Photocatalyst Based on Nanostructured TiO2 with Improved Photocatalytic and Antibacterial Properties
Abstract
:1. Introduction
2. Experimental
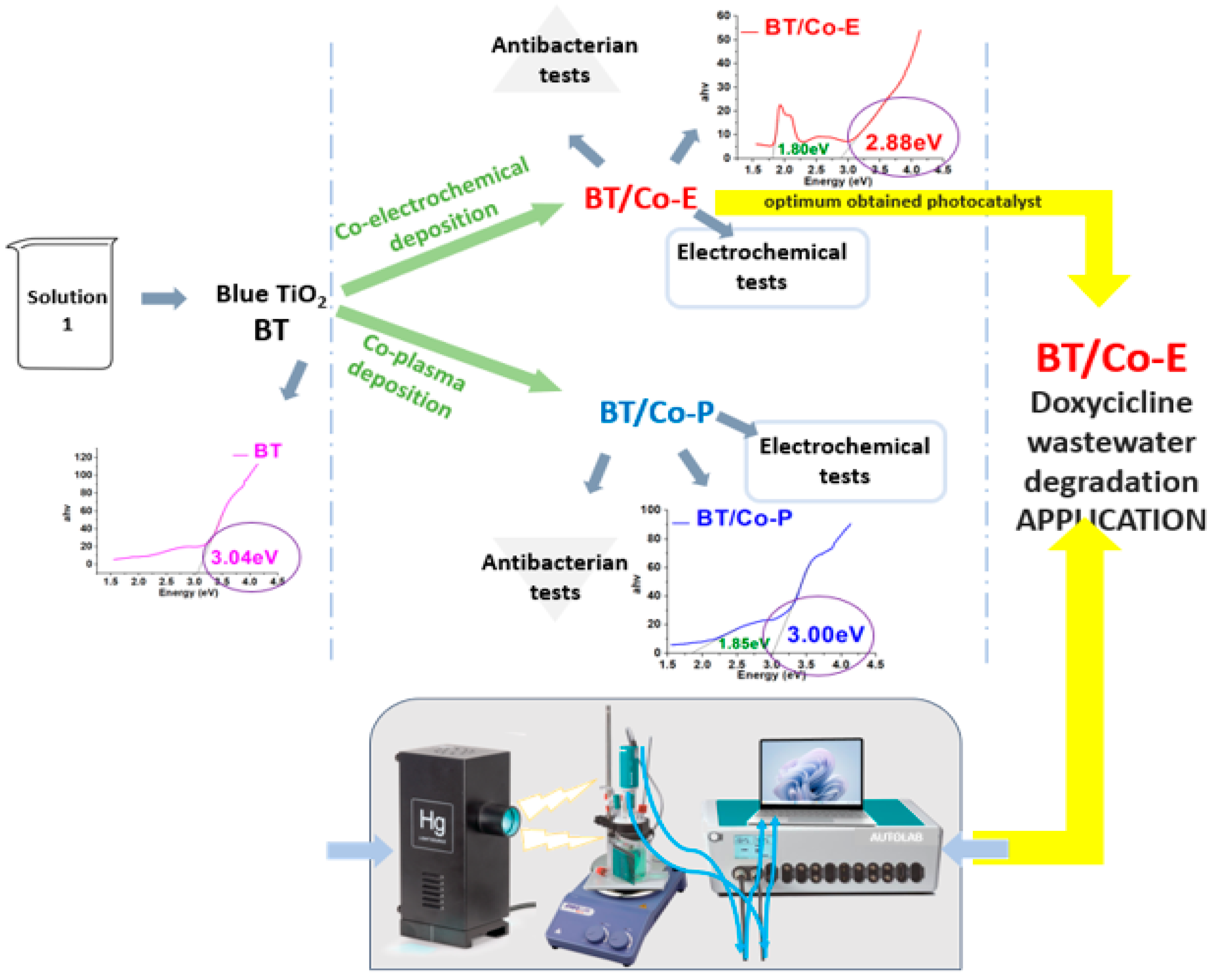
2.1. Catalyst Preparation
2.1.1. Blue TiO2 Nanostructures Electrochemically Obtained on Titanium Plates
2.1.2. Cobalt Deposition on the Blue TiO2 Nanostructures
2.2. Catalyst Characterization and Applicability
3. Results
3.1. Characterization of the Synthesized Electrodes
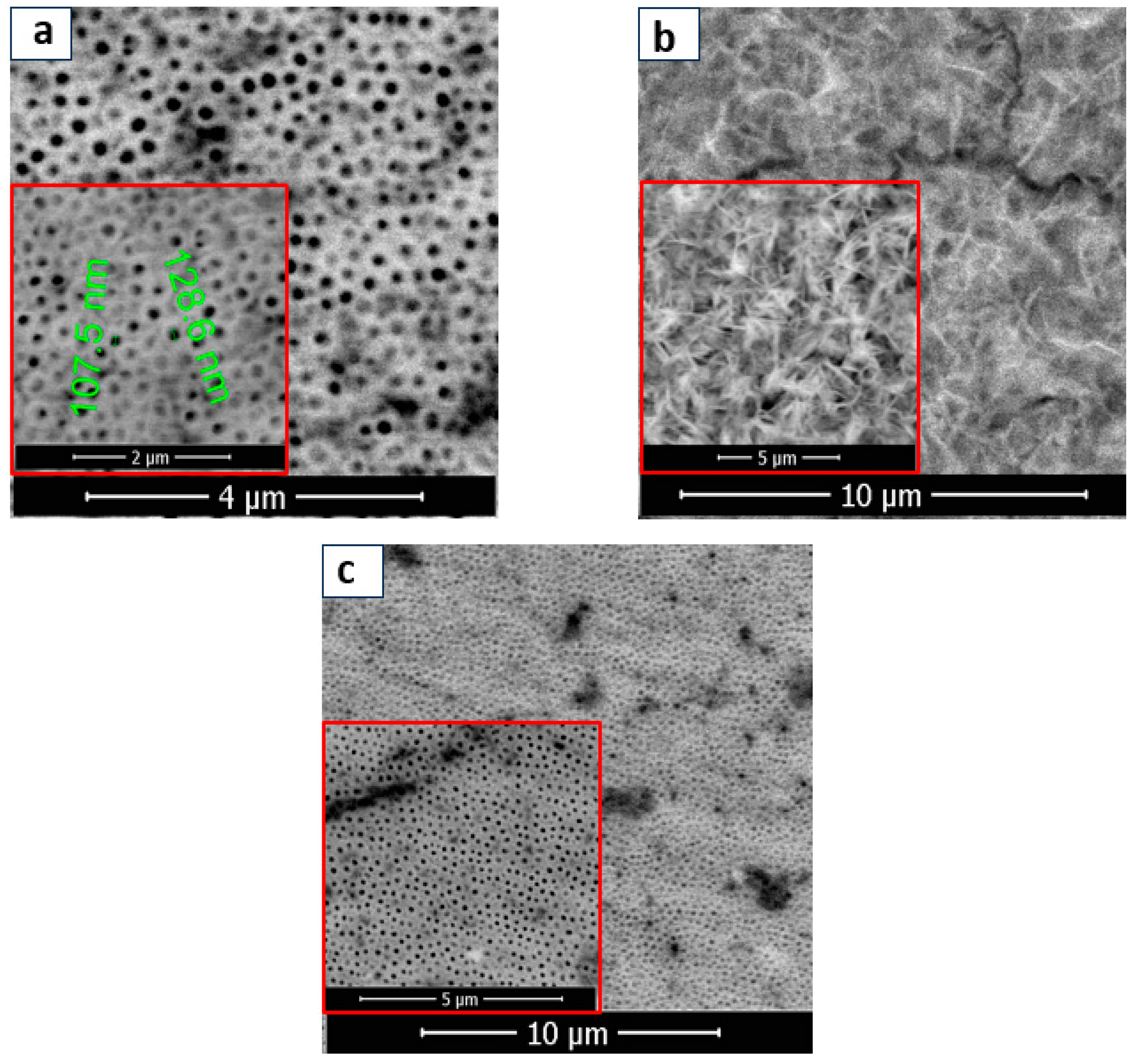
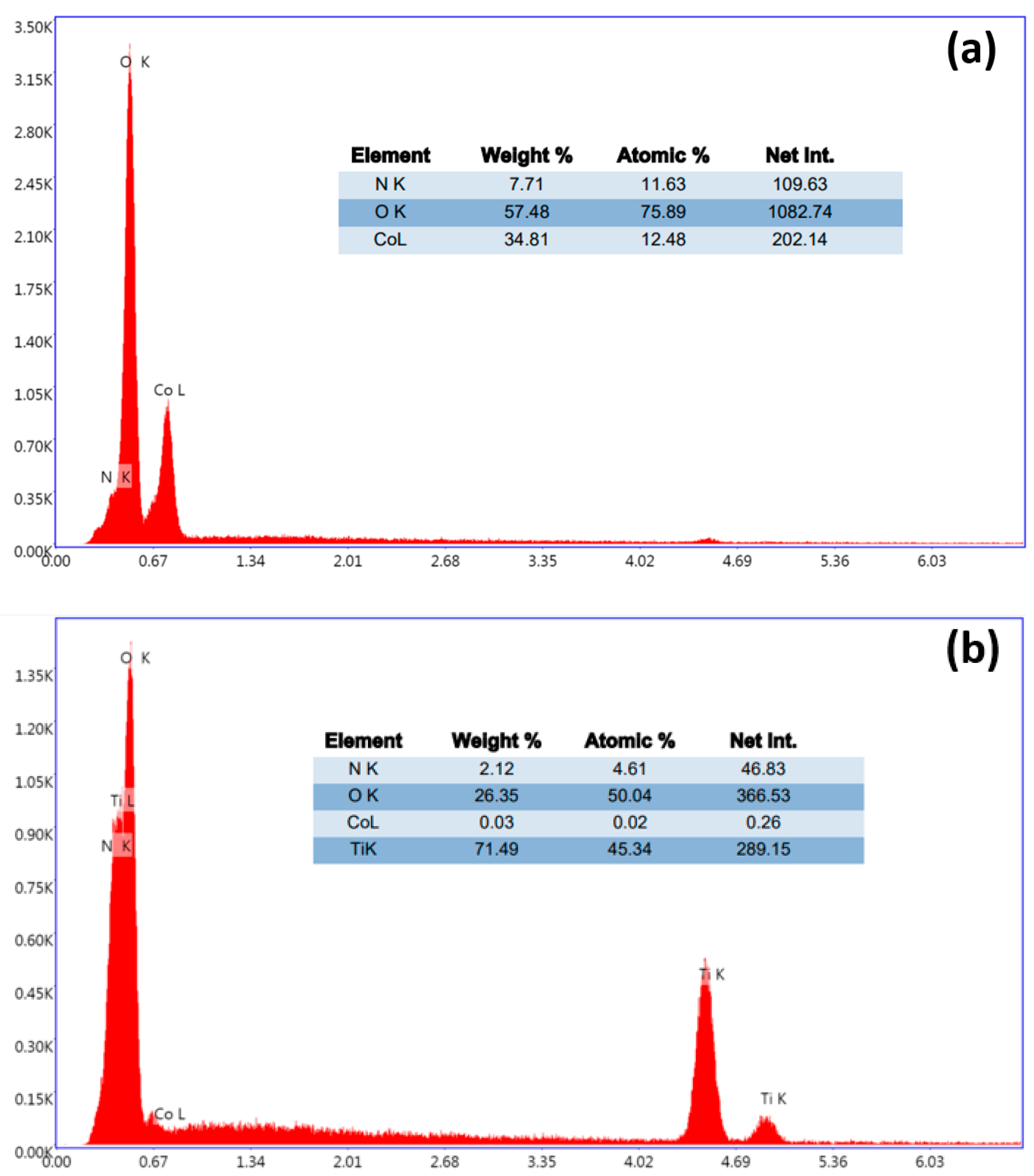
3.1.1. Physicochemical Characterization of BT, BT/Co-E, and BT-Co-P Electrodes by SEM, EDX, and Wettability
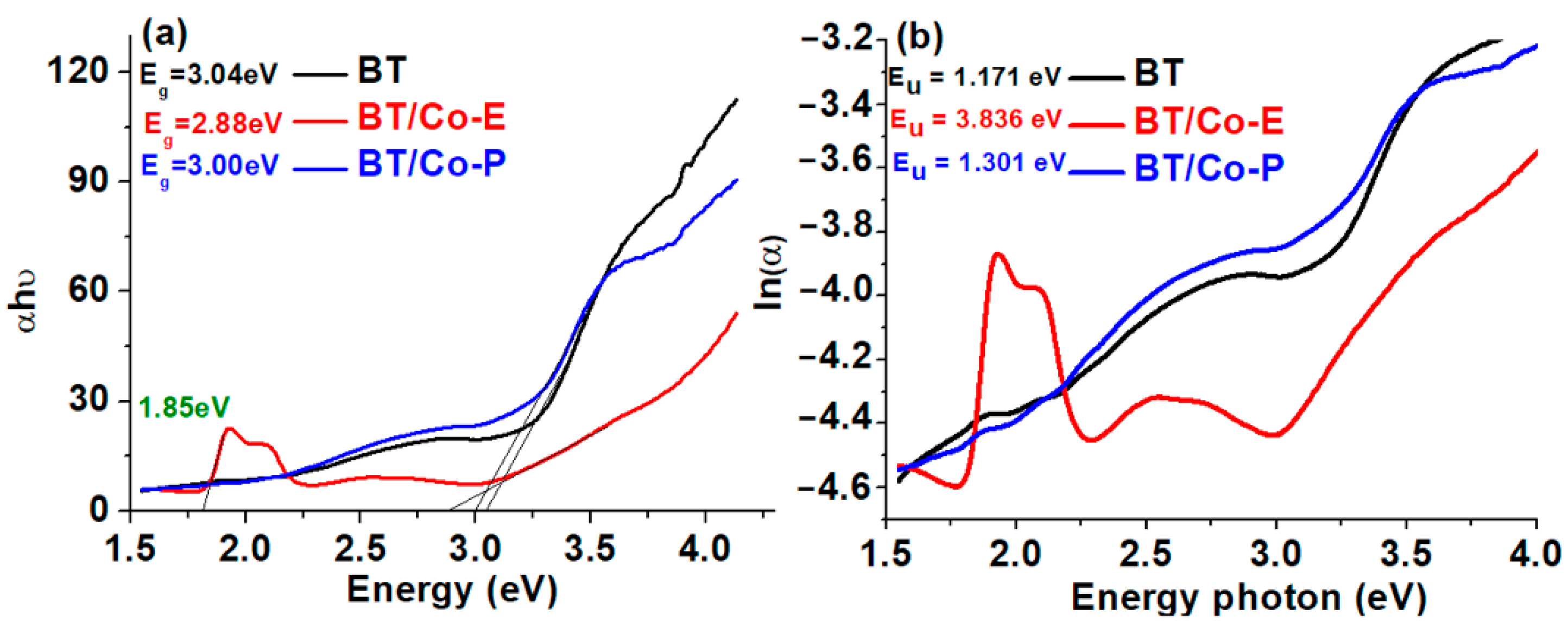
3.1.2. Optical Parameters—Band Gap Energy and Urbach Energy
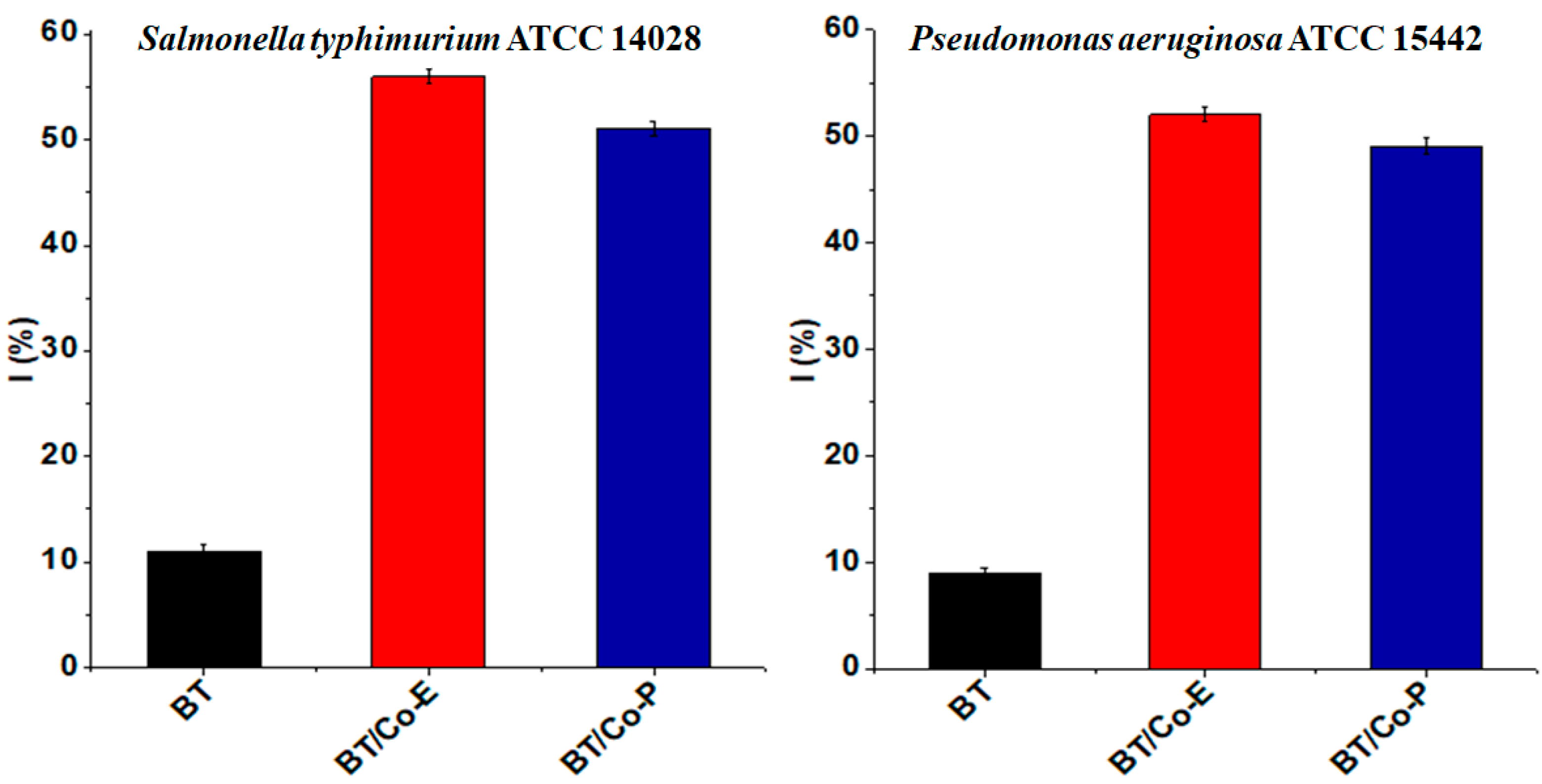
3.1.3. Antibacterial Activity
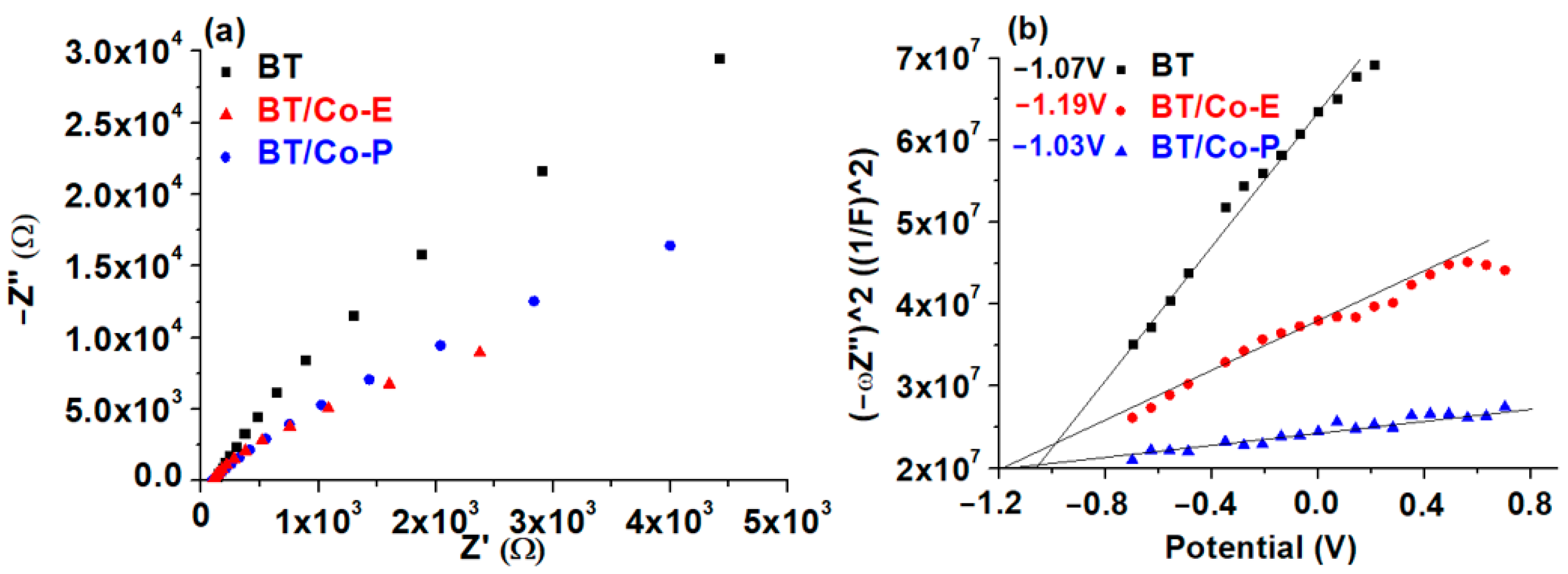
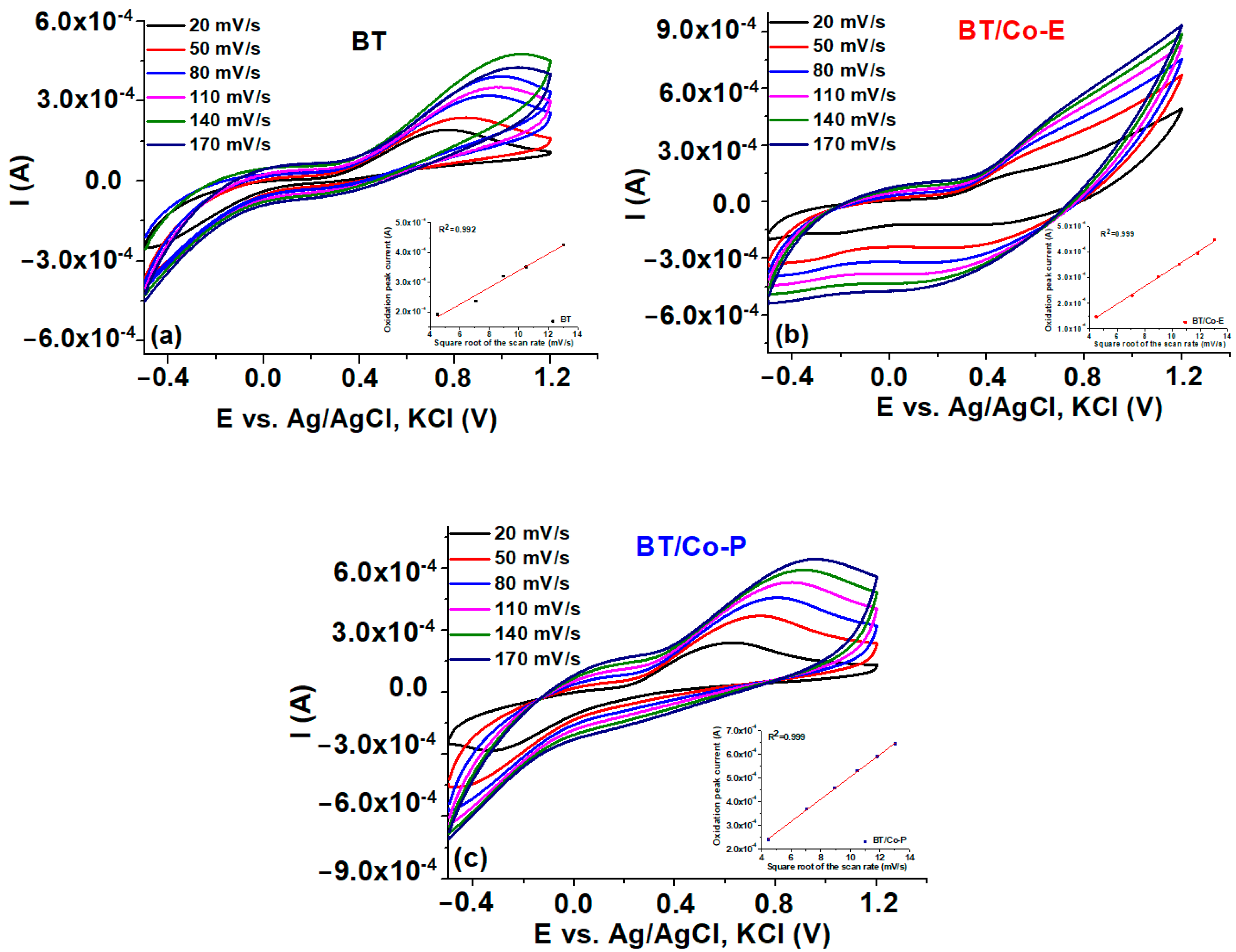
3.1.4. Electrochemical Features of the Developed Electrodes
3.1.5. Proposed Energy Band Levels
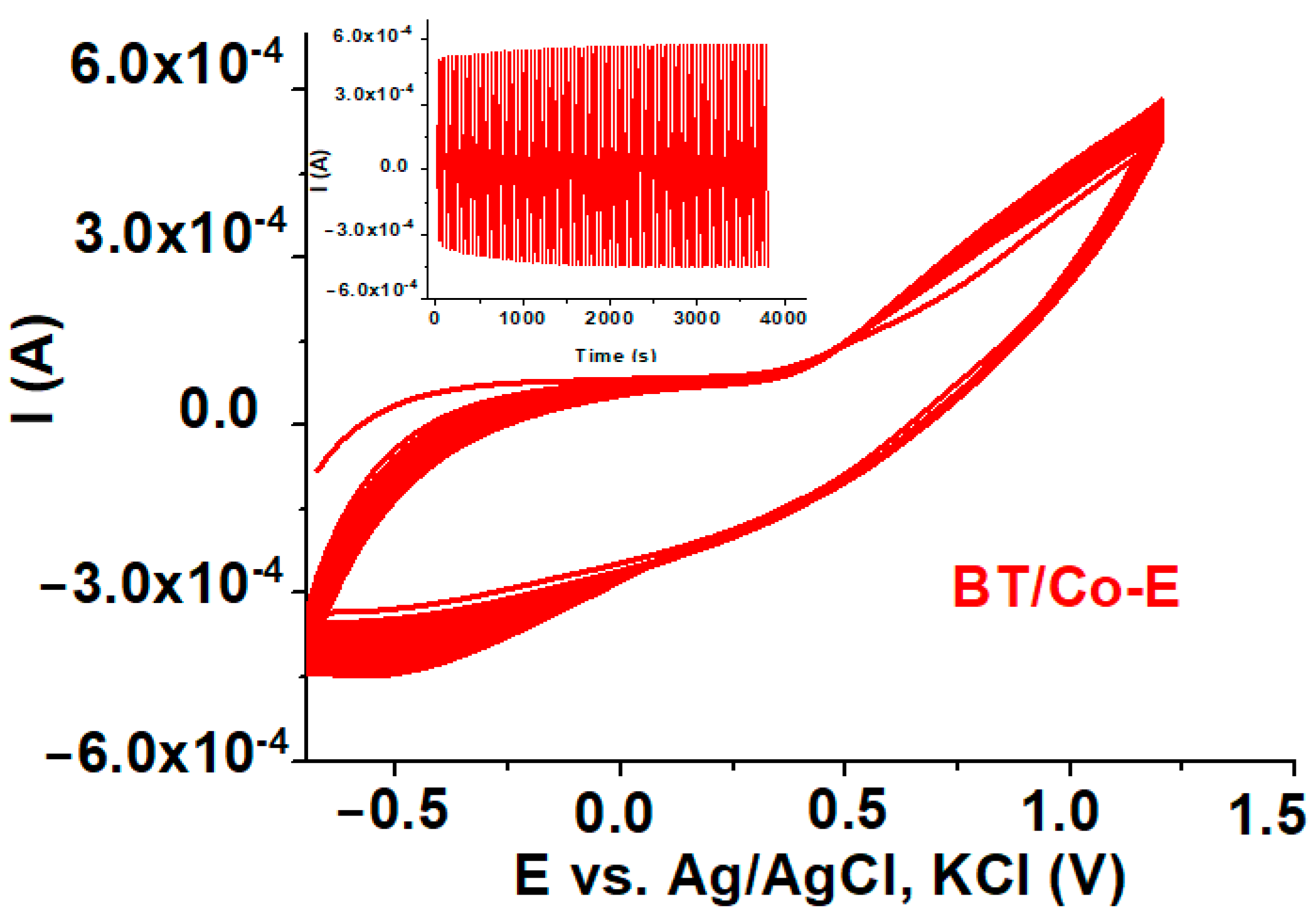
3.1.6. Electrochemical Stability
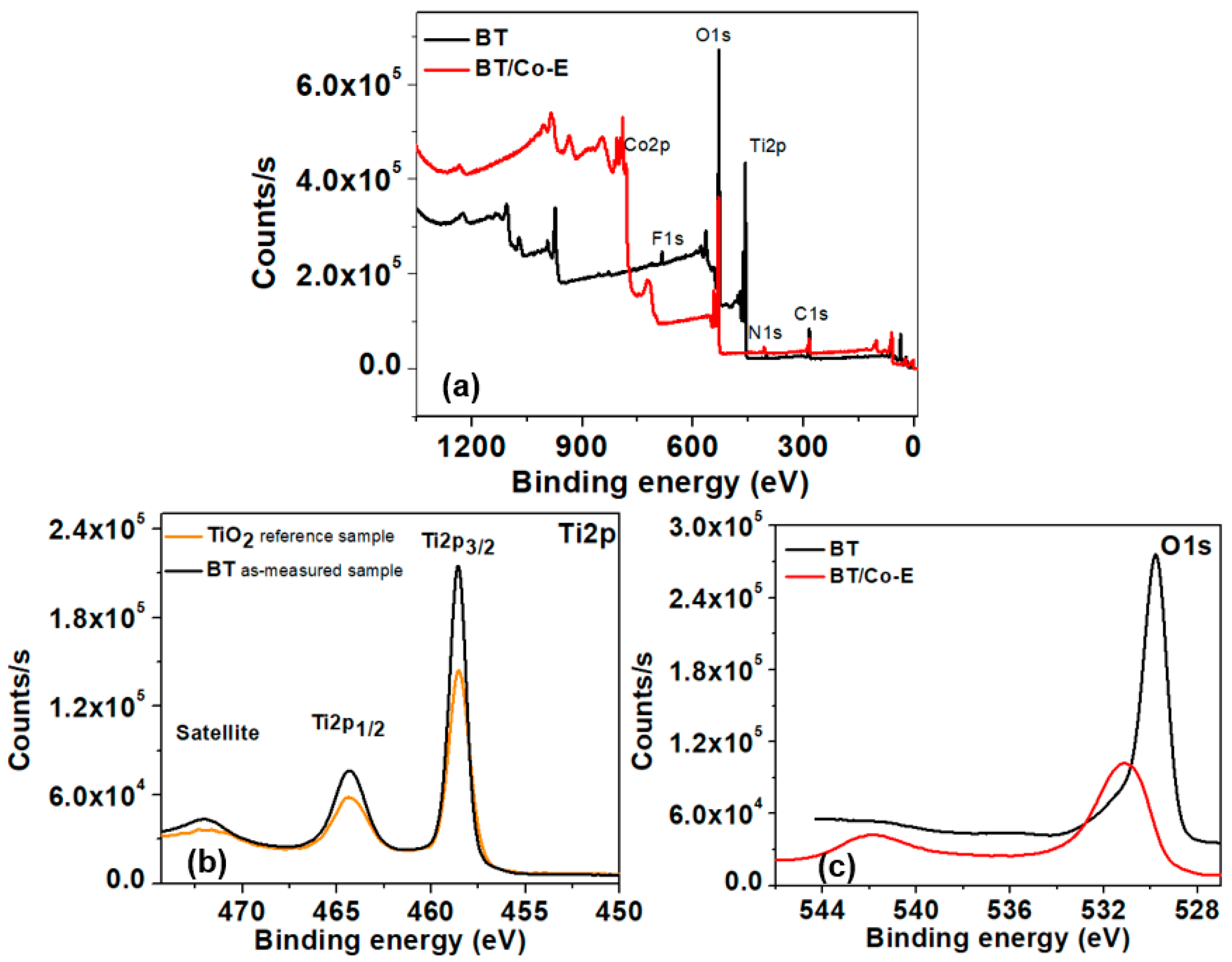
3.1.7. X-ray Photoelectron Spectroscopy (XPS)
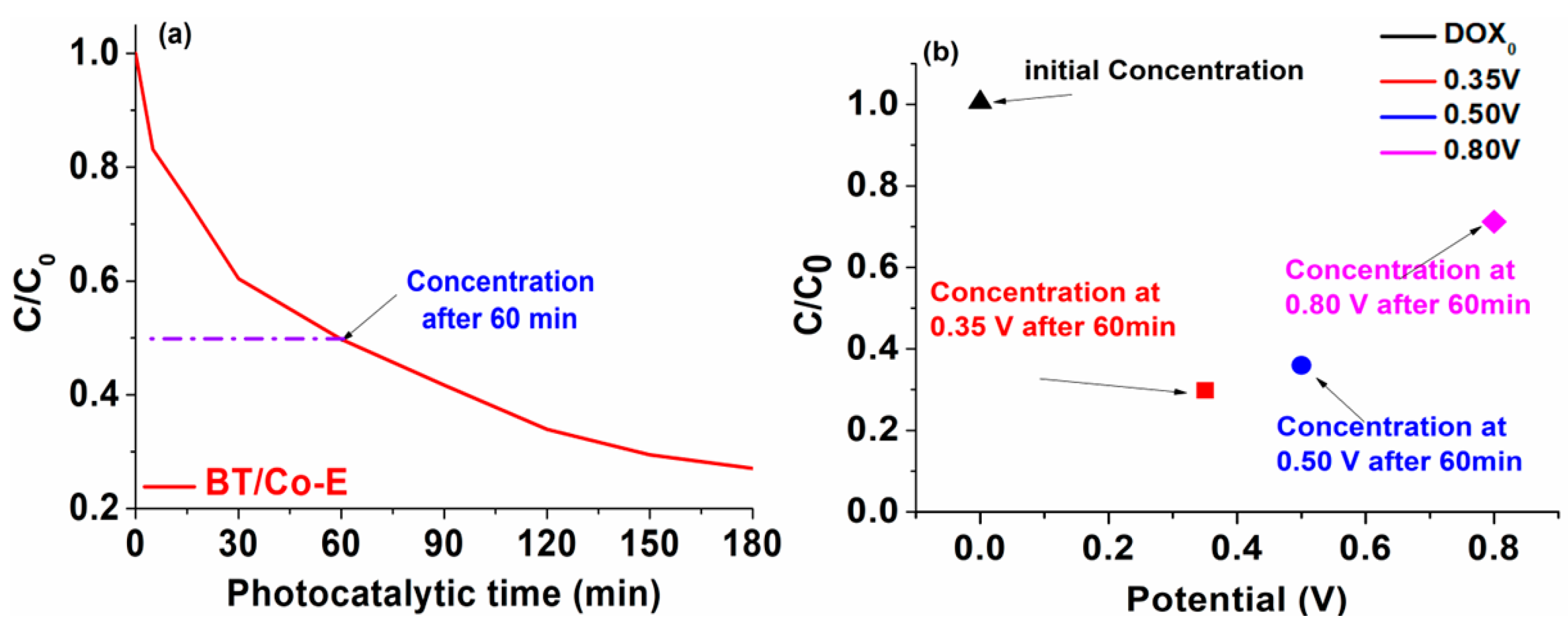
3.2. Applicability of the BT/Co-E Catalyst
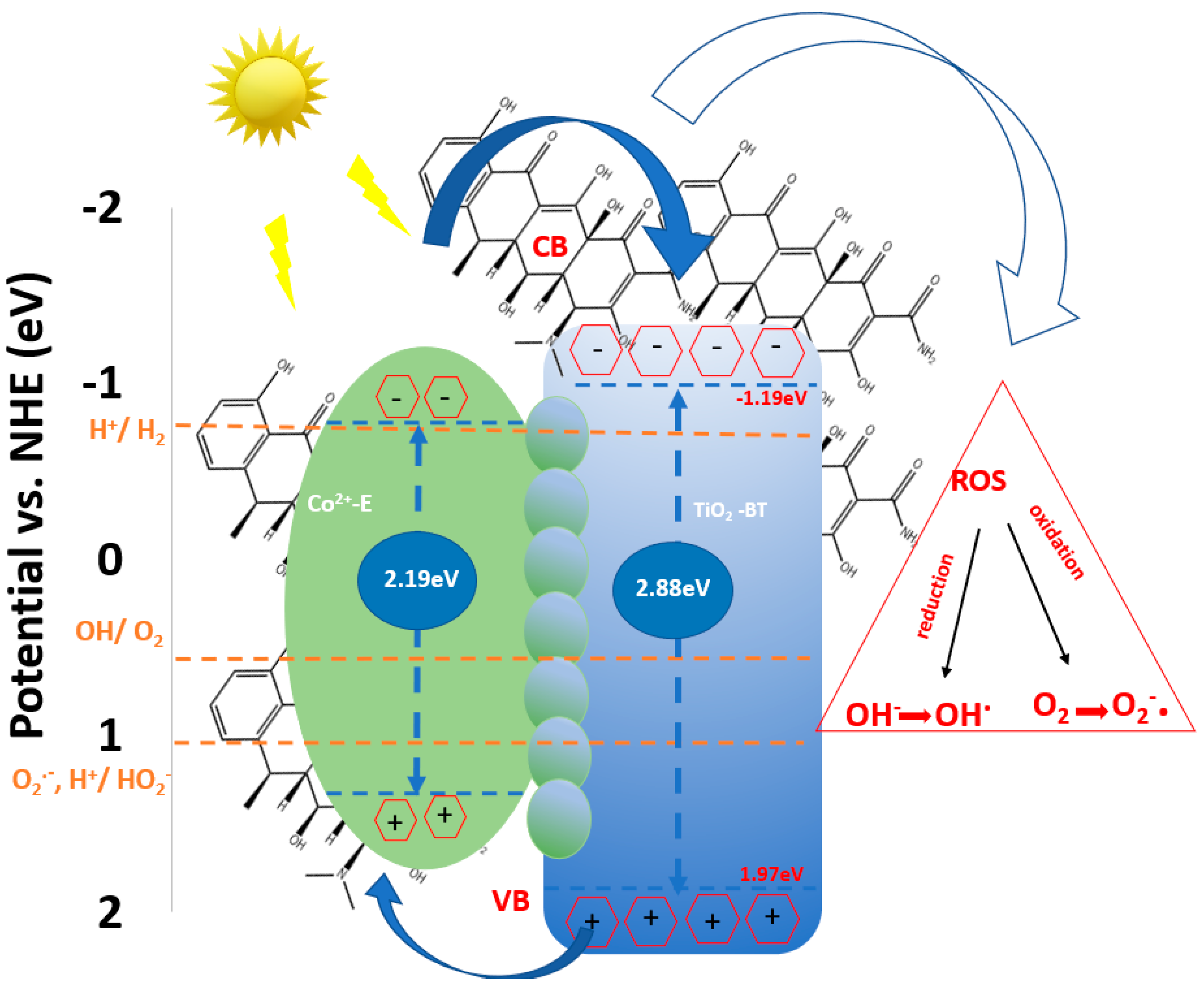
Proposed Mechanism of DOX Degradation in the Presence of BT/Co-E
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Gandhi, T.N.; Petty, L.A.; Patel, P.K.; Prescott, H.C.; Malani, A.N.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S.A. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): A multi-hospital cohort study. Clin. Infect. Dis. 2021, 72, e533–e541. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-Y.; Sun, K.; Liu, Z.-K.; Liu, X.; Dong, W.; Zuo, X.; Shao, R.; Tao, J. Identification of Dynamic Active Sites Among Cu Species Derived from MOFs@ CuPc for Electrocatalytic Nitrate Reduction Reaction to Ammonia. Nano-Micro Lett. 2023, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Zhang, B.; Siqin, L.; Liu, G.; Wu, Q.; Xue, S.; Shao, T.; Zhang, F.; Zhang, W.; Liu, X. Designing advanced s-scheme CdS QDs/La-Bi2WO6 photocatalysts for efficient degradation of rhb. In Exploration; Wiley Online Library: Hoboken, NJ, USA, 2023; p. 20230050. [Google Scholar]
- VT Nair, D.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. Foods 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, G.M.B.; Reiche, T.; Tennfjord, C.E.; Mehli, L. Antibiotic Resistance Properties among Pseudomonas spp. Associated with Salmon Processing Environments. Microorganisms 2022, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.O.; Fricovsky, E.; Ceballos, G.; Villarreal, F. Tetracyclines: A pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Physiol.-Cell Physiol. 2010, 299, C539–C548. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Giuffrida, R.; Zalaudek, I.; Di Meo, N. Doxycycline, a widely used antibiotic in dermatology with a possible anti-inflammatory action against IL-6 in COVID-19 outbreak. Dermatol. Ther. 2020, 33, e13437. [Google Scholar] [CrossRef]
