Abstract
The shielding and spectroscopic properties of Pr+3 and Pr3+/Ho3+-codoped tellurite glass were investigated. The intensity parameters (Ω2 = 3.24-, Ω4 = 1.64-, Ω6 = 1.10 × 10−20 cm2) as well as the radiative lifetimes of 3F4 + 5S2 and 5I6 excited states of Ho3+ ions were equal to 301 μs and 3.0 μs, respectively. The former value appears to be much higher than that obtained from the lifetime measurement, indicating the presence of various energy transfer processes. The NIR spectrum of Pr3+/Ho3+-co-doped tellurite glass is dominated by strong Ho3+: 5I6 emission at around 1200 nm, being the result of the energy transfer from Pr3+ to Ho3+ ions. The shielding effectiveness of the prepared glasses showed good performance against high-energy photons. These findings suggest that the prepared glasses could be used in laser technology such as photodynamic therapy (PDT) treatment procedures and as shielding for radiation protection.
Keywords:
tellurite glasses; absorption; emission; rare earth; spectroscopic; Judd–Ofelt; shielding materials 1. Introduction
Tellurite glasses with rare earth (RE) doping are promising materials for photonic applications such as solid-state lasers and lighting, optical amplifiers, and medical and sensing technologies. The applications of tellurite glasses are connected with their high transmittance from the visible to mid-infrared ranges (0.4–6 μm), high refractive index (n > 2), and low maximal phonon energy (~750 cm−1) [1,2]. One of the most frequently used dopants for tellurite glasses is the Ho3+ ion, which exhibits both visible and infrared emissions due to its unique energy level structure [3]. In particular, these materials are expected to show an emission in the IR region at 1.2 μm, corresponding to the 5I6 → 5I8 transition. In addition, the emissions are also observed at 1.38 and 1.46 μm, for the 5F4+5S2→5I6 and 5F5→5I6 transitions, respectively. Emission from levels 5I6 → 5I8 and 5F4+5S2 → 5I5 of Ho3+ ions may be promising for obtaining a signal band gain of 1.2–1.4 μm [4,5,6].
Ho3+ions can induce interesting emission and luminescence decays. Therefore, glasses doped with Ho3+ions exhibit visible green and red emissions at exited level (5F4, 5S2)→5I8, and 5F5→5I8, respectively [6,7,8,9,10]. The relationship between green and red emission intensities strongly depends on the base matrix and the concentration of Ho3+ ions. Host materials containing Pr3+ ions have a broader luminescent range than other trivalent rare-earth ions [10,11]. Glasses that are co-doped with holmium and praseodymium have been studied as sources of applications of lasers in the mid-infrared and white light radiation. Double doping with Pr3+ and Ho3+ ions is mainly used to increase mid-infrared emissions. As for NIR emission, which is usually weak for Ho3+-only doped materials, a significant increase in PL intensity can be achieved by co-doping with Pr3+ ions, as signaled in Ref. [12]
In addition to the enhanced luminescence properties, the tellurite glasses have been widely investigated for different medical radiation applications such as shielding material and photodynamic therapy (PDT) treatment procedures [13,14,15,16,17,18,19,20,21,22]. For use in photodynamic therapy (PDT) treatment procedures and medical radiation technologies, laser glasses doped with rare earth elements such (Pr3+, Sm3+, Ho3+, and Tm3+) have been developed [20,21,22]. Light sources for PDT include incandescent lamps and xenon lamps with appropriate filters. However, their progress and use are limited by the relatively low light intensity of these sources, making lasers, which can be precisely directed and produce strong light, the preferred illumination method of choice. For application as a safety replacement device in medical workplaces such as PDT, X-ray, and atomic projects in the area of innovations, V-ray equipment, gamma camera rooms, and computed tomography (CT) examination workplaces, researchers analyzed the lasing and shielding qualities of such glasses [21,22]. According to J. Yang et al. [21], the radiant flux and quantum yield for the red fluorescence of Pr3+ is calculated to be 219W and 11.80%, respectively, under the commercial blue LED illumination. The excitation band of the majority of photosensitizers (PS) matches the wavelength range of 85.24% of the fluorescence photons in the visible region, showing significant potential for photodynamic therapy (PDT) treatment and clinical studies [21].
The aim of this study is to investigate spectroscopic analyses of novel, multicomponent tellurite glasses co-doped with Pr3+/Ho3+, to be employed as a laser material in radiology rooms for photodynamic therapy surgery (PDT). Moreover, the shielding effectiveness of the prepared glasses has been investigated due to the advantages of tellurite glasses doped rare earth ions as shielding material.
2. Experimental Work
The core six tellurite glass samples were prepared by melting 25 g batches of highly pure (99.99%) chemicals in gold crucibles at 850 °C in an ambient atmosphere. The resulting material has the molar composition 78TeO2- 10Nb2O5- 5PbO- 1PbF2- 5Li2O-1La2O3. To prevent vaporization losses, a platinum plate was placed on top of the crucibles. While melting, the melts were periodically agitated to prevent inhomogeneity. The melts were then placed onto plates that had been warmed to 400 °C, generating layers that were a few mm thick, and these layers were then annealed between 320 and 340 °C. The concentrations of lanthanide ions (NLn) of Pr2O3 and Ho2O3 incorporated into the host matrix have been calculated by the equation reported in Ref [23]. The density of the prepared glasses was determined using Archimedes’ law [23]. The glass samples were sliced and polished to a size of around 5’5’2 mm3 for spectroscopic measurements. The M-2000 Woollam ellipsometer was used to collect the ellipsometric data, and the Perkin Elmer Lambda 900 spectrophotometer was used to capture the transmittance and reflectance spectra. The luminescence decay curves were obtained after a short pulse stimulation delivered by an optical parametric oscillator powered by a third harmonic of a Nd:YAG laser [24]. The photoluminescence spectra were measured using an Optron Dong Woo fluorometer system. Table 1 shows the sample code, composition, molar mass, density, and concentrations of lanthanide ions. The shielding parameters of the prepared samples such mass and linear attenuation coefficients (MAC and LAC), half-value layer (HVL), and mean free path (MFP), were investigated using MIKE software [25].

