Freeze Drying of Polymer Nanoparticles and Liposomes Exploiting Different Saccharide-Based Approaches
Abstract
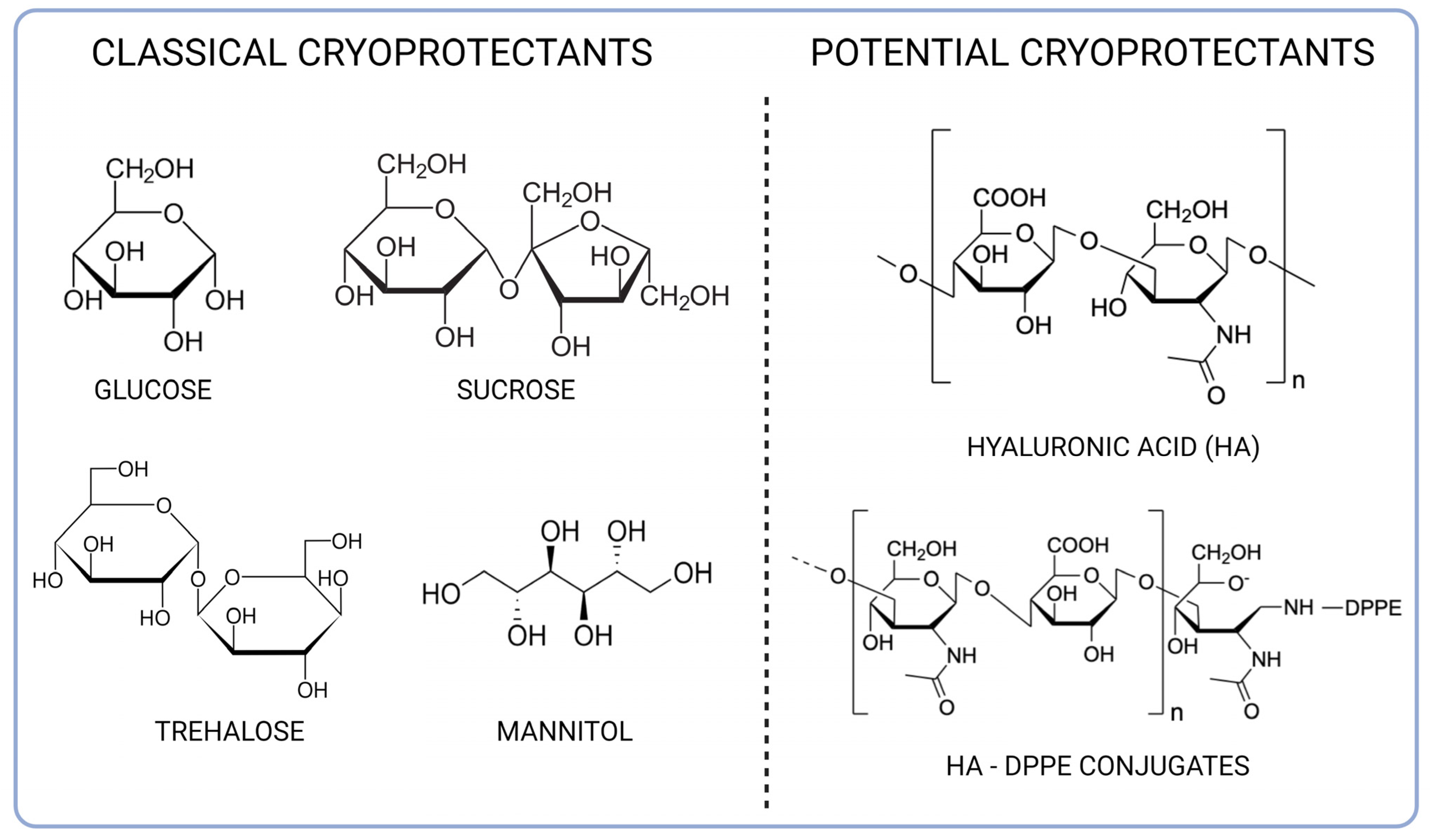
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles and Liposomes
2.3. Preparation of HA-Decorated Nanoparticles and Liposomes
2.4. Freeze-Drying Studies
2.5. Physicochemical Characterization of Nanoparticles and Liposomes
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent Advances in Nanoparticle-Based Cancer Drug and Gene Delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Friess, W. Freeze-drying of nanoparticles: How to overcome colloidal instability by formulation and process optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef]
- Kelkawi, A.H.A.; Hashemzadeh, H.; Pashandi, Z.; Tiraihi, T.; Naderi-Manesh, H. Differentiation of PC12 cell line into neuron by Valproic acid encapsulated in the stabilized core-shell liposome-chitosan Nano carriers. Int. J. Biol. Macromol. 2022, 210, 252–260. [Google Scholar] [CrossRef]
- Mohammady, M.; Mohammadi, Y.; Yousefi, G. Freeze-Drying of Pharmaceutical and Nutraceutical Nanoparticles: The Effects of Formulation and Technique Parameters on Nanoparticles Characteristics. J. Pharm. Sci. 2020, 109, 3235–3247. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczko, D.; Kariko, K.; Schreiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol. Ther. 2022, 30, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyazaki, T.; Muto, H.; Kubara, K.; Mukai, Y.; Watari, R.; Sato, S.; Kondo, K.; Tsukumo, S.I.; Yasutomo, K.; et al. Design and lyophilization of lipid nanoparticles for mRNA vaccine and its robust immune response in mice and nonhuman primates. Mol. Ther. Nucleic Acids 2022, 30, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, Y.; Gu, Y.; Liu, Y.; Zhao, J.; Yan, B.; Wang, Y. Cryoprotectant choice and analyses of freeze-drying drug suspension of nanoparticles with functional stabilisers. J. Microencapsul. 2018, 35, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, B.; Porfire, A.; Van Bockstal, P.J.; Porav, S.; Achim, M.; Beer, T.; Tomuta, I. Formulation Optimization of Freeze-Dried Long-Circulating Liposomes and In-Line Monitoring of the Freeze-Drying Process Using an NIR Spectroscopy Tool. J. Pharm. Sci. 2018, 107, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, W.; Degobert, G.; Fessi, H. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur. J. Pharm. Biopharm. 2006, 63, 87–94. [Google Scholar] [CrossRef]
- Umerska, A.; Paluch, K.J.; Santos-Martinez, M.J.; Corrigan, O.I.; Medina, C.; Tajber, L. Freeze drying of polyelectrolyte complex nanoparticles: Effect of nanoparticle composition and cryoprotectant selection. Int. J. Pharm. 2018, 552, 27–38. [Google Scholar] [CrossRef]
- de la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronan-based nanocarriers for transmucosal delivery of macromolecules. Macromol. Biosci. 2008, 8, 441–450. [Google Scholar] [CrossRef]
- Fonte, P.; Reis, S.; Sarmento, B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J. Control. Release 2016, 225, 75–86. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Fessi, H. A pilot study of freeze drying of poly(epsilon-caprolactone) nanocapsules stabilized by poly(vinyl alcohol): Formulation and process optimization. Int. J. Pharm. 2006, 309, 178–188. [Google Scholar] [CrossRef]
