Formation of Metal-Oxide Nanocomposites with Highly Dispersed Co Particles from a Co-Zr Powder Blend by Mechanical Alloying and Hydrogen Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
3. Results
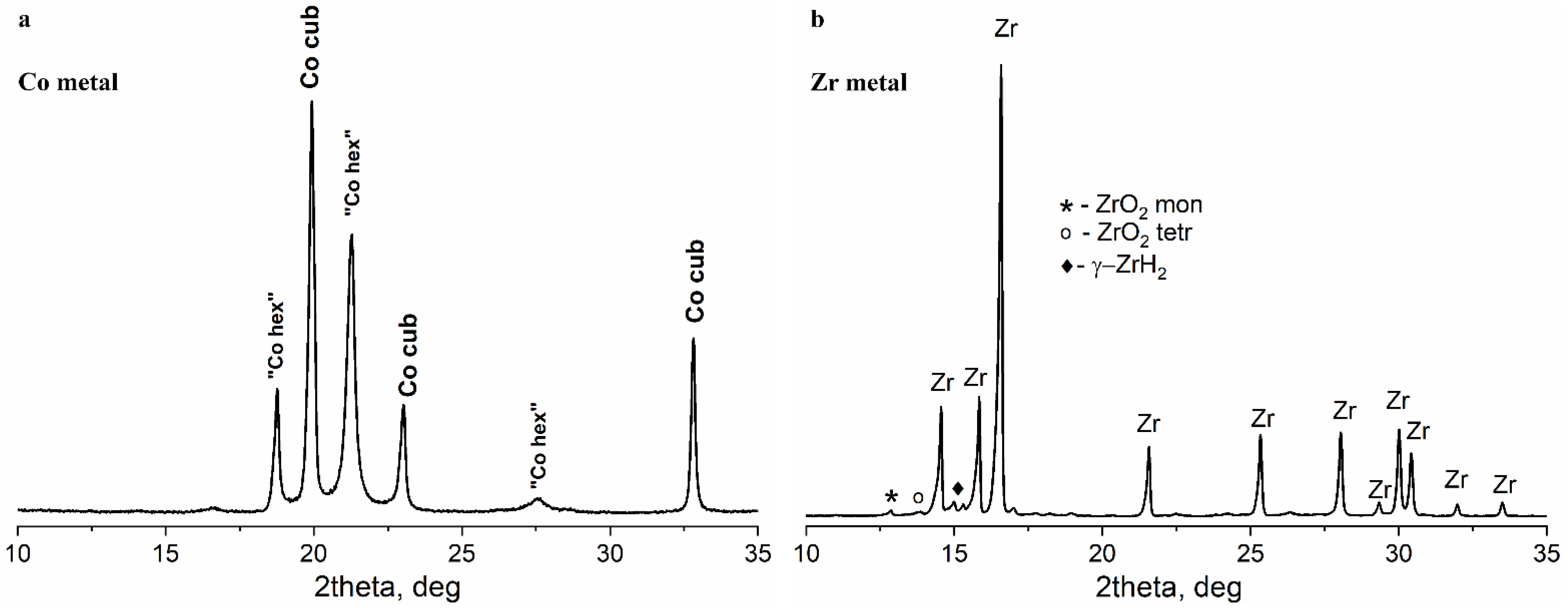
3.1. Initial Co and Zr Metal Powders
3.1.1. XRD and SEM
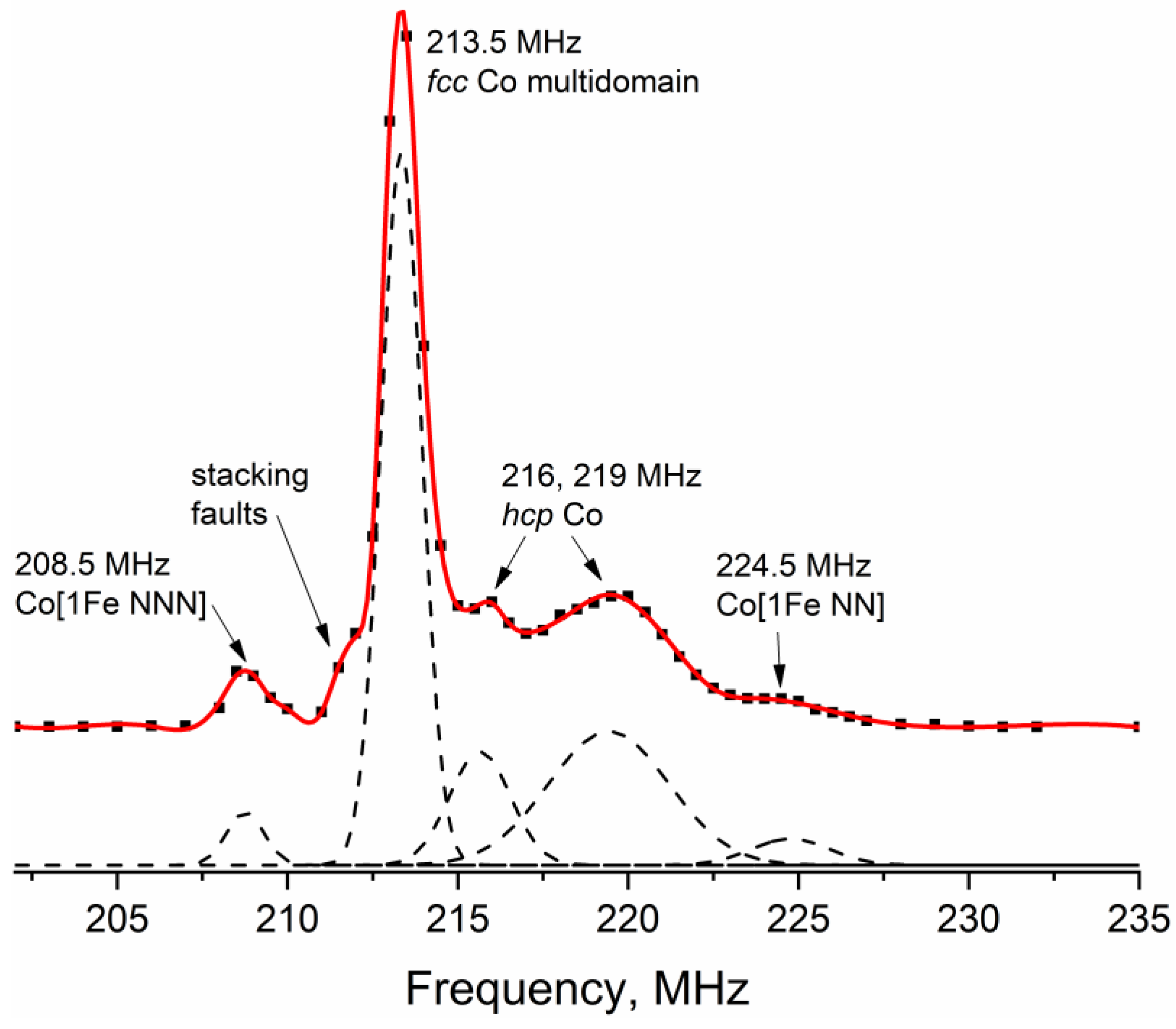
3.1.2. 59Co Internal Field NMR
3.2. Products of Mechanical Alloying
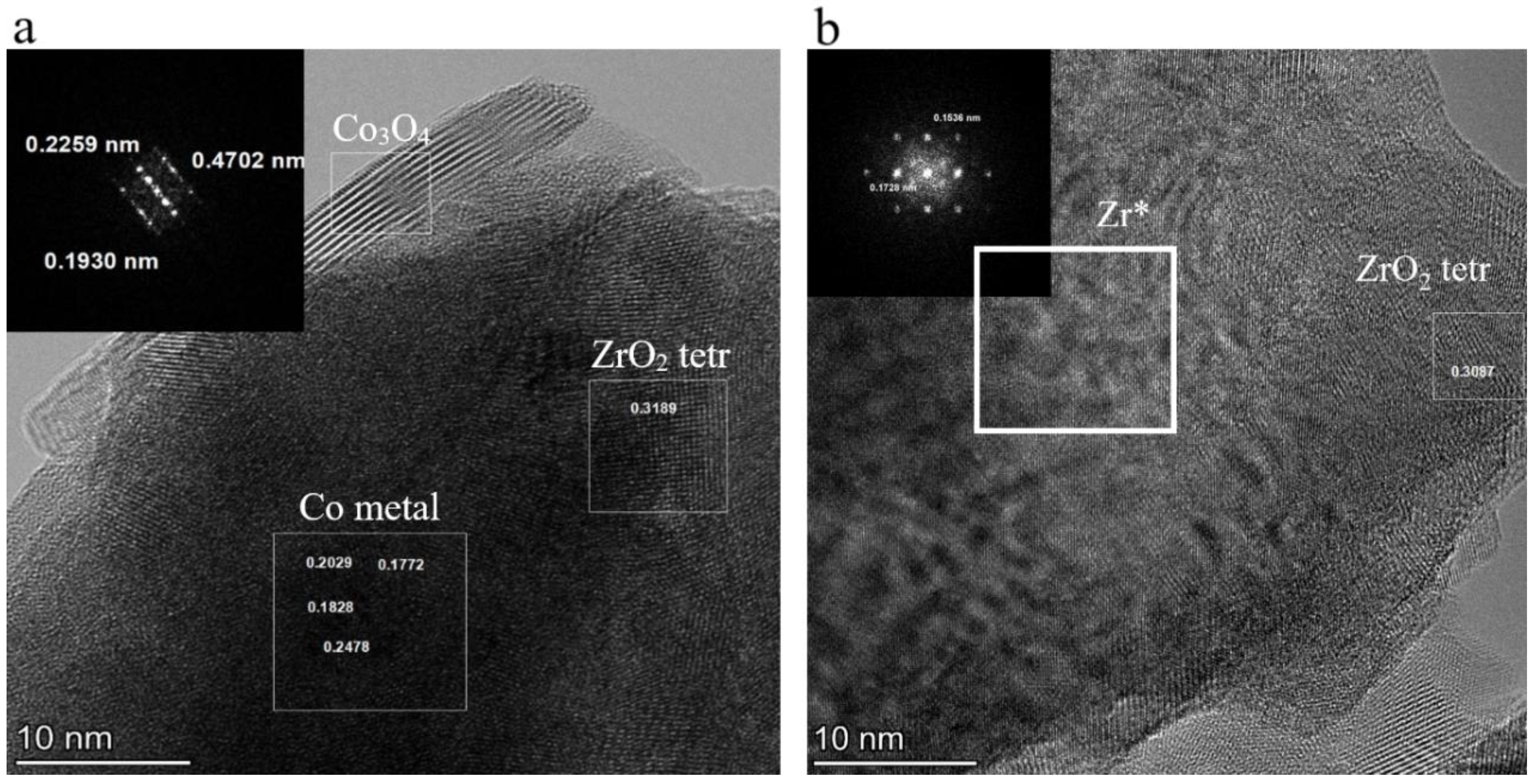
3.2.1. XRD and SEM
3.2.2. HRTEM and EDX Mapping
3.2.3. 59Co Internal Field NMR
3.3. Hydrogen Treated Sample
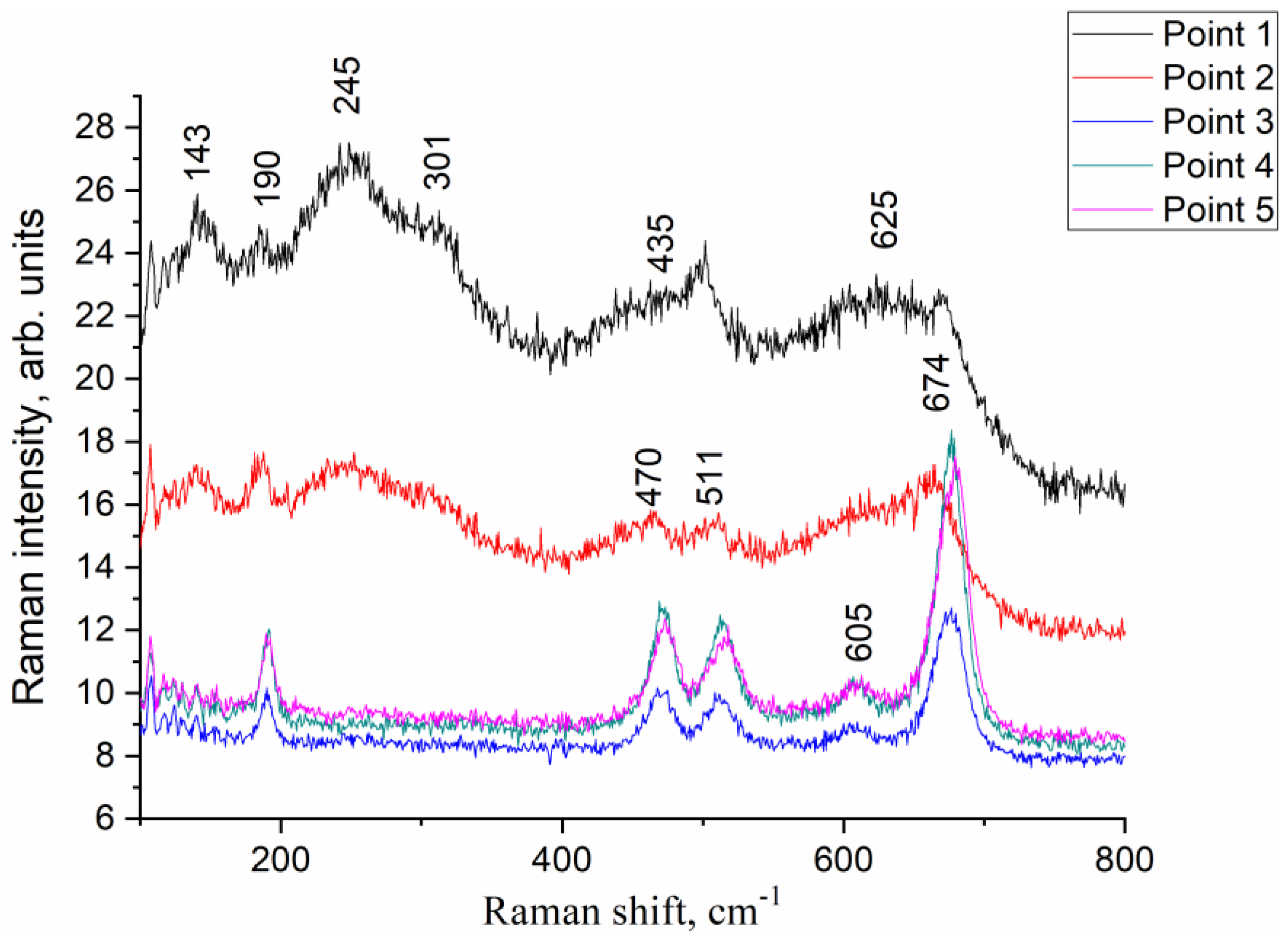
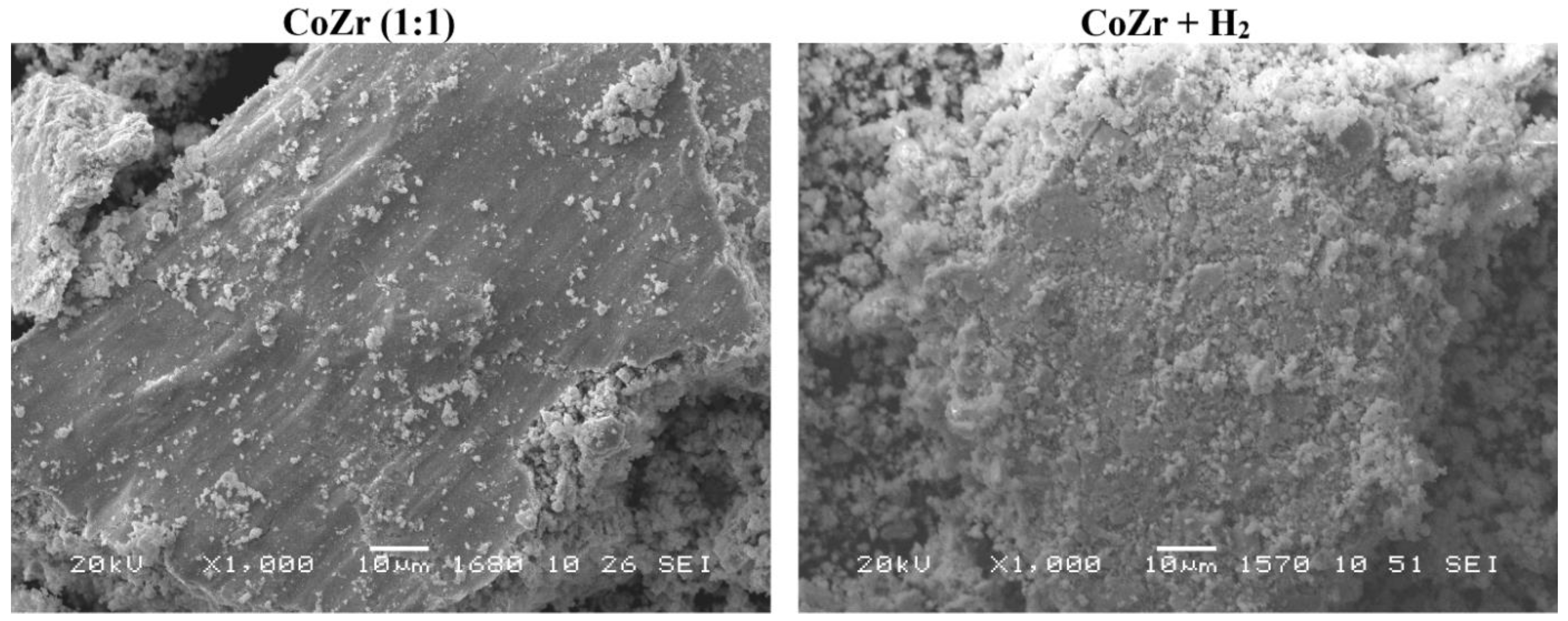
3.3.1. XRD and SEM
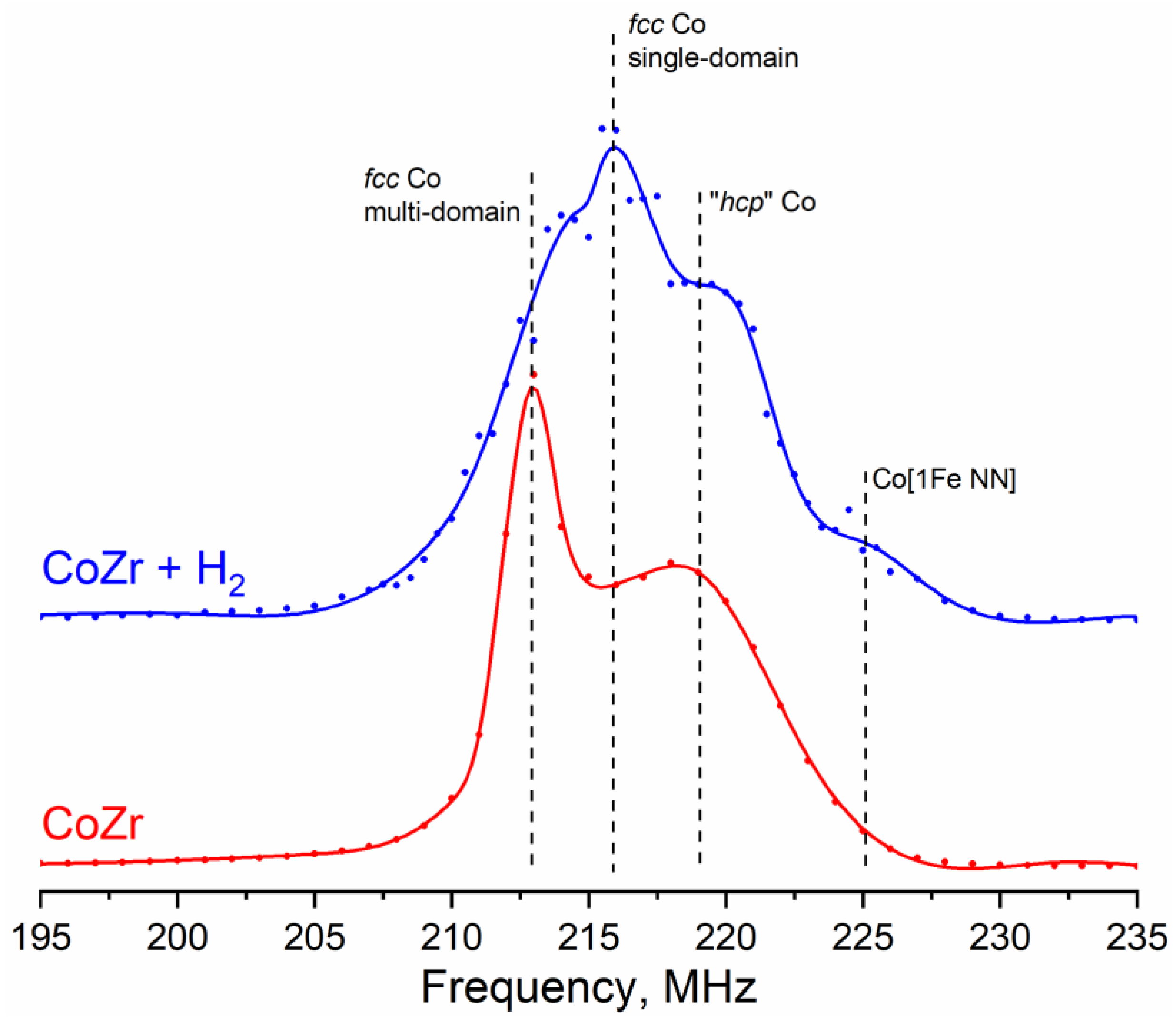
3.3.2. 59Co Internal Field NMR
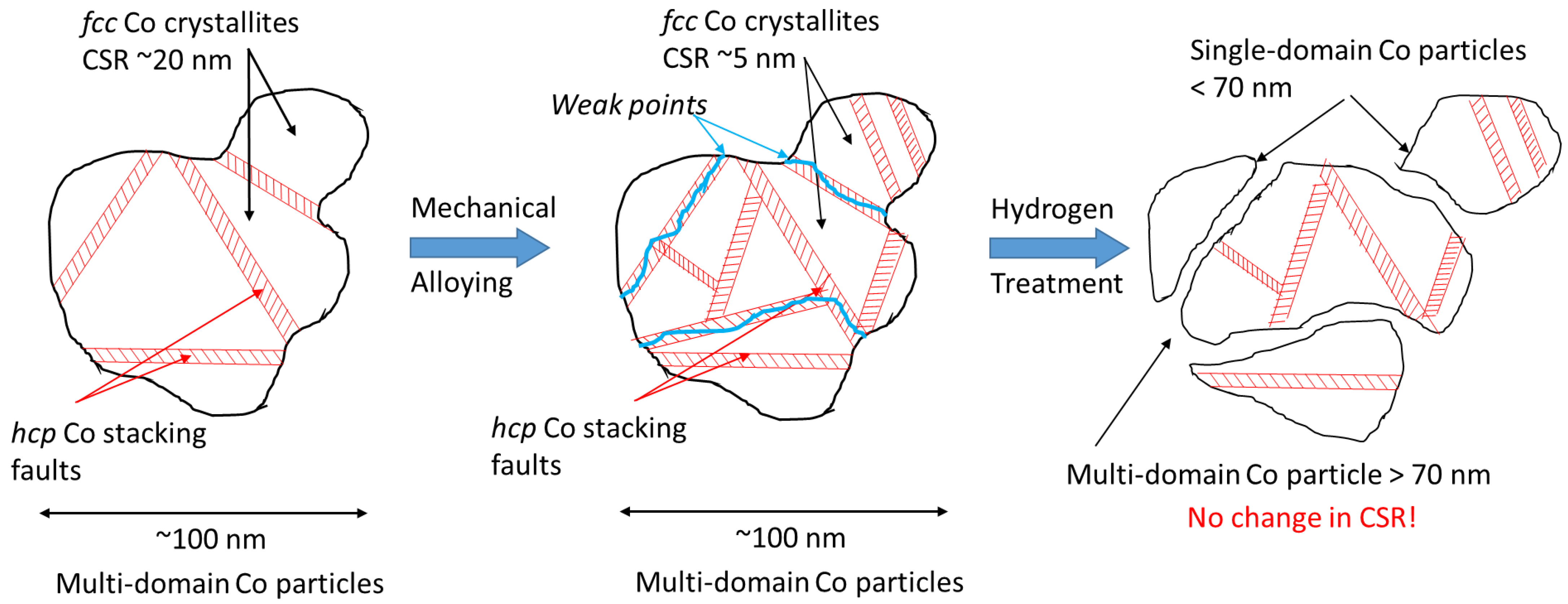
4. Discussion
4.1. “Fukushima” Effect
4.2. Formation of Single-Domain Cobalt Particles during Hydrogenation
4.3. Metal-Oxide Nanocomposite as a Precursor for the Fischer–Tropsch Catalyst
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kokanović, I.; Leontić, B.; Lukatela, J. The Resistivity and the Magnetoresistivity of Hydrogen-Doped Zr67Co33 Metallic Glass. J. Non-Cryst. Solids 1996, 205–207, 673–677. [Google Scholar] [CrossRef]
- Al Alam, A.F.; Matar, S.F.; Jammal, A.; Ouaini, N. Drastic Changes of Electronic Structure, Bonding Properties and Crystal Symmetry in Zr2Cu by Hydrogenation, from Ab Initio. Intermetallics 2014, 45, 5–10. [Google Scholar] [CrossRef]
- Matar, S.F. Drastic Changes in Electronic, Magnetic, Mechanical and Bonding Properties from Zr2CoH5 to Mg2CoH5. J. Solid State Chem. 2013, 200, 209–214. [Google Scholar] [CrossRef]
- Novak, M.; Kokanović, I. Effects of Absorbed Hydrogen on the Electronic Properties of (Zr2Fe)1−xHx Metallic Glasses. J. Phys. Condens. Matter 2012, 24, 235701. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Kokanović, I. Magnetoresistivity of Hydrogen-Doped Zr2(3d) Metallic Glasses. J. Non-Cryst. Solids 2013, 376, 86–89. [Google Scholar] [CrossRef]
