The Influence of Adhesive Strategy, Type of Dental Composite, and Polishing Time on Marginal Gap Formation in Class I-like Cavities
Abstract
1. Introduction
2. Materials and Methods
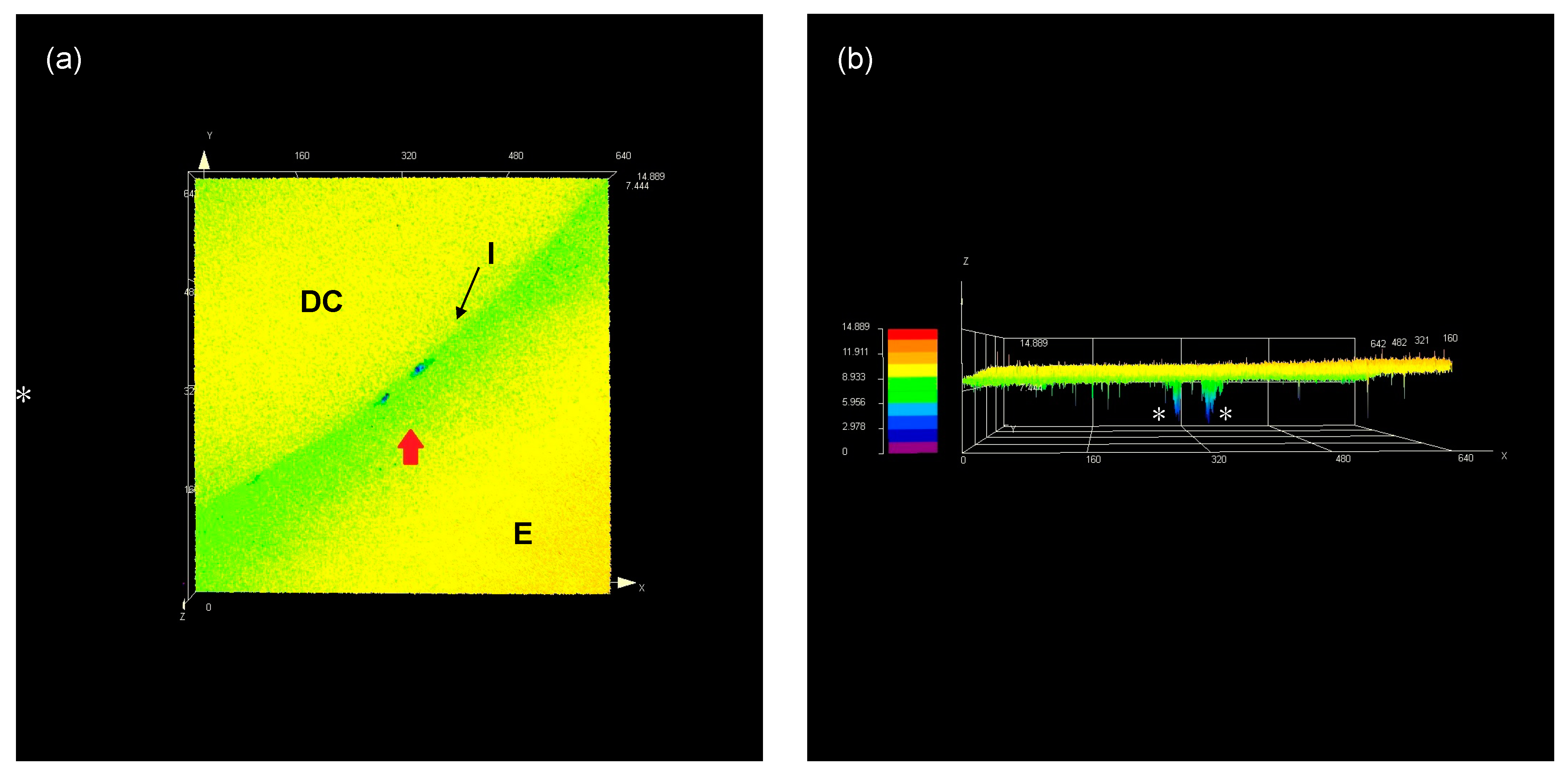
2.1. Percentage of Marginal Gap (%MG)
2.2. Flexural Modulus (FM)
2.3. Degree of Conversion (DC%)
2.4. Statistical Analysis
3. Results
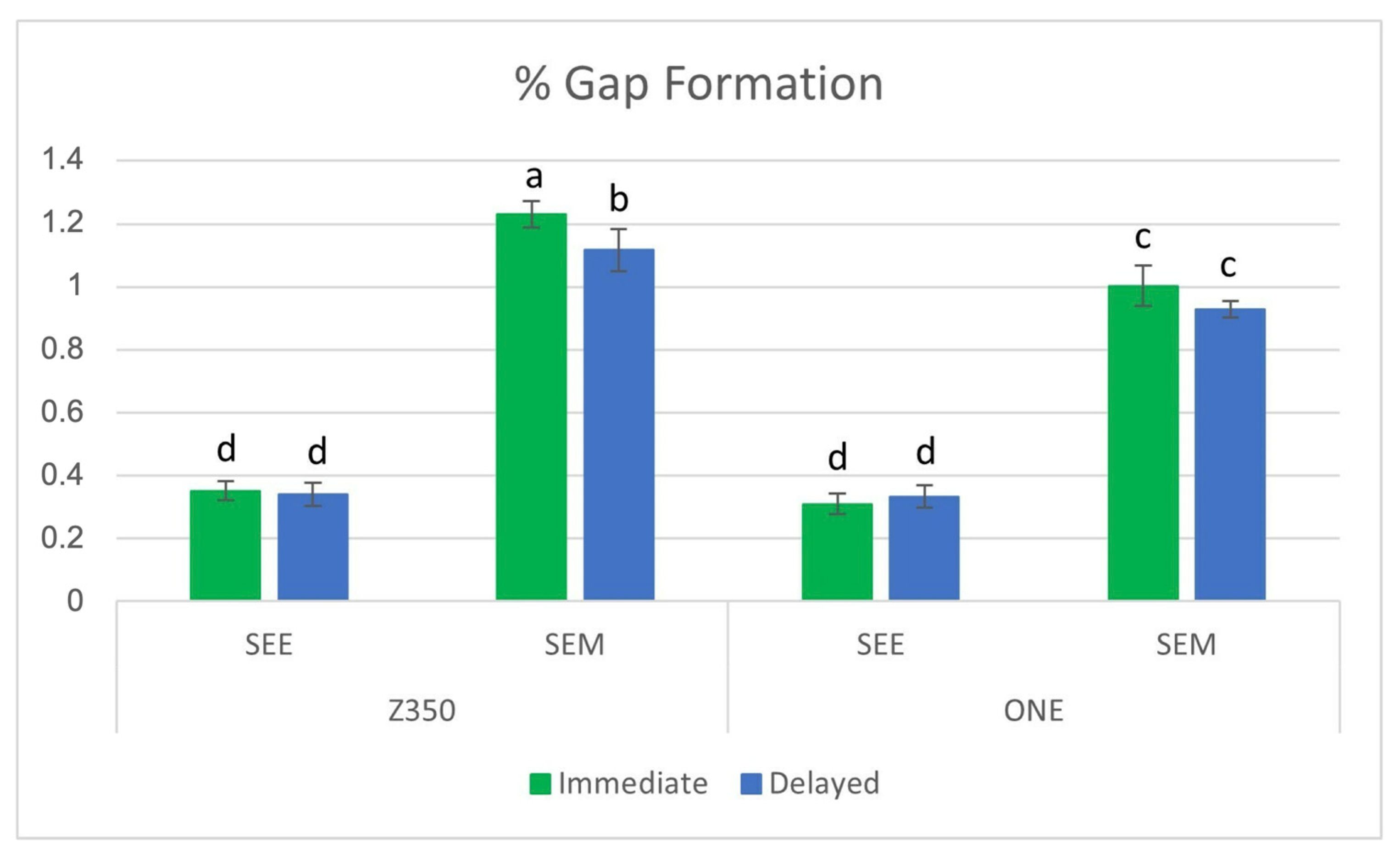
3.1. Percentage of Marginal Gap (%MG)
3.2. Flexural Modulus (FM)
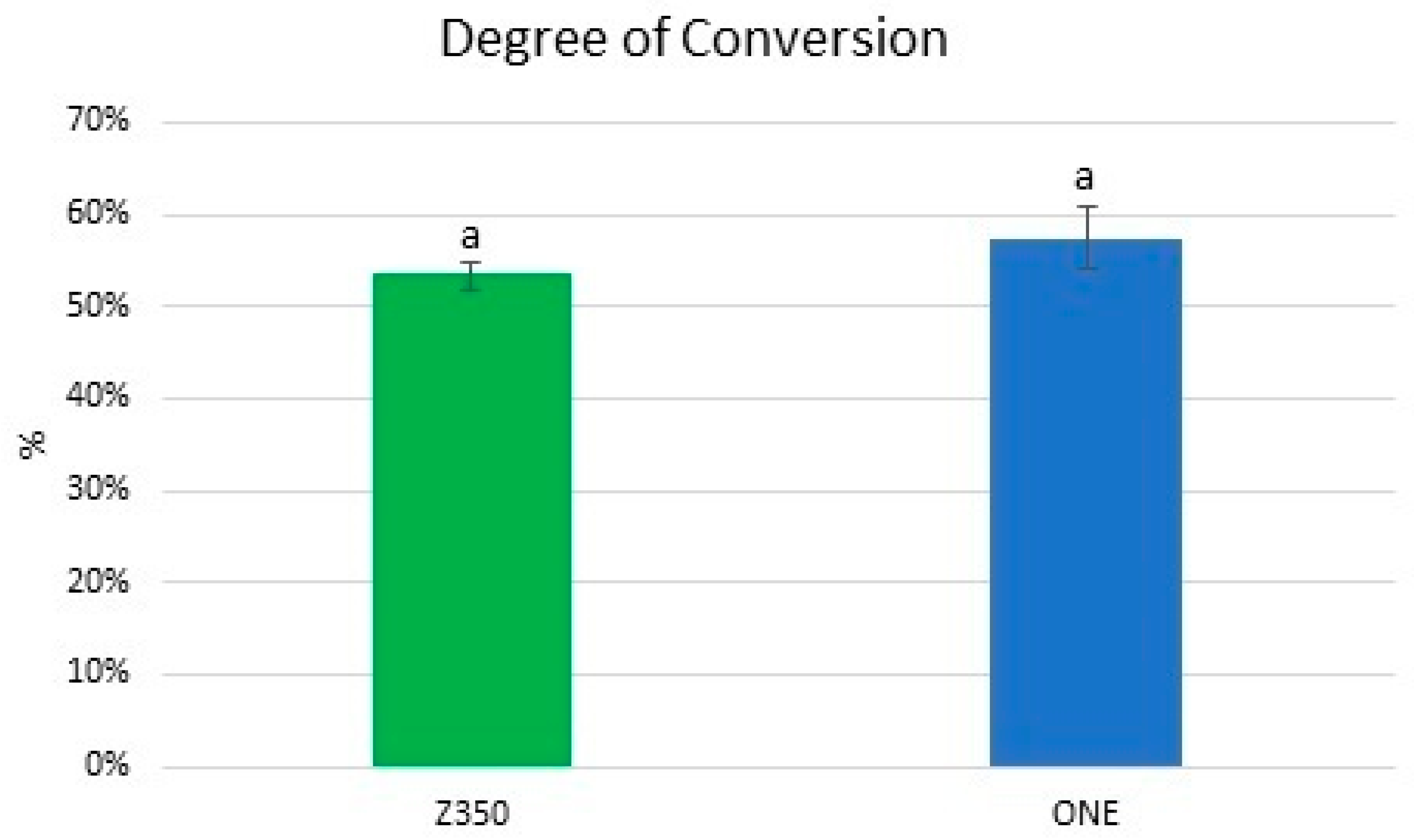
3.3. Degree of Conversion (DC%)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso, R.C.B.; Maria Correr, G.M.; Cunha, L.G.; Borges, A.F.S.; Puppin-Rontani, R.M.; Sinhoreti, M.A.C. Dye staining gap test: An alternative method for assessing marginal gap formation in composite restorations. Acta Odontol. Scand. 2006, 64, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.J.; Faria, E.S.A.L.; Rodrigues, M.P.; Vilela, A.B.F.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization shrinkage stress of composite resins and resin cements—What do we need to know? Braz. Oral. Res. 2017, 31, e62. [Google Scholar] [CrossRef] [PubMed]
- Burrer, P.; Par, M.; Fürer, L.; Stübi, M.; Marovic, D.; Tarle, Z.; Attin, T.; Tauböck, T.T. Effect of polymerization mode on shrinkage kinetics and degree of conversion of dual-curing bulk-fill resin composites. Clin. Oral. Investig. 2023, 27, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Gregor, L.; Bortolotto, T.; Feilzer, A.J.; Krejci, I. Shrinkage kinetics of a methacrylate- and a silorane-based resin composite: Effect on marginal integrity. J. Adhes. Dent. 2013, 15, 245–250. [Google Scholar] [CrossRef]
