Functional Acrylic Resins Prepared via Photo-Induced Telomerization Using Tetrabromomethane as Telogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- (a)
- n-butyl acrylate (BA), (BASF, Ludwigshafen, Germany),
- (b)
- (acrylic acid (AA), (BASF, Ludwigshafen, Germany),
- (c)
- 4-acryloylooxybenzophenone (ABP, Chemitec, Scandiccy, Italy),
- (d)
- ethyl(2,4,6-trimethylbenzoyl)-phenyl phosphinate (Omnirad TPOL; IGM Resins, Waalwijk, The Netherlands),
- (e)
- tetrabromomethane (CBr4; Merck, Warsaw, Poland).
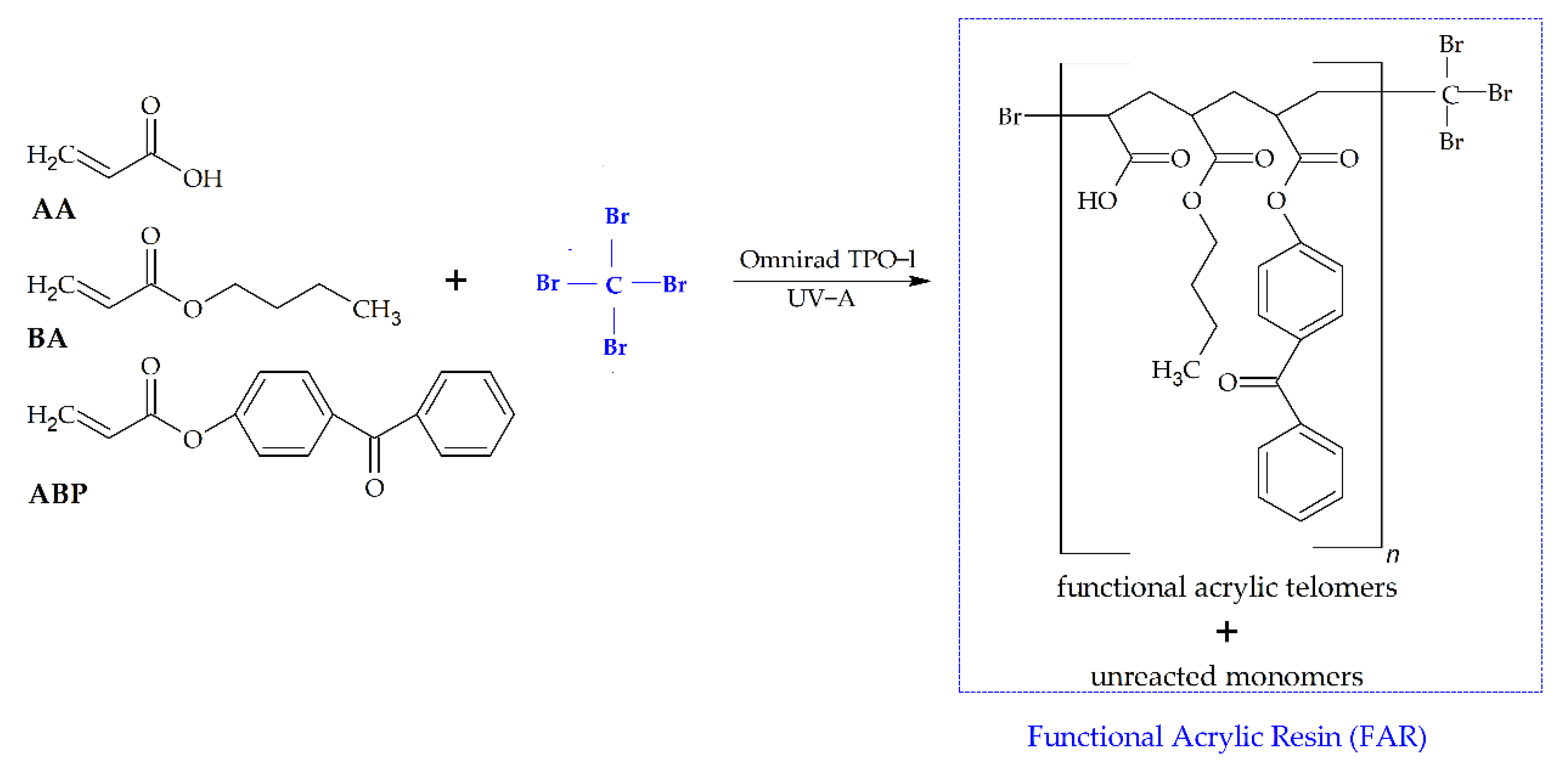
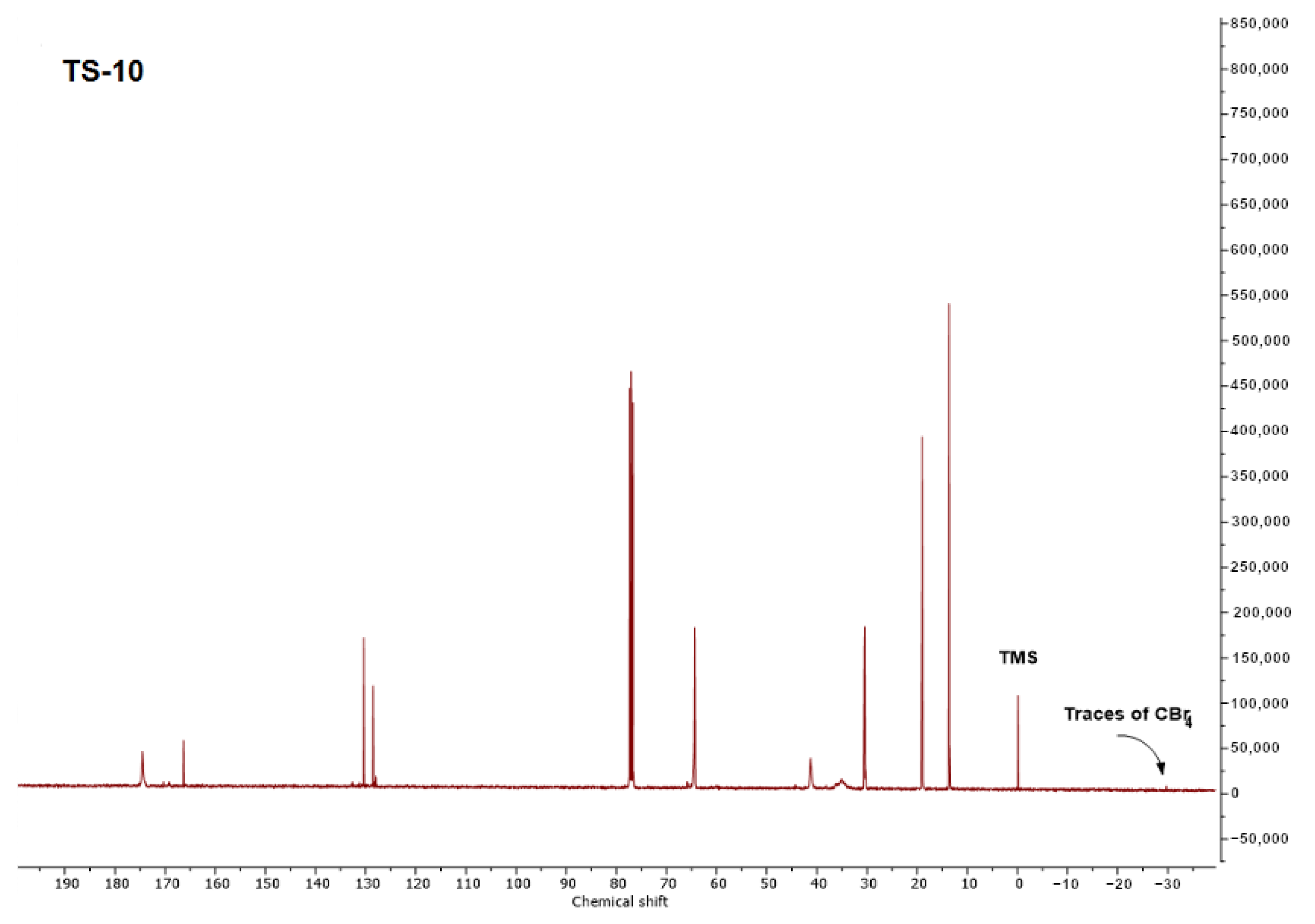
2.2. Synthesis and Characterization of Telomers Syrups
3. Results
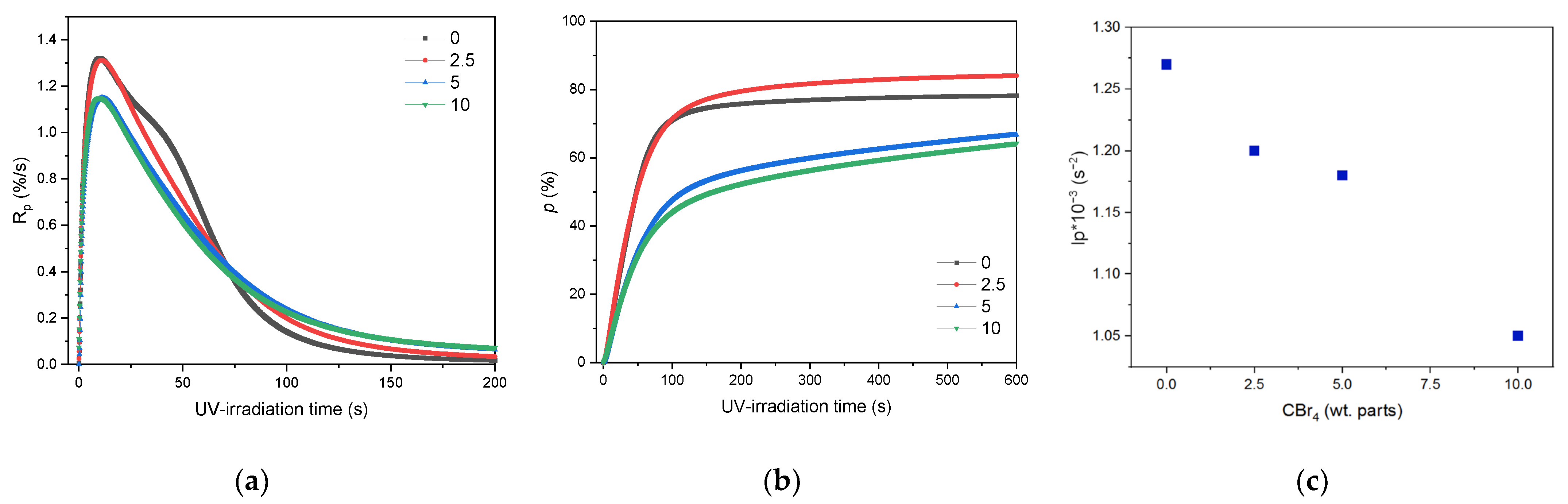
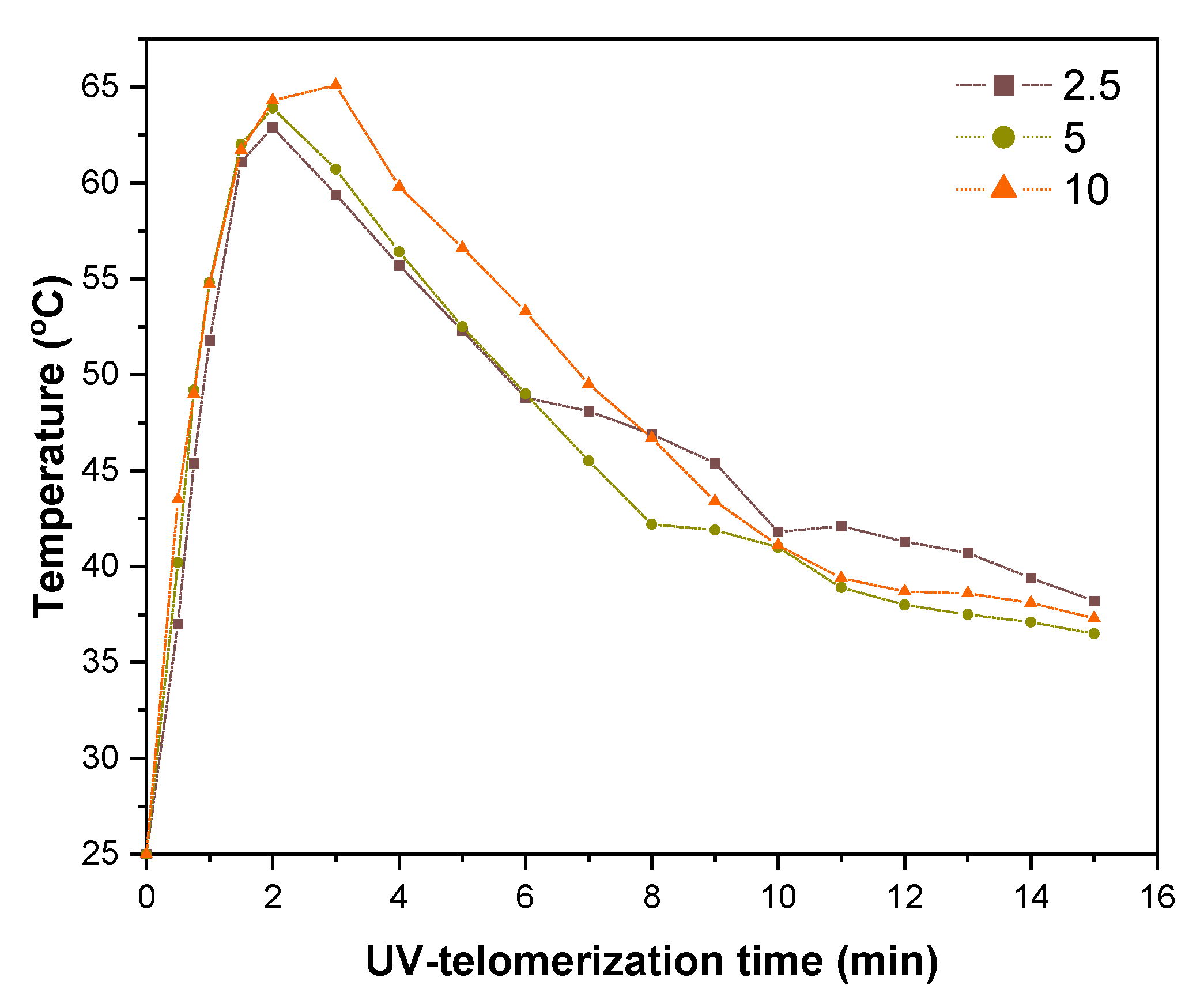
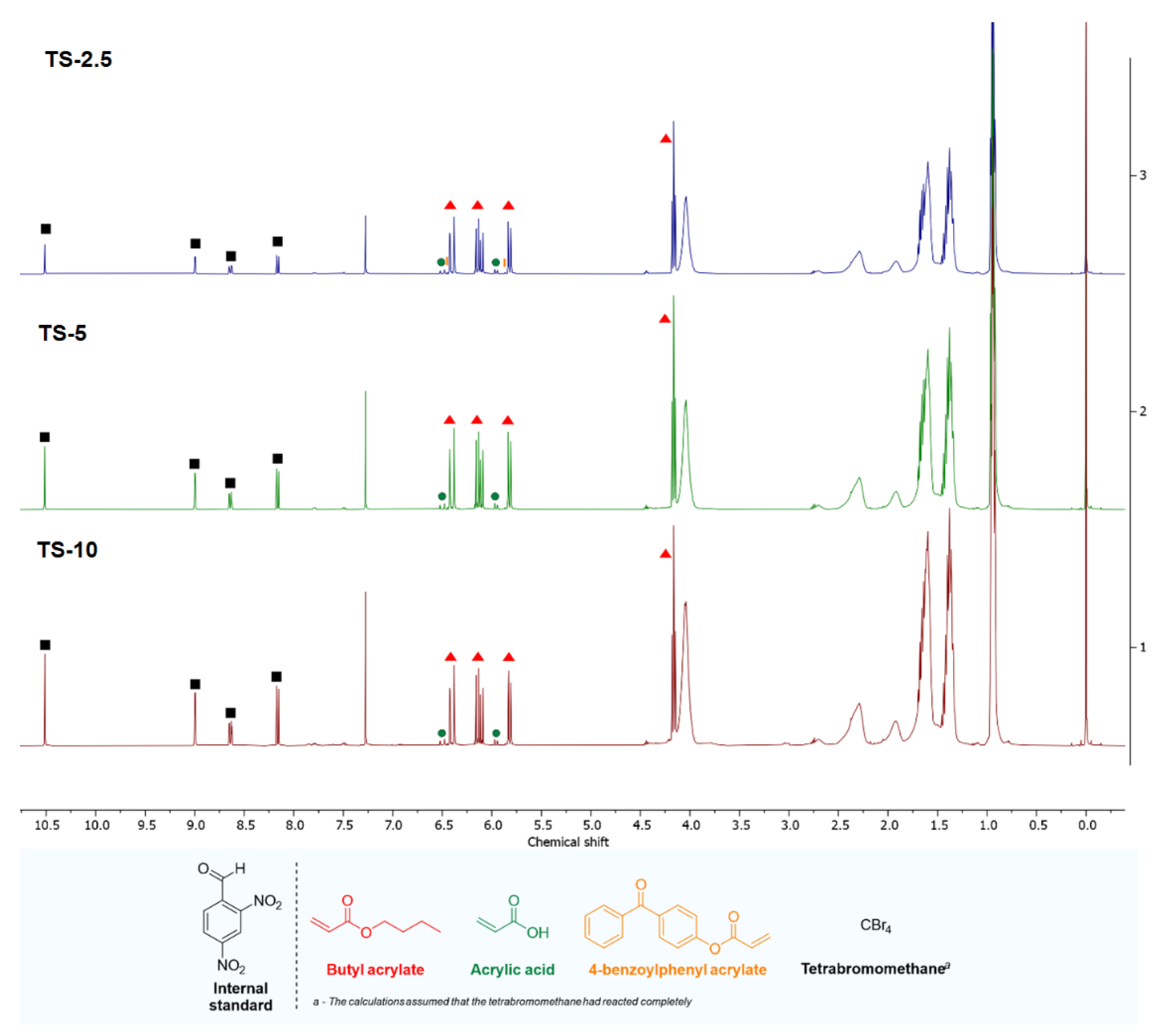
3.1. Kinetics of the UV-Telomerization
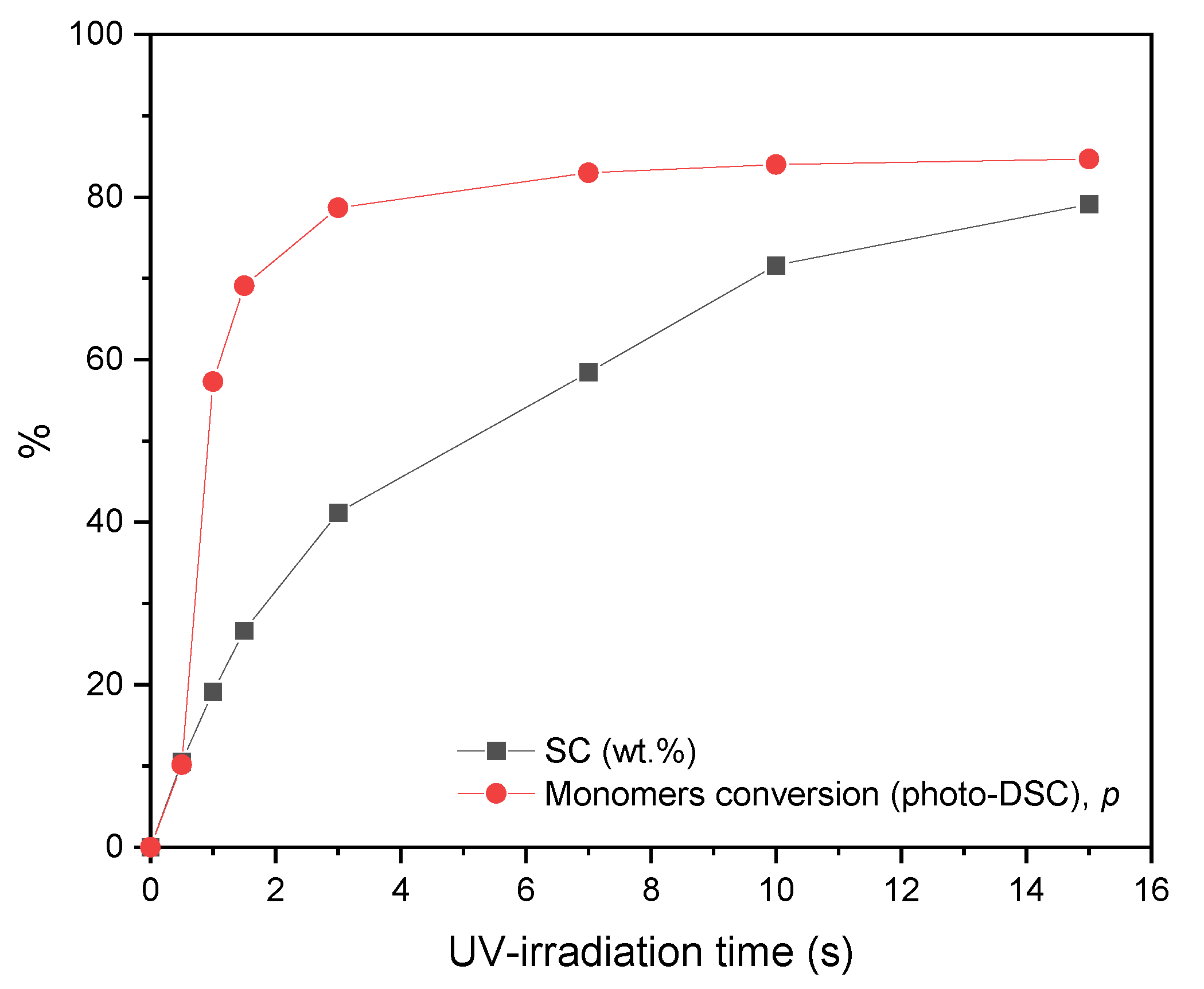
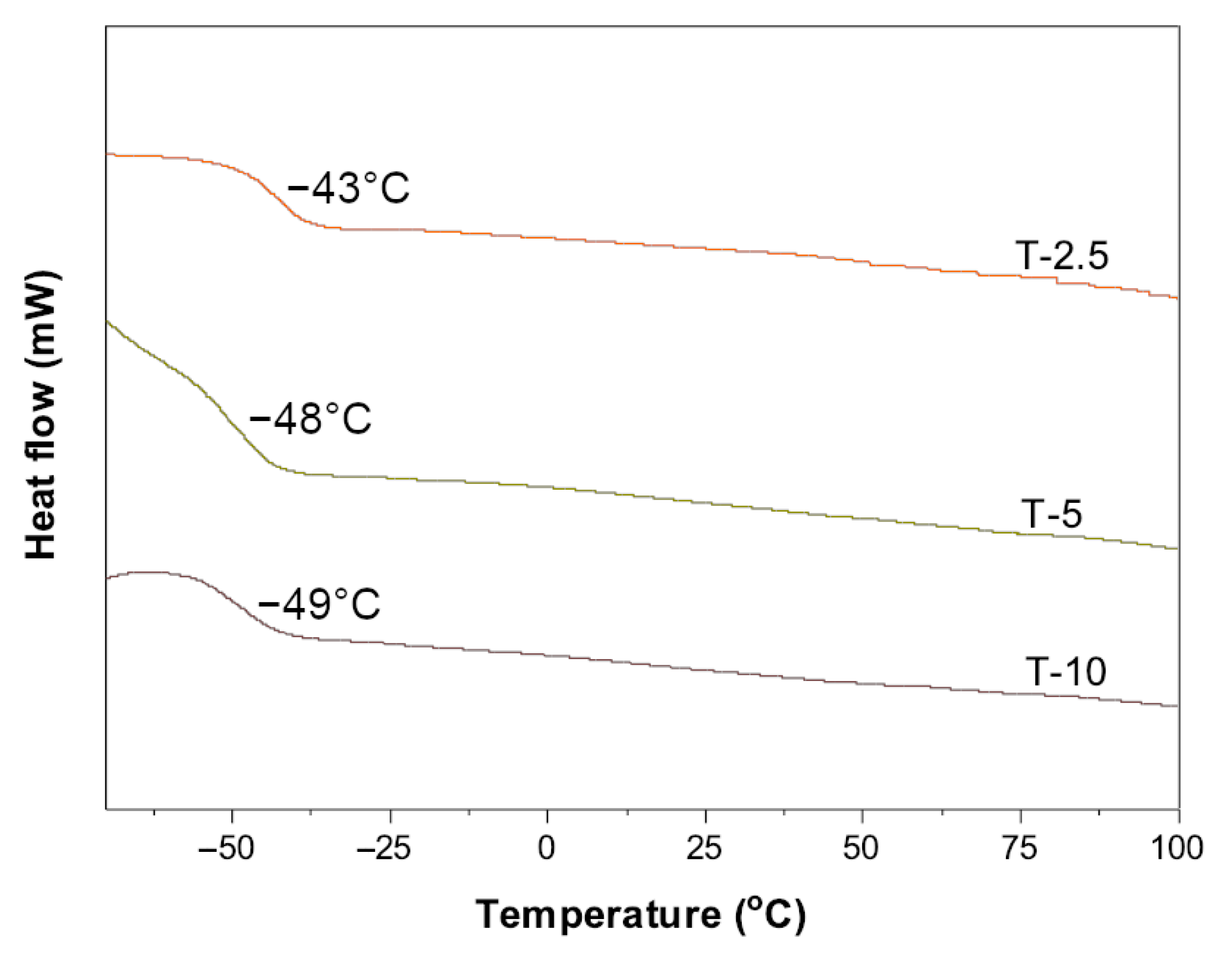
3.2. The Physicochemical Properties of the Functional Acrylic Resins
4. Conclusions
- -
- A higher concentration of CBr4 in the UV-telomerization process no longer affects the maximum reaction rate but reduces the initiating capacity of the two-component initiating system.