- Kokanović, I.; Leontić, B.; Lukatela, J. Transport Properties of Hydrogen-Doped (Zr803d20)1−xHx (3d = Co, Ni) Metallic Glasses. Phys. Status Solidi B 2004, 241, 908–915. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Li, J.; Kou, H.; Hu, R.; Xue, X. Microstructure and Hydrogenation Properties of a Melt-Spun Non-Stoichiometric Zr-Based Laves Phase Alloy. Mater. Charact. 2016, 111, 53–59. [Google Scholar] [CrossRef]
- Matar, S.F. Drastic Changes of Electronic, Magnetic, Mechanical and Bonding Properties in Zr2Co by Hydrogenation. Intermetallics 2013, 36, 25–30. [Google Scholar] [CrossRef]
- Gabay, A.M.; Zhang, Y.; Hadjipanayis, G.C. Cobalt-Rich Magnetic Phases in Zr–Co Alloys. J. Magn. Magn. Mater. 2001, 236, 37–41. [Google Scholar] [CrossRef]
- Zhang, H.; Su, R.; Chen, D.; Shi, L. Thermal Desorption Behaviors of Helium in Zr-Co Films Prepared by Sputtering Deposition Method. Vacuum 2016, 130, 174–178. [Google Scholar] [CrossRef]
- Valdrè, G.; Zacchini, D.; Berti, R.; Costa, A.; Alessandrini, A.; Zucchetti, P.; Valdrè, U. Nitrogen Sorption Tests, SEM-Windowless EDS and XRD Analysis of Mechanically Alloyed Nanocrystalline Getter Materials. Nanostructured Mater. 1999, 11, 821–829. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Fattahzadeh, M.; Kaflou, A.; Dashtizad, V. Effect of Milling and Adding Yttrium on Sorption Characteristics of Zr-Co Based Nanostructure Chemical Getter. J. Alloys Compd. 2020, 846, 155329. [Google Scholar] [CrossRef]
- Heidary Moghadam, A.; Dashtizad, V.; Kaflou, A.; Yoozbashizadeh, H.; Ashiri, R. Development of a Nanostructured Zr3Co Intermetallic Getter Powder with Enhanced Pumping Characteristics. Intermetallics 2015, 57, 51–59. [Google Scholar] [CrossRef]