- Dhar, R.; Kirkpatrick, J.; Gilbert, L.; Khanna, A.; Modi, M.M.; Chawla, R.K.; Dalal, S.; Maturu, V.N.; Stern, M.; Keppler, O.T. Doxycycline for the prevention of progression of COVID-19 to severe disease requiring intensive care unit (ICU) admission: A randomized, controlled, open-label, parallel group trial (DOXPREVENT. ICU). PLoS ONE 2023, 18, e0280745. [Google Scholar] [CrossRef]
- Butler, C.C.; Yu, L.-M.; Dorward, J.; Gbinigie, O.; Hayward, G.; Saville, B.R.; Van Hecke, O.; Berry, N.; Detry, M.A.; Saunders, C.; et al. Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial. Lancet Respir. Med. 2021, 9, 1010–1020. [Google Scholar] [CrossRef]
- Dorobisz, K.; Dorobisz, T.; Janczak, D.; Zatoński, T. Doxycycline in the coronavirus disease 2019 therapy. Ther. Clin. Risk Manag. 2021, 17, 1023–1026. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H.; Burke, J. Water Pollution from Agriculture: A Global Review. In Executive Summary; FAO: Rome, Italy; IWMI: Colombo, Sri Lanka, 2017. [Google Scholar]
- Toze, R. Microbial Pathogens in Wastewater: Literature Review for Urban Water Systems Multi-Divisional Research Program; SIRO Land and Water: Clayton, Australia, 1997. [Google Scholar]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Mena, K.D.; Gerba, C.P. Risk assessment of Pseudomonas aeruginosa in water. Rev. Environ. Contam. Toxicol. 2009, 201, 71–115. [Google Scholar] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa biofilm: A review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Hu, H.; Chen, G.; Li, Z.; Li, A.; Zhang, J. Disinfectant resistance in bacteria: Mechanisms, spread, and resolution strategies. Environ. Res. 2021, 195, 110897. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.A.; Karoui, S.; Trabelsi, K.; Hajjaji, A.; Elfalleh, W.; Ghorbal, A.; Maghzaoui, M.; Assadi, A.A. Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) with Ag Silver Nanoparticles (Ag-NPs): Photocatalytic Performance for Wastewater Treatment under Visible Light. Materials 2022, 15, 1463. [Google Scholar] [CrossRef]
- Mîndroiu, V.M.; Stoian, A.B.; Irodia, R.; Trușcă, R.; Vasile, E. Titanium Dioxide Thin Films Produced on FTO Substrate Using the Sol–Gel Process: The Effect of the Dispersant on Optical, Surface and Electrochemical Features. Materials 2023, 16, 3147. [Google Scholar] [CrossRef]
