Effect of Mo Content on Hydrogen Evolution Reaction of 1400 MPa-Grade High-Strength Bolt Steel
Abstract
1. Introduction
2. Experimental Methods
2.1. Tested Materials
2.2. Electrochemical Investigation Methods
2.3. Thermal Desorption Spectroscopy (TDS)
2.4. Computational Details and Structural Models
3. Results
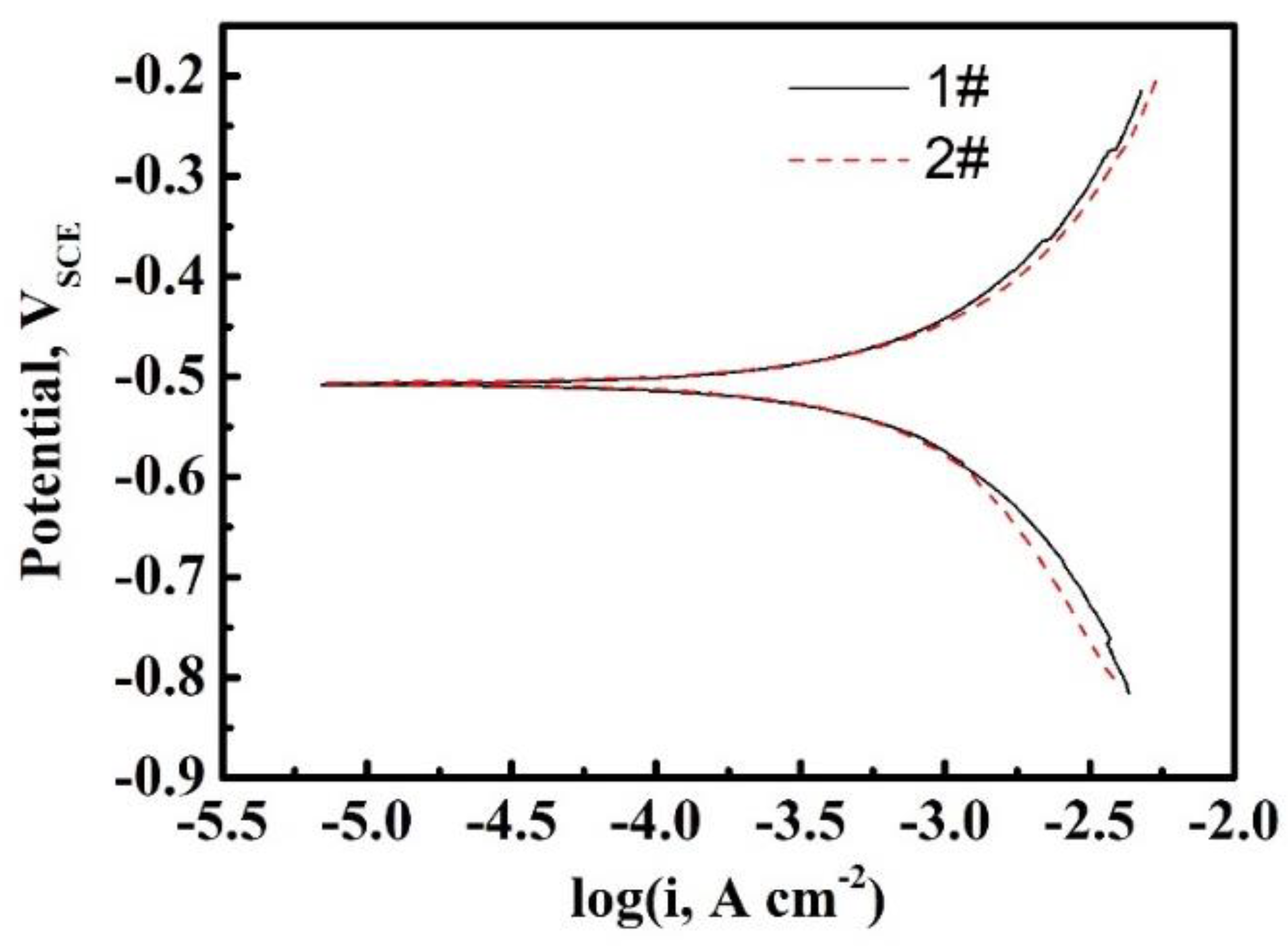
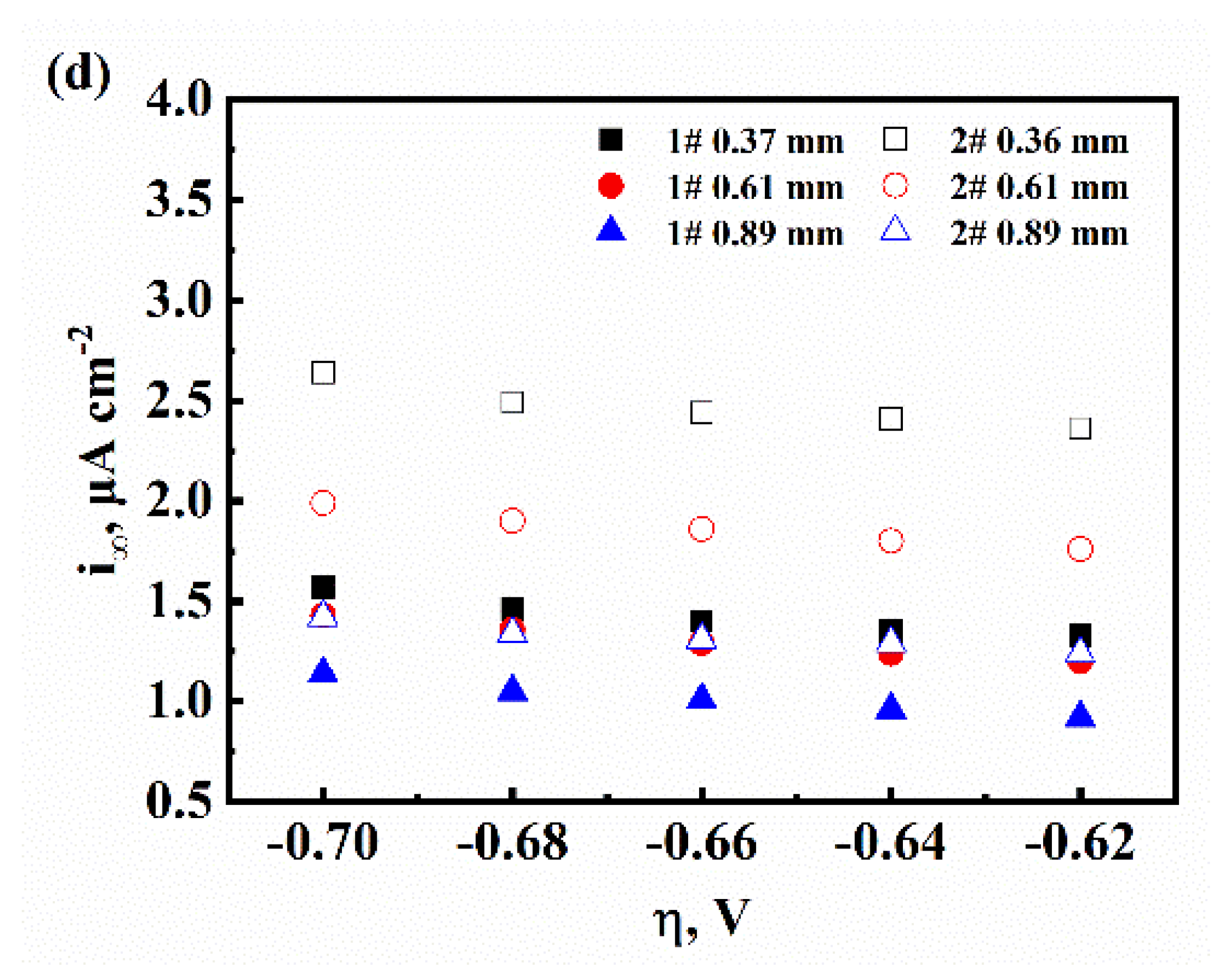
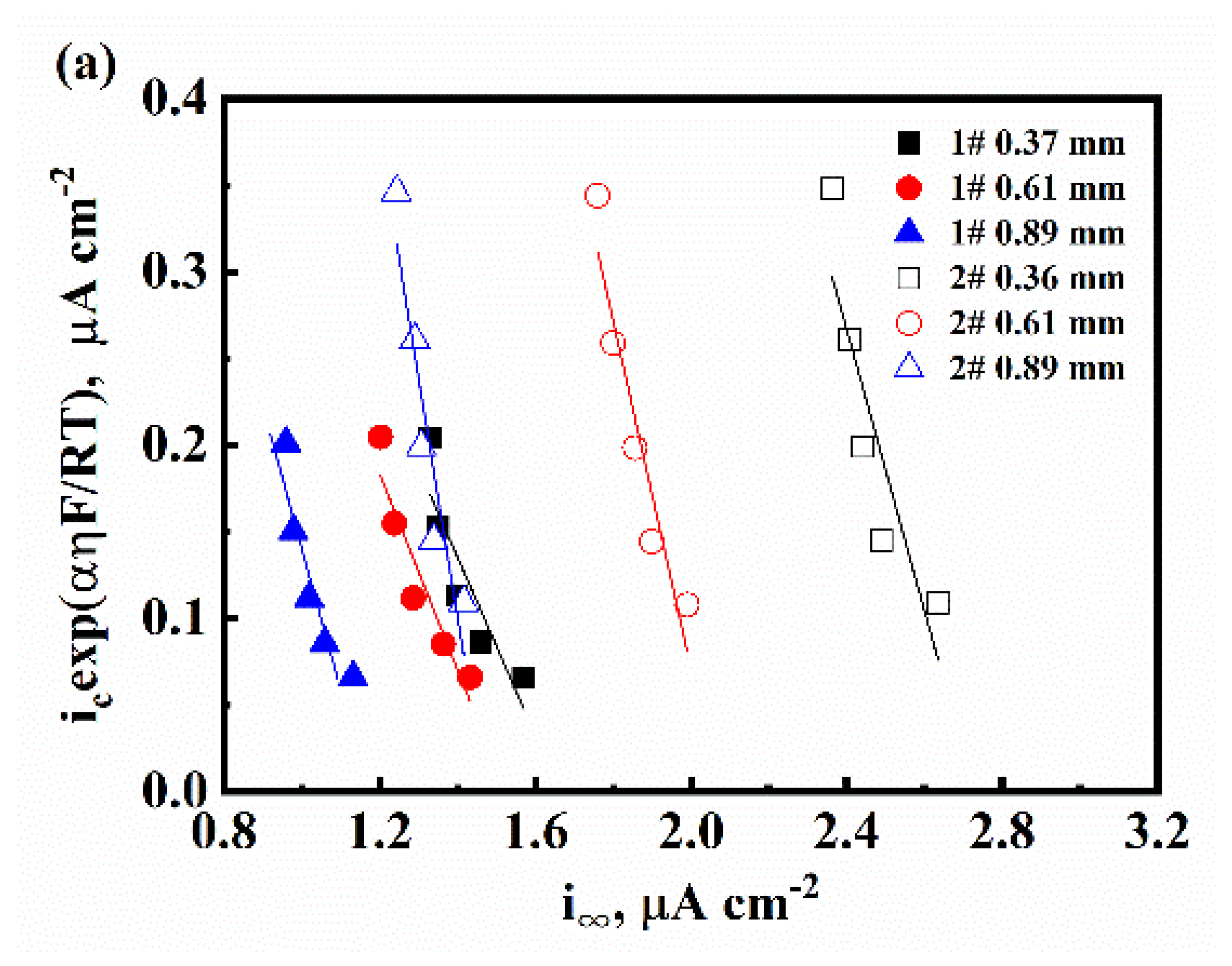
3.1. The Characteristic of Hydrogen Diffusion and the HER
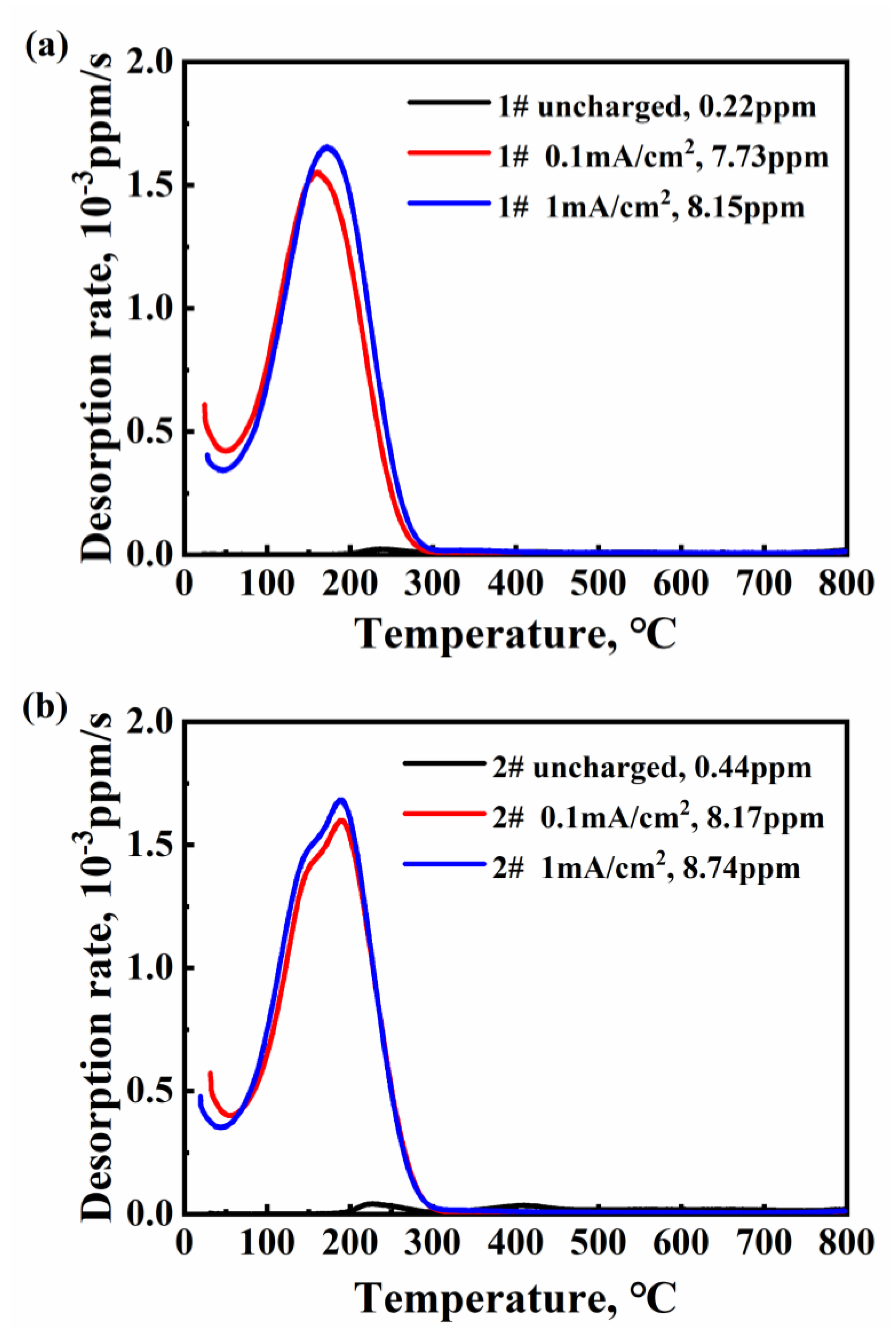
3.2. The Characteristic of Hydrogen Desorption Behavior
3.3. The First-Principal Calculation of Hydrogen Adsorption
