Recent Advances in Bromine Complexing Agents for Zinc–Bromine Redox Flow Batteries
Abstract
1. Introduction
1.1. RFB Description
1.2. RFB Classification
| Redox Couples | Redox Reaction | Eo (V) | Electrolyte |
|---|---|---|---|
| All-vanadium | anode: V2+ ←→ V3+ + e− cathode: VO2+ + e− ←→ VO2+ | 1.4 | H2SO4/H2SO4 |
| V/polyhalide | anode: V2+ ←→ V3+ + e− cathode: ½Br2 + e− ←→ Br− | 1.3 | VCl3-HCl/NaBr-HCl |
| Fe/Cr | anode: Fe2+ ←→ Fe3+ + e− cathode: Cr3+ + e− ←→ Cr2+ | 1.2 | HCl/HCl |
| Br/polysulphure | anode: 2S22− ←→ S42− + e− cathode: Br2 + 2e− ←→ 2Br− | 1.5 | NaS2/NaBr |
| H2/Br2 | anode: H2 ←→ 2H+ + e− cathode: Br2 + 2e− ←→ 2Br− | 1.1 | PEM+-HBr |
| Zn/Br | anode: Zn ←→ Zn2+ + 2e− cathode: Br2 + 2e− ←→ 2Br− | 1.8 | ZnBr2/ZnBr2 |
| Zn/Ce | anode: Zn ←→ Zn2+ + 2e− cathode: 2Ce4+ + 2e− ←→ 2Ce3+ | 2.4 | CH3SO3H/CH3SO3H |
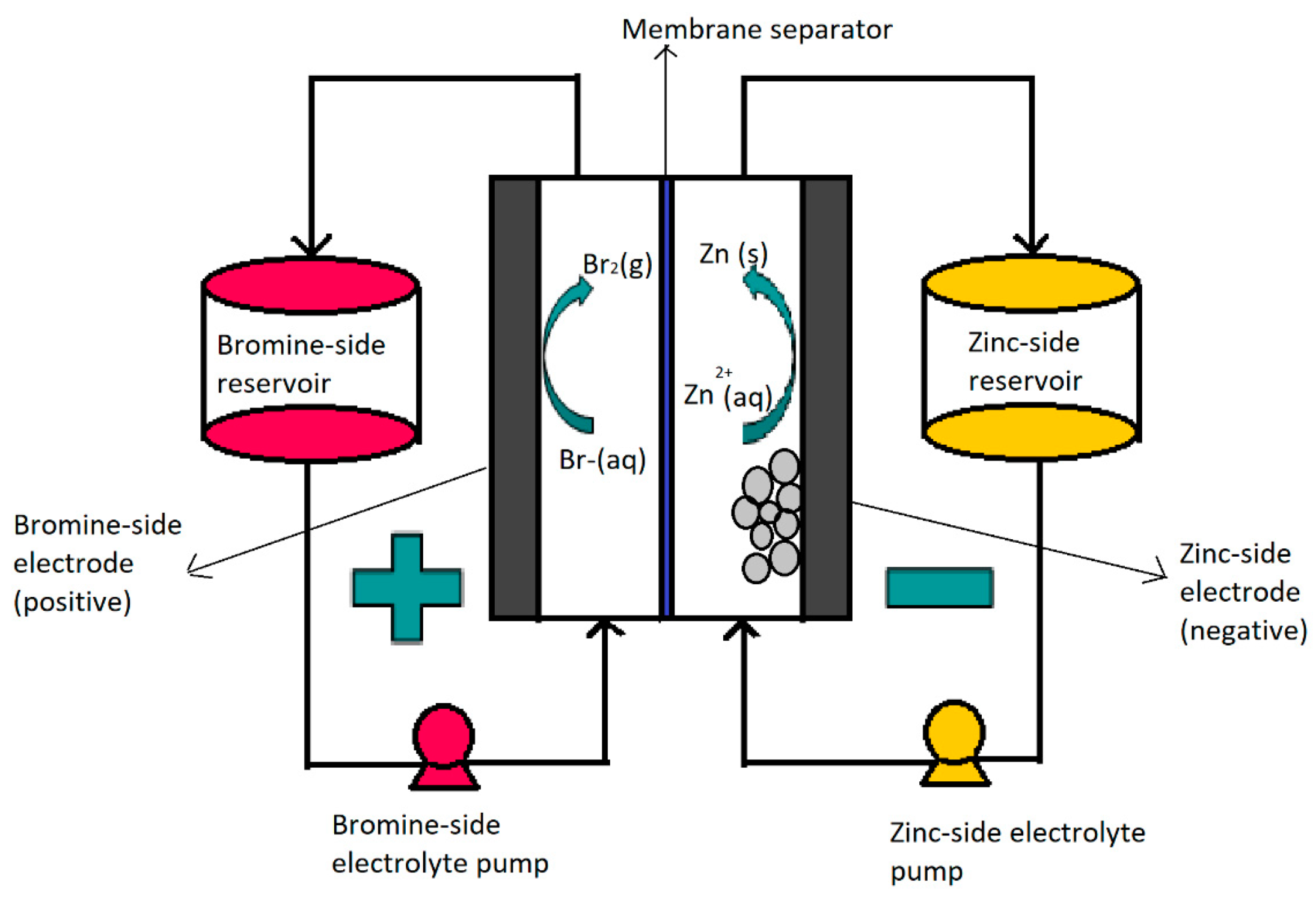
2. Zinc–Bromine Flow Batteries (ZBFBs)
ZFBF Components
3. Bromine Complexing Agents (BCAs)
3.1. Morpholinium-Based BCAs
3.1.1. N-ethyl-N-methylmorpholinium Bromide (MEM-Br)
3.1.2. N-methyl-N-propylmorpholinium Bromide (MPM-Br)
3.1.3. 1-(2-Carboximethyl)-1-methylmorpholinium Bromide (CMMM-Br)
3.2. Pirrolidinium-Based BCAs
3.2.1. 1-Ethyl-1-methylpyrrolidinium Bromide (MEP-Br)
3.2.2. 1-(2-Carboxymethyl)-1-methylpyrrolidinium Bromide (CMMP-Br)
3.3. Imidazolium-Based BCAs
3.3.1. 1-Ethyl-3-methylimidazolium Bromide (EMI-Br)
3.3.2. 1,2-Dimethyl-ethylimidazolium Bromide (DMEI-Br)
3.3.3. 1-(2-Hydroxyethyl)-3-methylimidazolium Bromine (C2OHMI-Br)
3.4. Piperidinium-Based BCAs
1-Ethyl-3-methylpiperidinium Bromide (C2MPip-Br)
3.5. Pyridinium-Based BCAs
3.5.1. 1-Ethylpyridinium Bromide (C2Py-Br)
3.5.2. 1-(2-Hydroxyethyl)-pyridinium Bromide (C2OHPy-Br)
3.5.3. 1-(Carboxymethyl)-pyridinium Bromide (CMMPy-Br)
3.6. Summary of All Salts Reported in This Review
4. Study of Bromine Complexing Agents in ZBFBs
4.1. Bromine Complexation Capacity
4.2. Influence on Zinc Electrodeposition
4.3. Electrochemical Properties: Cyclic Voltammetry (CV) Measurements and Electrochemical Impedance Spectroscopy (EIS)
4.4. Cell Performance in ZBFBs
5. Overview and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, K.; Dong, X.; Jiang, Q.; Zhao, J. Assessing energy resilience and its greenhouse effect: A global perspective. Energy Econ. 2021, 104, 105659. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, G. Influence of spectral characteristics of the Earth’s surface radiation on the greenhouse effect: Principles and mechanisms. Atmos. Environ. 2021, 244, 117908. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Jiao, Y.; Månsson, D. Greenhouse gas emissions from hybrid energy storage systems in future 100% renewable power systems—A Swedish case based on consequential life cycle assessment. J. Energy Storage 2023, 57, 106167. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Abo-Khalil, A.G.; Maghrabie, H.M.; Elsaid, K.; Abdelkareem, M.A. Battery energy storage systems and SWOT (strengths, weakness, opportunities, and threats) analysis of batteries in power transmission. Energy 2022, 254, 123987. [Google Scholar] [CrossRef]
- Arabkoohsar, A. Chapter One—Classification of energy storage systems. In Mechanical Energy Storage Technologies; Arabkoohsar, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–12. [Google Scholar] [CrossRef]
