Development of TiO2 Nanosheets with High Dye Degradation Performance by Regulating Crystal Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of TiO2 Nanosheets
2.2. Dye Degradation Assay
2.3. Chemical Analysis of ROS Formation in the Presence of TiO2
2.4. Statistical Analysis
3. Results
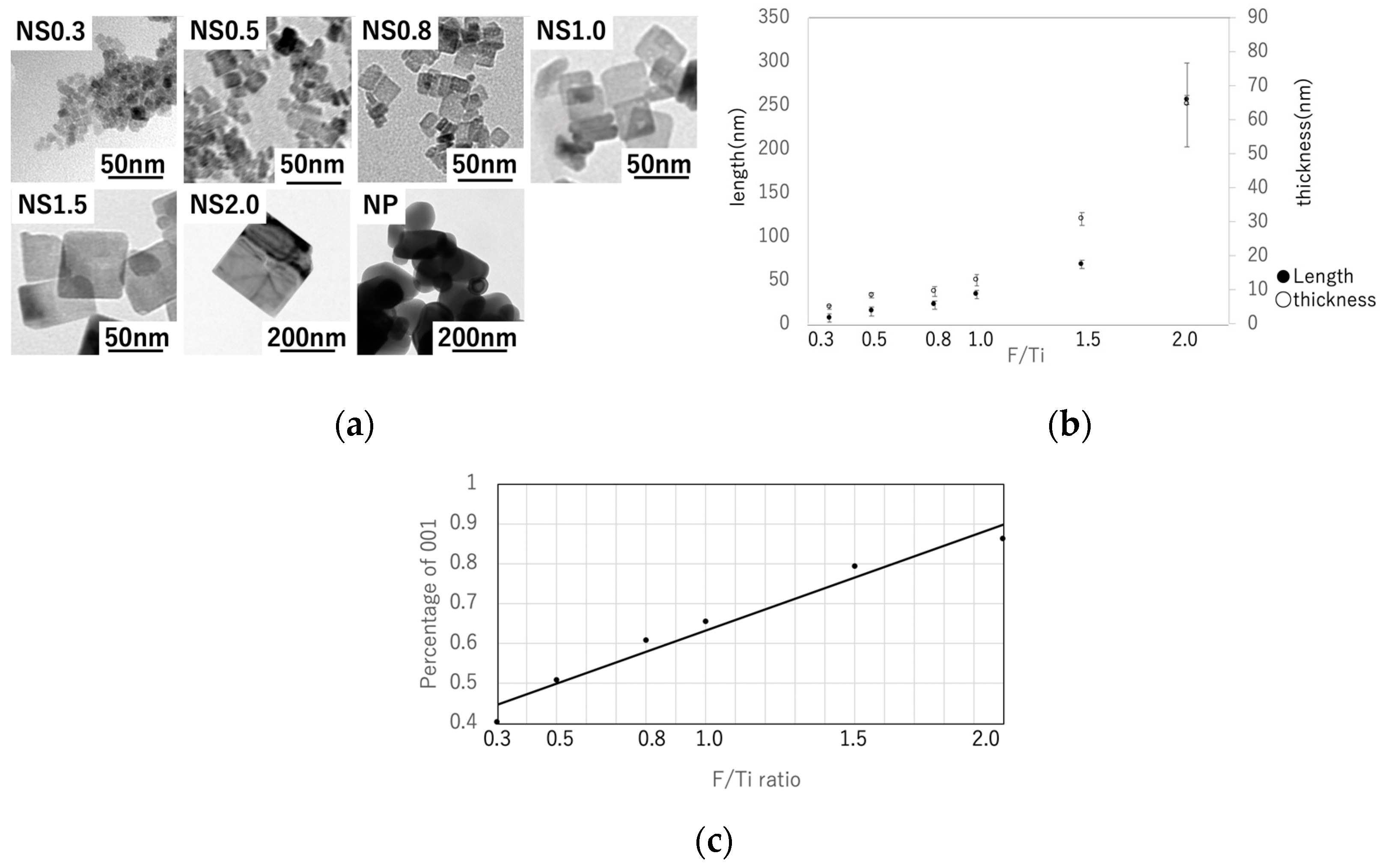
3.1. Characterization of TiO2 Nanosheets
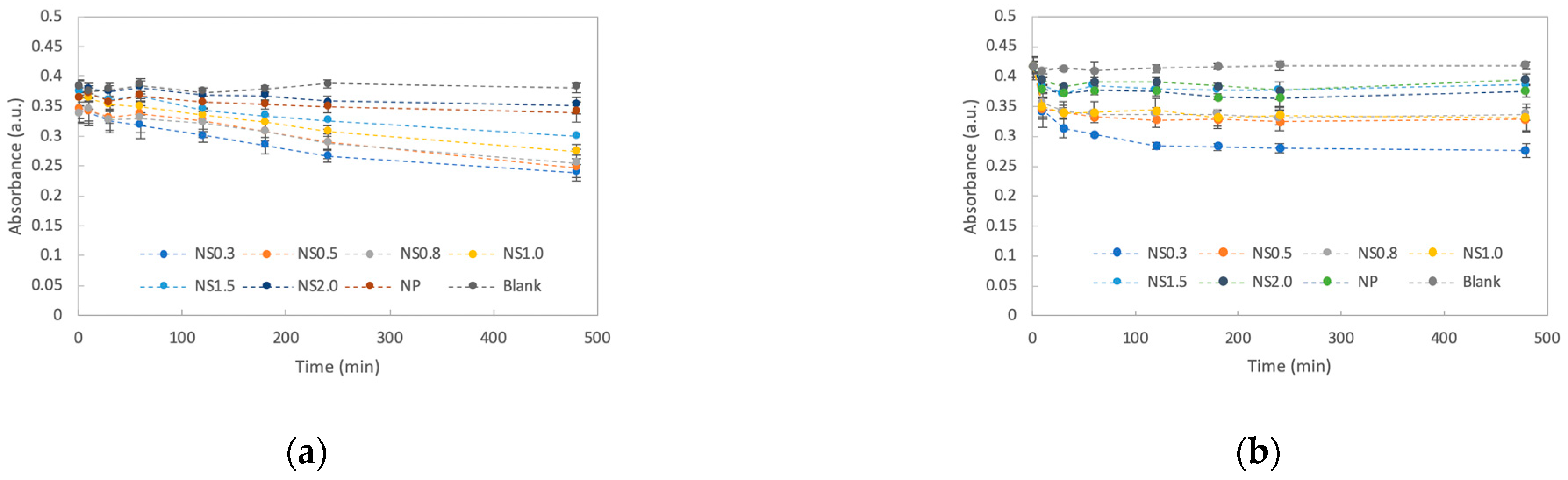
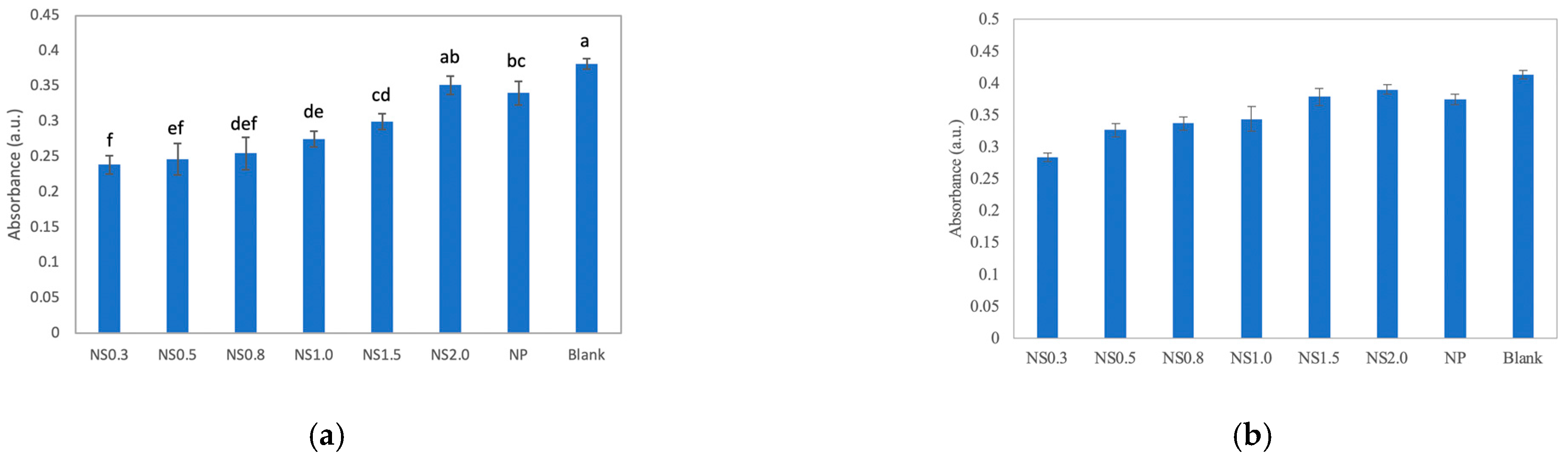
3.2. Assay of Dye Degradation by TiO2 Nanosheets
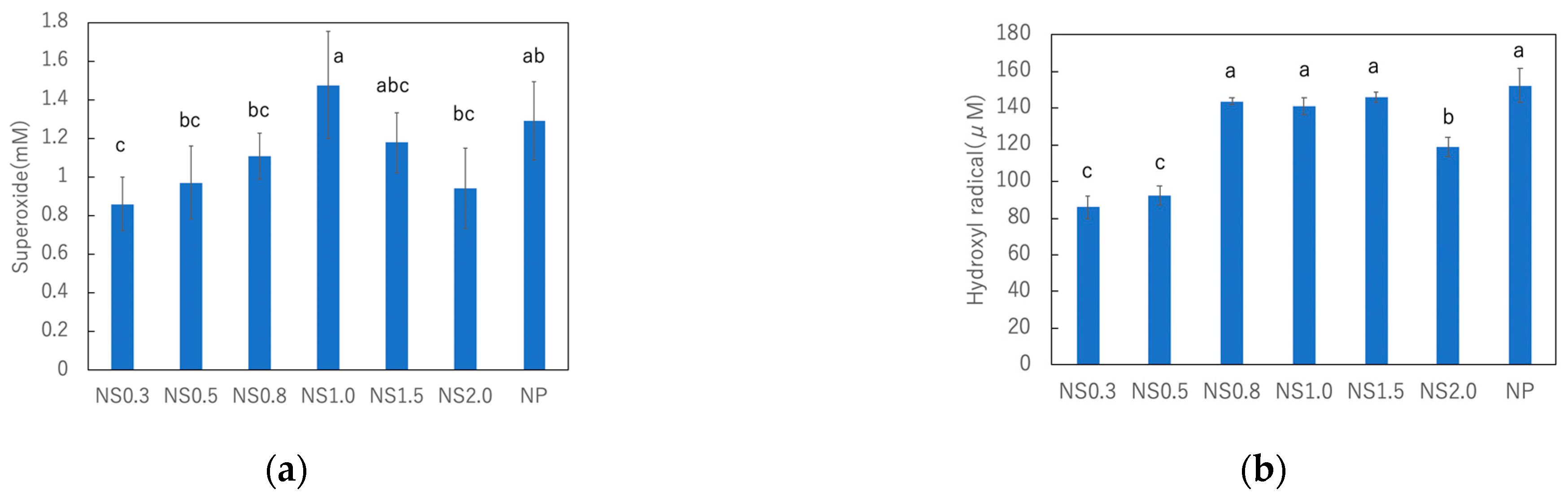
3.3. Comparison of ROS Generation on TiO2 Nanosheets under UV Irradiation
4. Discussion
5. Conclusions
- The dye was decolorized more strongly under UV irradiation than in the dark;
- NS0.3 had the best decolorizing power, followed by NS0.5, NS1.0, NS0.8, NS1.5, and NS2.0.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazuhito, H.; Hiroshi, I.; Akira, F. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar]
- Amit, B.; Anish, S.; Indranath, M.; Susmita, B. Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration. Acta Biomater. 2019, 96, 686–693. [Google Scholar]
- Dongjie, C.; Yanling, C.; Nan, Z.; Paul, C.; Yunpu, W.; Kun, L.; Huo, S.; Pengfei, C.; Peng, P.; Renchuang, Z.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar]
- Amir, Z.; Nauman, A.; Fazle, S.; Natasha, A.; Muhammad, I.A.S.; Muhammad, A.; Zahid, H.; Khair, Z.; Momin, K. Suitable energy platform significantly improves charge separation of g-C3N4 for CO2 reduction and pollutant oxidation under visible-light. Prog. Nat. Sci. Mater. Int. 2019, 29, 138–144. [Google Scholar]