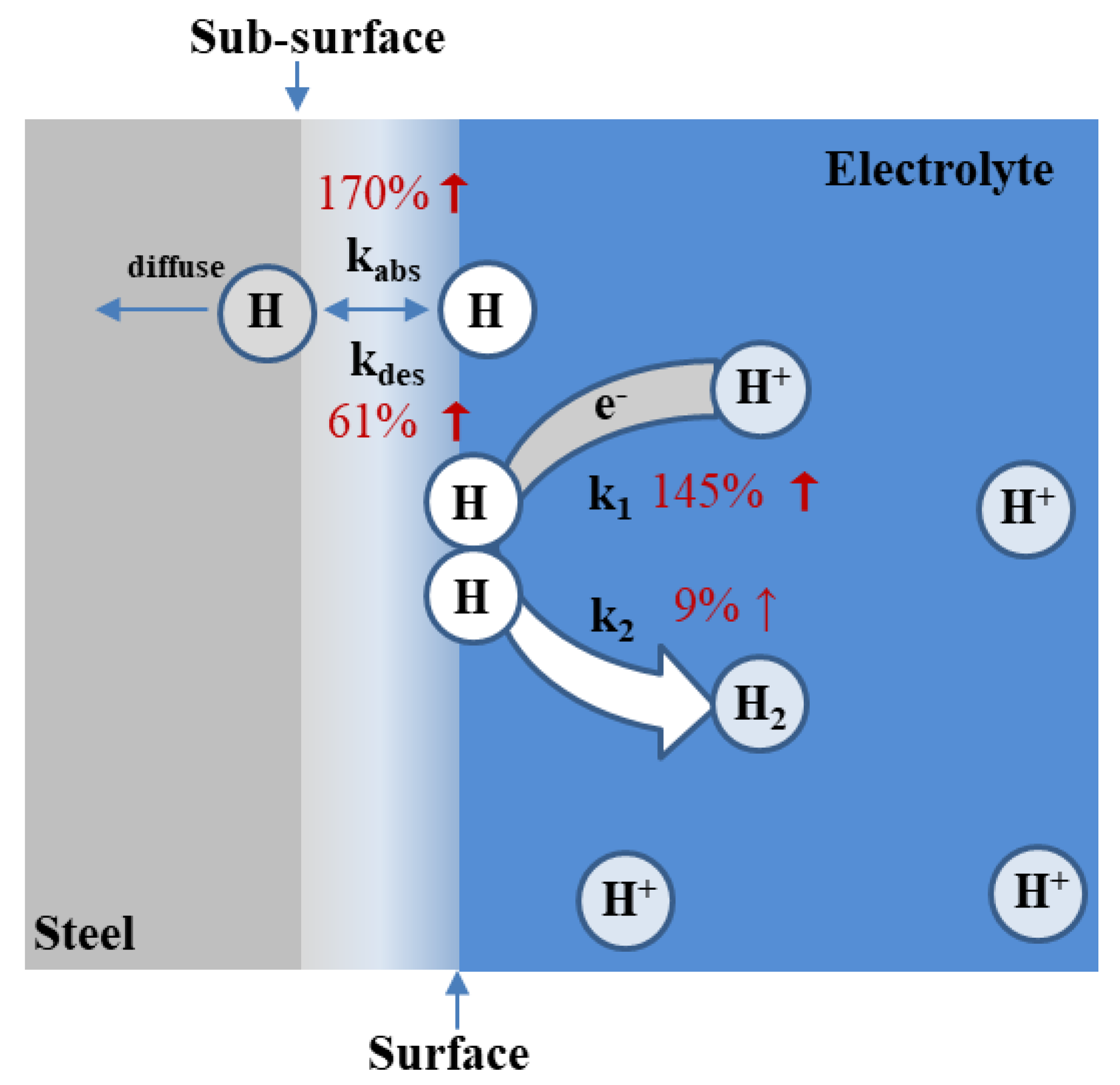
4. Discussion
5. Conclusions
- (1)
- With increased Mo content, the hydrogen-charging-current density and steady-state current density increased, while the hydrogen diffusion coefficient decreased.
- (2)
- Due to the stronger bonding ability of Mo-H than Fe-H, the adsorption energy decreased with the increase in Mo content. It induced the promotion of the hydrogen adsorption reaction and increased hydrogen surface coverage.
- (3)
- The increase in Mo content slightly promoted hydrogen recombination and significantly advanced the forward reaction of hydrogen absorption and desorption.
- (4)
- On the whole, increasing the Mo content facilitated hydrogen entry behavior and increased the hydrogen content in 1400 MPa-grade high-strength bolt steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GB/T 3098.1-2010; Mechanical Properties of Fasteners: Beijing, China, 2010.