- Dixit, M.; Muralidharan, N.; Parejiya, A.; Essehli, R.; Belharouak, I.; Amin, R. 9—Electrochemical energy storage systems. In Emerging Trends in Energy Storage Systems and Industrial Applications; Kumar, N.P., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 259–282. [Google Scholar] [CrossRef]
- Bhattacharjee, U.; Ghosh, S.; Bhar, M.; Martha, S.K. 6—Electrochemical energy storage part I: Development, basic principle and conventional systems. In Emerging Trends in Energy Storage Systems and Industrial Applications; Kumar, N.P., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 151–188. [Google Scholar] [CrossRef]
- Miller, J.R.; Butler, S. Storage system design based on equivalent-circuit-model simulations: Comparison of eight different electrochemical capacitor storage systems. J. Power Sources 2021, 491, 229441. [Google Scholar] [CrossRef]
- Tang, L.; Leung, P.; Mohamed, M.R.; Xu, Q.; Dai, S.; Zhu, X.; Flox, C.; Shah, A.A.; Liao, Q. Capital cost evaluation of conventional and emerging redox flow batteries for grid storage applications. Electrochim. Acta 2023, 437, 141460. [Google Scholar] [CrossRef]
- He, H.; Tian, S.; Tarroja, B.; Ogunseitan, O.A.; Samuelsen, S.; Schoenung, J.M. Flow battery production: Materials selection and environmental impact. J. Clean. Prod. 2020, 269, 121740. [Google Scholar] [CrossRef]
- Tian, S.; He, H.; Kendall, A.; Davis, S.J.; Ogunseitan, O.A.; Schoenung, J.M.; Samuelsen, S.; Tarroja, B. Environmental benefit-detriment thresholds for flow battery energy storage systems: A case study in California. Appl. Energy 2021, 300, 117354. [Google Scholar] [CrossRef]
- Xiong, D.; Yang, L.; Cao ZLi, F. In Situ Construction of High-Density Solid Electrolyte Interphase from MOFs for Advanced Zn Metal Anodes. Adv. Funct. Mater. 2023, 33, 2301530. [Google Scholar] [CrossRef]
- Liu, C.; Deng, L.; Li, X.; Wu, T.; Zhang, W.; Cui, H. Metal–organic frameworks for solid-state electrolytes: A mini review. Electrochem. Commun. 2023, 150, 107491. [Google Scholar] [CrossRef]
- Tao, S.; Momem, R.; Luo, Z.; Zhu, Y.; Xiao, X.; Cao, Z.; Xiong, D.; Deng, W.; Liu, Y.; Hou, H.; et al. Trapping Lithium Selenides with Evolving Heterogeneous Interfaces for High-Power Lithium-Ion Capacitors. Small 2023, 19, e2207975. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.T.; Shehata, N.; Abdelkareem, M.A.; Ramadan, M.; Olabi, A.-G. Redox Flow Batteries. In Encyclopedia of Smart Materials; Olabi, A.-G., Ed.; Elsevier: Oxford, UK, 2022; pp. 176–185. [Google Scholar] [CrossRef]
- Tang, L.; Leung, P.; Xu, Q.; Mohamed, M.R.; Dai, S.; Zhu, X.; Flox, C.; Shah, A.A. Future perspective on redox flow batteries: Aqueous versus nonaqueous electrolytes. Curr. Opin. Chem. Eng. 2022, 37, 100833. [Google Scholar] [CrossRef]
- Adeniran, A.; Bates, A.; Schuppert, N.; Menon, A.; Park, S. Recent advances in aqueous redox flow battery research. J. Energy Storage 2022, 56, 106000. [Google Scholar] [CrossRef]
- Yang, I.; Lee, S.; Jang, D.; Lee, J.-E.; Cho, S.Y.; Lee, S. Enhancing energy efficiency and long-term durability of vanadium redox flow battery with catalytically graphitized carbon fiber felts as electrodes by boron doping. Electrochim. Acta 2022, 429, 141033. [Google Scholar] [CrossRef]
- Doetsch, C.; Pohlig, A. 13—The Use of Flow Batteries in Storing Electricity for National Grids. In Future Energy, 3rd ed.; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 263–277. [Google Scholar] [CrossRef]