Table 1.
The sample codes, compositions, molar weight, experimental densities, and the calculated concentrations of lanthanide ions of the investigated samples.
Shielding Properties
The possibility of photons interacting with a barrier is characterized as the mass attenuation coefficient (MAC) of a shielding material, and it is expressed as follows for a combination of elements or any chemical molecule [26].
where wi represents the fractional weight of individual components in each compound, and indicate the density of material, µ/ is mass attenuation of the individual components. The LAC can then be estimated by using the following relationship:
The average distance a photon can go through the barrier without interacting is known as the mean free path (MFP), and it is determined by the reciprocal of the linear attenuation [27]:
The thickness of the interaction target at which the attenuated intensities account for 50% of the narrow photon beam intensity is defined by the half-value layer (HVL). The necessary thickness of shielding material is inversely proportional to the HVL value. The HVL can be calculated using the following equation [27]:
3. Results and Discussion
3.1. Absorption Spectra and Judd–Ofelt Analysis
Figure 1 shows the net absorption bands of Pr3+ and Ho3+ ions, obtained by subtraction of the monotonic background absorption of the glass matrix (shown for sample T0 in [28]) from the total absorption determined for samples TPr and TPrHo.
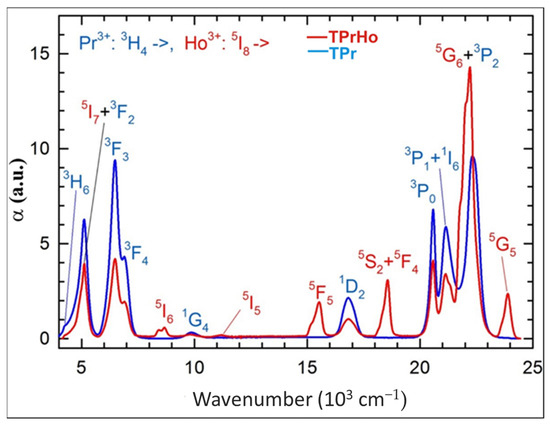
Figure 1.
Net absorption spectra of samples TPr and TPrHo.
As seen in Figure 1, the spectra of samples TPr and TPrHo show the existence of several single absorption bands attributed to transitions from the ground states of both ions, Pr3+:3H4 and Ho3+:5I8, to subsequent excited states, as indicated elsewhere [29].
In addition, the spectra of sample TPrHo show overlapping absorption bands associated with transitions to excited levels of Ho3+:5I7 and Pr3+:3F2 in the NIR region as well as transitions to levels of Ho3+:5G6 and Pr3+:3P2 in the visible region.
One can see in Figure 1 five Ho3+ absorption bands, that do not overlap with Pr3+ bands allowing one to perform the standard J-O analysis for the former ion. However, it appears that such an analysis results in a negative, unphysical value of the Ω2 J-O intensity parameter, due to the neglect of the strong Ho3+:5G6 absorption band. In order to include this band, we have subtracted from the sample TPrHo absorption a contribution from the Pr3+ absorption, which is equal to half of that measured for sample TPr, considering the difference in Pr3+ ion concentrations between the two samples as shown in Table 1. For completeness’ sake, we have also extracted the Ho3+:5I7 absorption band, and both bands are shown in Figure 2.
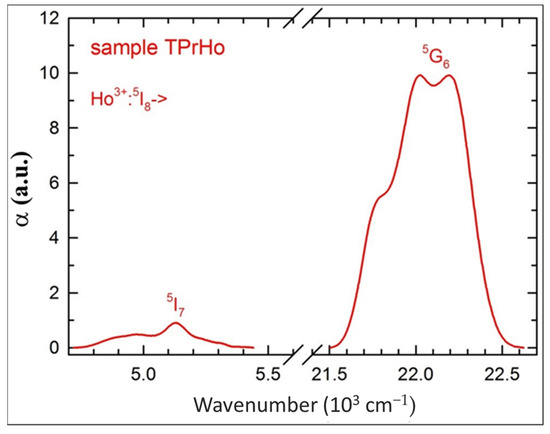
Figure 2.
Extracted absorption bands of Ho3+ ions for sample TPrHo.
It appears that the extracted absorption bands from Figure 2 are very similar to those determined for a single Ho3+-doped tellurite glass [30], justifying the extraction procedure.
Finally, we have applied the standard Judd–Ofelt theory to six electric dipole transitions specified in Table 2, calculating their oscillator strengths by numerical integration of the corresponding absorption bands; in order to minimize the root-mean-square deviation, the transition 5I8→5I7 has been omitted in the analysis due to a significant, magnetic dipole contribution to the oscillator strength and uncertainty connected with the extraction procedure [31]. The oscillator strength values of the prepared glass were calculated using Equation reported in Ref [23].

Table 2.
The experimental and calculated oscillator strengths and Judd–Ofelt intensity parameters for Ho3+ ions in sample TPrHo.
The refractive index dispersion of samples T0 and TPr, obtained from ellipsometric data, is presented in our previous work [28], as being very well described by the Sellmeier model of the form n(λ) = [A + Bλ2/(λ2 − C2) − Dλ2]1/2, where A, B, C, and D are the fitting parameters. Adopting the same procedure for sample TPrHo, the values of the fitting parameters have been found as A = 2.729, B = 1.764, C = 244.16 nm, and D = 2.52 × 10−9 nm−2. As for the reduced matrix elements for the electric dipole transitions, we have used the data from Ref. [32]. Table 2 shows the calculated Judd–Ofelt parameter values along with the root-mean square deviation (δrms).
