Peculiarities of Fe and Ni Diffusion in Polycrystalline Cu
Abstract
1. Introduction
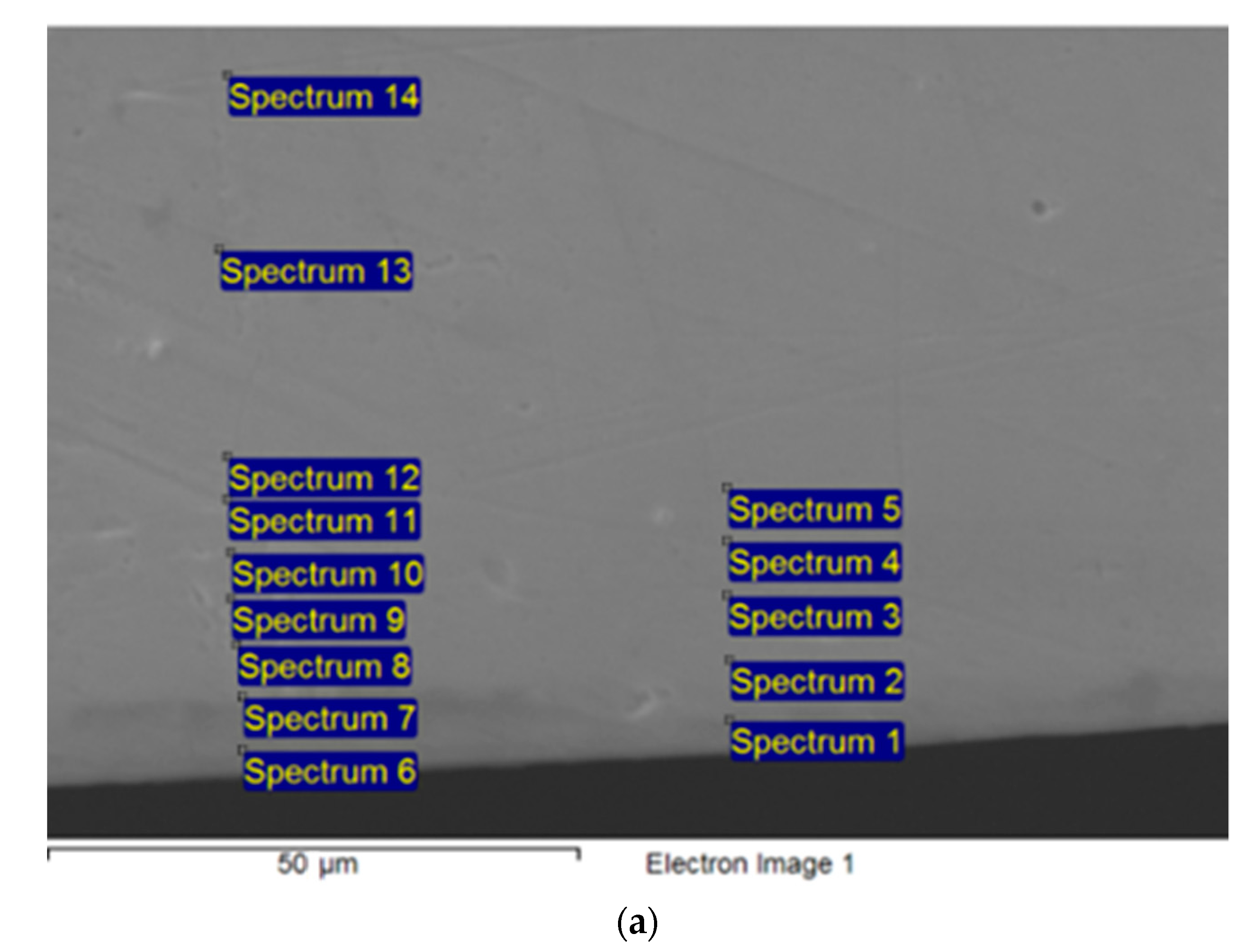