- Jian, L.; Wang, G.; Liu, X.; Ma, H. Unveiling an S-scheme F-Co3O4@ Bi2WO6 heterojunction for robust water purification. eScience 2023, 100206. [Google Scholar] [CrossRef]
- Sihor, M.; Gowrisankaran, S.; Martaus, A.; Motola, M.; Mailhot, G.; Brigante, M.; Monfort, O. Anodic TiO2 Nanotube Layers for Wastewater and Air Treatments: Assessment of Performance Using Sulfamethoxazole Degradation and N2O Reduction. Molecules 2022, 27, 8959. [Google Scholar] [CrossRef]
- Jian, L.; Li, M.; Liu, X.; Wang, G.; Zhang, X.; Kim, M.G.; Fu, Y.; Ma, H. Unveiling hierarchical dendritic Co3O4–SnO2 heterostructure for efficient water purification. Nano Lett. 2023, 23, 3739–3747. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.H.; Ismail, N.A.; Yusoff, M. Study on Band Gap Energy of F Doped TiO2 Nanotubes; Materials Science Forum; Trans Tech Publications, Ltd.: Stafa-Zurich, Switzerland, 2017; pp. 234–238. [Google Scholar]
- Liu, B.; Guo, W.; Jia, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Jiang, J.; Ren, N. Novel nonradical oxidation of sulfonamide antibiotics with Co (II)-doped g-C3N4-activated peracetic acid: Role of high-valent cobalt–oxo species. Environ. Sci. Technol. 2021, 55, 12640–12651. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Wang, T.; Qi, D.; Liu, J.; Liao, T.; Yamauchi, Y.; Sun, Z. Three-dimensional fast Na-ion transport in sodium titanate nanoarchitectures via engineering of oxygen vacancies and bismuth substitution. ACS Nano 2021, 15, 13604–13615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Yang, S.; Quispe-Cardenas, E.; Hoffmann, M.R. Application of heterojunction Ni–Sb–SnO2 anodes for electrochemical water treatment. ACS Es&T Eng. 2021, 1, 1236–1245. [Google Scholar]
- Akpan, U.; Hameed, B. The advancements in sol–gel method of doped-TiO2 photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Kurtz, S.R.; Gordon, R.G. Chemical vapor deposition of doped TiO2 thin films. Thin Solid Films 1987, 147, 167–176. [Google Scholar] [CrossRef]
- Dargahi, Z.; Asgharzadeh, H.; Maleki-Ghaleh, H. Synthesis of Mo-doped TiO2/reduced graphene oxide nanocomposite for photoelectrocatalytic applications. Ceram. Int. 2018, 44, 13015–13023. [Google Scholar] [CrossRef]
- Roose, B.; Pathak, S.; Steiner, U. Doping of TiO2 for sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8326–8349. [Google Scholar] [CrossRef]
- Yadav, S.; Jaiswar, G. Review on undoped/doped TiO2 nanomaterial; synthesis and photocatalytic and antimicrobial activity. J. Chin. Chem. Soc. 2017, 64, 103–116. [Google Scholar] [CrossRef]