- Jiménez-Blasco, U.; Moreno, E.; Cólera, M.; Díaz-Carrasco, P.; Arrebola, J.C.; Caballero, A.; Morales, J.; Vargas, O.A. Enhanced Performance of Zn/Br Flow Battery Using N-Methyl-N-Propylmorpholinium Bromide as Complexing Agent. Int. J. Mol. Sci. 2021, 22, 9288. [Google Scholar] [CrossRef]
- Arenas, L.F.; de León, C.P.; Walsh, F.C. Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, J.; Feng, J.-K. Research progress in preparation of electrolyte for all-vanadium redox flow battery. J. Ind. Eng. Chem. 2023, 118, 33–43. [Google Scholar] [CrossRef]
- Trovò, A.; Poli, N.; Guarnieri, M. New strategies for the evaluation of Vanadium Flow Batteries: Testing prototypes. Curr. Opin. Chem. Eng. 2022, 37, 100853. [Google Scholar] [CrossRef]
- Flox, C.; Monllor-Satoca, D. Hybrid Flow Batteries: Advances, Scientific Challenges and Market. In Encyclopedia of Energy Storage; Cabeza, L.F., Ed.; Elsevier: Oxford, UK, 2022; pp. 443–452. [Google Scholar] [CrossRef]
- Krishna, M.; Fraser, E.J.; Wills, R.G.A.; Walsh, F.C. Developments in soluble lead flow batteries and remaining challenges: An illustrated review. J. Energy Storage 2018, 15, 69–90. [Google Scholar] [CrossRef]
- Chaabene, N.; Ngo, K.; Turmine, M.; Vivier, V. Ionic liquid redox flow membraneless battery in microfluidic system. J. Energy Storage 2023, 57, 106270. [Google Scholar] [CrossRef]
- Wang, X.; Lashgari, A.; Chai, J.; Jiang, J. A membrane-free, aqueous/nonaqueous hybrid redox flow battery. Energy Storage Mater. 2022, 45, 1100–1108. [Google Scholar] [CrossRef]
- Leung, P.; Li, X.; de León, C.P.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in redox flow batteries, remaining challenges and their applications in energy storage. RSC Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Jin, C.; Lei, H.; Liu, M.; Tan, A.; Piao, J.; Fu, Z.; Liang, Z.; Wang, H. Low-dimensional nitrogen-doped carbon for Br2/Br− redox reaction in zinc-bromine flow battery. Chem. Eng. J. 2020, 380, 122606. [Google Scholar] [CrossRef]
- Archana, K.S.; Naresh, R.P.; Enale, H.; Rajendran, V. Effect of positive electrode modification on the performance of zinc-bromine redox flow batteries. J. Energy Storage 2020, 29, 101462. [Google Scholar] [CrossRef]
- Wu, M.C.; Zhao, T.S.; Wei, L.; Jiang, H.R.; Zhang, R.H. Improved electrolyte for zinc-bromine flow batteries. J. Power Sources 2018, 384, 232–239. [Google Scholar] [CrossRef]
- Lee, Y.; Yun, D.; Park, J.; Hwang, G.; Chung, D.; Kim, M.; Jron, J. An organic imidazolium derivative additive inducing fast and highly reversible redox reactions in zinc-bromine flow batteries. J. Power Sources 2022, 547, 232007. [Google Scholar] [CrossRef]
- Schneider, M.; Rajarathnam, G.P.; Easton, M.E.; Masters, A.F.; Maschmeyer, T.; Vassallo, A.M. The influence of novel bromine sequestration agents on zinc/bromine flow battery performance. RSC Adv. 2016, 6, 110548–110556. [Google Scholar] [CrossRef]
- Wu, M.C.; Zhang, R.H.; Liu, K.; Sun, J.; Chan, K.Y.; Zhao, T.S. Mesoporous carbon derived from pomelo peel as a high-performance electrode material for zinc-bromine flow batteries. J. Power Sources 2019, 442, 227255. [Google Scholar] [CrossRef]
- Wu, M.C.; Jiang, H.R.; Zhang, R.H.; Wei, L.; Chan, K.Y.; Zhao, T.S. N-doped graphene nanoplatelets as a highly active catalyst for Br2/Br− redox reactions in zinc-bromine flow batteries. Electrochim. Acta 2019, 318, 69–75. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Lai, Q.; Li, X.; Cheng, Y. Development of carbon coated membrane for zinc/bromine flow battery with high power density. J. Power Sources 2013, 227, 41–47. [Google Scholar] [CrossRef]