- -
- A higher telogen content has a positive effect on the increase in the total conversion of monomers and the reduction in the viscosity of the obtained resins.
- -
- A high telogen content does not affect the molecular weights of telomeres but improves their unimodality (Mw/Mn ca. 1.47); the glass transition temperature of telomeres does not change either.
- -
- The UV-telomerization method of basic and functional monomers to obtain functionalized polymers is very promising because it is relatively simple and quick to perform (approx. 30 min). Additionally, it allows the acquisition of resins with a very low content of unreacted monomers (high-solid systems) and characterized by low dynamic viscosity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambourne, R.; Strivens, T.A. Paint and Surface Coatings Theory and Practice, 2nd ed.; Woodhead Publishing: Sawston, UK, 1999. [Google Scholar]
- Prieto, J.; Kiene, J. Wood Coatings; Vincentz Network: Hanover, Germany, 2018; ISBN 3748600380. [Google Scholar]
- Wagner, O. Polymerdispersionen in Silikatsystemen. Farbe Lack 1991, 97, 109–113. [Google Scholar]
- Vukušić, T.; Vesel, A.; Holc, M.; Ščetar, M.; Jambrak, A.R.; Mozetič, M. Modification of Physico-Chemical Properties of Acryl-Coated Polypropylene Foils for Food Packaging by Reactive Particles from Oxygen Plasma. Materials 2018, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.; Groteklaes, M.; Mischke, P. European Coatings Handbook; Vincentz Network: Hanover, Germany, 2014; ISBN 3748602251. [Google Scholar]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Kraśkiewicz, A. The Effect of Type-I Photoinitiators on the Kinetics of the UV-Induced Cotelomerization Process of Acrylate Monomers and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2021, 14, 4563. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Gziut, K. Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2020, 13, 5661. [Google Scholar] [CrossRef] [PubMed]
- Poth, U.; Schwalm, R.; Schwartz, M.; Baumstark, R. Acrylic Resins; Vincentz Network: Hanover, Germany, 2011; 294p, ISBN 978-3-86630-809-1. [Google Scholar]
- Glöckner, P.; Jung, T.; Struck, S.; Studer, K. Radiation Curing; Vincentz: Hanover, Germany, 2009. [Google Scholar]