- Liu, C.; Wang, F.; Qiu, Y.; Liang, Q.; Mitsuzak, N.; Chen, Z. Facile electrodeposition of cobalt hydroxide on anodic TiO2 nanotubes arrays for enhanced photoelectrochemical application. J. Photochem. Photobiol. A Chem. 2018, 353, 200–205. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.M.; Liu, C.; Andrews, S.C.; Hahn, C.; Yang, P. Large-scale synthesis of transition-metal-doped TiO2 nanowires with controllable overpotential. J. Am. Chem. Soc. 2013, 135, 9995–9998. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Dhir, A. Transition metal doped TiO2 mediated photocatalytic degradation of anti-inflammatory drug under solar irradiations. J. Environ. Chem. Eng. 2016, 4, 1267–1273. [Google Scholar] [CrossRef]
- Khairy, M.; Zakaria, W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Castillo-Deltell, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M.d.C. TiO2 modification with transition metallic species (Cr, Co, Ni, and Cu) for photocatalytic abatement of acetic acid in liquid phase and propene in gas phase. Materials 2018, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Vijayalakshmi, S.; Venkataraj, S.; Jayavel, R. Effect of cobalt doping on the structural and optical properties of TiO2 films prepared by sol–gel process. Thin Solid Films 2008, 516, 3776–3782. [Google Scholar] [CrossRef]
- Hosseini-Zori, M. Co-doped TiO2 nanostructures as a strong antibacterial agent and self-cleaning cover: Synthesis, characterization and investigation of photocatalytic activity under UV irradiation. J. Photochem. Photobiol. B Biol. 2018, 178, 512–520. [Google Scholar] [CrossRef]
- Altın, İ.; Sökmen, M.; Bıyıklıoğlu, Z. Sol gel synthesis of cobalt doped TiO2 and its dye sensitization for efficient pollutant removal. Mater. Sci. Semicond. Process. 2016, 45, 36–44. [Google Scholar] [CrossRef]
- Boutlala, A.; Bourfaa, F.; Mahtili, M.; Bouaballou, A. Deposition of Co-doped TiO2 Thin Films by sol-gel method. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; p. 012048. [Google Scholar]
- Li, J.-G.; Buchel, R.; Isobe, M.; Mori, T.; Ishigaki, T. Cobalt-doped TiO2 nanocrystallites: Radio-frequency thermal plasma processing, phase structure, and magnetic properties. J. Phys. Chem. C 2009, 113, 8009–8015. [Google Scholar] [CrossRef]
- Kondo, H.; Machmudah, S.; Kanda, H.; Zhao, Y.; Goto, M. Synthesis of titanium dioxide nanoparticle by means of discharge plasma over an aqueous solution under high-pressure gas environment. Alex. Eng. J. 2022, 61, 3805–3820. [Google Scholar]
- Vasilyeva, M.; Lukiyanchuk, I.; Sergeev, A.; Ustinov, A.Y.; Sergeeva, K.; Kuryavyi, V. Ti/TiO2-CoWO4-Co3 (PO4)2 composites: Plasma electrolytic synthesis, optoelectronic properties, and solar light-driven photocatalytic activity. J. Alloys Compd. 2021, 863, 158066. [Google Scholar] [CrossRef]
