“To Be or Not to Be” of a Polymer Nanogel—Unravelling the Relationship of Product Properties vs. Synthesis Conditions Governing the Radiation Crosslinking of Poly(acrylic acid) Using GPC/SEC—MALLS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparative Pulse Radiolysis of Poly(acrylic acid)
2.3. Gel Permeation Chromatography/Size Exclusion Chromatography (GPC/SEC)
2.4. Dynamic Light Scattering (DLS)
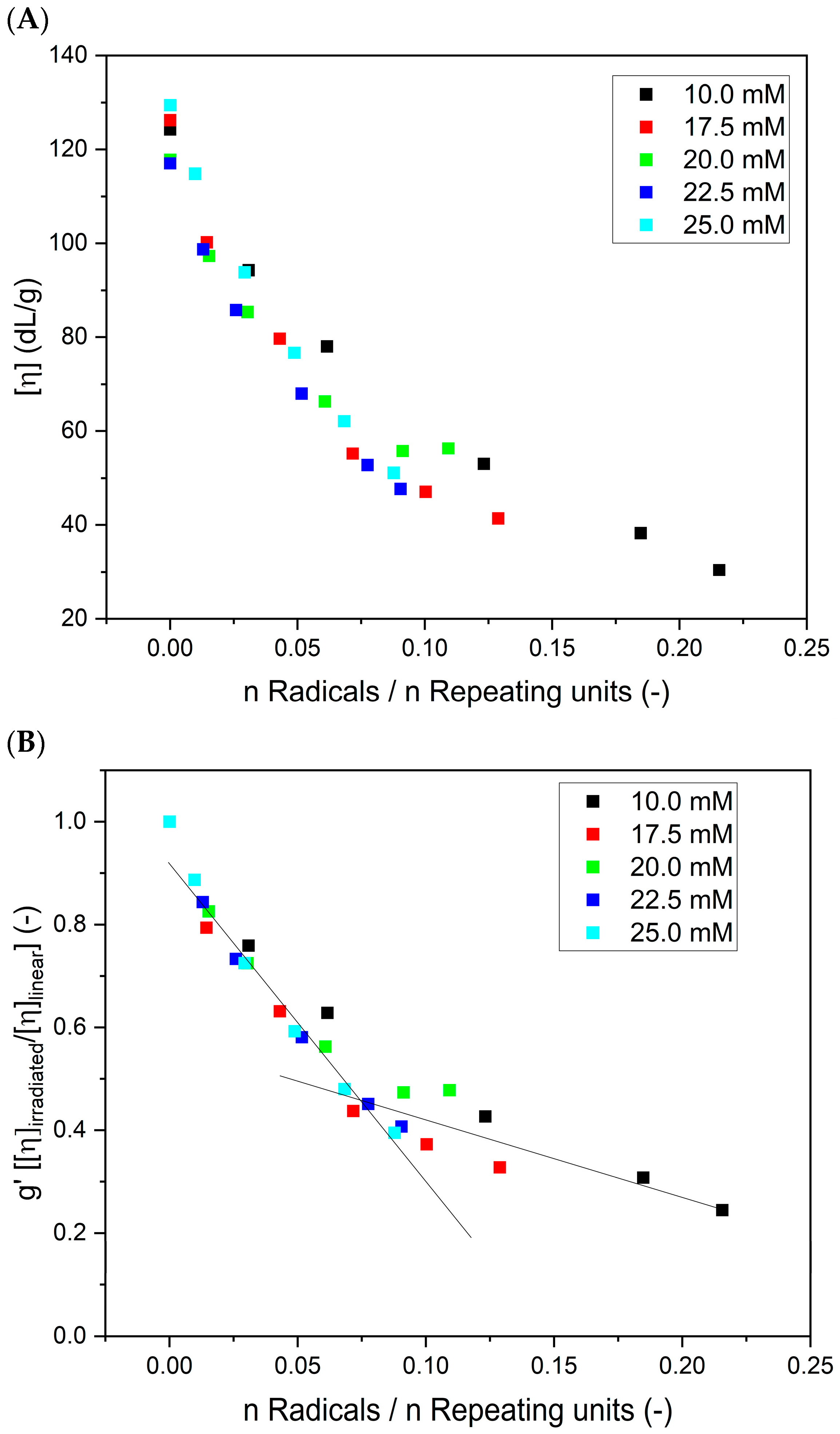
3. Results
- Insights into the structure of the product using the Mark–Houwink equation evaluation;
- Early detection of structure changes as a function of the irradiation by close monitoring the alpha parameter in the Mark–Houwink equation;
- Determination of whether soft nanogels or more tightly packed particles are formed;
- Indication of the processes (e.g., intermolecular crosslinking, looping and branching) that are involved in nanogel formation.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels … A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.M.; Moorjani, S.K.; Scranton, A.B. Methods for synthesis of hydrogel networks: A review. J. Macromol. Sci. Part C 1996, 36, 405–430. [Google Scholar] [CrossRef]
- Roy, A.; Manna, K.; Pal, S. Recent advances in various stimuli-responsive hydrogels: From synthetic designs to emerging healthcare applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.; He, J.; Kan, D.; Chen, K.; Lu, J. Synthesis of hydrogels and their progress in environmental remediation and antimicrobial application. Gels 2023, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, classification, properties and potential applications—A brief review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel trends in hydrogel development for biomedical applications: A review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Lopérgolo, L.C.; Lugão, A.B.; Catalani, L.H. Direct UV photocrosslinking of poly(N-vinyl-2-pyrrolidone) (PVP) to produce hydrogels. Polymer 2003, 44, 6217–6222. [Google Scholar] [CrossRef]