The obtained values of the Ωi (I = 2,4,6) parameters follow the same pattern as those observed for different Ho3+ -doped tellurite glasses and follow the same sequence, namely Ω2 > Ω4 > Ω6 [29]. On the other hand, the sequence is different with Tellurite glasses doped with Pr+3 [24,28].
The J-O parameters have been used to estimate the transition probability Wr of 5I6 (323 s−1), 5S2 (4549 s−1) and 5F4 (2060 s−1) excited states of Ho3+ ions and subsequently the total radiative lifetimes τrad = 1/Wr. The levels 5S2 (lower) and 5F4 (higher) are very close to each other, leading to one absorption band, as shown in Figure 1. The occupation of these levels is governed by the Boltzmann factor, exp(-ΔE/kBT), where ΔE is the energy separation between the levels, kB is the Boltzmann constant, and T is temperature. To calculate ΔE, we have fitted two Gaussians to the (5S2 + 5F4) absorption band, which yields ΔE = 128 cm−1. The common transition probability for these levels is given by [33]
where J′ = 2 and J″ = 4 are the total momenta of 5S2 and 5F4 excited levels, respectively.
3.2. Emission Spectra
In addition, the PL signal of both samples (in arbitrary units) has been divided by NPr; to illustrate the impact of Pr3+ ion concentration (NPr) on the emission spectra as shown in Table 1. Emission spectra were obtained under identical conditions, permitting comparison of their relative intensities.
A significant number of excited states of Pr3+ and Ho3+ ions and their relatively high concentrations in the investigated samples create many possible radiative and non-radiative energy transfer (ET) processes between the Pr-Pr, Ho-Ho, and Pr-Ho pairs as discussed below. Figure 3 shows the visible PL spectrum for samples TPr and TPrHo under 445 nm (22,472 cm−1) excitation, corresponding to Pr3+: 3H4→3P2 and Ho3+: 5I8→5G6 absorption bands, as shown in Figure 1.
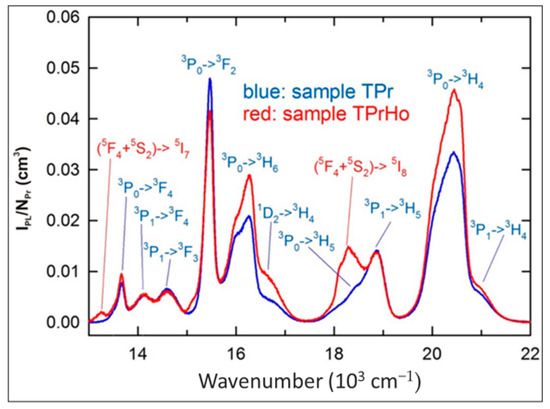
Figure 3.
Visible emission spectra of sample TPr and TPrHo (λexc = 445 nm).
When comparing the emission spectra from Figure 3, it is clear that the spectrum of sample TPrHo consists of many Pr3+ emission bands that are completed with two Ho3+ bands, namely with a relatively strong (5F4+5S2)→5I8 emission at 18,290 cm−1 and a weak (5F4+5S2)→5I7 emission at 13,250 cm−1. With the exception of the 3P0→3H6 and 3P0→3H4 bands, many Pr3+ emission bands are generally proportional to NPr, indicating that the concentration has been quenched by cross-relaxation and energy migration processes [30,34]. Similar cross-relaxation pathways result in numerous transitions between the energy levels of this ion in glasses doped exclusively with Ho3+ ions [30,35,36,37,38]. The energy level diagram of Ho+3/Pr+3 ion codoped in the present host matrix were shown in Figure 4.
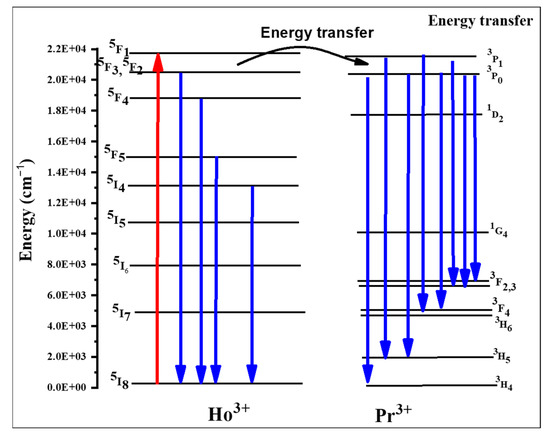
Figure 4.
The energy level diagram of Ho+3/Pr+3 ion.
Figure 5 displays the NIR emission spectra of the samples TPr and TPrHo, normalized as scale from the visible spectra in Figure 3.
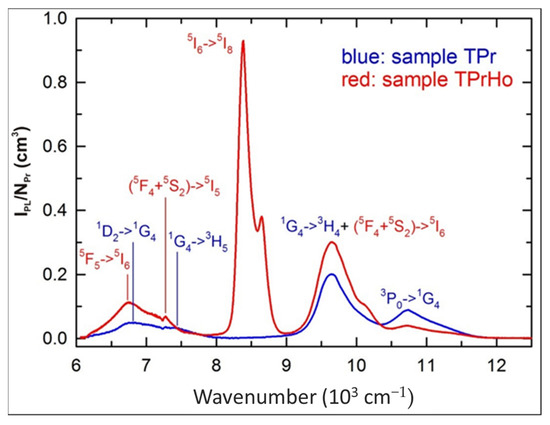
Figure 5.
NIR emission spectra of samples TPr and TPrHo (λexc = 445 nm).