- Rajarathnam, G.P.; Vassallo, A.M. The Zinc/Bromine Flow Battery. In SpringerBriefs in Energy; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Wu, M.C.; Zhao, T.S.; Jiang, H.R.; Zeng, Y.K.; Ren, Y.X. High-performance zinc bromine flow battery via improved design of electrolyte and electrode. J. Power Sources 2017, 355, 62–68. [Google Scholar] [CrossRef]
- Hua, L.; Lu, W.; Li, T.; Xu, P.; Zhang, H.; Li, X. A highly selective porous composite membrane with bromine capturing ability for a bromine-based flow battery. Mater. Today Energy 2021, 21, 100763. [Google Scholar] [CrossRef]
- Yang, H.S.; Park, J.H.; Ra, H.W.; Jin, C.-S.; Yang, J.H. Critical rate of electrolyte circulation for preventing zinc dendrite formation in a zinc–bromine redox flow battery. J. Power Sources 2016, 325, 446–452. [Google Scholar] [CrossRef]
- Kim, M.; Yun, D.; Jeon, J. Effect of a bromine complex agent on electrochemical performances of zinc electrodeposition and electrodissolution in Zinc–Bromide flow battery. J. Power Sources 2019, 438, 227020. [Google Scholar] [CrossRef]
- Tomazic, G.; Skyllas-Kazacos, M. Chapter 17—Redox Flow Batteries. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Moseley, P.T., Garche, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 309–336. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Trovò, A.; Guarnieri, M. Battery Management Systems for Redox Flow Batteries and Controllers for Fuel Cells. In Encyclopedia of Energy Storage; Cabeza, L.F., Ed.; Elsevier: Oxford, UK, 2022; pp. 557–567. [Google Scholar] [CrossRef]
- Bryans, D.; McMillan, B.G.; Spicer, M.; Wark, A.; Berlouis, L. Complexing Additives to Reduce the Immiscible Phase Formed in the Hybrid ZnBr 2 Flow Battery. J. Electrochem. Soc. 2017, 164, A3342–A3348. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Pérez, G.; Ortiz, A.; Ortiz, I. The roles of ionic liquids as new electrolytes in redox flow batteries. Sep. Purif. Technol. 2020, 252, 117436. [Google Scholar] [CrossRef]
- Ghalami-Choobar, B.; Fallahkar, T.N. Thermophysical properties of 1-ethyl-3-methylimidazolium bromide ionic liquid in water + ethylene carbonate mixtures at T = (298.2, 308.2 and 318.2) K. Fluid Phase Equilibria 2019, 496, 42–60. [Google Scholar] [CrossRef]
- Malek, N.I.; Ijardar, S.P. Binary mixtures of ([C4mim][NTf2]+molecular organic solvents): Thermophysical, acoustic and transport properties at various compositions and temperatures. J. Chem. Thermodyn. 2016, 93, 75–85. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, pp. 4786–4815. [Google Scholar] [CrossRef]
- Alvarez-Guerra, E.; Irabien, A.; Ventura, S.P.M.; Coutinho, J.A.P. Ionic liquid recovery alternatives in ionic liquid-based three-phase partitioning (ILTPP). AIChE J. 2014, 60, 3577–3586. [Google Scholar] [CrossRef]
- Khan, I.; Taha, M.; Pinho, S.P.; Coutinho, J.A.P. Interactions of pyridinium, pyrrolidinium or piperidinium based ionic liquids with water: Measurements and COSMO-RS modelling. Fluid Phase Equilibria 2016, 414, 93–100. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, S.R. Ionic liquids as environmental friendly cutting fluids—A review. Mater. Today Proc. 2021, 37, 2121–2125. [Google Scholar] [CrossRef]
