Kinetics of Oxygen Exchange and N2O Decomposition Reaction over MeOx/CeO2 (Me = Fe, Co, Ni) Catalysts
Abstract
1. Introduction
- (1)
- N2O + S ↔ N2O-S
- (2)
- N2O-S → N2 + O-S
- (3a)
- 2O-S ↔ O2 + 2S
- (3b)
- O-S + N2O → N2 + O2 + S
- (3c)
- O2-S ↔ O2 + S
- (4)
- N2O + O-S N2 + O2-S
2. Materials and Methods
2.1. Samples Preparation and Characterization
2.2. Temperature-Programmed Isotopic Exchange (TPIE) of O2
2.3. Catalytic Activity Measurement
3. Results and Discussion
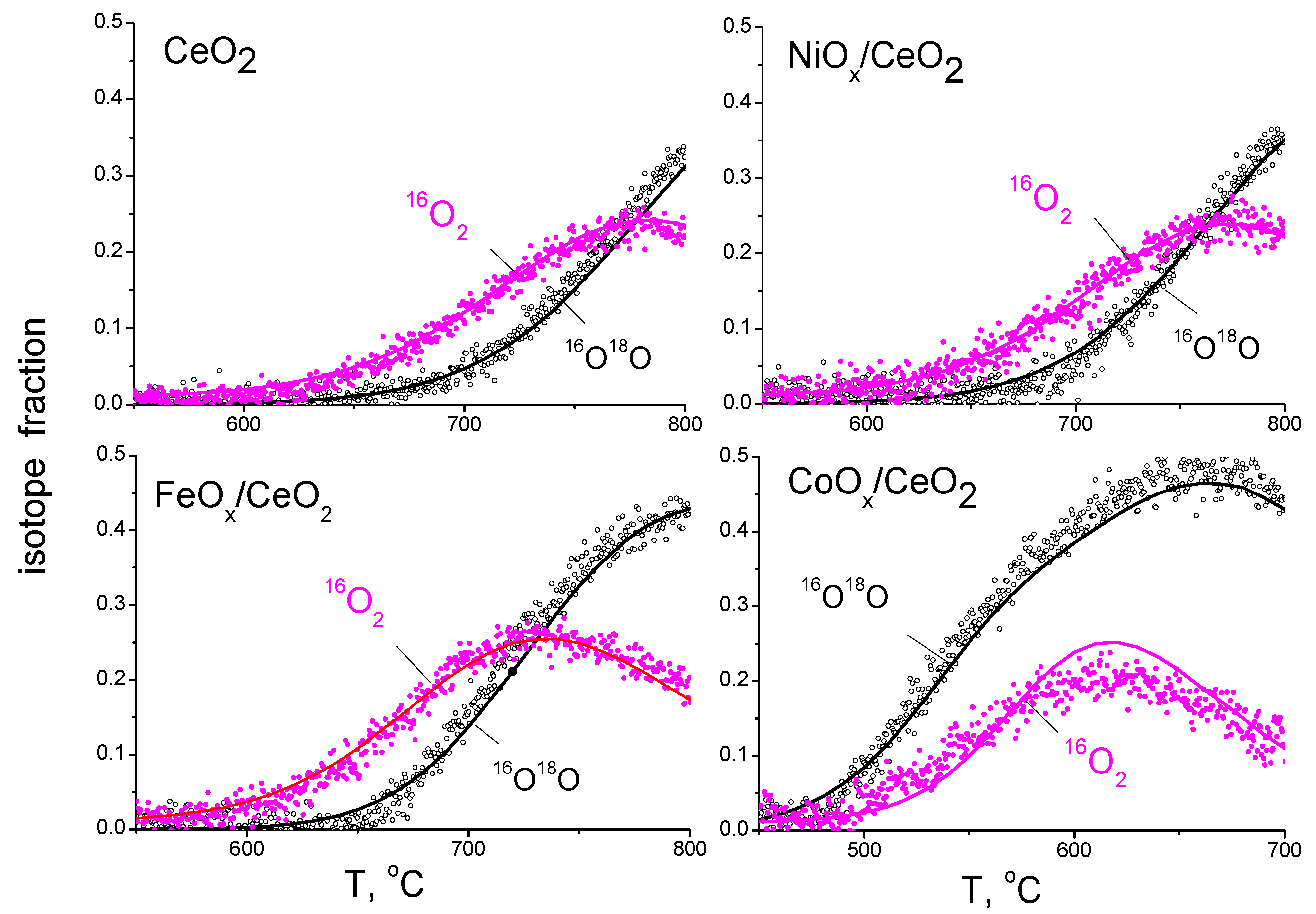
3.1. Qualitative Analysis of Oxygen Isotope Exchange Process
3.2. Quantitative Estimates of Oxygen Isotope Exchange Rates
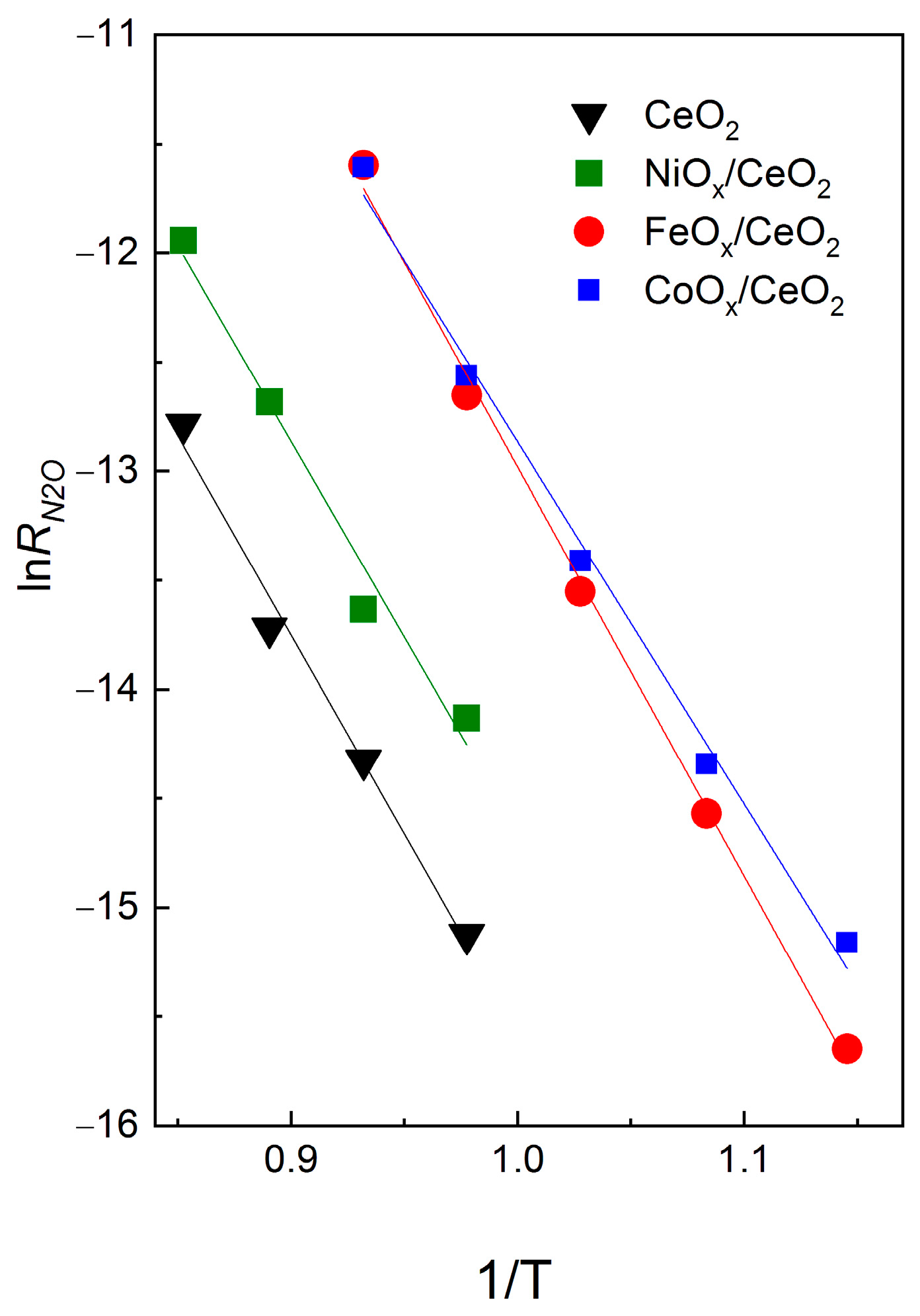
3.3. Comparison of the Rates of N2O Decomposition and Oxygen Isotope Exchange
3.4. Mechanism and Form of Rate Equation for Oxygen Isotope Exchange in Oxides
3.5. Mechanism and Equation Rate for N2O Decomposition Reaction
3.6. Mobility of Surface Oxygen and Rate Determining Steps of Two Atomic Isotope Exchange and N2O Decomposition Reaction
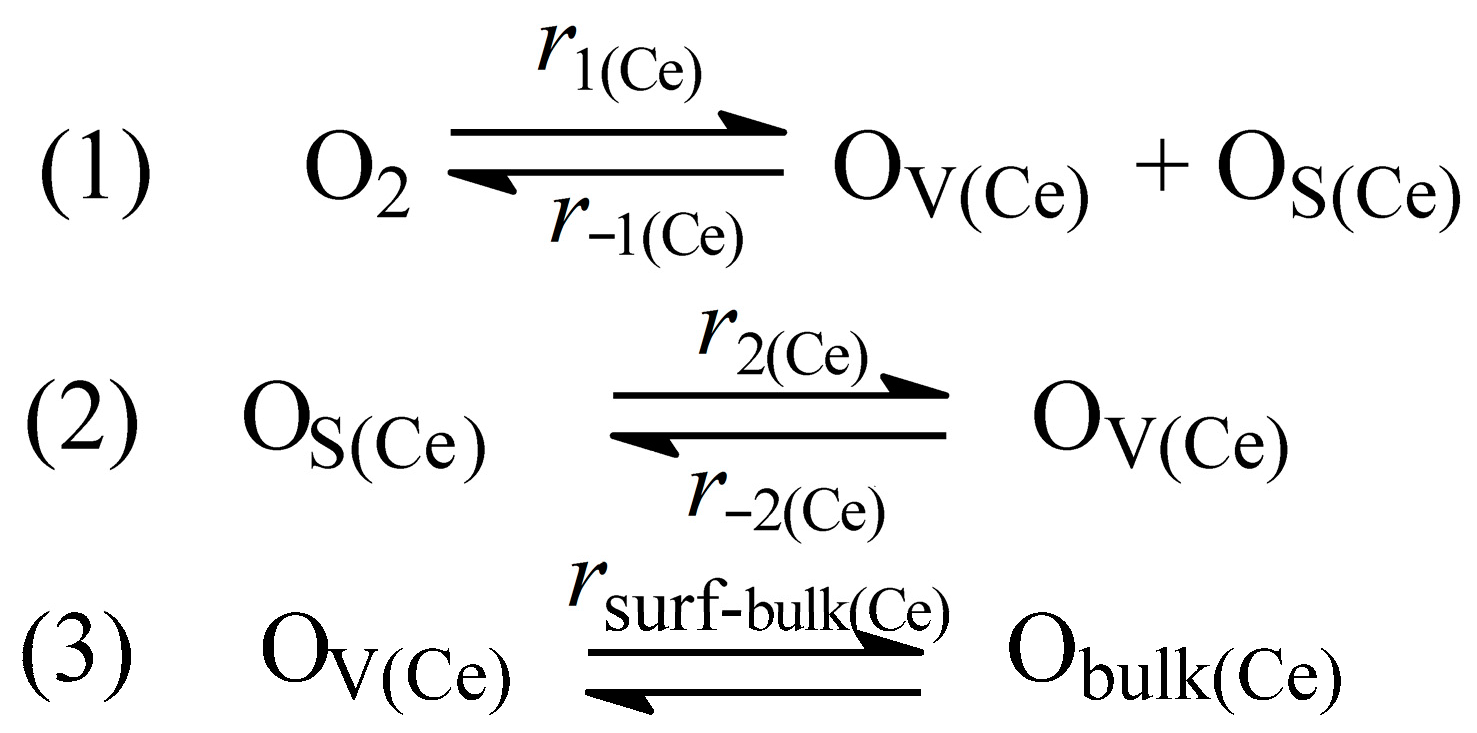
3.7. Role of MeOx–CeO2 Interface in the Reactions of Isotope Exchange and N2O Decomposition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Numerical Analysis of Temperature Programmed Isotope Exchange of Oxygen
Appendix B. Derivation of an Equation for the Rate Oxygen Two-Atom Exchange
Appendix C. Derivation of an Equation for Steady State Rate of N2O Decomposition
Appendix D. Determination of the Order of deN2O Reaction Rate on FeOx/CeO2

References