- Tseng, P.C.; Chuang, S.F.; Kaisarly, D.; Kunzelmann, K.H. Simulating the shrinkage-induced interfacial damage around Class I composite resin restorations with damage mechanics. Dent. Mater. 2023, 39, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Cenci, M.S.; Montagner, A.F.; de Lima, V.P.; Correa, M.B.; Moraes, R.R.; Opdam, N.J.M. Longevity of composite restorations is definitely not only about materials. Dent. Mater. 2023, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moreira da Silva, E.; dos Santos, G.O.; Guimarães, J.G.; Barcellos Ade, A.; Sampaio, E.M. The influence of C-factor, flexural modulus and viscous flow on gap formation in resin composite restorations. Oper. Dent. 2007, 32, 356–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, H.Y.; Manhart, J.; Hickel, R.; Kunzelmann, K.H. Polymerization contraction stress in light-cured packable composite resins. Dent. Mater. 2001, 17, 253–259. [Google Scholar] [CrossRef]
- Goncalves, F.; Azevedo, C.L.; Ferracane, J.L.; Braga, R.R. BisGMA/TEGDMA ratio and filler content effects on shrinkage stress. Dent. Mater. 2011, 27, 520–526. [Google Scholar] [CrossRef]
- Van Ende, A.; De Munck, J.; Lise, D.P.; Van Meerbeek, B. Bulk-Fill Composites: A Review of the Current Literature. J. Adhes. Dent. 2017, 19, 95–109. [Google Scholar] [CrossRef]
- Boaro, L.C.; Fróes-Salgado, N.R.; Gajewski, V.E.; Bicalho, A.A.; Valdivia, A.D.; Soares, C.J.; Miranda Júnior, W.G.; Braga, R.R. Correlation between polymerization stress and interfacial integrity of composites restorations assessed by different in vitro tests. Dent. Mater. 2014, 30, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Alex, G. Universal adhesives: The next evolution in adhesive dentistry? Compend. Contin. Educ. Dent. 2015, 36, 15–26, quiz 28, 40. [Google Scholar] [PubMed]