- Ding, H.; Li, B.; Jiang, Y.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. pH-responsive UV crosslinkable chitosan hydrogel via “thiol-ene” click chemistry for active modulating opposite drug release behaviors. Carbohydr. Polym. 2021, 251, 117101. [Google Scholar] [CrossRef] [PubMed]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.A.G.; Fechine, G.J.M.; Alcantara, M.R.; Catalani, L.H. Poly(N-vinyl-2-pyrrolidone) hydrogels produced by Fenton reaction. Polymer 2006, 47, 8414–8419. [Google Scholar] [CrossRef]
- Fechine, G.J.M.; Barros, J.A.G.; Catalani, L.H. Poly(N-vinyl-2-pyrrolidone) hydrogel production by ultraviolet radiation: New methodologies to accelerate crosslinking. Polymer 2004, 45, 4705–4709. [Google Scholar] [CrossRef]
- Revzin, A.; Russell, R.J.; Yadavalli, V.K.; Koh, W.-G.; Deister, C.; Hile, D.D.; Mellott, M.B.; Pishko, M. V Fabrication of poly(ethylene glycol) hydrogel microstructures using photolithography. Langmuir 2001, 17, 5440–5447. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Cuy, J.L.; Hauch, K.D.; Ratner, B.D. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials 2007, 28, 2978–2986. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S. Femtosecond laser nanofabrication of hydrogel biomaterial. MRS Bull. 2011, 36, 1028–1033. [Google Scholar] [CrossRef]
- Lekjinda, K.; Sunintaboon, P. Green synthesis of quaternized chitosan nanogel using emulsion-photopolymerization as redox-responsive drug carrier. Carbohydr. Polym. 2023, 304, 120495. [Google Scholar] [CrossRef]
- Naik, J.B.; Rajput, R.L.; Narkhede, J.S.; Mujumdar, A.; Patil, P.B. Synthesis and evaluation of UV cross-linked poly(acrylamide) loaded thymol nanogel for antifungal application in oral candidiasis. J. Polym. Res. 2021, 28, 15. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, G.; Ji, J. Preparation of reversibly photo-cross-linked nanogels from pH-responsive block copolymers and use as nanoreactors for the synthesis of gold nanoparticles. Eur. Polym. J. 2010, 46, 2120–2128. [Google Scholar] [CrossRef]
- Rosiak, J. Hydrogel dressings. In Radiation Effects on Polymers. ACS Symposium Series 475; Clough, R.C., Shalaby, S.W., Eds.; American Chemical Society: Washington, DC, USA, 1991; pp. 271–299. [Google Scholar] [CrossRef]
- Rosiak, J.M. Radiation formation of hydrogels for drug delivery. J. Control. Release 1994, 31, 9–19. [Google Scholar] [CrossRef]