- Kapteijn, F.; Marban, G.; Rodriguez-Mirasol, J.; Moulijn, J.A. Kinetic Analysis of the Decomposition of Nitrous Oxide over ZSM-5 Catalysts. J. Catal. 1997, 167, 256–265. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. NO-Assisted N2O Decomposition over Fe-Based Catalysts: Effects of Gas-Phase Composition and Catalyst Constitution. J. Catal. 2002, 208, 211–223. [Google Scholar] [CrossRef]
- Fu, C.M.; Korchak, V.N.; Hall, W.K. Decomposition of nitrous oxide on FeY zeolite. J. Catal. 1981, 68, 166–171. [Google Scholar] [CrossRef]
- Raj, S.L.; Srinivasan, V. Decompositi90049on of nitrous oxide on rare earth manganites. J. Catal. 1980, 65, 121–126. [Google Scholar] [CrossRef]
- Sazonov, L.A.; Moskvina, Z.V.; Artamonov, E.V. Investigation of catalytic properties of LnMeO3 oxides in oxygen homomolecular exchange reaction. Kinet. Catal. 1974, 15, 120–126. (In Russian) [Google Scholar]
- Winter, E.R.S. Exchange reactions of oxides. Part IX. J. Chem. Soc. A 1968, 2889–2902. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Sadovskaya, E.M.; Pinaeva, L.G.; Isupova, L.A. Influence of oxygen mobility on catalytic activity of La–Sr–Mn–O composites in the reaction of high temperature N2O decomposition. J. Catal. 2009, 267, 5–13. [Google Scholar] [CrossRef]
- Pinaeva, L.G.; Prosvirin, I.P.; Dovlitova, L.S.; Danilova, I.G.; Sadovskaya, E.M.; Isupova, L.A. MeOx/Al2O3 and MeOx/CeO2 (Me = Fe, Co, Ni) catalysts for high temperature N2O decomposition and NH3 oxidation. Catal. Sci. Technol. 2016, 6, 2150–2161. [Google Scholar] [CrossRef]
- Pinaeva, L.; Prosvirin, I.; Chesalov, Y.; Atuchin, V. High-Temperature Abatement of N2O over FeOx/CeO2-Al2O3 Catalysts: The Effects of Oxygen Mobility. Catalysts 2022, 12, 938. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Pérez-Ramírez, J. Mechanism and Kinetics of Direct N2O Decomposition over Fe−MFI Zeolites with Different Iron Speciation from Temporal Analysis of Products. J. Phys. Chem. B 2006, 110, 22586–22595. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Pérez-Ramírez, J. Micro-kinetic analysis of direct N2O decomposition over steam-activated Fe-silicalite from transient experiments in the TAP reactor. Catal. Today 2007, 121, 197–203. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Kondratenko, V.A.; Santiago, M.; Pérez-Ramírez, J. Mechanistic origin of the different activity of Rh-ZSM-5 and Fe-ZSM-5 in N2O decomposition. J. Catal. 2008, 256, 248–258. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Kondratenko, V.A.; Santiago, M.; Pérez-Ramírez, J. Mechanism and micro-kinetics of direct N2O decomposition over BaFeAl11O19 hexaaluminate and comparison with Fe-MFI zeolites. Appl. Catal. B Environ. 2010, 99, 66–73. [Google Scholar] [CrossRef]
- Boreskov, G.K.; Muzykantov, V.S. Investigation of Oxide-Type Oxidation Catalysts by Reactions of Oxygen Isotopic Exchange. Ann. N. Y. Acad. Sci. 1973, 213, 137–170. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Domen, K.; Maruya, K.; Onishi, T. Oxygen exchange reactions over cerium oxide: An FT-IR study. J. Catal. 1990, 123, 436–442. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Gross, M.S.; Ulla, M.A.; Querini, C.A.J. Diesel particulate matter combustion with CeO2 as catalyst. Part I: System characterization and reaction mechanism. Mol. Catal. A 2012, 352, 86–94. [Google Scholar] [CrossRef]
- Winter, E.R.S. The reactivity of oxide surfaces. Adv. Catal. 1958, 10, 196–241. [Google Scholar] [CrossRef]
- Duprez, D. Isotopes in Heterogeneous Catalysis; Imperial College Press: London, UK, 2006; pp. 133–181. [Google Scholar] [CrossRef]