- Luo, W.C.; O’Reilly Beringhs, A.; Kim, R.; Zhang, W.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of formulation on the quality and stability of freeze-dried nanoparticles. Eur. J. Pharm. Biopharm. 2021, 169, 256–267. [Google Scholar] [CrossRef]
- Amis, T.M.; Renukuntla, J.; Bolla, P.K.; Clark, B.A. Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles. Pharmaceutics 2020, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Vogel, V.; Mantele, W.; Schwartz, D.; Haase, W.; Langer, K. Physico-chemical characterisation of PLGA nanoparticles after freeze-drying and storage. Eur. J. Pharm. Biopharm. 2009, 72, 428–437. [Google Scholar] [CrossRef]
- Guimaraes, D.; Noro, J.; Silva, C.; Cavaco-Paulo, A.; Nogueira, E. Protective Effect of Saccharides on Freeze-Dried Liposomes Encapsulating Drugs. Front. Bioeng. Biotechnol. 2019, 7, 424. [Google Scholar] [CrossRef]
- Fonte, P.; Soares, S.; Costa, A.; Andrade, J.C.; Seabra, V.; Reis, S.; Sarmento, B. Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter 2012, 2, 329–339. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Franze, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef]
- Kumar, S.; Gokhale, R.; Burgess, D.J. Sugars as bulking agents to prevent nano-crystal aggregation during spray or freeze-drying. Int. J. Pharm. 2014, 471, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.C.; Hedtrich, S.; Friess, W. Lyophilization of Synthetic Gene Carriers. Methods Mol. Biol. 2019, 1943, 211–225. [Google Scholar] [CrossRef]
- Tonnis, W.F.; Mensink, M.A.; de Jager, A.; van der Voort Maarschalk, K.; Frijlink, H.W.; Hinrichs, W.L. Size and molecular flexibility of sugars determine the storage stability of freeze-dried proteins. Mol. Pharm. 2015, 12, 684–694. [Google Scholar] [CrossRef]
- Arpicco, S.; Lerda, C.; Dalla Pozza, E.; Costanzo, C.; Tsapis, N.; Stella, B.; Donadelli, M.; Dando, I.; Fattal, E.; Cattel, L.; et al. Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur. J. Pharm. Biopharm. 2013, 85, 373–380. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Stella, B.; Andreana, I.; Zonari, D.; Arpicco, S. Pentamidine-Loaded Lipid and Polymer Nanocarriers as Tunable Anticancer Drug Delivery Systems. J. Pharm. Sci. 2020, 109, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Susa, F.; Bucca, G.; Limongi, T.; Cauda, V.; Pisano, R. Enhancing the preservation of liposomes: The role of cryoprotectants, lipid formulations and freezing approaches. Cryobiology 2021, 98, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Vallorz, E.L.; Encinas-Basurto, D.; Schnellmann, R.G.; Mansour, H.M. Design, Development, Physicochemical Characterization, and In Vitro Drug Release of Formoterol PEGylated PLGA Polymeric Nanoparticles. Pharmaceutics 2022, 14, 638. [Google Scholar] [CrossRef]
- Fonte, P.; Araujo, F.; Seabra, V.; Reis, S.; van de Weert, M.; Sarmento, B. Co-encapsulation of lyoprotectants improves the stability of protein-loaded PLGA nanoparticles upon lyophilization. Int. J. Pharm. 2015, 496, 850–862. [Google Scholar] [CrossRef]