- Hult, A.; Malström, E.; Johansson, M. UV-curing of Acrylate Functional Hyperbranched Polyesters. Polym. Mater. Sci. Eng. 1995, 72, 528–529. [Google Scholar]
- Park, H.W.; Park, J.W.; Lee, J.H.; Kim, H.J.; Shin, S. Property Modification of a Silicone Acrylic Pres-sure-Sensitive Adhesive with Oligomeric Silicone Urethane Methacrylate. Eur. Polym. J. 2019, 112, 320–327. [Google Scholar] [CrossRef]
- Yu, Y.; Liao, B.; Li, G.; Jiang, S.; Sun, F. Synthesis and Properties of Photosensitive Silicone-Containing Poly-urethane Acrylate for Leather Finishing Agent. Ind. Eng. Chem. Res. 2014, 53, 564–571. [Google Scholar] [CrossRef]
- Mohan, P. A Critical Review: The Modification, Properties, and Applications of Epoxy Resins. Polym. Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Weisbrodt, M.; Kowalczyk, A.; Kowalczyk, K. Structural Adhesives Tapes Based on a Solid Epoxy Resin and Multifunctional Acrylic Telomers. Polymers 2021, 13, 3561. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical Addition–Fragmentation Chemistry in Polymer Synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Roy, A.; Sivaram, S. Synthesis of functional olymers of polar and nonpolar monomers by living and/or controlled polymerization. In Functional Polymers. Design, Synthesis, and Applications; Shunmugam, R., Ed.; Apple Academic Press: Waretown, NJ, USA, 2017; pp. 1–48. [Google Scholar]