- Boreskov, G.K. The catalysis of isotopic exchange in molecular oxygen. Adv. Catal. 1965, 15, 285–339. [Google Scholar] [CrossRef]
- Klier, K.; Nováková, J.; Jíru, P. Exchange Reactions of Oxygen between Oxygen Molecules and Solid Oxides. J. Catal. 1963, 2, 479–484. [Google Scholar] [CrossRef]
- Klier, K.; Kučera, E. Theory of exchange reactions between fluids and solids with tracer diffusion in the solid. J. Phys. Chem. Solids 1966, 27, 1087–1095. [Google Scholar] [CrossRef]
- Muzykantov, V.S. Isotopic Studies of Dioxygen Activation on Oxide Catalysts for Oxidation: Problems, Results and Perspectives. React. Kinet. Catal. Lett. 1987, 35, 437–447. [Google Scholar] [CrossRef]
- Muzykantov, V.S.; Kemnitz, E.; Sadykov, V.A.; Lunin, V.V. Interpretation of Isotope Exchange Data “without Time”: Nonisothermal Exchange of Dioxygen with Oxides. Kinet. Catal. 2003, 44, 319–322. [Google Scholar] [CrossRef]
- Borovskikh, L.; Mazo, G.; Kemnitz, E. Reactivity of oxygen of complex cobaltates La1−xSrxCoO3−δ and LaSrCoO4. Solid State Sci. 2003, 5, 409–417. [Google Scholar] [CrossRef]
- Galdikas, A.; Duprez, D.; Descorme, C. A novel dynamic kinetic model of oxygen isotopic exchange on a supported metal catalyst. Appl. Surf. Sci. 2004, 236, 342–355. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Pinaeva, L.G.; Isupova, L.A.; Sadovskaya, E.M.; Prosvirin, I.P.; Gerasimov, E.Y.; Yakovleva, I.S. Effect of surface decoration with LaSrFeO4 on oxygen mobility and catalytic activity of La0.4Sr0.6FeO3−δ in high-temperature N2O decomposition, methane combustion and ammonia oxidation. Appl. Catal. A 2013, 457, 42–51. [Google Scholar] [CrossRef]