- Rosiak, J.; Rucińska-Rybus, A.; Pękala, W. Method of Manufacturing Hydrogel Dressings. United States Patent 4871490, 3 October 1989. [Google Scholar]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amato, A.; San Biagio, P.L.; Mulè, F.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Adamo, G.; Grimaldi, N.; Campora, S.; Bulone, D.; Bondì, M.L.; Al-Sheikhly, M.; Sabatino, M.A.; Dispenza, C.; Ghersi, G. Multi-functional nanogels for tumor targeting and redox-sensitive drug and siRNA delivery. Molecules 2016, 21, 1594. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Sakr, T.M.; Rashed, H.M.; Hamed, A.A.; Abd El-Rehim, H.A. Polyethylene oxide–polyacrylic acid–folic acid (PEO-PAAc) nanogel as a 99mTc targeting receptor for cancer diagnostic imaging. J. Label. Compd. Radiopharm. 2021, 64, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R.; Narkhede, J.; Naik, J.B. Nanogels as nanocarriers for drug delivery: A review. Admet DMPK 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Kandil, R.; Merkel, O.M. Recent progress of polymeric nanogels for gene delivery. Curr. Opin. Colloid Interface Sci. 2019, 39, 11–23. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem.-Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; Sütekin, S.D.; Karacaoğlu, E.; Karahisar Turan, S.; İnci, Ö.G.; Güven, O.; Barsbay, M. Folic acid-modified biocompatible pullulan/poly(acrylic acid) nanogels for targeted delivery to MCF-7 cancer cells. Eur. J. Pharm. Biopharm. 2023, 184, 189–201. [Google Scholar] [CrossRef]
- Ges Naranjo, A.; Viltres Cobas, H.; Kumar Gupta, N.; Rodríguez López, K.; Martínez Peña, A.; Sacasas, D.; Álvarez Brito, R. 5-fluorouracil uptake and release from pH-responsive nanogels: An experimental and computational study. J. Mol. Liq. 2022, 362, 119716. [Google Scholar] [CrossRef]
- Sütekin, S.D.; Güven, O. Application of radiation for the synthesis of poly(n-vinyl pyrrolidone) nanogels with controlled sizes from aqueous solutions. Appl. Radiat. Isot. 2019, 145, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Rashed, E.R.; Abd El-Rehim, H.A.; El-Ghazaly, M.A. Potential efficacy of dopamine loaded-PVP/PAA nanogel in experimental models of Parkinsonism: Possible disease modifying activity. J. Biomed. Mater. Res.-Part A 2015, 103, 1713–1720. [Google Scholar] [CrossRef]