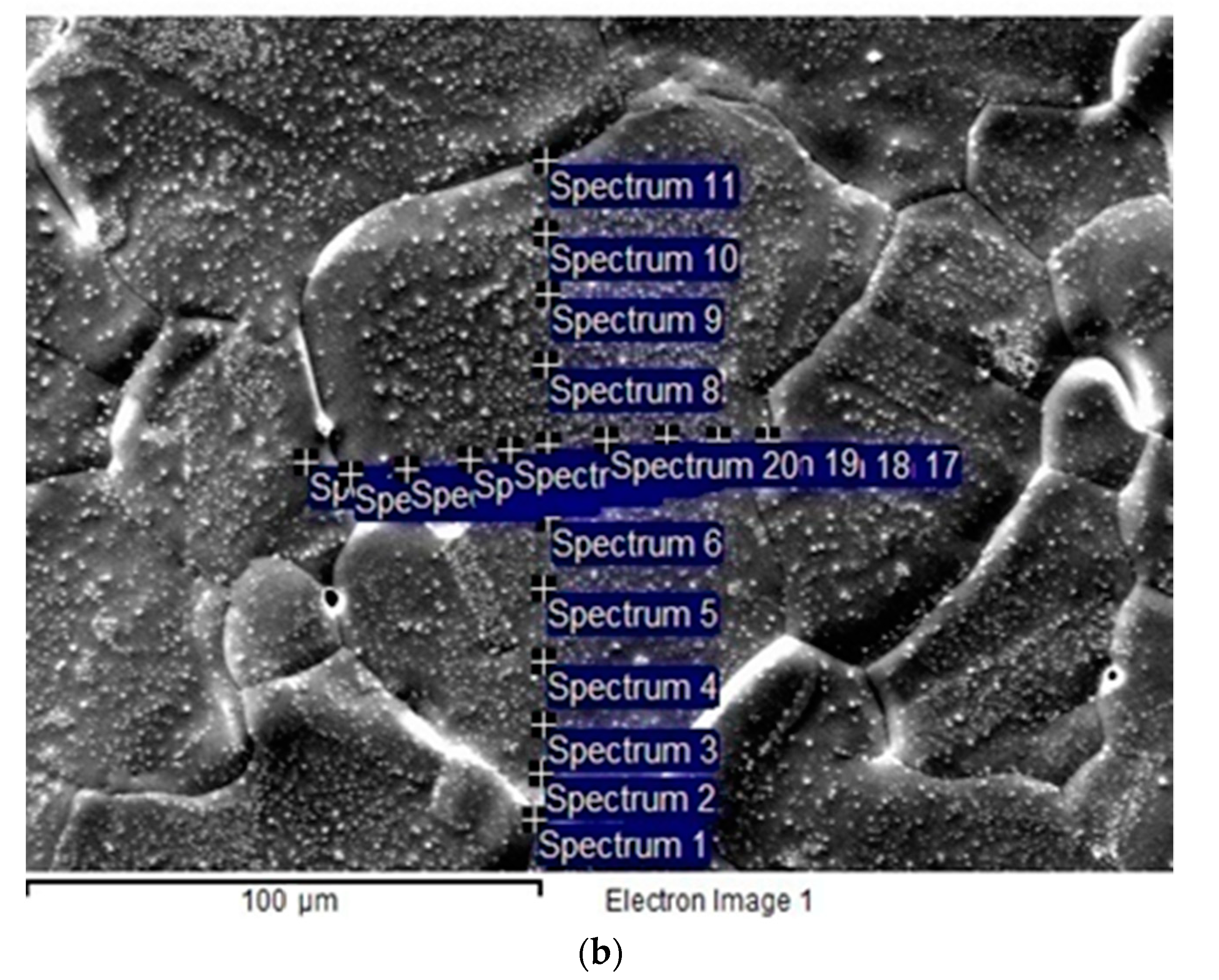
2. Materials and Methods
3. Results
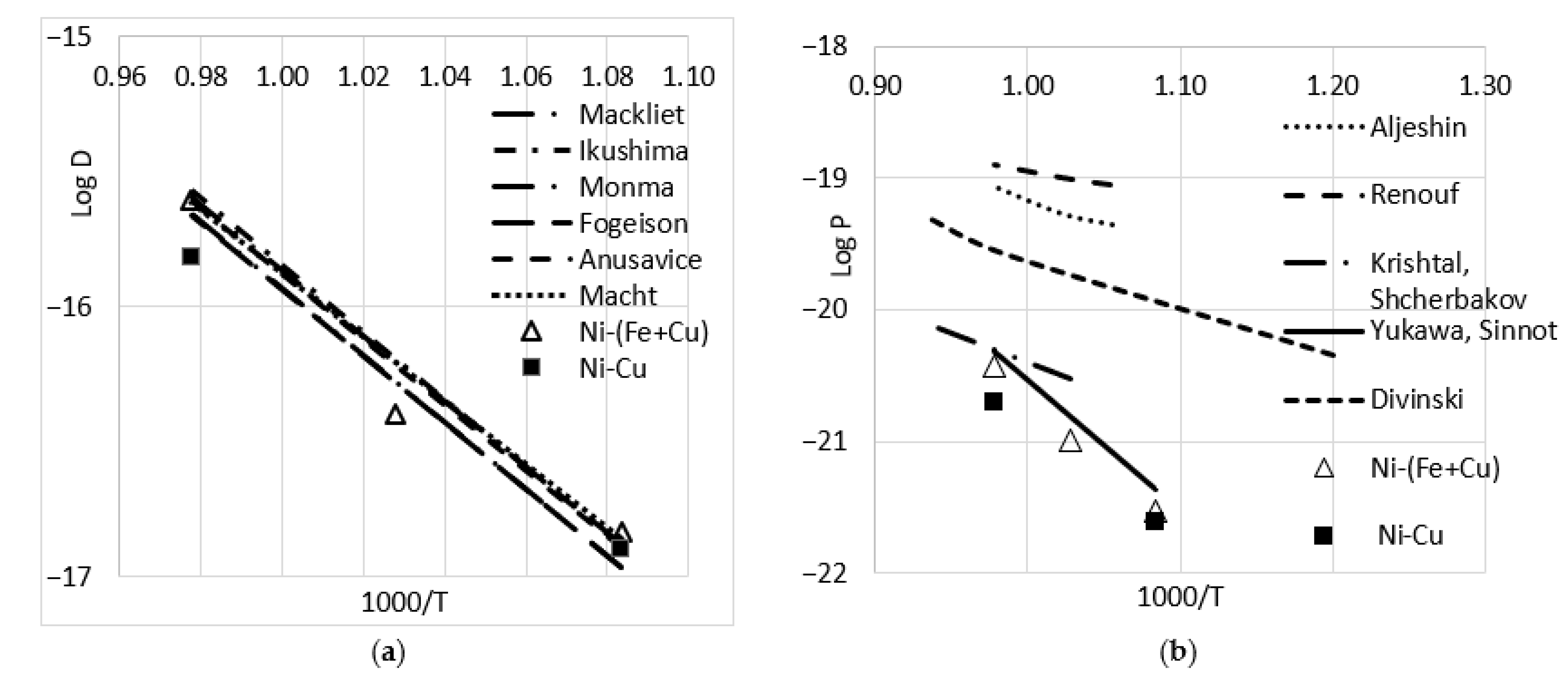
3.1. Ni Diffusion