- Ruan, Q.; Yao, M.; Yuan, D.; Dong, H.; Liu, J.; Yuan, X.; Fang, W.; Zhao, G.; Zhang, H. Ionic liquid crystal electrolytes: Fundamental, applications and prospects. Nano Energy 2023, 106, 108087. [Google Scholar] [CrossRef]
- Fradera, X.; Torrent, L.A.M.; Mestres, J.; Constans, P.; Besalú, E.; Martí, J.; Simon, S.; Lobato, M.; Oliva, J.M.; Luis, J.M.; et al. Analysis of the changes on the potential energy surface of Menshutkin reactions induced by external perturbations. J. Mol. Struct. THEOCHEM 1996, 371, 171–183. [Google Scholar] [CrossRef]
- Rocha, M.V.; Smits, N.W.; Wolters, L.P.; de Cózar, A.; Guerra, C.F.; Ramalho, T.C.; Bickelhaupt, F.M. Asymmetric identity SN2 transition states: Nucleophilic substitution at α-substituted carbon and silicon centers. Int. J. Mass Spectrom. 2017, 413, 85–91. [Google Scholar] [CrossRef]
- Giri, S.; Inostroza-Rivera, R.; Herrera, B.; Núñez, A.S.; Lund, F.; Toro-Labbé, A. The mechanism of Menshutkin reaction in gas and solvent phases from the perspective of reaction electronic flux. J. Mol. Model. 2014, 20, 2353. [Google Scholar] [CrossRef]
- Maran, U.; Karelson, M.; Pakkanen, T.A. A gas phase ab initio study of the Menshutkin reaction. J. Mol. Struct. THEOCHEM 1997, 397, 263–272. [Google Scholar] [CrossRef]
- Webb, S.P.; Gordon, M.S. Solvation of the Menshutkin Reaction: A Rigorous Test of the Effective Fragment Method. J. Phys. Chem. A 1999, 103, 1265–1273. [Google Scholar] [CrossRef][Green Version]
- Halls, M.D.; Schlegel, H.B. Chemistry Inside Carbon Nanotubes: the Menshutkin SN2 Reaction. J. Phys. Chem. B 2002, 106, 1921–1925. [Google Scholar] [CrossRef]
- Cathro, K.J.; Cedzynska, K.; Constable, D.C.; Hoobin, P.M. Selection of quaternary ammonium bromides for use in zinc/bromine cells. J. Power Sources 1986, 18, 349–370. [Google Scholar] [CrossRef]
- Li, X.; de Léon, C.P.; Walsh, F.C.; Wills, R.G.A.; Pletcher, D. Chapter 8—Zinc-based flow batteries for medium- and large-scale energy storage. In Advances in Batteries for Medium and Large-Scale Energy Storage; Menictas, C., Skyllas-Kazacos, M., Lim, T.M., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2015; pp. 293–315. [Google Scholar] [CrossRef]
- Cha, J.-H.; Kim, K.-S.; Choi, S.; Yeon, S.-H.; Lee, H.; Kim, H.S.; Kim, H. Thermal and electrochemical properties of morpholinium salts with bromide anion. Korean J. Chem. Eng. 2005, 22, 945–948. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Tian, X.-Y.; Zhang, Y.-C. Structure and hydrogen-bond properties of N-alkyl-N-methyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide and ethanol: A combination of FTIR and theoretical studies. J. Mol. Struct. 2022, 1265, 133488. [Google Scholar] [CrossRef]
- Lai, Q.; Zhang, H.; Li, X.; Zhang, L.; Cheng, Y. A novel single flow zinc–bromine battery with improved energy density. J. Power Sources 2013, 235, 1–4. [Google Scholar] [CrossRef]
- Domańska, U.; Karpińska, M.; Zawadzki, M. Activity coefficients at infinite dilution for organic solutes and water in 1-ethyl-1-methylpyrrolidinium lactate. J. Chem. Thermodyn. 2015, 89, 127–133. [Google Scholar] [CrossRef]
- Niu, H.; Ding, M.; Zhang, N.; Li, X.; Su, X.; Han, X.; Zhang, N.; Guan, P.; Hu, X. Preparation of imidazolium based polymerized ionic liquids gel polymer electrolytes for high-performance lithium batteries. Mater. Chem. Phys. 2023, 293, 126971. [Google Scholar] [CrossRef]