- An, J.-C.; Weaver, A.; Kim, B.; Barkatt, A.; Poster, D.; Vreeland, W.N.; Silverman, J.; Al-Sheikhly, M. Radiation-induced synthesis of poly(vinylpyrrolidone) nanogel. Polymer 2011, 52, 5746–5755. [Google Scholar] [CrossRef]
- Radwan, R.R.; Ali, H.E. Radiation-synthesis of chitosan/poly (acrylic acid) nanogel for improving the antitumor potential of rutin in hepatocellular carcinoma. Drug Deliv. Transl. Res. 2021, 11, 261–278. [Google Scholar] [CrossRef]
- Elliott, J.E.; MacDonald, M.; Nie, J.; Bowman, C.N. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Buxton, G.V. Radiation chemistry of the liquid state. Water and homogeneous aqueous solutions. In Radiation Chemistry: Principles and Applications; Farhataziz, I., Rodgers, M.A.J., Eds.; Verlag Chemie: Weinheim, Germany, 1987; pp. 321–349. [Google Scholar]
- Buxton, G. An overview of the radiation chemistry of liquids. In Radiation Chemistry: From Basics to Applications in Material and Life Sciences; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis, France, 2008; pp. 3–16. [Google Scholar]
- Coqueret, X.; Sabharwal, S.; Khairul Zaman, H.M.D.; Czechowska-Biskup, R.; Wach, R.A.; Rosiak, J.M.; Ulanski, P.; Gulrez, S.K.H.; Al-Assaf, S. Introduction to the Radiation Chemistry of Polymers Chapter 3. In The Radiation Chemistry of Polysaccharides; Al-Assaf, S., Coqueret, X., Khairul Zaman, H.M.D., Sen, M., Ulanski, P., Eds.; International Atomic Energy Agency: Vienna, Austria, 2016. [Google Scholar]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization reactions and modifications of polymers by ionizing radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Görlich, W.; Schnabel, W. Untersuchungen über den Einfluß der Ladungsdichte auf die gegenseitige Desaktivierung von Polyion-Makroradikalen. Makromol. Chem. 1973, 164, 225–235. [Google Scholar] [CrossRef]
- Ulański, P.; Janik, I.; Rosiak, J.M. Radiation formation of polymeric nanogels. Radiat. Phys. Chem. 1998, 52, 289–294. [Google Scholar] [CrossRef]