- Neelima, B.; Rama Rao, N.V.; Chary, V.R.; Pandian, S. Influence of Mechanical Milling on Structure, Particle Size, Morphology and Magnetic Properties of Rare Earth Free Permanent Magnetic Zr2Co11 Alloy. J. Alloys Compd. 2016, 661, 72–76. [Google Scholar] [CrossRef]
- Gerasimov, K.B.; Gusev, A.A.; Ivanov, E.Y.; Boldyrev, V.V. Tribochemical Equilibrium in Mechanical Alloying of Metals. J. Mater. Sci. 1991, 26, 2495–2500. [Google Scholar] [CrossRef]
- Lunin, V.V.; Khan, A.Z. Polymetallic Catalysts Derived from Intermetallic Hydrides. J. Mol. Catal. 1984, 25, 317–326. [Google Scholar] [CrossRef]
- Tikhov, S.F.; Kuz’min, A.E.; Bespalko, Y.N.; Kurkin, V.I.; Sadykov, V.A.; Bogolepova, E.I.; Tsybulya, S.V.; Kalinkin, A.V.; Zaikovskii, V.I.; Shavorsky, A.A.; et al. ZrFe Intermetallides for Fischer-Tropsch Synthesis: Pure and Encapsulated into Alumina-Containing Matrices. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 163, pp. 153–175. ISBN 978-0-444-52221-4. [Google Scholar]
- Borshch, V.N.; Pugacheva, E.V.; Zhuk, S.Y.; Sanin, V.N.; Andreev, D.E.; Yukhvid, V.I.; Eliseev, O.L.; Kazantsev, R.V.; Kolesnikov, S.I.; Kolesnikov, I.M.; et al. Polymetallic Catalysts for the Fischer–Tropsch Synthesis and Hydrodesulfurization Prepared Using Self-Propagating High-Temperature Synthesis. Kinet. Catal. 2015, 56, 681–688. [Google Scholar] [CrossRef]
- Lunin, V.V.; Solovetskii, Y.I. Formirovanie Aktivnoi Poverhnosti Katalizatorov Na Osnove Gidridov Intermetallidov Zr i Hf s Ni, Co i Fe. Kinet. Catal. 1985, 24, 694–698. (In Russian) [Google Scholar]
- Lunin, V.V.; Chetina, O.V. Vliyanie Okislitelno-Vosstanovitelnih Processov v Sisteme Intermetallid-Oksid Na Adsorbtsiyu Vodoroda. Zhurnal Fiz. Him. 1990, 64, 3019–3023. (In Russian) [Google Scholar]
- Gossard, A.C.; Portis, A.M. Observation of Nuclear Resonance in a Ferromagnet. Phys. Rev. Lett. 1959, 3, 164–166. [Google Scholar] [CrossRef]