- Hong, J.; Du, J.; Wang, B.; Zhang, Y.; Liu, C.; Xiong, H.; Sun, F.; Chen, S.; Li, J. Plasma-assisted preparation of highly dispersed cobalt catalysts for enhanced Fischer–Tropsch synthesis performance. ACS Catal. 2018, 8, 6177–6185. [Google Scholar] [CrossRef]
- Liu, N.; Ye, W. Electrodeposition of Co-P/TiO2 composite and its electrochemical properties and photocatalytic application for degradation of methyl orange in simulated wastewater. Int. J. Electrochem. Sci. 2023, 18, 20–25. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Z.; Zheng, S.; Chen, J.; He, Z. An Investigation into Electrodeposited Co−Ni−TiO2 Films with Improved Mechanical and Corrosion Properties. Coatings 2023, 13, 783. [Google Scholar] [CrossRef]
- Da Dalt, S.; Alves, A.; Bergmann, C. Photocatalytic degradation of methyl orange dye in water solutions in the presence of MWCNT/TiO2 composites. Mater. Res. Bull. 2013, 48, 1845–1850. [Google Scholar] [CrossRef]
- Yang, Y.; Hoffmann, M.R. Synthesis and stabilization of blue-black TiO2 nanotube arrays for electrochemical oxidant generation and wastewater treatment. Environ. Sci. Technol. 2016, 50, 11888–11894. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, S.; Choi, J.; Lee, J.; Kang, J.S.; Sung, Y.-E.; Lee, J.; Choi, W.; Yoon, J. Blue TiO2 Nanotube Array as an Oxidant Generating Novel Anode Material Fabricated by Simple Cathodic Polarization. Electrochim. Acta 2014, 141, 113–119. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A.; Maidul Islam, A.K.M.; Alagarsamy, P.; Mukherjee, M. Effect of oxygen vacancy and dopant concentration on the magnetic properties of high spin Co2+ doped TiO2 nanoparticles. J. Magn. Magn. Mater. 2011, 323, 440–446. [Google Scholar] [CrossRef]
- Siddiqa, A.; Masih, D.; Anjum, D.; Siddiq, M. Cobalt and sulfur co-doped nano-size TiO2 for photodegradation of various dyes and phenol. J. Environ. Sci. 2015, 37, 100–109. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Tsao, C.-W.; Fang, M.-J.; Hsu, Y.-J. Modulation of interfacial charge dynamics of semiconductor heterostructures for advanced photocatalytic applications. Coord. Chem. Rev. 2021, 438, 213876. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, D.; Bastia, S.; Chaudhary, Y.S. Band-structure Tunability via Modulation of Excitons in Semiconductor Nanostructures: Manifestation in Photocatalytic Fuel Generation. Nanoscale 2023, 15, 10939–10974. [Google Scholar] [CrossRef] [PubMed]
- Augello, C.; Liu, H. Surface modification of magnesium by functional polymer coatings for neural applications. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 335–353. [Google Scholar]