For the sample TPrHo, the weak overlapping emission bands of prepared glasses doped with Pr3+ and Pr3+/Ho3+ ions in the 6000–8000 cm−1 region were attributed. Furthermore, beyond 9000 cm−1, there is another overlapping emission band of present glasses that appeared at 9648 cm−1. The sample TPrHo spectra is characterized by strong emission at 8386 cm−1 attributed to Ho3+: 5I6 transition. When comparing the emission of sample TPrHo to the transition Pr3+:3P0→1G4 emission from sample TPr at 10,735 cm−1, the energy transfer (ET) from Ho3+ to Pr3+ ions is observed. After 445 nm, the photons excite the Pr3+:3P2 level, and there is a non-radiative transition to the 3P0 level, followed by cross-relaxation and the 1G4 level as an intermediate state. This mechanism populates the Ho3+:5I6 level by the pathways Pr3+(3P0→1G4)→Ho3+(5I8→5I6) and Pr3+(1G4→3H4)→ Ho3+(5I8→5I6). The Ho3+:5I6→5I8 emission, which is preceded by the non-radiative and radiative transitions 5G6→(5F4+5S2) and (5F4+5S2)→5I6, is amplified by this down-conversion mechanism [35].
3.3. Lifetime
Table 3 includes the lifetimes of the 3P0 excited state of the Pr3+ ions as well as the predicted lifetime values and experimental data in order to compute the ET efficiency from these ions to Ho3+ ions. The 3P0 Lifetimes of TPr are taken from our previous work [28]. The (3F4+5S2) lifetime of TPrHo was measured and calculated as shown in Table 3. The 5I6 lifetime is only calculated in the present study.

Table 3.
Lifetimes of Pr3+ and Ho3+ emission bands obtained experimentally (τeff) as well as the calculated values using the J-O approach (τrad).
Figure 6 and Figure 7, respectively, show the excited levels of Ho3+:(5F4 + 5S2) and Pr3+:3P0’s PL decays. Since the decay data are non-exponential, we have established that the experimental lifespan is equal to the effective lifetime, which is defined as follows:
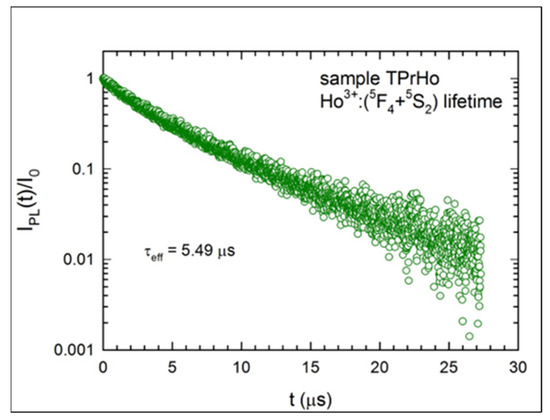
Figure 6.
Luminescence decay of the Ho3+:(5F4+5S2) emission for sample TPrHo.
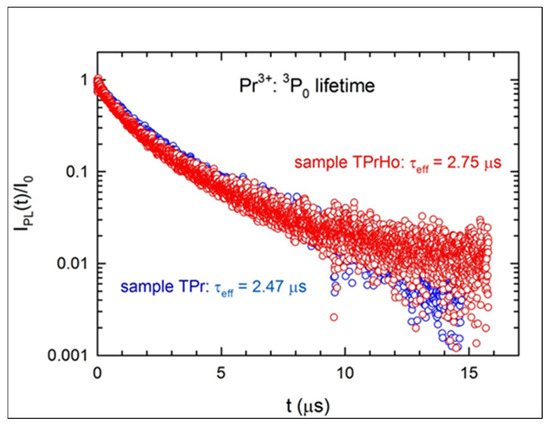
Figure 7.
Luminescence lifetimes of the Pr3+:3P0 emission for samples TPr and TPrHo.
There are numerous potential energy transfer mechanisms between the ions when Pr3+ and Ho3+ ions are present in sample TPrHo at relatively high and comparable concentrations. As a result, the measured lifetime of Ho3+:(5F4 + 5S2) emission is significantly shorter (5.49 μs) than that predicted from the J-O analysis (301μs; Table 3).
As was mentioned before while describing the sample’s emission spectra, sample TPr has a high concentration of Pr3+ ions, which results in an effective cross-relaxation (CR). When compared to the J-O radiative lifetime (9.43 μs [28]), the Pr3+:3P0 effective lifetime is significantly reduced by the CR mechanism, going down to τeff = 2.47 μs. The concentration of Pr3+ ions in sample TPrHo is two times lower than in sample TPr, so one would expect that τeff would be significantly higher and equal to 3.92 μs [28]. However, when the former sample is codoped with Ho3+ ions, an additional energy transfer (ET) occurs between the two ions, which reduces the Pr3+:3P0 lifetime to 2.75 μs.
The ET efficiency () from Pr3+ to Ho3+ ions can be estimated using the following equation, calculated as [29]
where τPr-Ho and τPr are the effective lifetimes with and without Ho3+ co-doping, respectively, for the same concentration of Pr3+ ions. Using the value τPr = 3.92 μs, corresponding to NPr =3.16×1020 cm−3, we have obtained the ET efficiency = 29% that can be compared with the values 19 and 32.3% found for tellurite glasses doped with 1 mol% of Pr3+ ions, and codoped with 0.5 and 1 mol% of Ho3+ ions, respectively [28]. The observed increase of ET efficiency with the increasing NHo/NPr ratio is in accordance with the theoretical modeling of quantum cutting via a two-step ET [39].
3.4. 5I8→5I6 Transition
The absorption cross-section (ACS) and emission cross-section (ECS) of the 5I8↔ 5I6 transition are the key characteristics for use in optoelectronics, thus we will focus on them.
The absorption cross-section (ACS; σabs) of the 5I8→5I6 transition was determined in the range of 1100–1250 nm by dividing the absorption coefficient by the Ho3+ ion concentration. This makes it possible to use the McCumber formula to determine the emission cross-section (ECS; τem) of the 5I6→5I8 transition [40].
where h is the Planck constant., Emt is the mean transition energy between the 5I8 and 5I6 levels, which can be calculated by averaging the barycenter energies of the absorption and emission spectra. This results in Emt = 8519 cm−1.