- Ulanski, P.; Rosiak, J.M. The use of radiation technique in the synthesis of polymeric nanogels. Nucl. Instruments Methods Phys. Res. Sect. B 1999, 151, 356–360. [Google Scholar] [CrossRef]
- Kadlubowski, S.; Grobelny, J.; Olejniczak, W.; Cichomski, M.; Ulanski, P. Pulses of fast electrons as a tool to synthesize poly(acrylic acid) nanogels. Intramolecular cross-linking of linear polymer chains in additive-free aqueous solution. Macromolecules 2003, 36, 2484–2492. [Google Scholar] [CrossRef]
- Matusiak, M.; Kadlubowski, S.; Ulanski, P. Radiation-induced synthesis of poly(acrylic acid) nanogels. Radiat. Phys. Chem. 2018, 142, 125–129. [Google Scholar] [CrossRef]
- Todaro, S.; Sabatino, M.A.; Walo, M.; Mangione, M.R.; Bulone, D.; Dispenza, C. Influence of gamma-irradiation on thermally-induced mesoscopic gelation of degalactosylated xyloglucans. Radiat. Phys. Chem. 2014, 94, 245–248. [Google Scholar] [CrossRef]
- Zheng, Y.; Thurecht, K.J.; Chen, X.; Roberts, C.J.; Irvine, D.J.; Howdle, S.M.; Wang, W. In situ formation of crosslinked core-corona polymeric nanoparticles from a novel hyperbranched core. Polym. Chem. 2012, 3, 2807–2814. [Google Scholar] [CrossRef]
- Mandal, P.; Banerjee, S.L.; Bhattacharya, K.; Singha, N.K. A superparamagnetic metallopolymer using tailor-made poly [2-(acetoacetoxy)ethyl methacrylate] bearing pendant β-keto ester functionality. Eur. Polym. J. 2018, 103, 31–39. [Google Scholar] [CrossRef]
- Amamoto, Y.; Kikuchi, M.; Masunaga, H.; Sasaki, S.; Otsuka, H.; Takahara, A. Intelligent build-up of complementarily reactive diblock copolymers via dynamic covalent exchange toward symmetrical and miktoarm star-like nanogels. Macromolecules 2010, 43, 1785–1791. [Google Scholar] [CrossRef]
- Mourey, T.H.; Leon, J.W.; Bennett, J.R.; Bryan, T.G.; Slater, L.A.; Balke, S.T. Characterizing property distributions of polymeric nanogels by size-exclusion chromatography. J. Chromatogr. A 2007, 1146, 51–60. [Google Scholar] [CrossRef]
- Min, K.; Gao, H.; Yoon, J.A.; Wu, W.; Kowalewski, T.; Matyjaszewski, K. One-pot synthesis of hairy nanoparticles by emulsion ATRP. Macromolecules 2009, 42, 1597–1603. [Google Scholar] [CrossRef]