- Appiah, W.A.; Li, H.; Lampkin, J.; García-Lastra, J.M. Towards understanding aluminum sulfur batteries with imidazolium-based electrolytes: A phenomenological model. J. Power Sources 2022, 529, 231254. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Wang, A.; Ji, W.; Zhang, L.; Zhang, T.; Li, J.; Zhang, H.; Tang, H.; Zhang, H. Novel N-alkylation synthetic strategy of imidazolium cations grafted polybenzimidazole for high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2023, 669, 121332. [Google Scholar] [CrossRef]
- Banjare, M.K.; Behera, K.; Kurrey, R.; Banjare, R.K.; Satnami, M.L.; Pandey, S.; Ghosh, K.K. Self-aggregation of bio-surfactants within ionic liquid 1-ethyl-3-methylimidazolium bromide: A comparative study and potential application in antidepressants drug aggregation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 199, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Patra, J.; Yang, C.-H.; Tseng, C.-M.; Majumder, S.B.; Dong, Q.-F. Electrolyte Optimization for Enhancing Electrochemical Performance of Antimony Sulfide/Graphene Anodes for Sodium-Ion Batteries–Carbonate-Based and Ionic Liquid Electrolytes. ACS Sustain. Chem. Eng. 2017, 5, 8269–8276. [Google Scholar] [CrossRef]
- Rajarathnam, G.P.; Vassallo, A. Half-Cell Electrochemical Performance of Hybridized Ionic Liquid Additives for Zinc/Bromine Flow Battery Applications. ECS Trans. 2016, 72, 33–55. [Google Scholar] [CrossRef]
- Rajarathnam, G.P.; Easton, M.E.; Schneider, M.; Masters, A.F.; Maschmeyer, T.; Vassallo, A.M. The influence of ionic liquid additives on zinc half-cell electrochemical performance in zinc/bromine flow batteries. RSC Adv. 2016, 6, 27788–27797. [Google Scholar] [CrossRef]
- Burrell, A.K.; Sesto, R.E.D.; Baker, S.N.; McCleskey, T.M.; Baker, G.A. The large scale synthesis of pure imidazolium and pyrrolidinium ionic liquids. Green Chem. 2007, vol. 9, 449–454. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Lee, Y.; Jeon, J. 1,2-Dimethylimidazole based bromine complexing agents for vanadium bromine redox flow batteries. Int. J. Hydrogen Energy 2019, 44, 12024–12032. [Google Scholar] [CrossRef]
- Amit, L.; Naar, D.; Gloukhovski, R.; la O’, G.J.; Suss, M.E. A Single-Flow Battery with Multiphase Flow. ChemSusChem 2021, 14, 1068–1073. [Google Scholar] [CrossRef]
- Fan, Z.; Yu, H. Determination of piperidinium ionic liquid cations in environmental water samples by solid phase extraction and hydrophilic interaction liquid chromatography. J. Chromatogr. A 2018, 1559, 136–140. [Google Scholar] [CrossRef]
- Lee, J.-M. Solvent properties of piperidinium ionic liquids. Chem. Eng. J. 2011, 172, 1066–1071. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, X.; Wang, X.; Huang, D.; Chen, X. Micelle formation by N-alkyl-N-methylpiperidinium bromide ionic liquids in aqueous solution. Colloids Surf. Physicochem. Eng. Asp. 2012, 412, 90–95. [Google Scholar] [CrossRef]
- Alrefaee, S.H. Effect of alkyl chain length and halide ions on the corrosion inhibition potential of imidazolium and pyridinium based ionic liquids: Computational studies. J. Mol. Liq. 2021, 344, 117848. [Google Scholar] [CrossRef]
- Bhatt, A.M.; Deshmukh, S.; Boda, A.; Chauhan, R.S.; Ali, S.M.; Sengupta, A. Synthesis and application of chloroacetamides in pyridinium based ionic liquid for high temperature extraction of uranyl ion: A novel and “green” approach for extractive mass transfer at elevated temperature. J. Mol. Liq. 2022, 365, 120222. [Google Scholar] [CrossRef]