- Doshi, K.; Nivedhitha, M.S.; Solete, P.; S, D.P.A.; Balasubramaniam, A.; Jacob, B.; Siddique, R. Effect of adhesive strategy of universal adhesives in noncarious cervical lesions—An updated systematic review and meta-analysis. BDJ Open 2023, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Assis, P.; Silva, C.; Nascimento, A.; Anníbal, H.; Júnior, S.; Soares, N.; Junior, R.; Braz, R. Does Acid Etching Influence the Adhesion of Universal Adhesive Systems in Noncarious Cervical Lesions? A Systematic Review and Meta-analysis. Oper. Dent. 2023, 48, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Bernales Sender, F.R.; Castañeda Vía, J.A.; Tay, L.Y. Influence of different phosphoric acids before application of universal adhesive on the dental enamel. J. Esthet. Restor. Dent. 2020, 32, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Haak, R.; Werner, M.S.; Schneider, H.; Häfer, M.; Schulz-Kornas, E. Clinical Outcomes and Quantitative Margin Analysis of a Universal Adhesive Using a Randomized Clinical Trial over Three Years. J. Clin. Med. 2022, 11, 6910. [Google Scholar] [CrossRef]
- Ma, K.S.; Wang, L.T.; Blatz, M.B. Efficacy of adhesive strategies for restorative dentistry: A systematic review and network meta-analysis of double-blind randomized controlled trials over 12 months of follow-up. J. Prosthodont. Res. 2023, 67, 35–44. [Google Scholar] [CrossRef]
- Irie, M.; Tjandrawinata, R.; Suzuki, K. Effect of delayed polishing periods on interfacial gap formation of Class V restorations. Oper. Dent. 2003, 28, 552–559. [Google Scholar]
- Yap, A.U.; Ang, H.Q.; Chong, K.C. Influence of finishing time on marginal sealing ability of new generation composite bonding systems. J. Oral. Rehabil. 1998, 25, 871–876. [Google Scholar] [CrossRef]