- Muzykantov, V.S.; Popovskii, V.V.; Boreskov, G.R. Kinetics of isotope exchange in a mocecular oxygen-solid oxide system. Kinet. Catal. 1964, 5, 624–629. (In Russian) [Google Scholar]
- Vasiliades, M.A.; Harris, D.; Stephenson, H.; Boghosian, S.; Efstathiou, A.M. A Novel Analysis of Transient Isothermal 18O Isotopic Exchange on Commercial CexZr1−xO2−δ-based OSC Materials. Top. Catal. 2019, 62, 219–226. [Google Scholar] [CrossRef]
- Cortés-Reyes, M.; Martínez-Munuera, J.C.; Herrera, C.; Larrubia, M.Á.; Alemany, L.J.; García-García, A. Isotopic study of the influence of oxygen interaction and surface species over different catalysts on the soot removal mechanism. Catal. Today 2022, 384–386, 33–44. [Google Scholar] [CrossRef]
- Artsiusheuski, M.A.; van Bokhoven, J.A.; Sushkevich, V.L. Oxygen Isotope Exchange over Copper-Containing Mordenite: The Effect of Copper Loading and Si/Al Ratio. J. Phys. Chem. C 2021, 125, 26512–26521. [Google Scholar] [CrossRef]
- Wilczkowska, E.; Krawczyk, K.; Petryk, J.; Sobczak, J.W.; Kaszkur, Z. Direct nitrous oxide decomposition with a cobalt oxide catalyst. Appl. Catal. A Gen. 2010, 389, 165–172. [Google Scholar] [CrossRef]
- Iwanek, E.K.; Krawczyk, J.; Petryk, J.W.; Sobczak, J.W.; Kaszkur, Z. Direct nitrous oxide decomposition with CoOx-CeO2 catalysts. Appl. Catal. B Environ. 2011, 106, 416–422. [Google Scholar] [CrossRef]
- Eley, D.D.; Rideal, E.K. The catalysis of the parahydrogen conversion by tungsten. Proc. R. Soc. Lond. 1941, 178, 429–451. [Google Scholar] [CrossRef]
- Farkas, A.; Farkas, L. Experiments on heavy hydrogen—Part I. Proc. R. Soc. A Math. Phys. Eng. Sci. 1934, 144, 467–480. [Google Scholar] [CrossRef]