As for the measured 5I6→5I8 emission, it is given in arbitrary units in Figure 5. To scale this emission, we have used the equation for ECS following the Füchtbauer–Ladenburg (F-L) approach and given by (see Ref [9])
with the branching ratio β = 0.91 and Wr = 323 s−1 that were calculated for 5I6 → 5I8 emission from the J-O analysis.
The ACS and ECS spectrums were calculated using Equations (8) and (9). The calculated spectrum is shown in Figure 8.
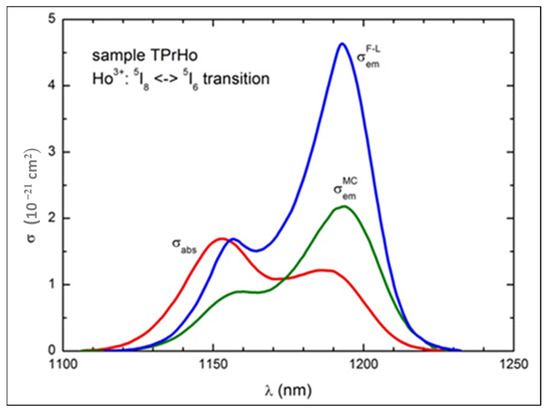
Figure 8.
The experimental absorption cross-section (σabs) and the emission cross-sections calculated for Ho3+: 5I6→5I8 emission using Equations (8) and (9), respectively.
As shown in Figure 8, there is a relatively small shift (107 cm−1) between the peak value of ACS at 1167 nm (10,246 cm−1) and that of ECS at 1181 nm (10,163 cm−1). The McCumber and Füchtbauer–Ladenburg (F-L) curves are quite similar in shape but very different in magnitude; the peak value of (4.63 × 10−21 cm2) is about two times greater than that of (× 10−21 cm2), suggesting effective ET from Pr3+ to Ho3+ ions, since the MC curve reflects emission of Ho3+ ions in the absence of Pr3+ ions, while the F-L curve represents the emission enhanced by codoping with the latter ions [39,40].
3.5. Shielding Properties
Over a broad energy range, ranging from 0.5 to 15 MeV, the shielding effectiveness of the produced glasses was examined. Using MIKE software, the radiation parameters of the investigated glasses were estimated. The mass attenuation coefficient and linear attenuation coefficients were investigated in the intermediate and high photon energy ranges, ranging between 500 keV and 15 MeV. The results of the mass attenuation coefficient and linear attenuation coefficients of the prepared glasses were compared to those of commercially available standard materials coded RS-253 G18, RS-520, and RS-360 [41], as shown in Figure 9a,b. As illustrated in Figure 9, the values of MAC and LAC decrease slowly as the photon energy increases. This trend is in fact due to the dominance of Compton scattering, which is directly proportional to the atomic number and inversely proportional to the photon energy [42]. As the photon energy increases above 5 MeV, a slight increase in the MAC and LAC is observed. This trend is mainly due to the contribution of the pair production process, which is directly proportional to the square of the atomic number and directly proportional to the photon energy [43]. For example, as shown in Table 4, the prepared glasses’ recoded LAC values at 6 MeV are 0.19588, 0.20125, and 0.20195 for T0, TPr, and TPrHo, respectively. As recoded, there is a slight increase in MAC and LAC values using different dopants; sample TPrHo recorded the highest values among the other prepared samples. In comparing the LAC values of the prepared glasses with some of the standard materials, namely RS-253 G18, RS-360, and RS-520, significant shielding performance was observed for the prepared glasses over the standard materials RS-253 G18 and RS-360 (0.069071 and 0.146893 at 6 MeV). On the other hand, the prepared glasses show a slightly lower performance compared with RS-520, this is obviously due to the higher lead oxide content in the RS-520 (70%) compared to 5% lead oxide in sample TPrHo (5%) in Table 4. The recoded MAC and LAC values of the prepared glasses at different energies range between 500 keV and 15 MeV. The good performance of the prepared glasses in terms of good optical, physical, and shielding properties such as good thermal stability, chemical durability, high values of linear and nonlinear refractive index, and shielding effectiveness are all due to the fact that the tellurite-based glass doped with suitable metal oxides and rare earths can form a high-efficiency glass material that can be used in different applications. [2,12,14].
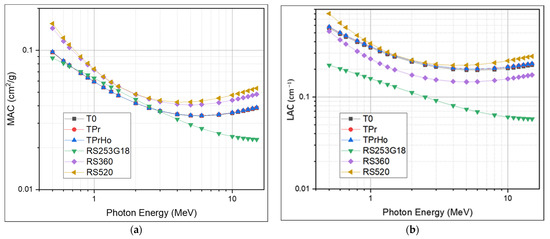
Figure 9.
(a) The mass attenuation coefficient of prepared glasses; (b) The linear attenuation coefficient of the prepared glasses.

Table 4.
The recoded MAC, LAC, HVL, and MFP values of the prepared glasses at 6 MeV.
The HVL represents the absorbance thickness necessary to halve the photon intensity. The mean free path (MFP) is the average distance a photon travels before colliding with a particle in a medium. The HVL and MFP values show how well the gamma radiation is slowed down by the shielding glass material. The HVL and MFP are inversely proportional to the shielding material’s linear attenuation coefficient (LAC); the lower the value, the more effective the material as a shield. The HVL and MFP of the prepared glasses are illustrated in Figure 10a,b. As shown in Figure 10a, there are two fundamental features of the HVL: First, the HVL of all prepared glasses increases with the increasing energy until reaching 6 MeV, it then decreases slowly with the increasing photon energy. For instance, as shown in Table 4, the recorded values at 6 MeV are equal to 3.54, 3.44, 3.43, 10.0, 4.71, and 3.08 cm for T0, TPr, TPrHo, RS254G18, RS360, and RS520, respectively. The recorded increase in HVL is caused by the decrease in the probability of interaction with high-energy photons, which increases the probability of penetrating the samples and necessitates a thicker sample to absorb the same amount of radiation. The second property of the HVL is that it decreases as the absorber density increases; at 6 MeV, the order of the HVL results is as follows: RS520 < TPrHo < TPr < T0 < RS360 < RS254G18. As discussed before, the prepared glasses have LAC values higher than standard materials RS360 and RS254G18, which explain the lower values recorded for the prepared glasses compared with the standard materials. Due to the toxicity of lead oxide, the prepared glasses have a better likelihood of being utilized as an alternative shielding material in medical applications such shielding glass windows and shielding materials used directly on patients undergoing X-ray examinations.