- Prakash, S.; Yeom, J. Chapter 4—Advanced Fabrication Methods and Techniques. In Nanofluidics and Microfluidics; Prakash, S., Yeom, J., Eds.; William Andrew Publishing: Norwich, NY, USA, 2014; pp. 87–170. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Ion, V.; Marascu, V.; Matei, E.; Stancu, C.; Dinescu, G. Combining Fluorinated Polymers with Ag Nanoparticles as a Route to Enhance Optical Properties of Composite Materials. Polymers 2020, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Khan, H.M.; Khan, A.A. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MRSA on isolates from skin infections. Biol. Med. 2011, 3, 141–146. [Google Scholar]
- Berdini, F.; Otalvaro, J.O.; Avena, M.; Brigante, M. Photodegradation of doxycycline in water induced by TiO2-MCM-41. Kinetics, TOC evolution and reusability. Results Eng. 2022, 16, 100765. [Google Scholar] [CrossRef]
- Irodia, R.; Mîndroiu, M.; Bîru, I.; Ioniţă, G.; Mihai, G.V.; Enăchescu, M.; Orbeci, C.; Pîrvu, C. Double S-Scheme Polydopamine/TiO2/Chlorophyll as Stable and Efficient Green Photoelectrocatalyst. ChemElectroChem 2023, e202300277. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.; Krishnan, V. Recent advances in plasmonic photocatalysis based on TiO2 and noble metal nanoparticles for energy conversion, environmental remediation, and organic synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef] [PubMed]
- Seelandt, B.; Wark, M. Electrodeposited Prussian Blue in mesoporous TiO2 as electrochromic hybrid material. Microporous Mesoporous Mater. 2012, 164, 67–70. [Google Scholar] [CrossRef]
- Akshay, V.R.; Arun, B.; Mandal, G.; Mutta, G.R.; Chanda, A.; Vasundhara, M. Observation of Optical Band-Gap Narrowing and Enhanced Magnetic Moment in Co-Doped Sol–Gel-Derived Anatase TiO2 Nanocrystals. J. Phys. Chem. C 2018, 122, 26592–26604. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen defect dependent variation of band gap, Urbach energy and luminescence property of anatase, anatase–rutile mixed phase and of rutile phases of TiO2 nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 56, 364–371. [Google Scholar] [CrossRef]
- Justicia, I.; Ordejón, P.; Canto, G.; Mozos, J.L.; Fraxedas, J.; Battiston, G.A.; Gerbasi, R.; Figueras, A. Designed Self-Doped Titanium Oxide Thin Films for Efficient Visible-Light Photocatalysis. Adv. Mater. 2002, 14, 1399–1402. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-Doped Ti3+ Enhanced Photocatalyst for Hydrogen Production under Visible Light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Q.; Qin, L.-C. Reduction in the electronic band gap of titanium oxide nanotubes. Solid State Commun. 2007, 141, 168–171. [Google Scholar] [CrossRef]