- Inoue, S.; Miwa, Y.; Aota, H.; Matsumoto, A.; Yokoyama, K.; Matoba, Y.; Shibano, M. Molecular design of core-shell type allylic nanoparticles based on crosslinking multiallyl/multivinyl copolymerizations. Macromol. Symp. 2011, 306–307, 99–106. [Google Scholar] [CrossRef]
- Burchard, W. Solution properties of branched macromolecules. Adv. Polym. Sci. 1999, 143, 114–194. [Google Scholar] [CrossRef]
- Schmidt, T.; Janik, I.; Kadłubowski, S.; Ulański, P.; Rosiak, J.M.; Reichelt, R.; Arndt, K.-F. Pulsed electron beam irradiation of dilute aqueous poly(vinyl methyl ether) solutions. Polymer 2005, 46, 9908–9918. [Google Scholar] [CrossRef]
- Lacík, I.; Stach, M.; Kasák, P.; Semak, V.; Uhelská, L.; Chovancová, A.; Reinhold, G.; Kilz, P.; Delaittre, G.; Charleux, B.; et al. SEC analysis of poly(acrylic acid) and poly(methacrylic acid). Macromol. Chem. Phys. 2015, 216, 23–37. [Google Scholar] [CrossRef]
- Duan, Q.-Y.; Zhu, Y.-X.; Jia, H.-R.; Wang, S.-H.; Wu, F.-G. Nanogels: Synthesis, properties, and recent biomedical applications. Prog. Mater. Sci. 2023, 139, 101167. [Google Scholar] [CrossRef]
- Suzuki, D. Nanogel/microgel science and beyond. Langmuir 2023, 39, 7525–7529. [Google Scholar] [CrossRef]
- Bhaladhare, S.; Bhattacharjee, S. Chemical, physical, and biological stimuli-responsive nanogels for biomedical applications (mechanisms, concepts, and advancements): A review. Int. J. Biol. Macromol. 2023, 226, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, F.-D.; Botezat, D.; Gardikiotis, I.; Uritu, C.-M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.-I.; Mihai, C.-T. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef]
- Funke, W.; Okay, O.; Joos-Müller, B. Microgels-intramolecularly crosslinked macromolecules with a globular structure. In Microencapsulation Microgels Iniferters; DiMari, S., Funke, W., Haralson, M.A., Hunkeler, D., Joos-Müller, B., Matsumoto, A., Okay, O., Otsu, T., Powers, A.C., Prokop, A., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 139–234. [Google Scholar]
- Saunders, B.R.; Vincent, B. Microgel particles as model colloids: Theory, properties and applications. Adv. Colloid Interface Sci. 1999, 80, 1–25. [Google Scholar] [CrossRef]