- Portis, A.M.; Gossard, A.C. Nuclear Resonance in Ferromagnetic Cobalt. J. Appl. Phys. 1960, 31, S205–S213. [Google Scholar] [CrossRef]
- Andreev, A.S.; Lapina, O.B.; Cherepanova, S.V. A New Insight into Cobalt Metal Powder Internal Field 59Co NMR Spectra. Appl. Magn. Reson. 2014, 45, 1009–1017. [Google Scholar] [CrossRef]
- Andreev, A.S.; Lapina, O.B.; d’Espinose de Lacaillerie, J.-B.; Khassin, A.A. EFfect of Alumina Modification on the Structure of Cobalt-Containing Fischer-Tropsch Synthesis Catalysts According to Internal-Field 59Co NMR Data. J. Struct. Chem. 2013, 54, 102–110. [Google Scholar] [CrossRef]
- Kazakova, M.A.; Andreev, A.S.; Selyutin, A.G.; Ishchenko, A.V.; Shuvaev, A.V.; Kuznetsov, V.L.; Lapina, O.B.; d’Espinose de Lacaillerie, J.-B. Co Metal Nanoparticles Deposition inside or Outside Multi-Walled Carbon Nanotubes via Facile Support Pretreatment. Appl. Surf. Sci. 2018, 456, 657–665. [Google Scholar] [CrossRef]
- Andreev, A.S.; Kazakova, M.A.; Ishchenko, A.V.; Selyutin, A.G.; Lapina, O.B.; Kuznetsov, V.L.; d’Espinose de Lacaillerie, J.-B. Magnetic and Dielectric Properties of Carbon Nanotubes with Embedded Cobalt Nanoparticles. Carbon 2017, 114, 39–49. [Google Scholar] [CrossRef]
- Yakovlev, I.V.; Yakushkin, S.S.; Kazakova, M.A.; Trukhan, S.N.; Volkova, Z.N.; Gerashchenko, A.P.; Andreev, A.S.; Ishchenko, A.V.; Martyanov, O.N.; Lapina, O.B.; et al. Superparamagnetic Behaviour of Metallic Co Nanoparticles According to Variable Temperature Magnetic Resonance. Phys. Chem. Chem. Phys. 2021, 23, 2723–2730. [Google Scholar] [CrossRef]
- Tikhov, S.F.; Andreev, A.S.; Salanov, A.N.; Cherepanova, S.V.; Lapina, O.B.; Sadykov, V.A.; Tanashev, Y.Y.; Bolotov, V.A. Ceramic Matrix Composites Prepared from CoAl Powders. J. Mater. Sci. 2016, 51, 10487–10498. [Google Scholar] [CrossRef]