- Gouliaev, A.H. Comprehensive Organic Functional Group Transformations; Elsevier: Amsterdam, The Netherlands, 1995; Volume 6, p. 211. [Google Scholar]
- Paul, C.; Pohnert, G. Production and role of volatile halogenated compounds from marine algae. Nat. Prod. Rep. 2011, 28, 186. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-J.; Vanderwal, C.D. Stereoselective halogenation in natural product synthesis. Angew. Chem. Int. Ed. 2016, 55, 4396. [Google Scholar] [CrossRef] [PubMed]
- Sutar, R.L.; Huber, S.M. Catalysis of organic reactions through halogen bonding. ACS Catal. 2019, 9, 9622. [Google Scholar] [CrossRef]
- Talukdar, R. Tracking down the brominated single electron oxidants in recent organic red-ox transformations: Photolysis and photocatalysis. Org. Biomol. Chem. 2020, 18, 8294. [Google Scholar] [CrossRef] [PubMed]
- Starks, C.M. Free Radical Telomerization; Academic Press: Cambridge, MA, USA, 1974; ISBN 9780126636505. [Google Scholar]
- Weisbrodt, M.; Kowalczyk, A. Self-Crosslinkable Pressure-Sensitive Adhesives from Silicone-(Meth)Acrylate Telomer Syrups. Materials 2022, 15, 8924. [Google Scholar] [CrossRef] [PubMed]
- Kraśkiewicz, A.; Kowalczyk, A. Phosphorus-Containing Telomers as UV-Curable Binders of Solvent-Free Varnish Coatings. Materials 2022, 15, 8991. [Google Scholar] [CrossRef]
- Chen, J.; Chalamet, Y.; Taha, M. Telomerization of Butyl Methacrylate and 1-Octadecanethiol by Reactive Extrusion. Macromol. Mater Eng. 2003, 288, 357–364. [Google Scholar] [CrossRef]
- Bolshakov, A.I.; Kuzina, S.I.; Kiryukhin, D.P. Tetrafluoroethylene Telomerization Initiated by Benzoyl Peroxide. Russ. J. Phys. Chem. A 2017, 91, 482–489. [Google Scholar] [CrossRef]
- Lambourne, R. 1—Paint Composition and Applications—A General Introduction. In Paint and Surface Coatings, 2nd ed.; Lambourne, R., Strivens, T.A., Eds.; Woodhead Publishing: Sawston, UK, 1999; pp. 1–18. ISBN 978-1-85573-348-0. [Google Scholar]
- Ameduri, B.; Boutevin, B.; Kostov, G. Synthesis and Copolymerization of Vinylidene Fluoride (VDF) with Trifluorovinyl Monomers. Macromol. Chem. Phys. 2002, 203, 1763–1771. [Google Scholar] [CrossRef]