- Picone, P.; Ditta, L.A.; Sabatino, M.A.; Militello, V.; San Biagio, P.L.; Di Giacinto, M.L.; Cristaldi, L.; Nuzzo, D.; Dispenza, C.; Giacomazza, D.; et al. Ionizing radiation-engineered nanogels as insulin nanocarriers for the development of a new strategy for the treatment of Alzheimer’s disease. Biomaterials 2016, 80, 179–194. [Google Scholar] [CrossRef]
- Teraoka, I. Polymer Solutions: An Introduction to Physical Properties; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Kadlubowski, S. Radiation-induced synthesis of nanogels based on poly(N-vinyl-2-pyrrolidone)—A review. Radiat. Phys. Chem. 2014, 102, 29–39. [Google Scholar] [CrossRef]
- Brasch, U.; Burchard, W. Preparation and solution properties of microhydrogels from poly(vinyl alcohol). Macromol. Chem. Phys. 1996, 197, 223–235. [Google Scholar] [CrossRef]
- Jeszka, J.K.; Kadlubowski, S.; Ulanski, P. Monte Carlo simulations of nanogels formation by intramolecular recombination of radicals on polymer chain. Dispersive kinetics controlled by chain dynamics. Macromolecules 2006, 39, 857–870. [Google Scholar] [CrossRef]
- Touloupidis, V.; Albrecht, A. Development of a Monte Carlo random walk model for the prediction of linear and branched polymer configuration and connection to radius of gyration and GPC-MALS experimental results. Macromol. React. Eng. 2022, 16, 2200002. [Google Scholar] [CrossRef]
- Gaborieau, M.; Castignolles, P. Size-exclusion chromatography (SEC) of branched polymers and polysaccharides. Anal. Bioanal. Chem. 2011, 399, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; DesLauriers, P.J.; Rohlfing, D.C. SEC-MALS method for the determination of long-chain branching and long-chain branching distribution in polyethylene. Polymer 2005, 46, 5165–5182. [Google Scholar] [CrossRef]
- Podzimek, S. The use of GPC coupled with a multiangle laser light scattering photometer for the characterization of polymers. On the determination of molecular weight, size and branching. J. Appl. Polym. Sci. 1994, 54, 91–103. [Google Scholar] [CrossRef]
- Laidler, K.J. A glossary of terms used in chemical kinetics, including reaction dynamics (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 149–192. [Google Scholar] [CrossRef]


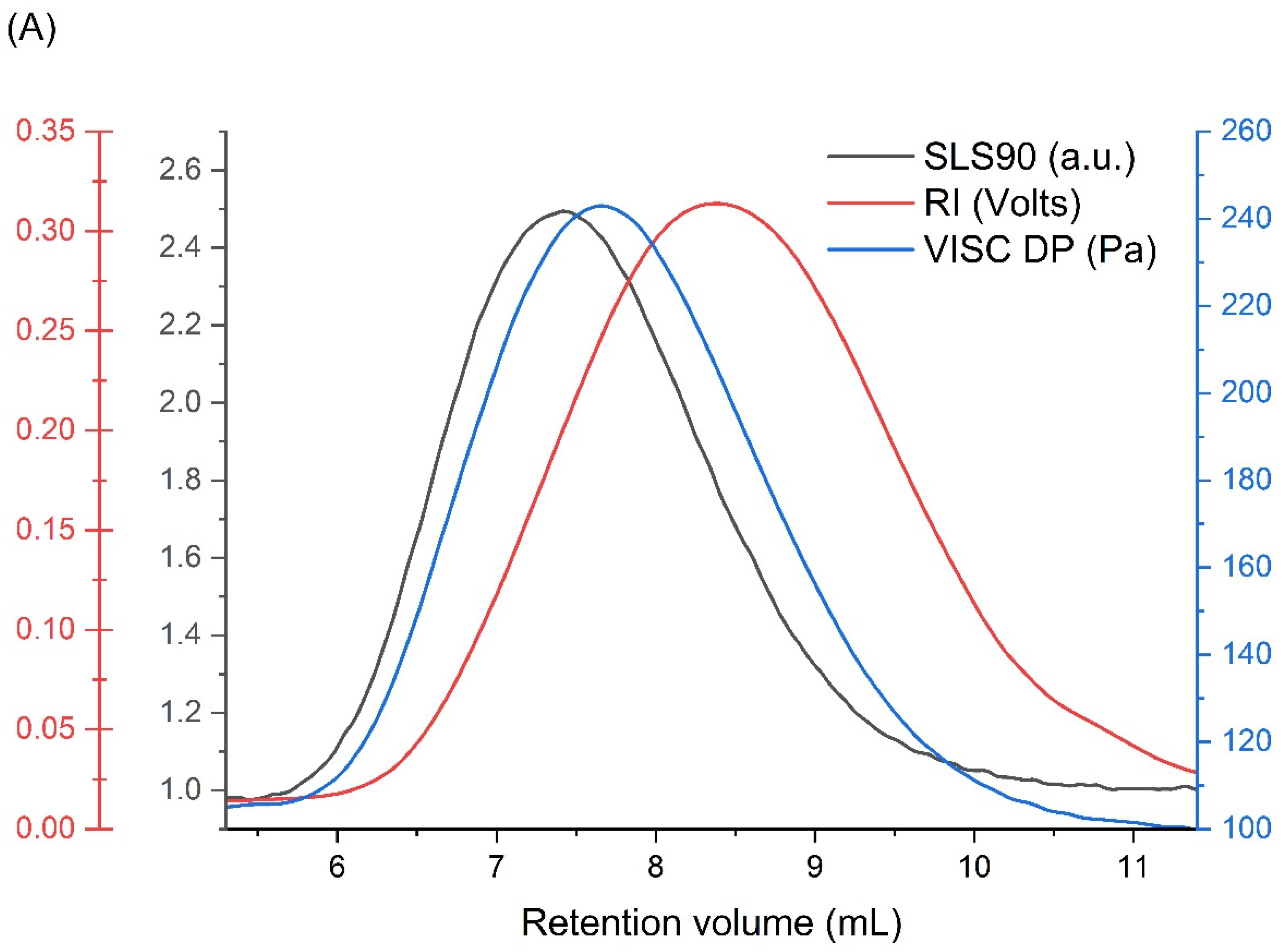
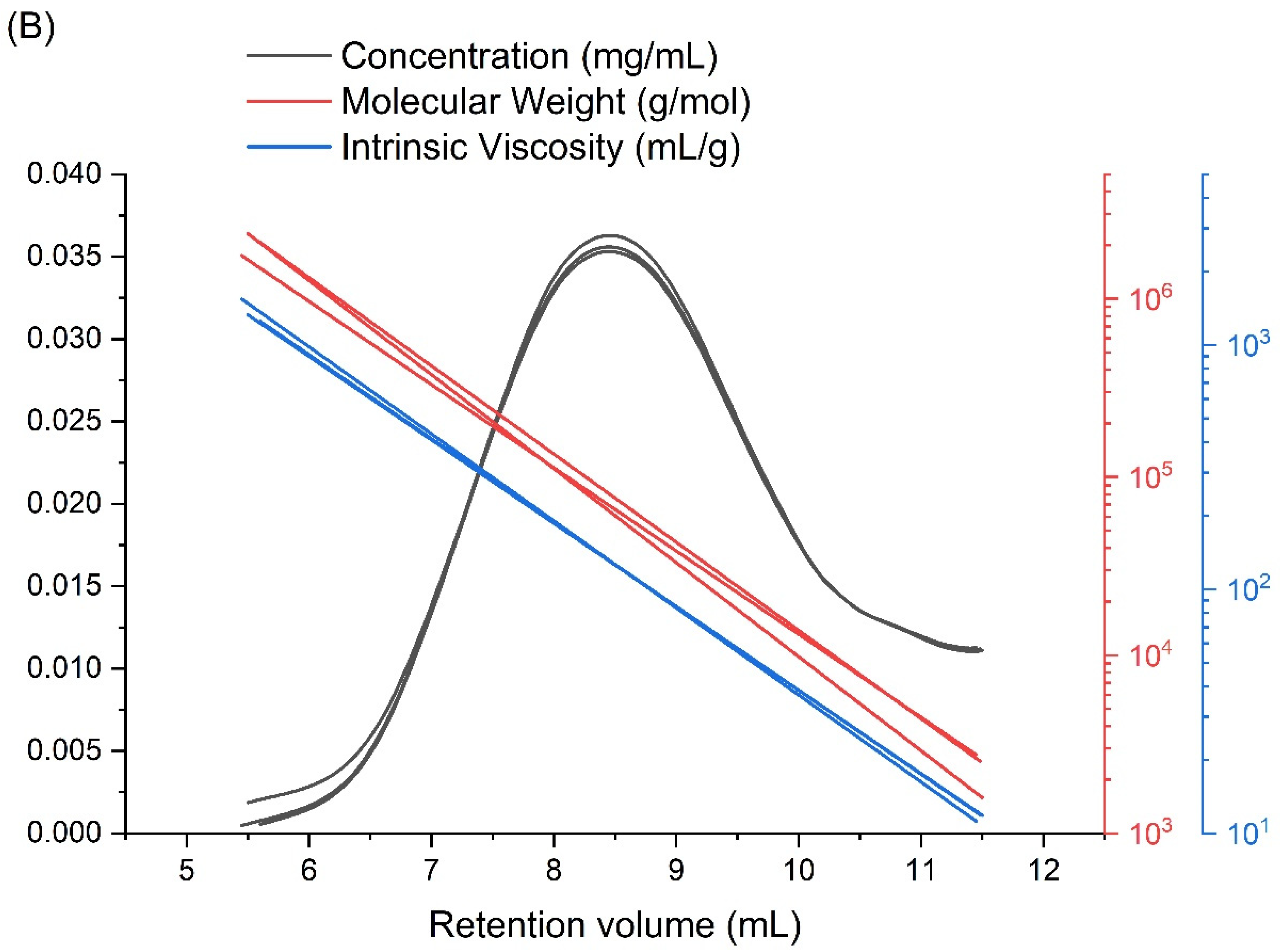