- Norouzi, F.; Abdolmaleki, A. CO2 conversion into carbonate using pyridinium-based ionic liquids under mild conditions. Fuel 2023, 334, 126641. [Google Scholar] [CrossRef]
- Matuszek, K.; Hatton, C.; Kar, M.; Pringle, J.M.; MacFarlane, D.R. Molecular patterns in the thermophysical properties of pyridinium ionic liquids as phase change materials for energy storage in the intermediate temperature range. J. Non-Cryst. Solids X 2022, 15, 100108. [Google Scholar] [CrossRef]
- Marcinkowski, Ł.; Olszewska, T.; Kloskowski, A.; Warmińska, D. Apparent Molar Volumes and Expansivities of Ionic Liquids Based on N-Alkyl-N-methylmorpholinium Cations in Acetonitrile. J. Chem. Eng. Data 2014, 59, 718–725. [Google Scholar] [CrossRef]
- Edwin, B.; Amalanathan, M.; Chadha, R.; Maiti, N.; Kapoor, S.; Joe, I.H. Structure activity relationship, vibrational spectral investigation and molecular docking analysis of anti-neuronal drug 4-(2-Aminoethyl) morpholine. J. Mol. Struct. 2017, 1148, 459–470. [Google Scholar] [CrossRef]
- Moosavi-Zare, A.R.; Zolfigol, M.A.; Salehi-Moratab, R.; Noroozizadeh, E. Catalytic application of 1-(carboxymethyl)pyridinium iodide on the synthesis of pyranopyrazole derivatives. J. Mol. Catal. Chem. 2016, 415, 144–150. [Google Scholar] [CrossRef]
- Takemoto, K.; Yamada, H. Development of rechargeable lithium–bromine batteries with lithium ion conducting solid electrolyte. J. Power Sources 2015, 281, 334–340. [Google Scholar] [CrossRef]
- Haller, H.; Schröder, J.; Riedel, S. Structural Evidence for Undecabromide [Br11]−. Angew. Chem. Int. Ed. 2013, 52, 4937–4940. [Google Scholar] [CrossRef] [PubMed]
- Easton, M.E.; Ward, A.J.; Hudson, T.; Turner, P.; Masters, A.F.; Maschmeyer, T. The Formation of High-Order Polybromides in a Room-Temperature Ionic Liquid: From Monoanions ([Br5]− to [Br11]−) to the Isolation of [PC16H36]2[Br24] as Determined by van der Waals Bonding Radii. Chem.—Eur. J. 2015, 21, 2961–2965. [Google Scholar] [CrossRef] [PubMed]
- Easton, M.E.; Ward, A.J.; Chan, B.; Radom, L.; Masters, A.F.; Maschmeyer, T. Factors influencing the formation of polybromide monoanions in solutions of ionic liquid bromide salts. Phys. Chem. Chem. Phys. 2016, 18, 7251–7260. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Q.; Liu, Z.; Wang, D.; Guo, Y.; Li, X.; Tang, Y.; Li, H.; Dong, B. Dendrites in Zn-Based Batteries. Adv. Mater. 2020, 32, 2001854. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Z.; Zhang, S.; Cheng, M.; Chen, F.; Xu, Y. A Low-Cost Neutral Aqueous Redox Flow Battery with Dendrite-Free Tin Anode. J. Electrochem. Soc. 2021, 168, 110547. [Google Scholar] [CrossRef]
- Thieu, N.A.; Li, W.; Chen, X.; Hu, S.; Tian, H.; Tran, H.N.N.; Li, W.; Reed, D.M.; Li, X. An Overview of Challenges and Strategies for Stabilizing Zinc Anodes in Aqueous Rechargeable Zn-Ion Batteries. Batteries 2023, 9, 41. [Google Scholar] [CrossRef]
- Yang, S.; Du, H.; Li, Y.; Wu, X.; Xiao, B.; He, Z.; Zhang, Q. Advances in the structure design of substrate materials for zinc anode of aqueous zinc ion batteries. Green Energy Environ. 2022, 8, 1531–1552. [Google Scholar] [CrossRef]
- Yin, Y.; Yuan, Z.; Li, X. Rechargeable aqueous zinc–bromine batteries: An overview and future perspectives. Phys. Chem. Chem. Phys. 2021, 23, 26070–26084. [Google Scholar] [CrossRef]





| Temp. (°C) | Solvent (mL/g reac.) | Adduct Excess (%) | N2 | Reflux | Crystallization (h) | Yeld (%) |