- Fonte, P.; Soares, S.; Sousa, F.; Costa, A.; Seabra, V.; Reis, S.; Sarmento, B. Stability study perspective of the effect of freeze-drying using cryoprotectants on the structure of insulin loaded into PLGA nanoparticles. Biomacromolecules 2014, 15, 3753–3765. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W. Freeze-drying of drug-free and drug-loaded solid lipid nanoparticles (SLN). Int. J. Pharm. 1997, 157, 171–179. [Google Scholar] [CrossRef]
- Sameti, M.; Bohr, G.; Ravi Kumar, M.N.; Kneuer, C.; Bakowsky, U.; Nacken, M.; Schmidt, H.; Lehr, C.M. Stabilisation by freeze-drying of cationically modified silica nanoparticles for gene delivery. Int. J. Pharm. 2003, 266, 51–60. [Google Scholar] [CrossRef]
- Craig, D.Q.M. A review of thermal methods used for the analysis of the crystal form, solution thermodynamics and glass transition behaviour of polyethylene glycols. Thermochimica. Acta 1995, 248, 189–203. [Google Scholar] [CrossRef]
- Chaudhury, A.; Das, S.; Lee, R.F.; Tan, K.B.; Ng, W.K.; Tan, R.B.; Chiu, G.N. Lyophilization of cholesterol-free PEGylated liposomes and its impact on drug loading by passive equilibration. Int. J. Pharm. 2012, 430, 167–175. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oldenhof, H.; Tablin, F.; Crowe, J.H. Preservation of dried liposomes in the presence of sugar and phosphate. Biochim. Biophys. Acta 2004, 1661, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Slade, L.; Levine, H. Glass transitions and water-food structure interactions. Adv. Food Nutr. Res. 1995, 38, 103–269. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Yu, S.; Zhou, Y. Protective effect of hyaluronic acid on cryopreserved boar sperm. Int. J. Biol. Macromol. 2016, 87, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; McMorrow, J.; Umerska, A.; Patel, H.B.; Kornerup, K.N.; Tajber, L.; Murphy, E.P.; Perretti, M.; Corrigan, O.I.; Brayden, D.J. An intra-articular salmon calcitonin-based nanocomplex reduces experimental inflammatory arthritis. J. Control. Release 2013, 167, 120–129. [Google Scholar] [CrossRef]
- Arpicco, S.; De Rosa, G.; Fattal, E. Lipid-Based Nanovectors for Targeting of CD44-Overexpressing Tumor Cells. J. Drug Deliv. 2013, 2013, 860780. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chung, H.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 2010, 31, 106–114. [Google Scholar] [CrossRef]
- Franze, S.; Marengo, A.; Stella, B.; Minghetti, P.; Arpicco, S.; Cilurzo, F. Hyaluronan-decorated liposomes as drug delivery systems for cutaneous administration. Int. J. Pharm. 2018, 535, 333–339. [Google Scholar] [CrossRef]
- Pandolfi, L.; Marengo, A.; Japiassu, K.B.; Frangipane, V.; Tsapis, N.; Bincoletto, V.; Codullo, V.; Bozzini, S.; Morosini, M.; Lettieri, S.; et al. Liposomes Loaded with Everolimus and Coated with Hyaluronic Acid: A Promising Approach for Lung Fibrosis. Int. J. Mol. Sci. 2021, 22, 7743. [Google Scholar] [CrossRef]
- Franze, S.; Rama, F.; Rocco, P.; Debernardi, M.; Bincoletto, V.; Arpicco, S.; Cilurzo, F. Rationalizing the Design of Hyaluronic Acid-Decorated Liposomes for Targeting Epidermal Layers: A Combination of Molecular Dynamics and Experimental Evidence. Mol. Pharm. 2021, 18, 3979–3989. [Google Scholar] [CrossRef]