- Andreev, A.S.; Tikhov, S.F.; Salanov, A.N.; Cherepanova, S.V.; Lapina, O.B.; Bolotov, V.A.; Tanashev, Y.Y.; d’Espinose de Lacaillerie, J.B.; Sadykov, V.A. Design of Al2O3/CoAlO/CoAl Porous Ceramometal for Multiple Applications as Catalytic Supports. AMR 2013, 702, 79–87. [Google Scholar] [CrossRef]
- Jędryka, E.; Wójcik, M.; Nadolski, S.; Stobiecki, T.; Czapkiewicz, M. Structural Studies in Co/Zr Multilayers Using NMR. J. Magn. Magn. Mater. 1996, 156, 38–40. [Google Scholar] [CrossRef]
- Wójcik, M.; Jȩdryka, E.; Nadolski, S.; Stobiecki, T.; Czapkiewicz, M. NMR Study in Amorphous CoZr Thin Film Alloys. J. Magn. Magn. Mater. 1996, 157–158, 220–222. [Google Scholar] [CrossRef]
- Frictional Planetary Discrete Activator AGO-3. Available online: http://www.solid.nsc.ru/developments/equipments/ago3/ (accessed on 9 January 2023).
- Gossard, A.C.; Portis, A.M.; Rubinstein, M.; Lindquist, R.H. Ferromagnetic Nuclear Resonance of Single-Domain Cobalt Particles. Phys. Rev. 1965, 138, A1415–A1421. [Google Scholar] [CrossRef]
- Andreev, A.S.; d’Espinose de Lacaillerie, J.-B.; Lapina, O.B.; Gerashenko, A. Thermal Stability and Hcp–Fcc Allotropic Transformation in Supported Co Metal Catalysts Probed near Operando by Ferromagnetic NMR. Phys. Chem. Chem. Phys. 2015, 17, 14598–14604. [Google Scholar] [CrossRef]
- Jay, J.P.; Wójcik, M.; Panissod, P. Hyperfine Field and Ordering in Bcc CoFe Bulk Alloys Studied by 59Co NMR and Monte-Carlo Simulation. Z. Phys. B Condens. Matter 1996, 101, 471–486. [Google Scholar] [CrossRef]
- Wojcik, M.; Jay, J.P.; Panissod, P.; Jedryka, E.; Dekoster, J.; Langouche, G. New Phases and Chemical Short Range Order in Co-Deposited CoFe Thin Films with Bcc Structure: An NMR Study. Z. Phys. B Condens. Matter 1997, 103, 5–12. [Google Scholar] [CrossRef]