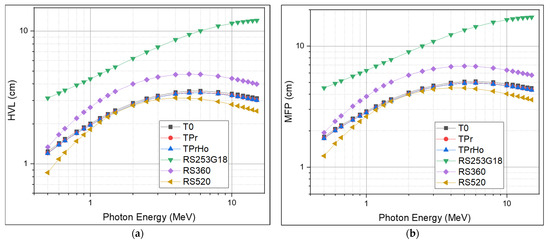
Figure 10.
(a) Half-value layer of prepared glasses; (b) Mean Free Path of prepared glasses.
The shielding effectiveness of the prepared glasses can also be investigated in terms of radiation protection efficiency (RPE) [44].
Figure 11 shows the RPE percentage of the prepared glasses with a thickness of 10 cm at photon energies ranging between 0.1 and 10 MeV. As shown in Figure 11, the RPE decreases with the increasing energy. For instance, the RPE percent decreased from 100 to 87.2% for T0, 100 to 87.9% for TPr, 100 to 88% for TPrHo, from 100 to 45.4% for RS-253G18, from 100 to 79.3% for RS-360, and from 100 to 91.5% for RS-520. As shown in Figure 11, the prepared glasses have good shielding efficiency compared to RS-254G18 and RS-360 and are slightly lower than RS-520. The 10 cm thickness of the prepared glasses has a shielding efficiency above 90% for energies up to 10 MeV.
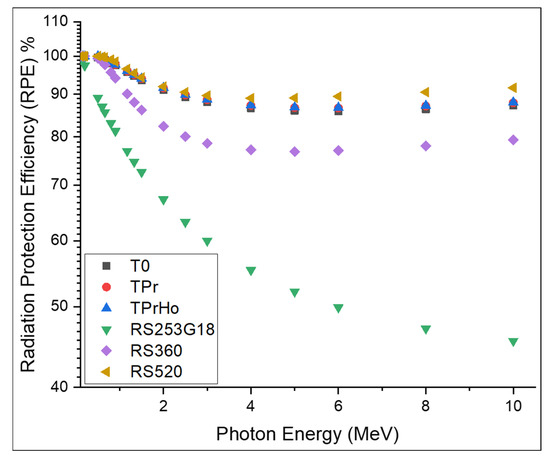
Figure 11.
RPE% with photon energy (in MeV) of the prepared glasses.
4. Conclusions
The spectroscopic properties of Pr3+-doped and Pr3+/Ho3+-co-doped multicomponent tellurite glass were investigated. Analysis of experimental data shows that there is energy transfer (ET) from Pr3+ to Ho3+ ions, which increases the emission of the latter ions by a factor of two at 1200 nm. The Pr3+−Ho3+ down-conversion ET, realized by two successive processes by cross-relaxation (CR), with the Pr3+:1G4 level as an intermediate state, is the cause of the extra emission, which was seen under the 445 nm illumination. This mechanism populates the Ho3+:5I6 level by Pr3+(3P0→1G4)→Ho3+(5I8→5I6) and Pr3+(1G4→3H4)→ Ho3+(5I8→5I6) pathways, with the excess energies dissipated in the glass matrix. A relatively low ET efficiency (29%) obtained for our Pr3+/Ho3+ co-doped tellurite glass can be significantly increased by optimizing the concentrations of both ions. The results of luminesces properties of the prepared glasses showed good performance as a laser source for photodynamic therapy (PDT) treatment procedures. In addition to that, the prepared glasses can also be considered as shielding material.