- Pradhan, A.; Vishwakarma, M.; Dwivedi, S. A review: The impact of hydrogen embrittlement on the fatigue strength of high strength steel. Mater. Today Proc. 2020, 26, 3015–3019. [Google Scholar] [CrossRef]
- Venezuela, J.; Lim, F.; Liu, L.; James, S.; Zhou, Q.; Knibbe, R.; Zhang, M.; Li, H.; Dong, F.; Dargusch, M.; et al. Hydrogen embrittlement of an automotive 1700 MPa martensitic advanced high-strength steel. Corros. Sci. 2020, 171, 108726. [Google Scholar] [CrossRef]
- Martin, M.; Weber, S.; Theisen, W.; Michler, T.; Naumann, J. Effect of alloying elements on hydrogen environment embrittlement of AISI type 304 austenitic stainless steel. Int. J. Hydrogen Energy 2011, 36, 15888–15898. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J.; Sattler, E. Influence of copper as an alloying element on hydrogen environment embrittlement of austenitic stainless steel. Int. J. Hydrogen Energy 2012, 37, 12765–12770. [Google Scholar] [CrossRef]
- Zamanzade, M.; Vehoff, H.; Barnoush, A. Cr effect on hydrogen embrittlement of Fe3Al-based iron aluminide intermetallics: Surface or bulk effect. Acta Mater. 2014, 69, 210–223. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jeon, S.-H.; Yang, W.-S.; Yoo, B.-G.; Chung, Y.-D.; Ha, H.-Y.; Chung, H.-Y. Effects of titanium content on hydrogen embrittlement susceptibility of hot-stamped boron steels. J. Alloys Compd. 2018, 735, 2067–2080. [Google Scholar] [CrossRef]
- Johnson, D.F.; Carter, E.A. First-principles assessment of hydrogen absorption into FeAl and Fe3Si: Towards prevention of steel embrittlement. Acta Mater. 2010, 58, 638–648. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, L.; Ma, H.; Xiong, X.; Su, Y. Carbon influence on hydrogen absorption and adsorption on Fe-C alloy surfaces. Surf. Sci. 2020, 697, 121606. [Google Scholar] [CrossRef]
- Seo, H.J.; Heo, Y.-U.; Kim, J.N.; Lee, J.; Choi, S.; Lee, C.S. Effect of V/Mo ratio on the evolution of carbide precipitates and hydrogen embrittlement of tempered martensitic steel. Corros. Sci. 2020, 176, 108929. [Google Scholar] [CrossRef]
- Li, S.; Akiyama, E.; Yuuji, K.; Tsuzaki, K.; Uno, N.; Zhang, B. Hydrogen embrittlement property of a 1700-MPa-class ultrahigh-strength tempered martensitic steel. Sci. Technol. Adv. Mater. 2010, 11, 025005. [Google Scholar] [CrossRef]
- Yamasaki, S.; Bhadeshia, H.K.D.H. Modelling and characterisation of Mo2C precipitation and cementite dissolution during tempering of Fe–C–Mo martensitic steel. Mater. Sci. Technol. 2003, 19, 723–731. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.; Kwon, Y.J.; Mun, D.; Yoo, J.; Lee, C.S. Role of Mo/V carbides in hydrogen embrittlement of tempered martensitic steel. Corros. Rev. 2015, 33, 433–441. [Google Scholar] [CrossRef]
- Wei, F.G.; Tsuzaki, K. Hydrogen trapping phenomena in martensitic steels. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Woodhead Publishing: Soston, UK, 2012; pp. 493–525. [Google Scholar]
- Elhamid, M.H.A.; Ateya, B.G.; Pickering, H.W. Determination of the Rate Constants of Hydrogen Absorption into Metals. J. Electrochem. Soc. 2000, 147, 2959–2963. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The Mechanism of Hydrogen Evolution on Iron in Acid Solutions by Determination of Permeation Rates. J. Electrochem. Soc. 1964, 111, 619–623. [Google Scholar] [CrossRef]