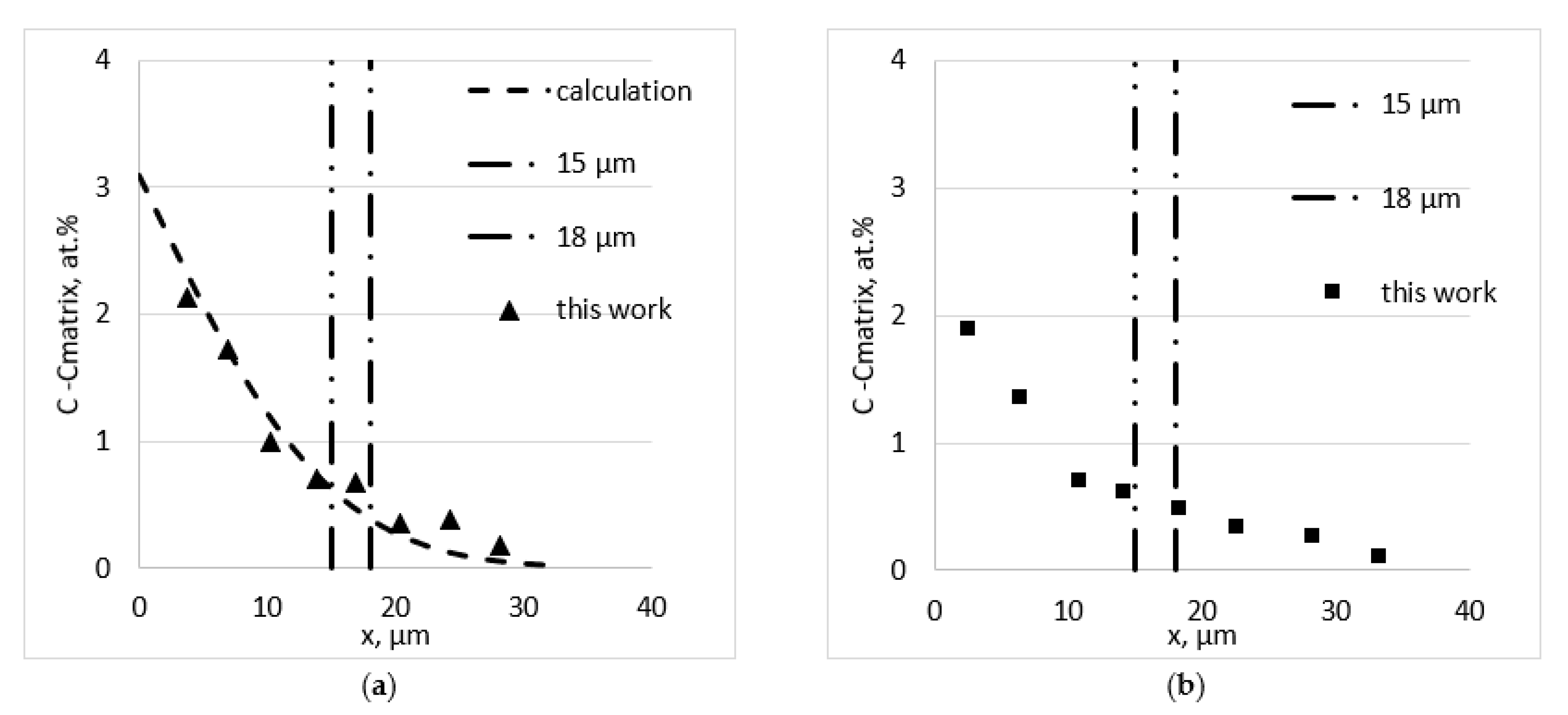
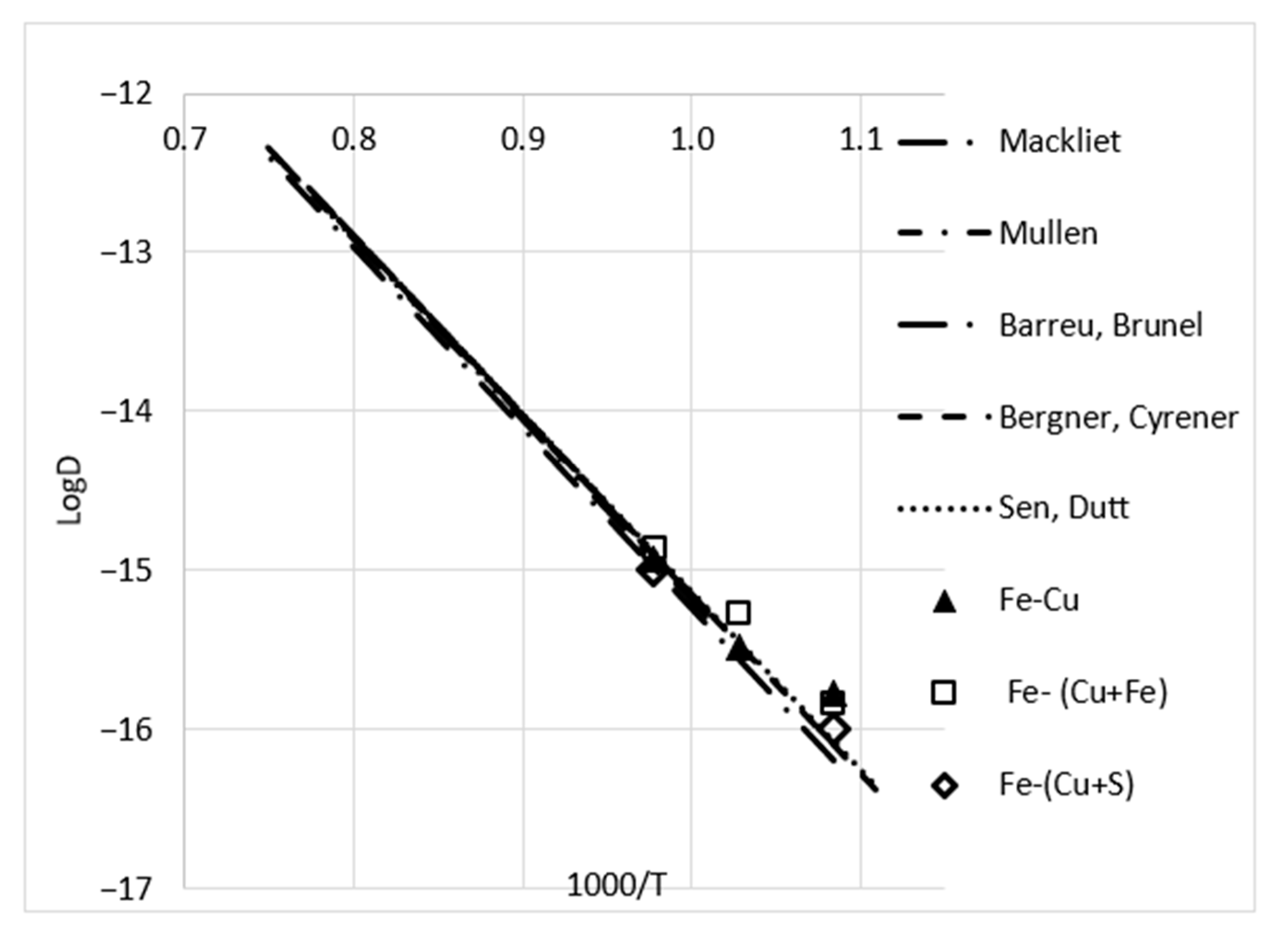
3.2. Results of Bulk and Grain Boundary Diffusion of Iron into Copper and Copper-Based Alloys
- The measured values of the concentration profiles were significantly above the solubility limit at the temperatures of annealing (the solubility at different temperatures is given in Table 3).