- Solomon, E.I.; Lever, A.B.P. Inorganic Electronic Structure and Spectroscopy; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Landi, S.; Segundo, I.R.; Afonso, C.; Lima, O.; Costa, M.F.M.; Freitas, E.; Carneiro, J. Evaluation of band gap energy of TiO2 precipitated from titanium sulphate. Phys. B Condens. Matter 2022, 639, 414008. [Google Scholar] [CrossRef]
- Derbali, A.; Attaf, A.; Saidi, H.; Benamra, H.; Nouadji, M.; Aida, M.S.; Attaf, N.; Ezzaouia, H. Investigation of structural, optical and electrical properties of ZnS thin films prepared by ultrasonic spray technique for photovoltaic applications. Optik 2018, 154, 286–293. [Google Scholar] [CrossRef]
- Attaf, A.; Derbali, A.; Saidi, H.; Benamra, H.; Aida, M.S.; Attaf, N.; Ezzaouia, H.; Derbali, L. Physical properties of Pb doped ZnS thin films prepared by ultrasonic spray technique. Phys. Lett. A 2020, 384, 126199. [Google Scholar] [CrossRef]
- Chen, M.C.; Koh, P.W.; Ponnusamy, V.K.; Lee, S.L. Titanium dioxide and other nanomaterials based antimicrobial additives in functional paints and coatings. Prog. Org. Coat. 2022, 163, 106660. [Google Scholar] [CrossRef]
- Schutte-Smith, M.; Erasmus, E.; Mogale, R.; Marogoa, N.; Jayiya, A.; Visser, H. Using visible light to activate antiviral and antimicrobial properties of TiO2 nanoparticles in paints and coatings: Focus on new developments for frequent-touch surfaces in hospitals. J. Coat. Technol. Res. 2023, 20, 789–817. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, F.; Li, M.; Wang, P.; Fu, Y.; Wang, G.; Liu, X. Construction of hollow binary oxide heterostructures by Ostwald ripening for superior photoelectrochemical removal of reactive brilliant blue KNR dye. Adv. Powder Mater. 2023, 2, 100117. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S. Flat-Band Potential of a Semiconductor: Using the Mott Schottky Equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]
- Wang, S.; Pan, L.; Song, J.-J.; Mi, W.; Zou, J.-J.; Wang, L.; Zhang, X. Titanium-Defected Undoped Anatase TiO2 with p-Type Conductivity, Room-Temperature Ferromagnetism, and Remarkable Photocatalytic Performance. J. Am. Chem. Soc. 2015, 137, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Tan, H.; Zhang, X.; Wu, Y.; Ma, C.; Burda, C. Effect of Quantum Dot Deposition on the Interfacial Flatband Potential, Depletion Layer in TiO2 Nanotube Electrodes, and Resulting H2 Generation Rates. J. Phys. Chem. C 2012, 116, 18633–18640. [Google Scholar] [CrossRef]
- Fan, X.; Ohlckers, P.; Chen, X. Tunable synthesis of hollow Co3O4 nanoboxes and their application in supercapacitors. Appl. Sci. 2020, 10, 1208. [Google Scholar] [CrossRef]