|---|---|---|---|---|---|---|
| 70 | 1.41 | 14 | Yes | Yes | 12 | 68 |
| 70 | 1.11 | 60 | Yes | Yes | 12 | 85 |
| 70 | 0 | 60 | Yes | Yes | 12 | 19 |
| 50 | 1.11 * | 60 | Yes | Yes | 12 | 10 |
| 70 | 0.55 | 60 | Yes | Yes | 2 | 90 |
| 25 | 0.59 | 50 | No | No | 120 | 89 |
| BCA | Molecule | Precursor | Synthesis Conditions | Dried under Vacuum | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| T (°C) | Time (h) | Refl. | Atm | |||||
| MEM-Br |  | -N-methylmorpholinium -bromoethane -acetonitrile | 65 | 5 | Yes | N2 | Yes | [64,85,86] |
| MPM-Br | 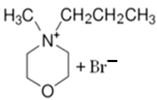 | -4-methylmorpholinium -1-bromopropane -acetonitrile | Room T | 120 | No | No | Yes | [21] |
| CMMM-Br |  | -4-methylmorpholinium -3-bromopropanoic acid | Room T | 4 | No | No | Yes | [46,87] |
| MEP-Br | 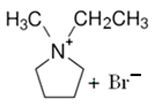 | -N-methypyrrolidinium -bromoethane -acetonitrile | Room T | 24 | No | No | Yes | [60,67] |
| CMMP-Br | 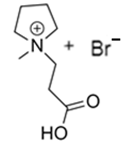 | -N-metilpyrrolidinium -3-bromopropanoic acid | Room T | 4 | No | No | Yes | [46,87] |
| EMI-Br | 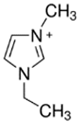 | -3-methilimidazolium -bromoethane | Room T | 24 | Yes | No | Yes | [34,75] |
| DMEI-Br |  | -1,2-dimethylimidazolium -bromoethane | 45 | 12 | Yes | No | No | [33,76,77] |
| C2OHMI-Br |  | -3-methilimidazolium -bromoethanol | Room T | 24 | Yes | No | Yes | [34,75] |
| C2MPip-Br | 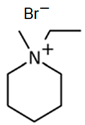 | -N-methylpiperidinium -bromoethane | 75–80 | 48 | Yes | N2 | Yes | [74,75,80] |
| C2Py-Br | 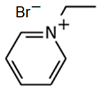 | -pyridine -bromoethane | Room T | 24 | Yes | No | Yes | [34,75] |
| C2OHPy-Br |  | -pyridine -bromoethanol | Room T | 24 | Yes | No | Yes | [34,75] |
| CMMPy-Br |  | -pyridine -2-bromoacetic acid | - | 4 | No | No | Yes | [46] |
| Polybromide Species | Raman Shift (cm−1) |
|---|---|
| Tribromide (Br3−) | 163–198 |
| Pentabromide (Br5−) | 231–380 |
| Free bromine (Br2) | 310 |
| BCA | Structure | Coulombic Efficiency (%) | Voltaic Efficiency (%) | Energy Efficiency (%) |
|---|---|---|---|---|
| MEM-Br |  | 90.7 | 80 | 78.1 |
| MEP-Br | 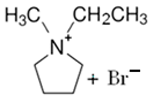 | 91.1 | 58.1 | 52.8 |
| MPM-Br | 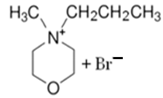 | 90 | - | 70 |
| DMEI-Br |  | 95 | 85 | 80 |
| C2OHPy-Br |  | 83.4 | 67.8 | 56.6 |
| C2OHMI-Br |  | 71.4 | 71.7 | 51.3 |
| C2MPip-Br |  | 80.3 | 47.4 | 38.4 |
| C2Py-Br |  | 91.1 | 61.1 | 55.8 |
| EMI-Br | 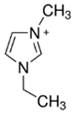 | 90.6 | 69.5 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Blasco, U.; Arrebola, J.C.; Caballero, A. Recent Advances in Bromine Complexing Agents for Zinc–Bromine Redox Flow Batteries. Materials 2023, 16, 7482. https://doi.org/10.3390/ma16237482
Jiménez-Blasco U, Arrebola JC, Caballero A. Recent Advances in Bromine Complexing Agents for Zinc–Bromine Redox Flow Batteries. Materials. 2023; 16(23):7482. https://doi.org/10.3390/ma16237482
Chicago/Turabian StyleJiménez-Blasco, Uxua, José Carlos Arrebola, and Alvaro Caballero. 2023. "Recent Advances in Bromine Complexing Agents for Zinc–Bromine Redox Flow Batteries" Materials 16, no. 23: 7482. https://doi.org/10.3390/ma16237482
APA StyleJiménez-Blasco, U., Arrebola, J. C., & Caballero, A. (2023). Recent Advances in Bromine Complexing Agents for Zinc–Bromine Redox Flow Batteries. Materials, 16(23), 7482. https://doi.org/10.3390/ma16237482