- Andreev, A.S.; Krasnikov, D.V.; Zaikovskii, V.I.; Cherepanova, S.V.; Kazakova, M.A.; Lapina, O.B.; Kuznetsov, V.L.; d’Espinose de Lacaillerie, J. Internal Field 59Co NMR Study of Cobalt-Iron Nanoparticles during the Activation of CoFe2/CaO Catalyst for Carbon Nanotube Synthesis. J. Catal. 2018, 358, 62–70. [Google Scholar] [CrossRef]
- Andreev, A. Internal field 59Co Nuclear Magnetic Resonance, Application to Catalysts and Related Structures. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 2015. [Google Scholar] [CrossRef]
- Iskhakov, R.S.; Kuzovnikova, L.A.; Denisova, E.A.; Komogortsev, S.V.; Balaev, A.D. Co-Cu Alloys Produced by Mechanical Alloying of Powder Precursors Characterized by Different Contact Surface and Energy Excess. Phys. Met. Metallogr. 2009, 107, 478–483. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of Crystal Structure of Nanosized ZrO2 on Photocatalytic Degradation of Methyl Orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Diallo, A.; Beye, A.C.; Doyle, T.B.; Park, E.; Maaza, M. Green Synthesis of Co3O4 Nanoparticles via Aspalathus Linearis: Physical Properties. Green Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Speight, R.; Wong, A.; Ellis, P.; Hyde, T.; Bishop, P.T.; Smith, M.E. A 59Co NMR Study to Observe the Effects of Ball Milling on Small Ferromagnetic Cobalt Particles. Solid State Nucl. Magn. Reson. 2009, 35, 67–73. [Google Scholar] [CrossRef]
- Leslie-Pelecky, D.L.; Rieke, R.D. Magnetic Properties of Nanostructured Materials. Chem. Mater. 1996, 8, 1770–1783. [Google Scholar] [CrossRef]
- Neeb, K.-H. The Radiochemistry of Nuclear Power Plants with Light Water Reactors; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1997; ISBN 978-3-11-013242-7. [Google Scholar]
- Arutyunyan, R.V.; Bolshov, L.A.; Borovoi, A.A.; Velikhov, E.P. System Analysis of Causes and Consequences of the Fukushima-1 NPP Accident; Nuclear Safety Institute of RAS: Moscow, Russia, 2018; ISBN 978-5-9907220-5-7. [Google Scholar]