- Kaptein, F.; Rodrigues-Mirasol, J.; Moulijn, J.A. Heterogeneous catalytic decomposition of nitrous oxide. Appl. Catal. B Environ. 1996, 9, 25–64. [Google Scholar] [CrossRef]
- Boreskov, G.K.; Kasatkina, L.A. Catalysis of Isotope Exchange in Molecular Oxygen and Its Application to the Study of Catalysts. Russ. Chem. Rev. 1968, 37, 613–628. [Google Scholar] [CrossRef]
- Popovskij, V.V.; Boreskov, G.K. Kinetics of isotope exchange of molecular oxygen and oxygen of the surface of iron, cobalt, nickel and copper oxides. Kinet. Catal. 1960, 1, 566–575. (In Russian) [Google Scholar]
- Pinaeva, L.; Isupova, L.; Prosvirin, I.; Sadovskaya, E.; Danilova, I.; Ivanov, D.; Gerasimov, E. La–Fe–O/CeO2 Based Composites as the Catalysts for High Temperature N2O Decomposition and CH4 Combustion. Catal. Lett. 2013, 143, 1294–1303. [Google Scholar] [CrossRef]
- Solsona, B.; Concepción, P.; Hernández, S.; Demicol, B.; López Nieto, J.M. Oxidative dehydrogenation of ethane over NiO–CeO2 mixed oxides catalysts. Catal. Today 2012, 180, 51–58. [Google Scholar] [CrossRef]
- Nagasawa, T.; Kobayashi, A.; Sato, S.; Kosaka, H.; Kim, K.; You, H.M.; Hanamura, K.; Terada, A.; Mishima, T. Visualization of oxygen storage process in Pd/CeO2-ZrO2 three-way catalyst based on isotope quenching technique. Chem. Eng. J. 2023, 453, 139937. [Google Scholar] [CrossRef]
- Zasada, F.; Grybos, J.; Budiyanto, E.; Janas, J.; Sojka, Z. Oxygen species stabilized on the cobalt spinel nano-octahedra at various reaction conditions and their role in catalytic CO and CH4 oxidation, N2O decomposition and oxygen isotopic exchange. J. Catal. 2019, 371, 224–235. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Noronha, F.B.; Toniolo, F.S. Pt nanoparticles embedded in CeO2 and CeZrO2 catalysts for biogas upgrading: Investigation on carbon removal mechanism by oxygen isotopic exchange and DRIFTS. J. CO2 Util. 2021, 49, 101572. [Google Scholar] [CrossRef]