- Lopes, G.C.; Franke, M.; Maia, H.P. Effect of finishing time and techniques on marginal sealing ability of two composite restorative materials. J. Prosthet. Dent. 2002, 88, 32–36. [Google Scholar] [CrossRef]
- Souza-Junior, E.J.; de Souza-Régis, M.R.; Alonso, R.C.; de Freitas, A.P.; Sinhoreti, M.A.; Cunha, L.G. Effect of the curing method and composite volume on marginal and internal adaptation of composite restoratives. Oper. Dent. 2011, 36, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Maruo, Y.; Nishigawa, G. Performance of Class I composite restorations when polished immediately or after one-day water storage. PLoS ONE 2017, 12, e0183381. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, T.A.; Sadr, A.; Shimada, Y.; Tagami, J.; Sumi, Y. Non-invasive quantification of resin-dentin interfacial gaps using optical coherence tomography: Validation against confocal microscopy. Dent. Mater. 2011, 27, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, R.; Krämer, N.; Lohbauer, U.; Nikolaenko, S.A.; Reich, S.M. Marginal integrity: Is the clinical performance of bonded restorations predictable in vitro? J. Adhes. Dent. 2007, 9 (Suppl. S1), 107–116. [Google Scholar] [PubMed]
- Peutzfeldt, A.; Asmussen, E. Determinants of in vitro gap formation of resin composites. J. Dent. 2004, 32, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Reis, A.; Ballester, R.Y. Polymerization shrinkage: Effects of constraint and filling technique in composite restorations. Dent. Mater. 