Author Contributions
B.B.-G.: Conceptualization, Methodology, Investigation, Writing—original draft, Writing—review and editing; M.R.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—original draft; E.S.Y.: Methodology, Writing—review and editing, Visualization; J.C.: Formal analysis, Investigation, Writing—original draft, Writing—review and editing, Visualization; R.L.: Methodology, Formal analysis, Writing—review and editing, Visualization; B.J. Formal analysis, review and editing, Visualization; A.A.: Formal analysis, Visualization, Writing—review and editing; K.I.H.: Conceptualization, Methodology, Investigation, Funding acquisition, Writing—review and editing, Visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deputyship for Research & Innovation, Ministry of Education, Saudi Arabia, grant number IFP-KKU-2020/7.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education, Saudi Arabia, for funding this research through project number IFP-KKU-2020/7.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rivera, V.A.G.; Manzani, D. (Eds.) Technological Advances in Tellurite Glasses; Springer Series in Materials Science; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- EI-Mallawany, R. (Ed.) Tellurite Glass Smart Materials, Applications in Optics and Beyond, Shielding Properties of Tellurite Glasses, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 17–28. ISBN 9783319765679. [Google Scholar] [CrossRef]
- Zhou, B.; Pun, E.Y.B.; Lin, H.; Yang, D.; Huang, L. Judd–Ofelt analysis, frequency upconversion, and infrared photoluminescence of Ho3+-doped and Ho3+/Yb3+-codoped lead bismuth gallate oxide glasses. J. Appl. Phys. 2009, 106, 103105. [Google Scholar] [CrossRef]
- Lisiecki, R.; Dominiak-Dzik, G.; Ryba-Romanowski, W.; Lukasiewicz, T. Conversion of infrared radiation into visible emission in YVO4 crystals doped with ytterbium and holmium. J. Appl. Phys. 2004, 94, 6323. [Google Scholar] [CrossRef]
- He, J.; Zhan, H.; Zhou, Z.; Zhang, A.; Lin, A. Study on 1.19 and 1.36 μm fluorescence for Ho-doped water-free fluorotellurite glasses. Opt. Commun. 2014, 320, 68–72. [Google Scholar] [CrossRef]
- Gavrilović, T.V.; Jovanović, D.J.; Trandafilović, L.V.; Dramićanin, M.D. Effects of Ho3+ and Yb3+ doping concentrations and Li+ co-doping on the luminescence of GdVO4 powders. Opt. Mater. 2015, 45, 76–81. [Google Scholar] [CrossRef]
- Pang, T.; Huang, Z. A novel upconverison phosphor Gd2Mo4O15: Yb3+, Ho3+: Intense red emission and ratiometric temperature sensing under 980 nm excitation. Mater. Res. Express 2018, 5, 066204. [Google Scholar] [CrossRef]
- Bordj, S.; Satha, H.; Barros, A.; Zambon, D.; Jouart, J.-P.; Diaf, M.; Mahiou, R. Spectroscopic characterization by up conversion of Ho3+/Yb3+ codoped CdF2 single crystal. Opt. Mater. 2021, 118, 111249. [Google Scholar] [CrossRef]
- Klimesz, B.; Lisiecki, R.; Ryba-Romanowski, W. Thermal, spectroscopic and optical sensor properties of oxyfluorotellurite glasses doped with holmium and ytterbium. Mater. Res. Bull. 2022, 153, 111909. [Google Scholar] [CrossRef]
- Kuwik, M.; Kowalska, K.; Pisarska, J.; Pisarski, W.A. Spectroscopic Properties of Pr3+, Tm3+, and Ho3+ in Germanate-Based Glass Systems Modified by TiO2. Materials 2023, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, C.; Zhu, L.; Zhao, D.; Li, J.; Zhou, Y. Pr3+/Tm3+/Er3+ tri-doped tellurite glass with ultra-broadband luminescence in the optical communication band. Ceram. Int. 2022, 48, 8779–8782. [Google Scholar] [CrossRef]
- Peng, J.; Xia, H.; Wang, P.; Hu, H.; Tang, L.; Zhang, Y.; Jiang, H.; Chen, B. Optical spectra and gain properties of Ho3+/Pr3+ co-doped LiYF4 crystal. J. Mater. Sci. Technol. 2014, 30, 910–916. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao., J. Investigation on radiation shielding parameters of bismuth borosilicate glass from 1keV to 100GeV. Ann. Nucl. Energy 2013, 55, 23–28. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Kawa, M.K.; Gaikwad, D.K.; Agar, O.; Gawai, U.P.; Baki, S.O. Physical, structural, optical and gamma radiation shielding properties of borate glasses containing heavy metals (Bi2O3/MoO3). J. Non-Cryst. Solids 2019, 507, 30–37. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; Rammah, Y.S. Electronic polarizability, dielectric, and gamma-ray shielding properties of some tellurite-based glasses. Appl. Phys. A 2019, 125, 1–9. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; Mann, K.S. Radiation shielding investigations for selected tellurite-based glasses belonging to the TNW system. Mater. Res. Express 2019, 6, 105206. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Al-Buriahi, M.S.; Abouhaswa, A.S. B2O3–BaCO3–Li2O3 glass system doped with Co3O4: Structure, optical, and radiation shielding properties. Phys. B Condens. Matter 2019, 576, 411717. [Google Scholar] [CrossRef]
- Sayyed, M.; Tekin, H.; Altunsoy, E.E.; Obaid, S.S.; Almatari, M. Radiation shielding study of tellurite tungsten glasses with different antimony oxide as transparent shielding materials using MCNPX code. J. Non-Cryst. Solids 2018, 498, 167–172. [Google Scholar] [CrossRef]
- Tijani, S.A.; Kamal, S.M.; Al-Hadeethi, Y.; Arib, M.; Hussein, M.A.; Wageh, S.; Dim, L.A. Radiation shielding properties of transparent erbium zinc tellurite glass system determined at medical diagnostic energies. J. Alloys Compd. 2018, 741, 293–299. [Google Scholar] [CrossRef]