- The profiles near and far from the grain boundary were practically the same and a matrix concentration was reached at the same depth.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Merher, H. Handbook Diffusion in Solid Metals and Alloys; Springer Nature Switzerland AG: Cham, Switzerland, 1990; Volume 26. [Google Scholar]
- Kaur, I.; Gust, W. Handbook Grain and Interphase Boundary Diffusion Data; Ziegler Press: Stuttgart, Germany, 1989; Volume 1. [Google Scholar]
- Yan, X.; Wang, J.; Swart, H.; Terblans, J. AES study of Cu and S surface segregation in a ternary Ni-Cu(S) alloy in combination with a linear heating method. J. Alloys Compd. 2018, 768, 153–157. [Google Scholar] [CrossRef]
- Zhevnenko, S.; Rodin, A.; Smirnov, A. Surface phase transition in Cu–Fe solid solutions. Mater. Lett. 2016, 178, 1–4. [Google Scholar] [CrossRef]
- Liu, S.; Jie, J.; Guo, Z.; Yu, S.; Li, T. A comprehensive investigation on microstructure and magnetic properties of immiscible Cu-Fe alloys with variation of Fe content. Mater. Chem. Phys. 2019, 238, 121909. [Google Scholar] [CrossRef]
- Jeong, Y.B.; Jo, H.R.; Park, H.J.; Kato, H.; Kim, K.B. Mechanical properties and microstructural change in (Cu–Fe) immiscible metal matrix composite: Effect of Mg on secondary phase separation. J. Mater. Res. Technol. 2020, 9, 15989–15995. [Google Scholar] [CrossRef]
- Badiger, P.V.; Desai, V.; Ramesh, M.R.; Prajwala, B.K.; Raveendra, K. Effect of cutting parameters on tool wear, cutting force and surface roughness in machining of MDN431 alloy using Al and Fe coated tools. Mater. Res. Express 2018, 6, 016401. [Google Scholar] [CrossRef]
- Badiger, P.V.; Desai, V.; Ramesh, M.R.; Prajwala, B.K.; Raveendra, K. Tribological behaviour of monolayer and multilayer Ti-based thin solid films deposited on alloy steel. Mater. Res. Express 2019, 6, 026419. [Google Scholar] [CrossRef]
- Mackliet, C.A. Diffusion of Fe. Co, and Ni in single crystal of pure Cu. Phys. Rev. 1958, 109, 1964–1970. [Google Scholar] [CrossRef]
- Ikushima, A. Diffusion of Manganese in Single Crystals of Copper. J. Phys. Soc. Jpn. 1959, 14, 111–112. [Google Scholar] [CrossRef]
- Monma, K.; Suto, H.; Oikawa, H. Relation between high-temperature creep and diffusion in alloys (On the relation between high-temperature creep and diffusion in nickel-base solid solutions. VII). J. Jpn. Inst. Met. 1964, 28, 308–312. [Google Scholar] [CrossRef]
- Fogelson, R.L.; Ugay, Y.A.; Pokoev, A.V.; Akimova, I.A. X-ray diffraction method for bulk diffusion measurements in polycrystalline materials. Fiz. Tverd. Tela 1971, 13. [Google Scholar]
- Anusavice, K.J.; DeHoff, R.T. Diffusion of the tracers Cu67, Ni66, and Zn65 in copper-rich solid solutions in the system Cu-Ni-Zn. Metall. Trans. 1972, 3, 1279–1298. [Google Scholar] [CrossRef]
- Macht, M.P.; Naudorf, V.; Dohl, R. DIMETA-82, Diffusion in Metals and Alloys; Kedves, F.J., Beke, D.L., Eds.; Trans Tech Publications: Zurich, Switzerland, 1983; p. 516. [Google Scholar]