- Amir, Z.; Muhammad, K.; Zahid, H.; Muhammad, I.A.S.; Muhammad, A.; Mohib, U.; Nauman, A.; Shabana, S.; Humaira, Y.; Syed, N.A.S.; et al. Extended visible light driven photocatalytic hydrogen generation by electron induction from g-C3N4 nanosheets to ZnO through the proper heterojunction. Z. Phys. Chem. 2022, 236, 53–66. [Google Scholar]
- Huaqiao, T.; Zhao, Z.; Mang, N.; Chengyu, M.; Dapeng, C.; Daojian, C.; Pingyun, F.; Zaicheng, S. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 10216–10223. [Google Scholar]
- Sun, J.; Zhang, M.; Wang, Z.F.; Chen, H.Y.; Chen, Y.; Murakami, N.; Ohno, T. Synthesis of anatase TiO2 with exposed {001} and {101} facets and photocatalytic activity. Rare Metals 2019, 38, 287–291. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Zhou, H.; Zou, C.; Xu, K.; Luo, X.; Yu, T.; Zhang, W.; Liu, Y.; Yuan, C. A systematic study on the crystal facets-dependent gas sensing properties of anatase TiO2 with designed {010}, {101} and {001} facets. Ceram. Int. 2019, 45, 6282–6290. [Google Scholar] [CrossRef]
- Rui, S.; Yong, C. Controlled Formation of Defective Shell on TiO2 (001) Facets for Enhanced Photocatalytic CO2 Reduction. ChemCatChem 2019, 11, 2270–2276. [Google Scholar]
- Vinodgopal, K.; Idriss, B.; Prashant, V.K. Nanostructured Semiconductor Films for Photocatalysis. Photoelectrochemical Behavior of SnO2/TiO2 Composite Systems and Its Role in Photocatalytic Degradation of a Textile Azo Dye. Chem. Mater. 1996, 8, 2180–2187. [Google Scholar] [CrossRef]
- Hadi, N.C.D.; Juhana, J.; Nurul, W.; Hideto, M.; Saied, R.; Mohd, H.D.O.; Mukhlis, A.R.; Nurul, N.M.J.; Nuor, S.S.; Atikah, M.N.; et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar]