- Chen, B.J.; Chen, L.F.; Pun, E.Y.B.; Lin, H. Sm3+ doped germanate glass channel waveguide as light source for minimally invasive photodynamic therapy surgery. Opt. Express 2012, 20, 879–889. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.J.; Pun, E.Y.B.; Zhai, B.; Lin, H. Pr3+-doped heavy metal germanium tellurite glasses for irradiative light source in minimally invasive photodynamic therapy surgery. Opt. Express 2013, 21, 1030–1040. [Google Scholar] [CrossRef]
- Yang, D.L.; Gong, H.; Pun, E.Y.B.; Zhao, X.; Lin, H. Rare-earth ions doped heavy metal germanium tellurite glasses for fiber lighting in minimally invasive surgery. Opt. Express 2010, 18, 18997–19008. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; Marzouk, S.; El–Sherbiny, M.; Atef, M.; Damak, K.; Alqahtani, M.S.; Algarni, H.; Reben, M.; Yousef, E.S. Spectroscopic properties in simple cost glasses with alkaline oxides doped with Sm2O3 for display laser emission. Results Phys. 2021, 31, 104955. [Google Scholar] [CrossRef]
- Burtan-Gwizdala, B.; Reben, M.; Cisowski, J.; Lisiecki, R.; Ryba-Romanowski, W.; Jarząbek, B.; Mazurak, Z.; Nosidlak, N.; Grelowska, I. The influence of Pr3+ content on luminescence and optical behavior of TeO2-WO3-PbO-Lu2O3 glass. Opt. Mater. 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Hussein, K.; Alqahtani, M.; Algarni, H.; Zahran, H.; Yaha, I.; Grelowska, I.; Reben, M.; Yousef, E. MIKE: A new computational tool for investigating radiation, optical and physical properties of prototyped shielding materials. J. Instrum. 2021, 16, T07004. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Rammah, Y.S.; Laariedh, F.; Abouhaswa, A.S.; Badeche, T.-B. Effect of Bi2O3 on some optical and gamma-photon-shielding properties of new bismuth borate glasses. Appl. Phys. A 2019, 125, 649. [Google Scholar] [CrossRef]
- Tekin, H.; Altunsoy, E.; Kavaz, E.; Sayyed, M.; Agar, O.; Kamislioglu, M. Photon and neutron shielding performance of boron phosphate glasses for diagnostic radiology facilities. Results Phys. 2019, 12, 1457–1464. [Google Scholar] [CrossRef]
- Burtan-Gwizdala, B.; Reben, M.; Cisowski, J.; Lisiecki, R.; Nosidlak, N. Strong emission at 1000 nm from Pr3+/Yb3+-codoped multicomponent tellurite glass. Pure Appl. Chem. 2022, 94, 147–156. [Google Scholar] [CrossRef]
- Trindade, C.; Alves, R.; Silva, A.; Dantas, N.; Gouveia-Neto, A. Tunable greenish to reddish luminescence and two-way energy transfer in Ho3+ and Pr3+ doped TeO2:ZnO glass. Opt. Mater. 2020, 99, 109574. [Google Scholar] [CrossRef]
- Sobczyk, M.; Marek, Ł. Comparative study of optical properties of Ho3+ -doped RE2O3 Na2O-ZnO-TeO2 glasses. J. Lumin. 2019, 206, 308–318. [Google Scholar] [CrossRef]
- Tanimura, K.; Shinn, M.D.; Sibley, W.A. Optical transitions of Ho3+ ions in fluorozirconate glass. Phys. Rev. B 1984, 30, 2429–2437. [Google Scholar] [CrossRef]
- Rukmini, E.; Jayasankar, C.K. Spectroscopic properties of Ho3+ ions in zinc borosulphate glasses and comparative energy level analyses of Ho3+ ions in various glasses. Opt. Mater. 1995, 4, 529–546. [Google Scholar] [CrossRef]
- Georgescu, Ş.; Ştefan, A.; Toma, O.; Voiculescu, A.M. Judd–Ofelt analysis of Ho3+ doped in ceramic CaSc2O4. J. Lumin. 2015, 162, 174–179. [Google Scholar] [CrossRef]
- Vengala Rao, B.; Buddhudu, S. Emission analysis of Pr3+ & Ho3+: Ca4GdO(BO3)3 powder phosphors. J. Mater. Sci. 2008, 43, 233–236. [Google Scholar]
- Seshadri, M.; Barbosa, L.C.; Radha, M. Study on structural, optical and gain properties of 1.2 and 2.0 μm emission transitions in Ho3+ doped tellurite glasses. J. Non-Cryst. Solids 2014, 406, 62–72. [Google Scholar] [CrossRef]
- Ding, M.; Cui, S.; Fang, L.; Lin, Z.; Lu, C.; Yang, X. NIR-I-Responsive Single-Band Upconversion Emission through Energy Migration in Core–Shell–Shell Nanostructures. Angew. Chem. Int. Ed. 2022, 61, e202203631. [Google Scholar] [CrossRef]
- Mingye, D.; Bang, D.; Yi, L.; Xiaofei, Y.; Yongjun, Y.; Wangfeng, B.; Shiting, W.; Zhenguo, J.; Chunhua, L.; Kan, Z.; et al. Energy Manipulation in Lanthanide-Doped Core–Shell Nanoparticles for Tunable Dual-Mode Luminescence Toward Advanced Anti-Counterfeiting. Adv. Mater. 2020, 32, 2002121. [Google Scholar]
- Dong, B.; Yuan, Y.; Ding, M.; Bai, W.; Wu, S.; Ji, Z. Efficient dual-mode luminescence from lanthanide-doped core–shell nanoarchitecture for anti-counterfeiting applications. Nanotechnology 2020, 31, 365705. [Google Scholar] [CrossRef]
- Song, P.; Jiang, C. Modeling of downconverter based on Pr3+-Yb3+ codoped fluoride glasses to improve sc-Si solar cells efficiency. AIP Adv. 2012, 2, 042130. [Google Scholar] [CrossRef]
- Burtan-Gwizdala, B.; Manuela, R.; Cisowski, J.; Grelowska, I.; Algarni, H.; Lisiecki, R.; Nosidlak, N. Spectroscopic properties of Er3+-doped fluorotellurite glasses containing various modifiers. Opt. Mater. 2017, 73, 509–516. [Google Scholar] [CrossRef]
- SCHOTT Glass Made of Ideas. Available online: https://www.schott.com/advanced_optics/english/products/optical-materials/special-materials/radiation-shielding-glasses/index.html (accessed on 25 November 2022).
- Rammah, Y.S.; Mahmoud, K.A.; Kavaz, E.; Kumar, A.; El-Agawany, F.I. The role of PbO/Bi2O3 insertion on the shielding characteristics of novel borate glasses. Ceram. Int. 2020, 46, 23357–23368. [Google Scholar] [CrossRef]
- El-Agawany, F.I.; Tashlykov, O.L.; Mahmoud, K.A.; Rammah, Y.S. The radiation-shielding properties of ternary SiO2–SnO–SnF2 glasses: Simulation and theoretical study. Ceram. Int. 2020, 46, 23369–23378. [Google Scholar] [CrossRef]
- Kumar, A. Gamma ray shielding properties of PbO-Li2O-B2O3 glasses. Radiat. Phys. Chem. 2017, 136, 50–53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).