- Grzybek, G.; Stelmachowski, P.; Gudyka, S.; Indyka, P.; Sojka, Z.; Guillén-Hurtado, N.; Rico-Pérez, V.; Bueno-López, A.; Kotarba, A. Strong dispersion effect of cobalt spinel active phase spread over ceriafor catalytic N2O decomposition: The role of the interface periphery. Appl. Catal. B 2016, 180, 622–629. [Google Scholar] [CrossRef]
- Zhang, R.; Miller, J.T.; Baertsch, C.D. Identifying the active redox oxygen sites in a mixed Cu and Ce oxide catalyst by in situ X-ray absorption spectroscopy and anaerobic reactions with CO in concentrated H2. J. Catal. 2012, 294, 69–78. [Google Scholar] [CrossRef]

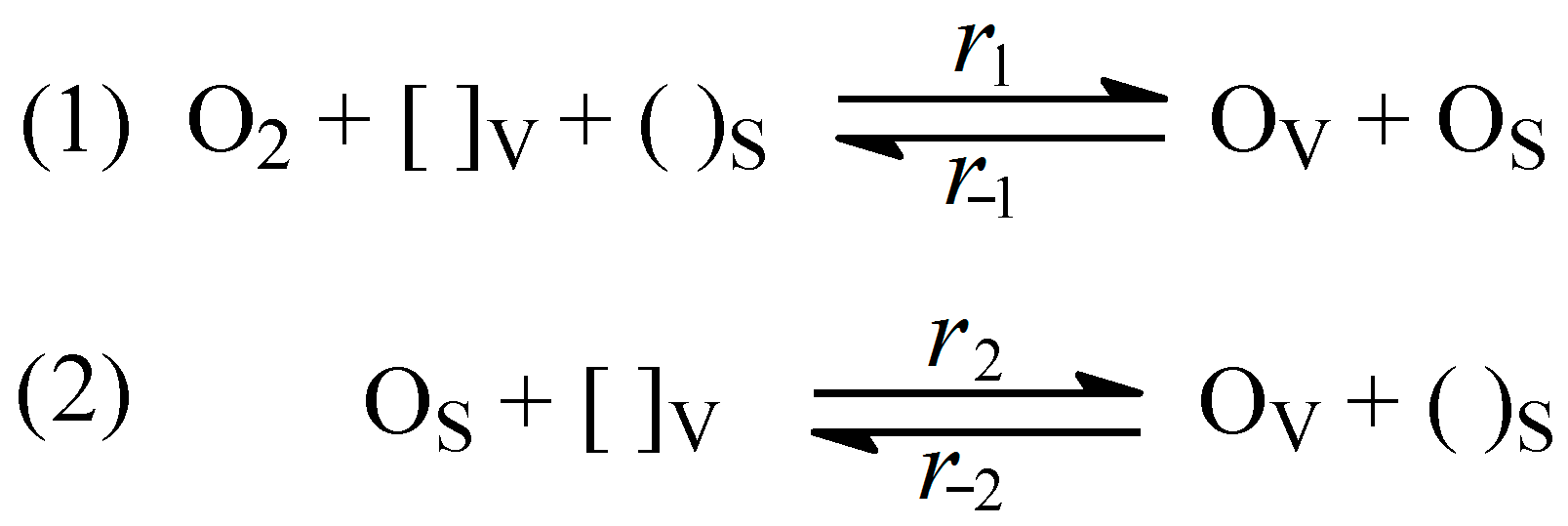


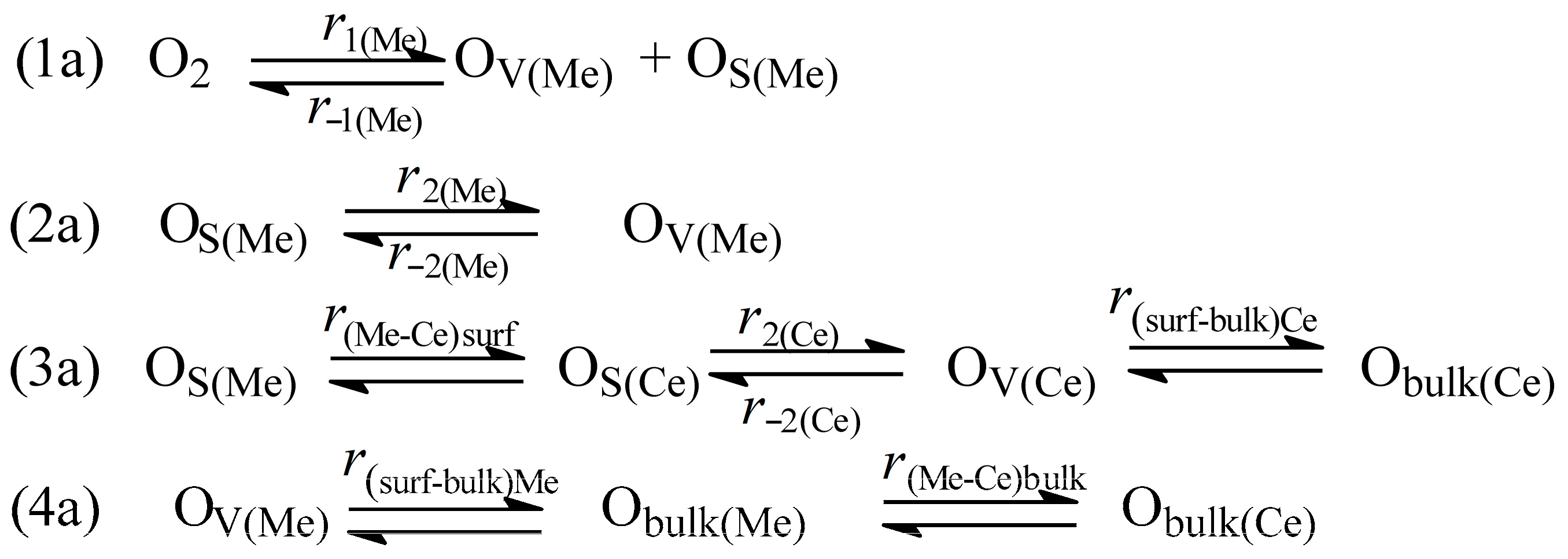

| Sample | NO | R × 105 | RI × 105 | RII × 105 | RII/R | ER |
|---|---|---|---|---|---|---|
| CeO2 | 4.5 1 | 0.42 (±0.02) | 0.03 (±0.01) | 0.4 (±0.02) | 0.95 (±0.05) | 170 (±10) |
| NiOx/CeO2 | 2.2 2 | 0.52 (±0.02) | 0.03 (±0.01) | 0.5 (±0.02) | 0.96 (±0.55) | 170 (±10) |
| FeOx/CeO2 | 5.4 1 | 1.4 (±0.08) | 0.1 (±0.02) | 1.3 (±0.08) | 0.93 (±0.04) | 175 (±10) |
| CoOx/CeO2 | 6.4 | 26 (±1) | 41 (±1) | 5.5 (±0.5) | 0.21 (±0.02) | 160 (±10) |
| Sample | RN2O × 106, mol/m2s | ERN2O, kJ/mole | RII/RN2O | R/RN2O |
|---|---|---|---|---|
| CeO2 | 0.27 | 152 | 15 | 16 |
| NiOx/CeO2 | 0.73 | 153 | 7 | 7 |
| FeOx/CeO2 | 3.2 | 156 | 5 | 5 |
| CoOx/CeO2 | 3.7 | 138 | 14 | 70 |
| Sample | N2O Conversion, % | |
|---|---|---|
| 0.15% N2O in He | 0.15% N2O + 3% O2 in He | |
| FeOx/CeO2 | 72.8 | 63.8 |
| CoOx/CeO2 | 70.8 | 65.4 |