- Jiaguo, Y.; Jingxiang, L.; Wei, X.; Peng, Z.; Mietek, J. Enhanced Photocatalytic CO2-Reduction Activity of Anatase TiO2 by Coexposed {001} and {101} Facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Maria, V.D.; Elena, S. Specific Facets-Dominated Anatase TiO2: Fluorine-Mediated Synthesis and Photoactivity. Catalysts 2013, 3, 455–485. [Google Scholar]
- Xun, L.C.; Ming, H.; Rong, H.; Ji, S.J. HF-Free Synthesis of Anatase TiO2 Nanosheets with Largely Exposed and Clean {001} facets and Their Enhanced Rate Performance As Anodes of Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2014, 6, 19176–19183. [Google Scholar]
- Tan, Z.; Sato, K.; Takami, S.; Numako, C.; Umetsu, M.; Soga, K.; Nakayama, M.; Sasaki, R.; Tanaka, T.; Ogino, C.; et al. Particle size for photocatalytic activity of anatase TiO2 nanosheets with highly exposed {001} facets. RSC Adv. 2013, 3, 19268–19271. [Google Scholar] [CrossRef]
- Hua, G.Y.; Cheng, H.S.; Shi, Z.Q.; Jin, Z.; Gang, L.; Sean, C.S.; Hui, M.C.; Gao, Q.L. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar]
- Wena, C.Z.; Ji, Z.Z.; Hai, B.J.; Qiu, H.H.; Shi, Z.Q.; Hua, G.Y. Synthesis of micro-sized titanium dioxide nanosheets wholly exposed with high-energy {001} and {100} facets. Chem. Commun. 2011, 47, 4400–4402. [Google Scholar] [CrossRef]
- Angeline, D.A.; Puspamitra, P. Tuning optical properties of TiO2 by dimension reduction: From 3D bulk to 2D sheets along {001} and {101} plane. Mater. Res. Express 2019, 6, 1250f1. [Google Scholar]
- Shaowen, C.; Franklin(Feng), T.; Yu, T.; Yuting, L.; Jiaguo, Y. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar]
- Masakazu, A.; Takahito, S.; Sukeya, K.; Yutaka, K. Photocatalytic hydrogenation of propyne with water on small particle titania: Size quantization effects and reaction intermediates. J. Phys. Chem. 1987, 91, 4305–4310. [Google Scholar]