- Behr, A.; Becker, M.; Beckmann, T.; Johnen, L.; Leschinski, J.; Reyer, S. Telomerization: Advances and Applications of a Versatile Reaction. Angew. Chem. Int. Ed. 2009, 48, 3598–3614. [Google Scholar] [CrossRef]
- Faßbach, T.A.; Vorholt, A.J.; Leitner, W. The Telomerization of 1,3-Dienes—A Reaction Grows Up. Chem-CatChem 2019, 11, 1153–1166. [Google Scholar] [CrossRef]
- David, G.; Boyer, C.; Tonnar, J.; Ameduri, B.; Lacroix-Desmazes, P.; Boutevin, B. Use of Iodocompounds in Radical Polymerization. Chem. Rev. 2006, 106, 3936–3962. [Google Scholar] [CrossRef]
- Améduri, B.; Boutevin, B.; Gramain, P. Synthesis of Block Copolymers by Radical Polymerization and Telomerization. Adv. Polym. Sci. 1997, 127, 87–142. [Google Scholar]
- Loubat, C.; Boutevin, B. Telomerization of Acrylic Acid with Mercaptans: Part 2. Kinetics of the Synthesis of Star-Shaped Macromolecules of Acrylic Acid. Polym. Int. 2001, 50, 375–380. [Google Scholar] [CrossRef]
- ISO 1628-1:1998; Determination of the Viscosity of Polymers in Dilute Solution Using Capillary Viscometers, Part 1: General Principles. The International Organization for Standardization (ISO): Geneva, Switzerland, 1998.