| Sample | S | (r1 = r−1) × 105 mol/m2s | (r2 = r−2) × 105 mol/m2s | r2(−2)/r1(−1) |
|---|---|---|---|---|
| CeO2 | 0.93 (±0.04) | 0.43 (±0.03) | 8 (±3) | 20 (±10) |
| NiOx/CeO2 | 0.94 (±0.04) | 0.53 (±0.03) | 15 (±7) | 30 (±20) |
| FeOx/CeO2 | 0.92 (±0.04) | 1.4 (±0.1) | 16 (±5) | 12 (±5) |
| CoOx/CeO2 | 0.12 (±0.01) | 46 (±1.5) | 7 (±1) | 0.15 |
| Sample | Me/Ce | RI (Me,Ce), s−1 | RII (Me,Ce), s−1 | (r1 = r−1) (Me,Ce), s−1 | (r2 = r−2) (Me,Ce), s−1 |
|---|---|---|---|---|---|
| CeO2 | 0.015 | 0.245 | 0.26 (±0.02) | 4.8 (±2) | |
| NiOx/CeO2 | 0.12 | 0.04 | 0.68 | 0.72 (±0.02) | 12 (±6) |
| FeOx/CeO2 | 0.13 | 0.4 | 4.3 | 4.7 (±0.05) | 48 (±15) |
| CoOx/CeO2 | 0.14 | 190 | 23 | 210 (±7) | 25 (±3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadovskaya, E.; Pinaeva, L.; Skazka, V.; Prosvirin, I. Kinetics of Oxygen Exchange and N2O Decomposition Reaction over MeOx/CeO2 (Me = Fe, Co, Ni) Catalysts. Materials 2023, 16, 929. https://doi.org/10.3390/ma16030929
Sadovskaya E, Pinaeva L, Skazka V, Prosvirin I. Kinetics of Oxygen Exchange and N2O Decomposition Reaction over MeOx/CeO2 (Me = Fe, Co, Ni) Catalysts. Materials. 2023; 16(3):929. https://doi.org/10.3390/ma16030929
Chicago/Turabian StyleSadovskaya, Ekaterina, Larisa Pinaeva, Valerii Skazka, and Igor Prosvirin. 2023. "Kinetics of Oxygen Exchange and N2O Decomposition Reaction over MeOx/CeO2 (Me = Fe, Co, Ni) Catalysts" Materials 16, no. 3: 929. https://doi.org/10.3390/ma16030929
APA StyleSadovskaya, E., Pinaeva, L., Skazka, V., & Prosvirin, I. (2023). Kinetics of Oxygen Exchange and N2O Decomposition Reaction over MeOx/CeO2 (Me = Fe, Co, Ni) Catalysts. Materials, 16(3), 929. https://doi.org/10.3390/ma16030929