- Kočía, K.; Obalová, L.; Matějová, L.; Plachá, D.; Lacný, Z.; Jirkovský, J.; Šolcová, O. Effect of TiO2 particle size on the photocatalytic reduction of CO2. Catal. B Environ. 2009, 89, 494–502. [Google Scholar] [CrossRef]
- Michela, M.; Maria, V.D.; Elena, S. Searching for facet-dependent photoactivity of shape-controlled anatase TiO2. J. Photochem. Photobiol. C Photochem. Rev. 2016, 28, 29–43. [Google Scholar]
- Yumei, J.; Ye, M.; Jie, L.; Hongguo, W.; Shunmugavel, S.; Hu, L. Engineering oxygen vacancy and crystal surfaces for TiO2-based photocatalysts for enhanced photocatalytic hydrogenation of bio-based carbonyls to biofuels. J. Environ. Chem. Eng. 2022, 10, 108837. [Google Scholar]
- Michela, M.; Maria, V.D.; Mauro, C.; Luca, A.; Gaetano, G.; Elena, S. Unraveling the Multiple Effects Originating the Increased Oxidative Photoactivity of {001}-Facet Enriched Anatase TiO2. ACS Appl. Mater. Interfaces 2016, 8, 9745–9754. [Google Scholar]
- Kenichiro, H.; Kosuke, N.; Zhenquan, T.; Kazuhisa, F.; Reina, N.; Kimihiro, Y.; Hiroyuki, M.; Keiji, I.; Satoshi, O. Enhanced Antibacterial Property of Facet-Engineered TiO2 Nanosheet in Presence and Absence of Ultraviolet Irradiation. Materials 2020, 13, 78. [Google Scholar]
- Zhang, J.; Zhang, L.; Yu, W.; Jiang, F.; Zhang, E.; Wang, H.; Kong, Z.; Xi, J.; Ji, Z. Novel dual heterojunction between MoS2 and anatase TiO2 with coexposed {101} and {001} facets. J. Am. Ceram. Soc. 2017, 100, 5274–5285. [Google Scholar] [CrossRef]
