, 299–312. [Google Scholar] [CrossRef]
- Dossi, S.; Maggi, F. Ignition of Mechanically Activated Aluminum Powders Doped with Metal Oxides. Propellants Explos. Pyrotech. 2019, 44, 1312. [Google Scholar] [CrossRef]
- Bao, Q.; Yang, Y.; Wen, X.; Guo, L.; Guo, Z. The preparation of spherical metal powders using the high-temperature remelting spheroidization technology. Mater. Des. 2021, 199, 109382. [Google Scholar] [CrossRef]
- Buchelnikov, V.D.; Louzguine-Luzgin, D.V.; Xie, G.; Yoshikawa, N.; Sato, M.; Anzulevich, A.P.; Bychkov, I.V.; Inoue, A. Heating of metallic powders by microwaves: Experiment and theory. J. Appl. Phys. 2008, 104, 113505. [Google Scholar] [CrossRef]
- Mishra, R.R.; Sharma, A.K. A Review of Research Trends in Microwave Processing of Metal-Based Materials and Opportunities in Microwave Metal Casting. Crit. Rev. Solid State Mater. Sci. 2016, 41, 217–255. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Cherepanova, V.A.; Sinayskiy, M.A.; Samokhin, A.V.; Ananikov, V.P. Systematic Study of the Behavior of Different Metal and Metal-Containing Particles under the Microwave Irradiation and Transformation of Nanoscale and Microscale Morphology. Nanomaterials 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Rao, K.J.; Vaidhyanathan, B.; Ganguli, M.; Ramakrishnan, P.A. Synthesis of inorganic solids using microwaves. Chem. Mater. 1999, 11, 882–895. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Semenov, V.E.; Egorov, S.V.; Eremeev, A.G.; Plotnikov, I.V.; Bykov, Y.V. Microwave heating of conductive powder materials. J. Appl. Phys. 2006, 99, 023506. [Google Scholar] [CrossRef]
- Mostovshchikov, A.V.; Korshunov, A.V.; Ilyin, A.P.; Kalinich, I.; Chumerin, P.Y. Influence of Microwave and Electron Beam Irradiation on Composition of Aluminum Nanopowder. KEM 2018, 769, 90–95. [Google Scholar] [CrossRef]
- Mostovshchikov, A.V.; Il’in, A.P.; Chumerin, P.Y.; Yushkov, Y.G. Parameters of Iron and Aluminum Nano- and Micropowder Activity upon Oxidation in Air under Microwave Irradiation. Techn. Phys. 2018, 63, 1223–1227. [Google Scholar] [CrossRef]
- Arkhipov, V.; Bondarchuk, S.; Goldin, V.; Zharova, I. The crystallization processes in the aluminum particles production technology. EPJ Web Conf. 2015, 82, 01016. [Google Scholar] [CrossRef] [Green Version]
- Gromov, A.A.; Teipel, U. Metal Nanopowders: Production, Characterization, and Energetic Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014; p. 440. [Google Scholar]
- Kwon, Y.-S.; Moon, J.-S.; Ilyin, A.P.; Gromov, A.A.; Popenko, E.M. Estimation of the reactivity of aluminum superfine powders for energetic applications. Combust. Sci. Tech. 2004, 176, 277–288. [Google Scholar] [CrossRef]
- Gromov, A.; Ilyin, A.; Förter-Barth, U.; Teipel, U. Characterization of aluminum powders: II. Aluminum nanopowders passivated by non-inert coatings. Propell. Explos. Pyrotech. 2006, 31, 401–409. [Google Scholar] [CrossRef]
- Ignatenko, M.; Tanaka, M. Effective permittivity and permeability of coated metal powders at microwave frequency. Phys. B Condens. Matter. 2010, 405, 352–358. [Google Scholar] [CrossRef]
- Auerkari, P. Mechanical and Physical Properties of Engineering Alumina Ceramics, VTT Tiedotteita—Meddelanden—Research Notes No. 1792; VTT Technical Research Centre of Finland: Espoo, Finland, 1996. [Google Scholar]
- Ivanov, G.V.; Tepper, F. ‘Activated’ aluminum as a stored energy source for propellants. Int. J. Energ. Mater. Chem. Propuls. 1997, 4, 636–645. [Google Scholar]
- Kuo, K.K.; Acharya, R. Applications of Turbulent and Multiphase Combustion; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 576. [Google Scholar]
- Mostovshchikov, A.V.; Ilyin, A.P.; Egorov, I.S. Effect of electron beam irradiation on the thermal properties of the aluminum nanopowder. Radiat. Phys. Chem. 2018, 153, 156–158. [Google Scholar] [CrossRef]
- Mostovshchikov, A.V.; Goldenberg, B.G.; Nazarenko, O.B. Effect of synchrotron radiation on thermochemical properties of aluminum micro- and nanopowders. Mater. Sci. Eng. B 2022, 285, 115961. [Google Scholar] [CrossRef]


| Mode | Power Flux Density (W/cm2) | Pulse Duration | Frequency (GHz) | Pulse Repetition Rate (Hz) |
|---|---|---|---|---|
| 1 | 8 | 25 ns | 2.85 | 25 |
| 2 | 8 | 3 µs | 2.85 | 25 |
| Frequency, GHz | μeff | εeff |
|---|---|---|
| 2.85 | 0.49 + 0.20i | 777.80 + 0.88i |
| 9.40 | 0.37 + 0.14i | 777.84 + 0.94i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostovshchikov, A.; Gubarev, F.; Nazarenko, O.; Pestryakov, A. Influence of Short-Pulse Microwave Radiation on Thermochemical Properties Aluminum Micropowder. Materials 2023, 16, 951. https://doi.org/10.3390/ma16030951
Mostovshchikov A, Gubarev F, Nazarenko O, Pestryakov A. Influence of Short-Pulse Microwave Radiation on Thermochemical Properties Aluminum Micropowder. Materials. 2023; 16(3):951. https://doi.org/10.3390/ma16030951
Chicago/Turabian StyleMostovshchikov, Andrei, Fedor Gubarev, Olga Nazarenko, and Alexey Pestryakov. 2023. "Influence of Short-Pulse Microwave Radiation on Thermochemical Properties Aluminum Micropowder" Materials 16, no. 3: 951. https://doi.org/10.3390/ma16030951
APA StyleMostovshchikov, A., Gubarev, F., Nazarenko, O., & Pestryakov, A. (2023). Influence of Short-Pulse Microwave Radiation on Thermochemical Properties Aluminum Micropowder. Materials, 16(3), 951. https://doi.org/10.3390/ma16030951