- Mullen, J.G. Isotope effect in intermetallic diffusion. Phys. Rev. 1961, 121, 1649. [Google Scholar] [CrossRef]
- Barreu, G.; Brunel, G.; Cizeron, G. Determination of the heterodiffusion coefficients at infinite dilution of iron and chromium in pure copper. C. R. Acad. Sci. 1971, 272, 1–5. [Google Scholar]
- Bergner, D.; Cyrener, E. Diffusion of foreign elements in aluminum solid solutions. Pt. 3. Investigations into the diffusion of silicon in aluminum using the microprobe. Neue Hutte 1973, 18, 356–361. [Google Scholar]
- Sen, S.K.; Dutt, M.B.; Barua, A.K. The diffusion of iron in copper and of nickel in silver. Phys. Status Solidi 1978, 45, 657–663. [Google Scholar] [CrossRef]
- Krishtal, M.; Shcherbakov, L.; Mokrov, A.; Markova, N. Parameters of Bulk and Boundary Diffusion in the Copper-Nickel System. Fiz. Met. Metalloved 1970, 29, 305. [Google Scholar]
- Yukawa, S.; Sinnot, M.J. Grain Boundary Diffusion of Nickel into Copper. Trans. AIME 1955, 203, 996. [Google Scholar]
- Renouf, T.J. The measurement of grain boundary diffusion by the method of autoradiography. Philos. Mag. 1970, 22, 359–375. [Google Scholar] [CrossRef]
- Divinski, S.; Ribbe, J.; Schmitz, G.; Herzig, C. Grain boundary diffusion and segregation of Ni in Cu. Acta Mater. 2007, 55, 3337–3346. [Google Scholar] [CrossRef]
- Aljeshin, A.N.; Prokofjev, S.I. Diffusion parameters and structure of grain boundaries, close to special Σ5 orientation. Poverkhnost Fiz. Khimiya Mech. 1986, 9, 131. [Google Scholar]
- Divinski, S.; Herzig, C. Grain Boundary Diffusion in Metals: Recent Developments. Mater. Trans. 2003, 44, 14–27. [Google Scholar]
- Ribbe, J.; Esin, V.A.; Divinski, S.V. Grain boundary diffusion of 59Fe in high-purity copper. Acta Mater. 2019, 165, 431–443. [Google Scholar] [CrossRef]
- Prokoshkina, D.; Rodin, A.; Esin, V. About Fe diffusion in Cu. Defect Diffus. Forum 2012, 323–325, 171–176. [Google Scholar] [CrossRef]
- Khairullin, A.; Nikulkina, V.; Zhevnenko, S.; Rodin, A. Peculiarity of grain boundary diffusion of Fe and Co in Cu. Defect Diffus. Forum 2017, 380, 135–140. [Google Scholar] [CrossRef]
- Rodin, A.; Khairullin, A. Diffusion and segregation behavior of Fe and Co in Cu. Mater. Lett. 2019, 239, 102–104. [Google Scholar] [CrossRef]
- Vengrenovitch, R.D. On the Ostwald ripening theory. Acta Metall. 1982, 30, 1079–1086. [Google Scholar] [CrossRef]
- Whipple, R. Concentration contours in grain boundary diffusion. Philos. Mag. 1954, 45, 1225–1236. [Google Scholar] [CrossRef]
- Fisher, J.C. Calculation of Diffusion Penetration Curves for Surface and Grain Boundary Diffusion. J. Appl. Phys. 1951, 22, 74–77. [Google Scholar] [CrossRef]
- Salje, G.; Feller-Kniepmeier, M. The diffusion and solubility of iron in copper. J. Appl. Phys. 1978, 49, 229. [Google Scholar] [CrossRef]