- Petru, A.; Cezar, C.; Carmen, T.; Sebastien, R. Mixed-phase TiO2 photocatalysts: Crystalline phase isolation and reconstruction, characterization and photocatalytic activity in the oxidation of 4-chlorophenol from aqueous effluents. Appl. Catal. B Environ. 2014, 160–161, 374–382. [Google Scholar]
- Lin, H.; Huang, C.P.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.H. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Catal. B 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Czoska, A.M.; Livraghi, S.; Chiesa, M.; Giamello, E.; Agnoli, S.; Granozzi, G.; Finazzi, E.; Valentin, C.D.; Pacchioni, G. The Nature of Defects in Fluorine-Doped TiO2. J. Phys. Chem. C 2008, 112, 8951–8956. [Google Scholar] [CrossRef]
- Fadhel, A.; Entesar, A.H.; Mona, A.; Yasser, A.; Ahmed, A.N.; Mohamed, O.A.; Metwally, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar]
- Parrino, F.; Livraghi, S.; Giamello, E.; Ceccato, R.; Palmisano, L. Role of Hydroxyl, Superoxide, and Nitrate Radicals on the Fate of Bromide Ions in Photocatalytic TiO2 Suspensions. ACS Catal. 2020, 10, 7922–7931. [Google Scholar] [CrossRef]
- Tianyong, Z.; Toshiyuki, O.; Satoshi, H.; Hisao, H.; Jincai, Z.; Nick, S. Photocatalyzed N-demethylation and degradation of methylene blue in titania dispersions exposed to concentrated sunlight. Sol. Energy Mater. Sol. Cells 2002, 73, 287–303. [Google Scholar]
- Chuanxi, Y.; Wenping, D.; Guanwei, C.; Yingqiang, Z.; Xifeng, S.; Xinyuan, X.; Bo, T.; Weiliang, W. Highly efficient photocatalytic degradation of methylene blue by P2ABSA-modified TiO2 nanocomposite due to the photosensitization synergetic effect of TiO2 and P2ABSA. RSC Adv. 2017, 7, 23699–23708. [Google Scholar]