- Kwon, Y.-S.; Gerasimov, K.B.; Yoon, S.-K. Ball Temperatures during Mechanical Alloying in Planetary Mills. J. Alloys Compd. 2002, 346, 276–281. [Google Scholar] [CrossRef]
- Du, H.; Jiang, M.; Zhu, H.; Huang, C.; Zhao, Z.; Dong, W.; Lu, W.; Liu, T.; Conrad Zhang, Z.; Ding, Y. Constructing Efficient Hcp-Co Active Sites for Fischer-Tropsch Reaction on an Activated Carbon Supported Cobalt Catalyst via Multistep Activation Processes. Fuel 2021, 292, 120244. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Fang, K.; Wang, Y.; Sun, Y. A Large Pore-Size Mesoporous Zirconia Supported Cobalt Catalyst with Good Performance in Fischer–Tropsch Synthesis. Catal. Commun. 2007, 8, 945–949. [Google Scholar] [CrossRef]




| Co:Zr ratio, at.% | Mechanical Alloying Conditions | Milling Time, h | Heat Treatment | Phases | Ref. |
|---|---|---|---|---|---|
| 34:66 | High energy planetary mill NARYA 250 Amin Co. Ar 3 atm, ball:powder ratio 15:1. 400 rpm. | 2 24 42 | No No No | Co, Zr Amorphous phases, Zr3Co Amorphous phases | [13] |
| 82:18 | High energy planetary mill, ball:powder ratio 30:1, toluene medium, 6 h at 200 rpm. | 8 | Preliminary melting and annealing at 1000° then crushed | Main phase Zr2Co11, minor phase Zr6Co23 | [15] |
| 32:68 | Planetary ball mill (Retsch PM 400), Ar, ball:powder ratio 15:1. 300 rpm | 2 8 16 | No No No | Zr, Co, ZrO2 Zr, Co, ZrO2, Zr3Co Amorphous phases | [14] |
| 33:67 (intermetallic) 50:50 (intermetallic) | High energy planetary mill, Ar, ball:powder ratio 10:1. 300 m s−2 600 m s−2 600 m s−2 900 m s−2 | Unknown | No No No No | Amorphous phases ZrCo, Zr3Co Amorphous phases Zr2Co, Zr3Co | [16] |
| Sample | Parameter | Phase | ||||
|---|---|---|---|---|---|---|
| Co (fcc, hcp) | ZrO2 Monoclinic | ZrO2 Tetrahedral | ZrH2 | Zr | ||
| Co | CSR (nm) | 21(1) | ||||
| wt% | 100 | |||||
| Zr | CSR (nm) | ≈30 | - | ≈30 | ≈100 | |
| wt% | 11 | 5 | 7 | 77 | ||