- Lyer, R.N.; Pickering, H.W. Analysis of Hydrogen Evolution and Entry into Metals for the Discharge-Recombination Process. J. Electrochem. Soc. 1989, 136, 2463–2470. [Google Scholar]
- Lyer, R.N.; Pickering, H.W. Mechanism and Kinetics of Electrochemical Hydrogen Entry and Degradation of Metallic Systems. Mater. Sci. 1990, 20, 299–338. [Google Scholar]
- Bockris, J.O.M.; McBreena, J.; Nanis, L. The Hydrogen Evolution Kinetics and Hydrogen Entry into α-iron. J. Electrochem. Soc 1965, 112, 1025–1031. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Jiang, D.E.; Carter, E.A. Diffusion of interstitial hydrogen into and through bcc Fe from first principles. Phys. Rev. B 2004, 70, 064102. [Google Scholar] [CrossRef]
- Kerr, J.A.; Huber, K.P.; Herzberg, G. Molecular spectra and molecular structure: IV constants of diatomic molecules: Von Nostrand—Reinhold, New York, 1979, pp. 716, price £20.65. Anal. Chim. Acta 1982, 144, 298. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Reddy, A.K.N. Modern Electrochemistry; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 1998. [Google Scholar]
- Zhang, T.Y.; Zheng, Y.P. Effects of absorption and desorption on hydrogen permeation—I. Theoretical modeling and room temperature verification. Acta Mater. 1998, 46, 5023–5033. [Google Scholar] [CrossRef]
- Wach, S.; Miodownik, A.P.; Mackowiak, J. The diffusion of hydrogen through pure iron membranes. Corros. Sci. 1966, 6, 271–285. [Google Scholar] [CrossRef]
- Merrill, P.B.; Madix, R.J. Hydrogen bonding on iron: Correlation of adsorption and desorption states on Fe(100) and perturbation of the Fe-H bond with coadsorbed CO. Surf. Sci. 1996, 347, 249–264. [Google Scholar] [CrossRef]
- Eder, M.; Terakura, K.; Hafner, J. Initial stages of oxidation of (100) and (110) surfaces of iron caused by water. Phys. Rev. B 2001, 64, 115426. [Google Scholar] [CrossRef]
- Walch, S.P. Model studies of the interaction of h atoms with bcc iron. Surf. Sci. 1984, 143, 188–203. [Google Scholar] [CrossRef]




| C | Si | Mn | Cr | Mo | V | |
|---|---|---|---|---|---|---|
| 1# | 0.41 | 0.22 | 0.31 | 1.00 | 0.70 | 0.34 |
| 2# | 0.42 | 0.19 | 0.31 | 1.00 | 1.09 | 0.35 |
| Sample | L, mm | D, ×107 cm2/s | k, ×10−11 mol cm−2 s−1 | k1, ×10−1 cm s−1 | k2, ×10−7 mol cm−2 s−1 | kabs, ×10−11 mol cm−2 s−1 | kdes, ×10−6 cm s−1 |
|---|---|---|---|---|---|---|---|
| 1# | 0.37 | 1.41 | 1.72 | 0.89 | 1.11 | 2.47 | 1.59 |
| 0.61 | 1.50 | 1.58 | |||||
| 0.89 | 1.51 | 1.24 | |||||
| 2# | 0.36 | 0.59 | 2.83 | 2.18 | 1.21 | 6.67 | 2.56 |
| 0.61 | 0.68 | 2.15 | |||||
| 0.89 | 0.78 | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Song, K.; Li, J.; Su, Y. Effect of Mo Content on Hydrogen Evolution Reaction of 1400 MPa-Grade High-Strength Bolt Steel. Materials 2023, 16, 1020. https://doi.org/10.3390/ma16031020
Xiong X, Song K, Li J, Su Y. Effect of Mo Content on Hydrogen Evolution Reaction of 1400 MPa-Grade High-Strength Bolt Steel. Materials. 2023; 16(3):1020. https://doi.org/10.3390/ma16031020
Chicago/Turabian StyleXiong, Xilin, Keke Song, Jinxu Li, and Yanjing Su. 2023. "Effect of Mo Content on Hydrogen Evolution Reaction of 1400 MPa-Grade High-Strength Bolt Steel" Materials 16, no. 3: 1020. https://doi.org/10.3390/ma16031020
APA StyleXiong, X., Song, K., Li, J., & Su, Y. (2023). Effect of Mo Content on Hydrogen Evolution Reaction of 1400 MPa-Grade High-Strength Bolt Steel. Materials, 16(3), 1020. https://doi.org/10.3390/ma16031020