- Thomas, R.G.; Matteo, C.; Taejong, P.; Filippo, M.; Ralph, T.W.; Paolo, F.; Christopher, B.M. Nonaqueous Synthesis of TiO2 Nanocrystals Using TiF4 to Engineer Morphology, Oxygen Vacancy Concentration, and Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar]
- Qian, W.; Min, L.; Zhijiao, W.; Yongliang, L.; Lingyu, P. Is Photooxidation Activity of {001} Facets Truly Lower Than That of {101} Facets for Anatase TiO2 Crystals? J. Phys. Chem. C 2012, 116, 26800–26804. [Google Scholar]



| TiO2 | Optical Bandgap (eV) |
|---|---|
| NS0.3 | 3.2 |
| NS0.5 | 3.22 |
| NS0.8 | 3.2 |
| NS1.0 | 3.2 |
| NS1.5 | 3.24 |
| NS2.0 | 3.29 |
| NP (control sample) | 3.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowaka, Y.; Nozaki, K.; Mihara, T.; Yamashita, K.; Miura, H.; Tan, Z.; Ohara, S. Development of TiO2 Nanosheets with High Dye Degradation Performance by Regulating Crystal Growth. Materials 2023, 16, 1229. https://doi.org/10.3390/ma16031229
Kowaka Y, Nozaki K, Mihara T, Yamashita K, Miura H, Tan Z, Ohara S. Development of TiO2 Nanosheets with High Dye Degradation Performance by Regulating Crystal Growth. Materials. 2023; 16(3):1229. https://doi.org/10.3390/ma16031229
Chicago/Turabian StyleKowaka, Yasuyuki, Kosuke Nozaki, Tomoyuki Mihara, Kimihiro Yamashita, Hiroyuki Miura, Zhenquan Tan, and Satoshi Ohara. 2023. "Development of TiO2 Nanosheets with High Dye Degradation Performance by Regulating Crystal Growth" Materials 16, no. 3: 1229. https://doi.org/10.3390/ma16031229
APA StyleKowaka, Y., Nozaki, K., Mihara, T., Yamashita, K., Miura, H., Tan, Z., & Ohara, S. (2023). Development of TiO2 Nanosheets with High Dye Degradation Performance by Regulating Crystal Growth. Materials, 16(3), 1229. https://doi.org/10.3390/ma16031229