| Lattice parameter (Å) | a = 5.23(1) b = 5.10(1) c = 5.34(1) β = 99.3(1) | - | a = 4.661) c = 4.79(1) | a = 3.231(1) c = 5.145(1) | ||
| Sample | Co Structures Relative Contribution, % | ||||
|---|---|---|---|---|---|
| Co [1Fe NNN] 208.5 MHz | fcc Co m. d. 213.5 MHz | fcc Co s. d. 216.5 MHz | hcp Co 214–221 MHz | Co [1Fe NN] 224.5 MHz | |
| Co | 3 | 52 | 41 | 4 | |
| CoZr | 33 | 67 | |||
| CoZr + H2 | 2 | 26 | 32 | 36 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakovlev, I.; Tikhov, S.; Gerasimov, E.; Kardash, T.; Valeev, K.; Salanov, A.; Chesalov, Y.; Lapina, O.; Lomovskii, O.; Dudina, D. Formation of Metal-Oxide Nanocomposites with Highly Dispersed Co Particles from a Co-Zr Powder Blend by Mechanical Alloying and Hydrogen Treatment. Materials 2023, 16, 1074. https://doi.org/10.3390/ma16031074
Yakovlev I, Tikhov S, Gerasimov E, Kardash T, Valeev K, Salanov A, Chesalov Y, Lapina O, Lomovskii O, Dudina D. Formation of Metal-Oxide Nanocomposites with Highly Dispersed Co Particles from a Co-Zr Powder Blend by Mechanical Alloying and Hydrogen Treatment. Materials. 2023; 16(3):1074. https://doi.org/10.3390/ma16031074
Chicago/Turabian StyleYakovlev, Ilya, Serguei Tikhov, Evgeny Gerasimov, Tatiana Kardash, Konstantin Valeev, Aleksei Salanov, Yurii Chesalov, Olga Lapina, Oleg Lomovskii, and Dina Dudina. 2023. "Formation of Metal-Oxide Nanocomposites with Highly Dispersed Co Particles from a Co-Zr Powder Blend by Mechanical Alloying and Hydrogen Treatment" Materials 16, no. 3: 1074. https://doi.org/10.3390/ma16031074
APA StyleYakovlev, I., Tikhov, S., Gerasimov, E., Kardash, T., Valeev, K., Salanov, A., Chesalov, Y., Lapina, O., Lomovskii, O., & Dudina, D. (2023). Formation of Metal-Oxide Nanocomposites with Highly Dispersed Co Particles from a Co-Zr Powder Blend by Mechanical Alloying and Hydrogen Treatment. Materials, 16(3), 1074. https://doi.org/10.3390/ma16031074