- Zerdoumi, R.; Rößner, L.; Armbrüster, M. Addressing the stability of bulk electrode materials in the electrochemical methanol oxidation. J. Electrochem. Soc. 2019, 166, F1079. [Google Scholar] [CrossRef]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt doped TiO2: A stable and efficient photocatalyst for continuous hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrogen Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Cánovas, R.; Sleegers, N.; van Nuijs, A.L.; De Wael, K. Tetracycline antibiotics: Elucidating the electrochemical fingerprint and oxidation pathway. Chemosensors 2021, 9, 187. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 Nanomaterials: A Review of Recent Advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Rani, S.; Garg, A.; Singh, N. Efficient degradation of doxycycline and ofloxacin in an aqueous environment using Fe and Cu doped TiO2-SiO2 photocatalyst under sunlight. Environ. Eng. Res. 2022, 27, 210282. [Google Scholar] [CrossRef]
- Saravan, R.S.; Muthukumaran, M.; Mubashera, S.; Abinaya, M.; Prasath, P.V.; Parthiban, R.; Mohammad, F.; Oh, W.C.; Sagadevan, S. Evaluation of the photocatalytic efficiency of cobalt oxide nanoparticles towards the degradation of crystal violet and methylene violet dyes. Optik 2020, 207, 164428. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, L.; Li, Y.; Wang, X.; Zhao, L.; Huang, P.; Chen, D.; Wang, J. Enhancement of Visible-Light Photocatalytic Degradation of Tetracycline by Co-Doped TiO2 Templated by Waste Tobacco Stem Silk. Molecules 2023, 28, 386. [Google Scholar] [CrossRef] [PubMed]

| Sample | Contact Angle (°) Water Solvent |
|---|---|
| BT | 30 ± 0.13 |
| BT/Co-P | 10 ± 0.04 |
| BT/Co-E | 6 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irodia, R.; Ungureanu, C.; Sătulu, V.; Mîndroiu, V.M. Photocatalyst Based on Nanostructured TiO2 with Improved Photocatalytic and Antibacterial Properties. Materials 2023, 16, 7509. https://doi.org/10.3390/ma16247509
Irodia R, Ungureanu C, Sătulu V, Mîndroiu VM. Photocatalyst Based on Nanostructured TiO2 with Improved Photocatalytic and Antibacterial Properties. Materials. 2023; 16(24):7509. https://doi.org/10.3390/ma16247509
Chicago/Turabian StyleIrodia, Roberta, Camelia Ungureanu, Veronica Sătulu, and Vasilica Mihaela Mîndroiu. 2023. "Photocatalyst Based on Nanostructured TiO2 with Improved Photocatalytic and Antibacterial Properties" Materials 16, no. 24: 7509. https://doi.org/10.3390/ma16247509
APA StyleIrodia, R., Ungureanu, C., Sătulu, V., & Mîndroiu, V. M. (2023). Photocatalyst Based on Nanostructured TiO2 with Improved Photocatalytic and Antibacterial Properties. Materials, 16(24), 7509. https://doi.org/10.3390/ma16247509