| Monomers | ||
|---|---|---|
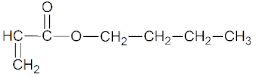 | 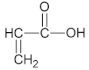 | 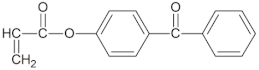 |
| BA | AA | ABP |
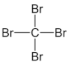 | 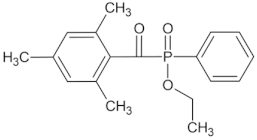 | |
| TPOL | ||
| Telomers Syrup | Monomers (wt. %) | Initiating System | |||||
|---|---|---|---|---|---|---|---|
| BA | AA | ABP | CBr4 * | TPOL * | |||
| wt. Parts | mmol | wt. Part | mmol | ||||
| TS-0 | 91.5 | 7.5 | 1.0 | 0 | 0 | 0.2 | 0.6 |
| TS-2.5 | 2.5 | 7.5 | |||||
| TS-5 | 5.0 | 15 | |||||
| TS-10 | 10 | 30 | |||||
| Sample | In Glass Reactor | In Thin Layer | |||
|---|---|---|---|---|---|
| SC (wt. %) | Tm (°C) | tTm (min.) | p (%) | Rpmax (%/s) | |
| TS-0 | — | — | — | 78 | 1.32 |
| TS-2.5 | 79 | 62 | 2 | 85 | 1.31 |
| TS-5 | 81 | 63 | 2 | 71 | 1.14 |
| TS-10 | 85 | 65 | 4 | 70 | 1.14 |
| Sample | Conversion (%) | ||||
|---|---|---|---|---|---|
| BA | AA | ABP | CBr4 | Total | |
| TS-2.5 | 78.4 | 86.3 | 58.2 | 100 | 79.5 |
| TS-5 | 80.7 | 87.5 | 100 | 100 | 82.7 |
| TS-10 | 87.6 | 89.7 | 100 | 100 | 88.3 |
| Sample | Mn (g/mol) | Mw (g/mol) | Đ | η (Pa·s) | K-Value |
|---|---|---|---|---|---|
| TS-2.5 | 19,000 | 28,700 | 1.51 | 13.8 | 26.3 |
| TS-5 | 17,000 | 25,000 | 1.47 | 7.3 | 18.1 |
| TS-10 | 16,900 | 25,000 | 1.47 | 6.8 | 17.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weisbrodt, M.; Kowalczyk, A.; Schmidt, B.; Idzik, T.J.; Sośnicki, J.G. Functional Acrylic Resins Prepared via Photo-Induced Telomerization Using Tetrabromomethane as Telogen. Materials 2023, 16, 7650. https://doi.org/10.3390/ma16247650
Weisbrodt M, Kowalczyk A, Schmidt B, Idzik TJ, Sośnicki JG. Functional Acrylic Resins Prepared via Photo-Induced Telomerization Using Tetrabromomethane as Telogen. Materials. 2023; 16(24):7650. https://doi.org/10.3390/ma16247650
Chicago/Turabian StyleWeisbrodt, Mateusz, Agnieszka Kowalczyk, Beata Schmidt, Tomasz J. Idzik, and Jacek G. Sośnicki. 2023. "Functional Acrylic Resins Prepared via Photo-Induced Telomerization Using Tetrabromomethane as Telogen" Materials 16, no. 24: 7650. https://doi.org/10.3390/ma16247650
APA StyleWeisbrodt, M., Kowalczyk, A., Schmidt, B., Idzik, T. J., & Sośnicki, J. G. (2023). Functional Acrylic Resins Prepared via Photo-Induced Telomerization Using Tetrabromomethane as Telogen. Materials, 16(24), 7650. https://doi.org/10.3390/ma16247650