- Brunel, G.; Cizeron, G.C.; Lacombe, R.C.R. Chemical Diffusion Study of the Matano and Hau Methods in Copper·Nickel Couples Between 800 and 1060°; The Variation of Activation Energy as a Function of Concentration. Acad. Sci. 1969, 296, 895. [Google Scholar]
- Rodin, A.; Prokoshkina, D.; Itckovitch, A.; Dolgopolov, N. About the Formation of the Supersaturated Solid Solutions by Diffusion Process. Defect Diffus. Forum 2015, 363, 35–39. [Google Scholar] [CrossRef]
- Itckovich, A.; Mendelev, M.; Rodin, A.; Bokstein, B. Effect of atomic complexes formation in grain boundaries on grain boundary diffusion. Defect Diffus. Forum 2018, 383, 103–111. [Google Scholar] [CrossRef]
- Zhevnenko, S.N.; Gershman, E. Grain boundary phase transformation in Cu–Co solid solutions. J. Alloys Compd. 2012, 536, 554–558. [Google Scholar] [CrossRef]
- Bokstein, B.; Rodin, A. Surface tension gradient as additional driving force for grain boundary diffusion. Equilibrium and non-equilibrium cases. Metallofiz. Noveishie Tekhnol. 2013, 35, 1223–1230. [Google Scholar]

| T, °C | Bulk Diffusion Coefficient D × 1016, m2/s | GB Diffusion Triple Product P × 1021, m3/s | ||||
|---|---|---|---|---|---|---|
| Pure Cu | Cu + 0.3% Fe | Cu + 0.4% Fe | Pure Cu | Cu + 0.3% Fe | Cu + 0.4% Fe | |
| 750 | 1.5 | 2.5 | 2.4 | 2 | 5 | 2.5 |
| 700 | - | 0.41 | 0.4 | - | 0.84 | 1.2 |
| 650 | 0.13 | 0.18 | 0.12 | 0.25 | 0.23 | 0.36 |
| Time | 24 h | 48 h | 72 h | 96 h | 192 h | 312 h |
|---|---|---|---|---|---|---|
| Cs, at.% | 2.9 | 3.1 | 3.2 | 3.1 | 2.7 | 3.4 |
| T, °C | Bulk Diffusion Coefficient D × 1016, m2/s | Max. Conc., Cs | Solubility, C0 [32] | ||||
|---|---|---|---|---|---|---|---|
| Pure Cu | Cu + 0.3% Fe | Cu + 0.4% Fe | Cu + 0.002% S [27] | [9] | |||
| 750 | 12 | 11 | 18 | 10.2 | 12.7 | 3.1 | 0.21 |
| 700 | 4.8 | 5.5 | 5.4 | - | 3.4 | 3.0 | 0.3 |
| 650 | 1.7 | 1.2 | 1.7 | 1 | 0.82 | 2.2 | 0.46 |
| Fe-Cu | Grain Boundary, at.% | Average Bulk, at.% | Calculation Bulk, at.% |
|---|---|---|---|
| 650 °C, 65 h | 0.4 | 0.3 | 0.3 |
| 650 °C, 120 h | 0.4 | 0.3 | 0.4 |
| 700 °C, 24 h | 0.3 | 0.3 | 0.3 |
| 700 °C, 45 h | 0.4 | 0.4 | 0.5 |
| 750 °C, 15 h | 0.4 | 0.4 | 0.3 |
| 750 °C, 27 h | 0.4 | 0.3 | 0.4 |
| 750 °C, 70 h | 0.5 | 0.4 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodin, A.; Khairullin, A. Peculiarities of Fe and Ni Diffusion in Polycrystalline Cu. Materials 2023, 16, 922. https://doi.org/10.3390/ma16030922
Rodin A, Khairullin A. Peculiarities of Fe and Ni Diffusion in Polycrystalline Cu. Materials. 2023; 16(3):922. https://doi.org/10.3390/ma16030922
Chicago/Turabian StyleRodin, Alexey, and Ainur Khairullin. 2023. "Peculiarities of Fe and Ni Diffusion in Polycrystalline Cu" Materials 16, no. 3: 922. https://doi.org/10.3390/ma16030922
APA StyleRodin, A., & Khairullin, A. (2023). Peculiarities of Fe and Ni Diffusion in Polycrystalline Cu. Materials, 16(3), 922. https://doi.org/10.3390/ma16030922