- Cannito, S.; Bincoletto, V.; Turato, C.; Pontisso, P.; Scupoli, M.T.; Ailuno, G.; Andreana, I.; Stella, B.; Arpicco, S.; Bocca, C. Hyaluronated and PEGylated Liposomes as a Potential Drug-Delivery Strategy to Specifically Target Liver Cancer and Inflammatory Cells. Molecules 2022, 27, 1062. [Google Scholar] [CrossRef]
- Tadros, T. General Principles of Colloid Stability and the Role of Surface Forces. In Colloid Stability: The Role of Surface Forces—Part I; Wiley: Hoboken, NJ, USA, 2011; Volume 1, pp. 1–22. [Google Scholar]
- Peer, D.; Florentin, A.; Margalit, R. Hyaluronan is a key component in cryoprotection and formulation of targeted unilamellar liposomes. Biochim. Biophys. Acta 2003, 1612, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]






| Before Freeze Drying | After Freeze Drying | ||||
|---|---|---|---|---|---|
| Formulation | Cryoprotectant % (w/w) | Hydrodynamic Diameter (nm) | PDI | Hydrodynamic Diameter (nm) | PDI |
| PLGA 75:25 | Sucrose 10 | 136 | 0.086 | 140 | 0.120 |
| PLGA 75:25 | Sucrose 20 | 127 | 0.005 | 132 | 0.172 |
| PLGA 75:25 | Trehalose 10 | 144 | 0.072 | 143 | 0.090 |
| PLGA 50:50 | Glucose 10 | 131 | 0.177 | 141 | 0.111 |
| PLGA 50:50 | Sucrose 20 | 124 | 0.087 | 129 | 0.106 |
| PLGA 75:25/PEG2000-PLGA | Glucose 20 | 104 | 0.090 | 147 | 0.338 |
| PLGA 75:25/PEG2000-PLGA | Trehalose 5 | 85 | 0.080 | 132 | 0.351 |
| PLGA 50:50/PEG2000-PLGA | Glucose 20 | 105 | 0.083 | 124 | 0.139 |
| Before Freeze Drying | After Freeze Drying | |||||
|---|---|---|---|---|---|---|
| Formulation | Cryoprotectant | Addition Method | Hydrodynamic Diameter (nm) | PDI | Hydrodynamic Diameter (nm) | PDI |
| Liposomes | Trehalose | Out * | 204 | 0.163 | 218 | 0.190 |
| Liposomes | Trehalose | In/Out ** | 210 | 0.139 | 221 | 0.143 |
| Liposomes | Sucrose | Out * | 200 | 0.138 | 190 | 0.163 |
| Liposomes | Sucrose | In/Out ** | 201 | 0.165 | 204 | 0.208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreana, I.; Bincoletto, V.; Manzoli, M.; Rodà, F.; Giarraputo, V.; Milla, P.; Arpicco, S.; Stella, B. Freeze Drying of Polymer Nanoparticles and Liposomes Exploiting Different Saccharide-Based Approaches. Materials 2023, 16, 1212. https://doi.org/10.3390/ma16031212
Andreana I, Bincoletto V, Manzoli M, Rodà F, Giarraputo V, Milla P, Arpicco S, Stella B. Freeze Drying of Polymer Nanoparticles and Liposomes Exploiting Different Saccharide-Based Approaches. Materials. 2023; 16(3):1212. https://doi.org/10.3390/ma16031212
Chicago/Turabian StyleAndreana, Ilaria, Valeria Bincoletto, Maela Manzoli, Francesca Rodà, Vita Giarraputo, Paola Milla, Silvia Arpicco, and Barbara Stella. 2023. "Freeze Drying of Polymer Nanoparticles and Liposomes Exploiting Different Saccharide-Based Approaches" Materials 16, no. 3: 1212. https://doi.org/10.3390/ma16031212
APA StyleAndreana, I., Bincoletto, V., Manzoli, M., Rodà, F., Giarraputo, V., Milla, P., Arpicco, S., & Stella, B. (2023). Freeze Drying of Polymer Nanoparticles and Liposomes Exploiting Different Saccharide-Based Approaches. Materials, 16(3), 1212. https://doi.org/10.3390/ma16031212