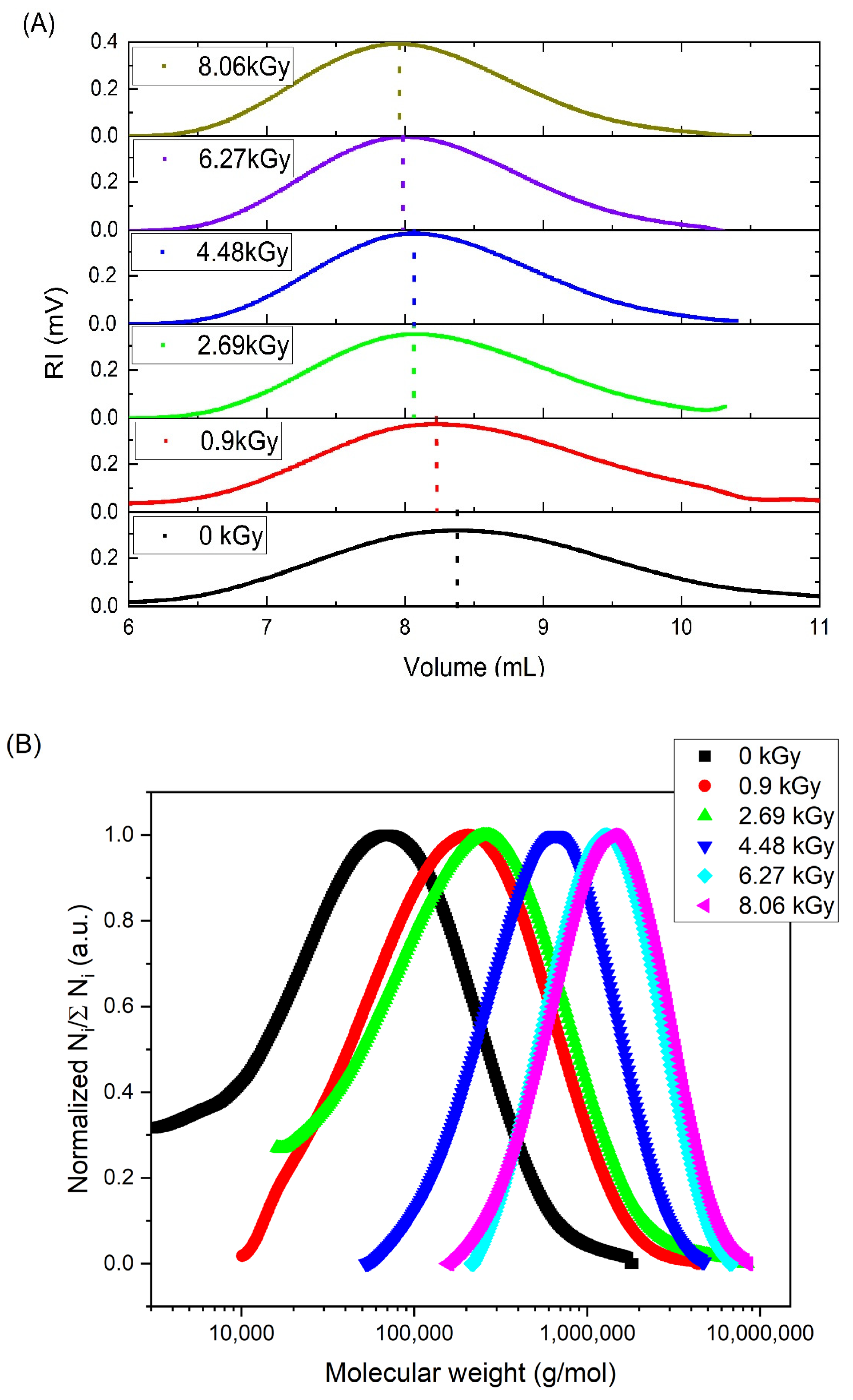
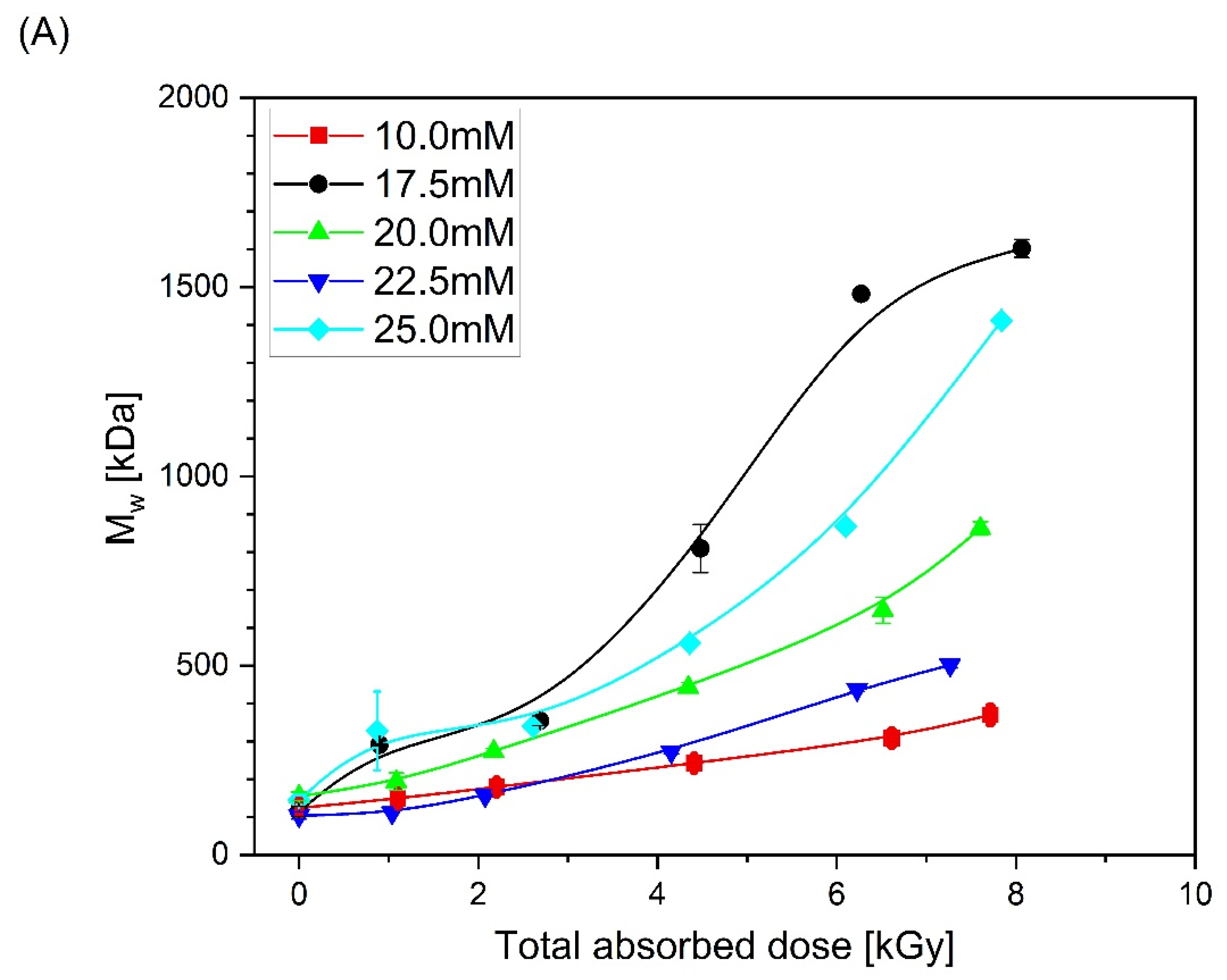
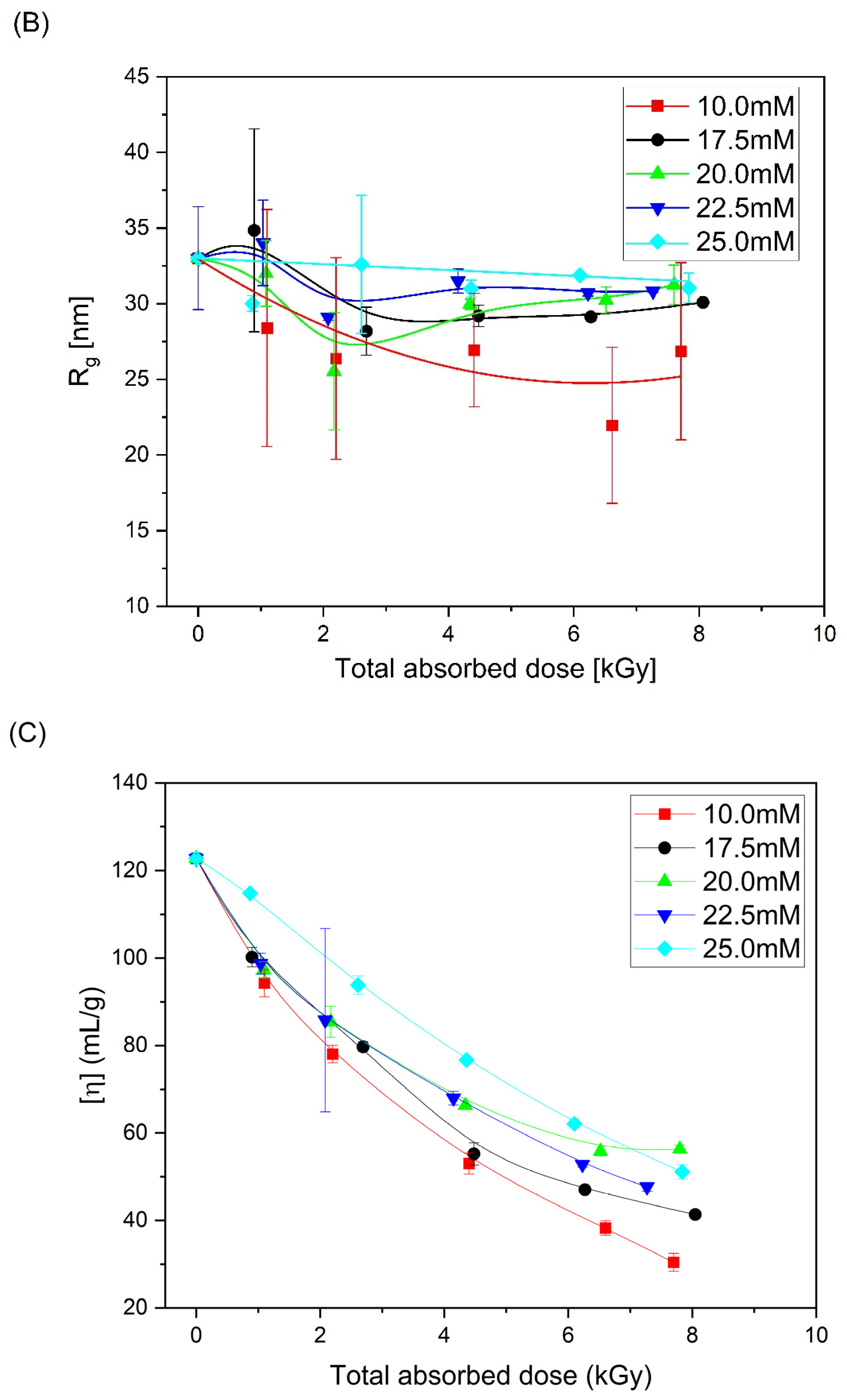
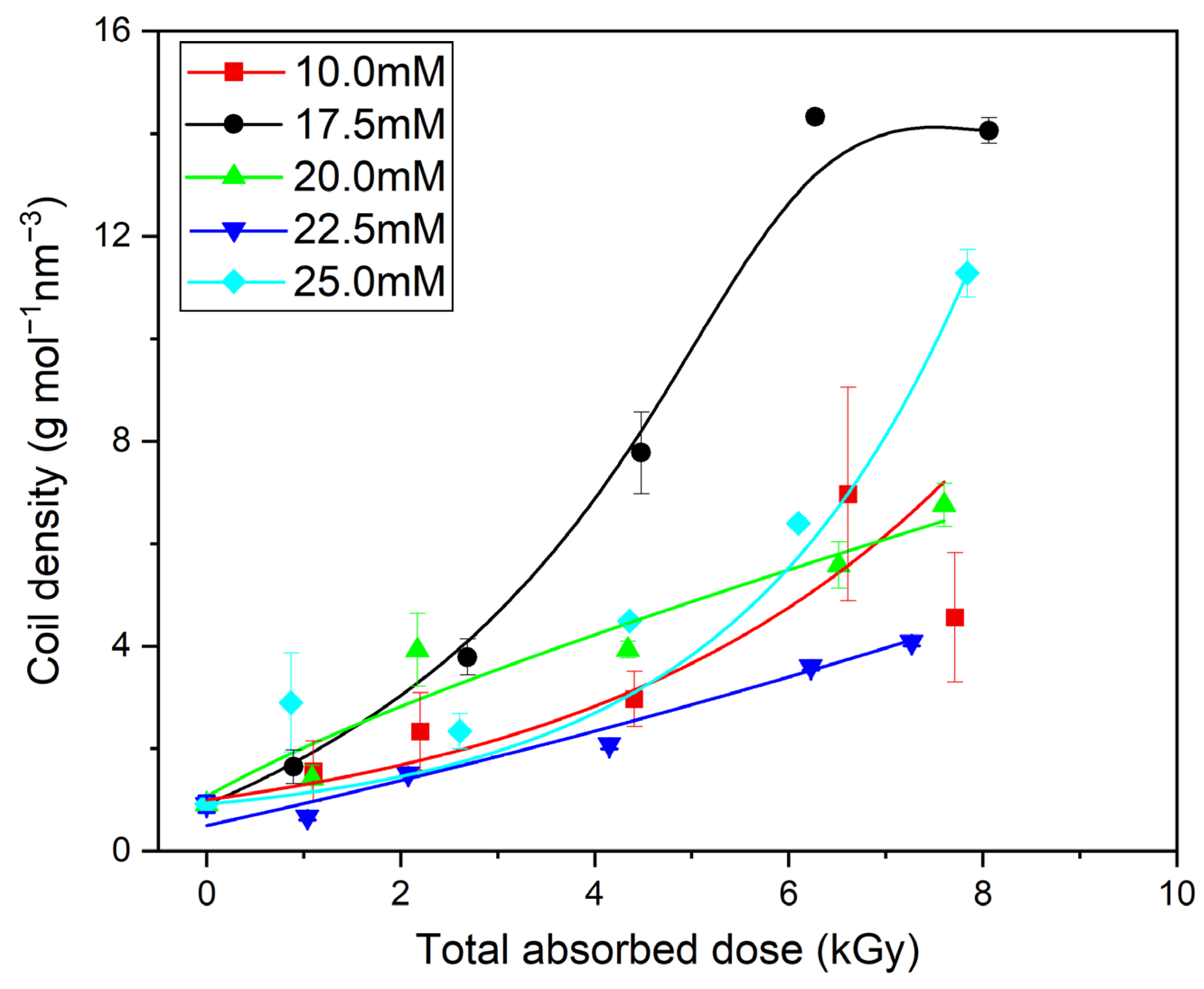
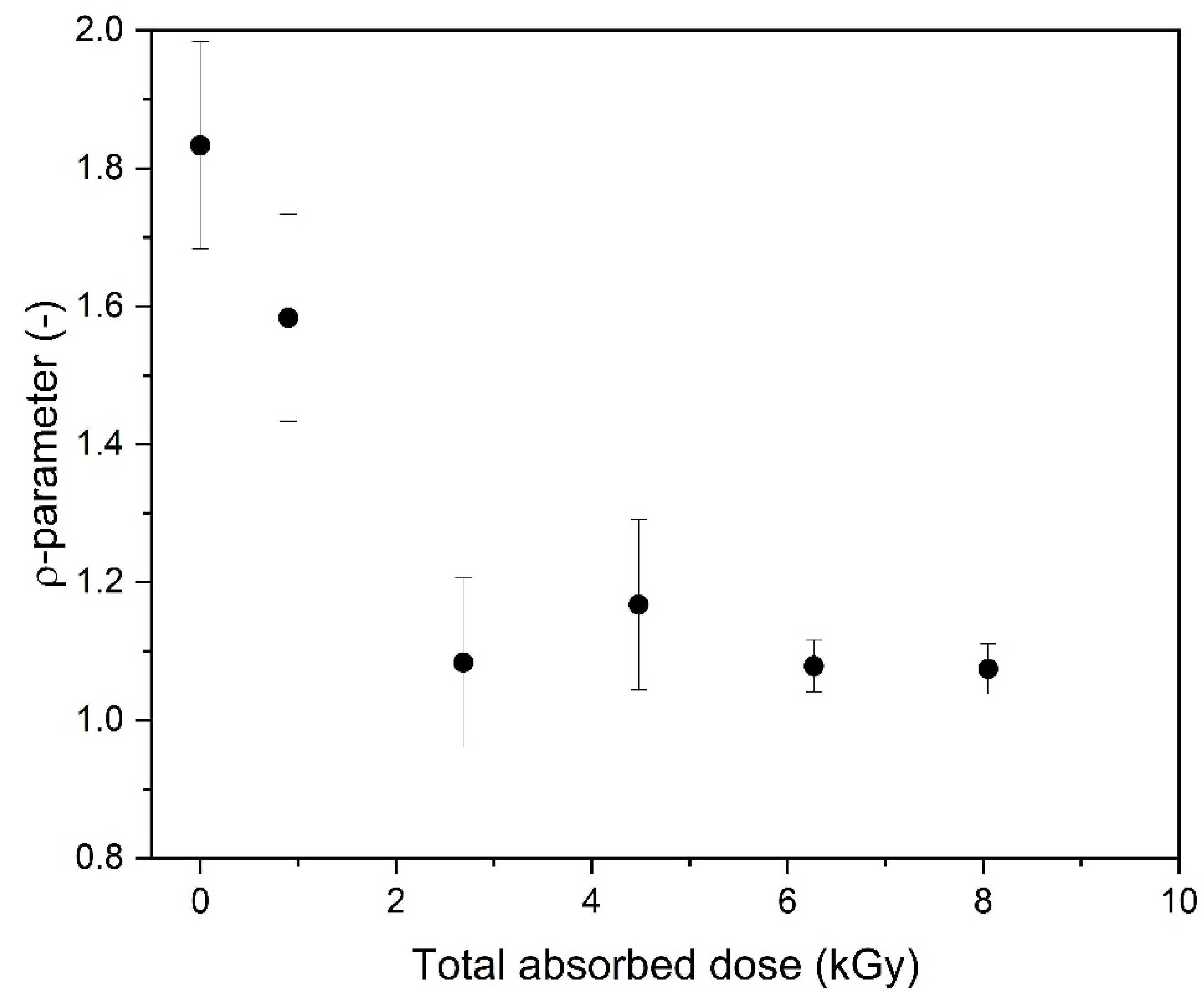
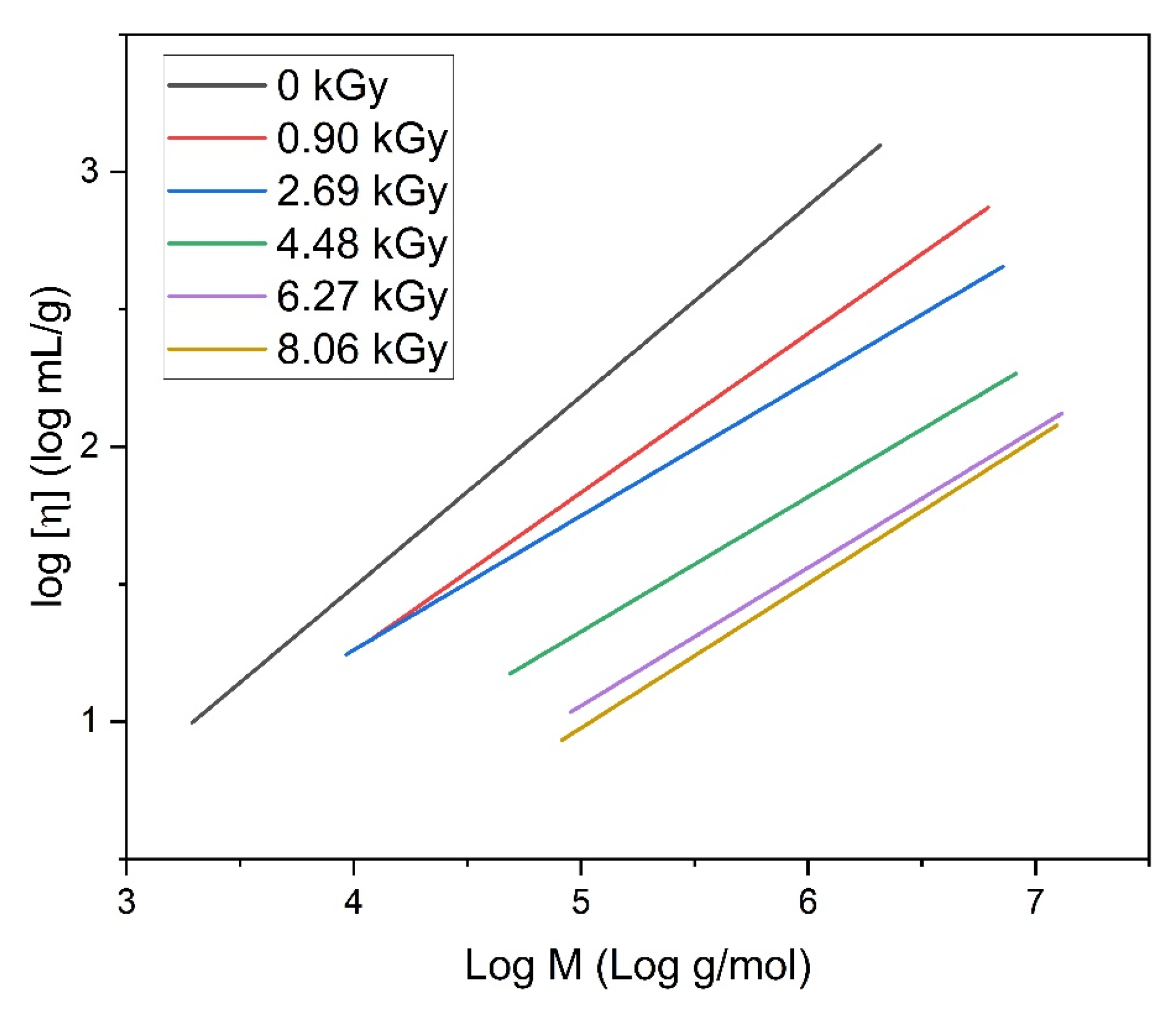

| Mw (kDa) | Mn (kDa) | Mw/Mn | Rg (nm) | [η] (mL/g) |
|---|---|---|---|---|
| 128 ± 21 | 42 ± 17 | 3.0 | 33 ± 4 | 123 ± 5 |
| 10.0 mM | 17.5 mM | 20.0 mM | 22.5 mM | 25.0 mM | |
|---|---|---|---|---|---|
| 1 cycle (ca. 1 kGy) | 0.57 | 0.60 | 0.71 | 0.64 | 0.56 |
| 2 cycles (ca. 2 kGy) | 0.59 | 0.53 | 0.53 | 0.57 | 0.63 |
| 3 cycles (ca. 4 kGy) | 0.54 | 0.52 | 0.41 | 0.66 | 0.61 |
| 4 cycles (ca. 6 kGy) | 0.45 | 0.50 | 0.44 | 0.61 | 0.61 |
| 5 cycles (ca. 8 kGy) | 0.43 | 0.52 | 0.36 | 0.60 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadłubowski, S.; Rurarz, B.P.; Raczkowska, J.; Dessy, C.; Ulański, P. “To Be or Not to Be” of a Polymer Nanogel—Unravelling the Relationship of Product Properties vs. Synthesis Conditions Governing the Radiation Crosslinking of Poly(acrylic acid) Using GPC/SEC—MALLS. Materials 2023, 16, 7467. https://doi.org/10.3390/ma16237467
Kadłubowski S, Rurarz BP, Raczkowska J, Dessy C, Ulański P. “To Be or Not to Be” of a Polymer Nanogel—Unravelling the Relationship of Product Properties vs. Synthesis Conditions Governing the Radiation Crosslinking of Poly(acrylic acid) Using GPC/SEC—MALLS. Materials. 2023; 16(23):7467. https://doi.org/10.3390/ma16237467
Chicago/Turabian StyleKadłubowski, Sławomir, Beata Paulina Rurarz, Joanna Raczkowska, Carlo Dessy, and Piotr Ulański. 2023. "“To Be or Not to Be” of a Polymer Nanogel—Unravelling the Relationship of Product Properties vs. Synthesis Conditions Governing the Radiation Crosslinking of Poly(acrylic acid) Using GPC/SEC—MALLS" Materials 16, no. 23: 7467. https://doi.org/10.3390/ma16237467
APA StyleKadłubowski, S., Rurarz, B. P., Raczkowska, J., Dessy, C., & Ulański, P. (2023). “To Be or Not to Be” of a Polymer Nanogel—Unravelling the Relationship of Product Properties vs. Synthesis Conditions Governing the Radiation Crosslinking of Poly(acrylic acid) Using GPC/SEC—MALLS. Materials, 16(23), 7467. https://doi.org/10.3390/ma16237467