2004, 20, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Putignano, A.; Tosco, V.; Monterubbianesi, R.; Vitiello, F.; Gatto, M.L.; Furlani, M.; Giuliani, A.; Orsini, G. Comparison of three different bulk-filling techniques for restoring class II cavities: μCT, SEM-EDS combined analyses for margins and internal fit assessments. J. Mech. Behav. Biomed. Mater. 2021, 124, 104812. [Google Scholar] [CrossRef] [PubMed]
- Scepanovic, D.; Par, M.; Attin, T.; Tauböck, T.T. Marginal Adaptation of Flowable vs Sonically Activated or Preheated Resin Composites in Cervical Lesions. J. Adhes. Dent. 2022, 24, 247–257. [Google Scholar] [CrossRef]
- Heintze, S.D.; Forjanic, M.; Jakob, G. Automatic gap detection at restoration margins with an optical sensor in vitro. J. Adhes. Dent. 2005, 7, 95–105. [Google Scholar]
- Tosco, V.; Vitiello, F.; Furlani, M.; Gatto, M.L.; Monterubbianesi, R.; Giuliani, A.; Orsini, G.; Putignano, A. Microleakage Analysis of Different Bulk-Filling Techniques for Class II Restorations: µ-CT, SEM and EDS Evaluations. Materials 2020, 14, 31. [Google Scholar] [CrossRef]
- Rosales-Leal, J.I.; del Castillo-Salmerón, R.; Molino-Serrano, M.A.; González-Moreira, H.; Cabrerizo-Vílchez, M.A. Effect of Hygroscopic Expansion of Resin Filling on Interfacial Gap and Sealing: A Confocal Microscopy Study. J. Adhes. Dent. 2013, 15, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- Rosa, W.L.; Piva, E.; Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Davidson, C.L.; Feilzer, A.J. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J. Dent. 1997, 25, 435–440. [Google Scholar] [CrossRef]
- Munksgaard, E.C.; Hansen, E.K.; Kato, H. Wall-to-wall polymerization contraction of composite resins versus filler content. Scand. J. Dent. Res. 1987, 95, 526–531. [Google Scholar] [CrossRef]
- Aw, T.C.; Nicholls, J.I. Polymerization shrinkage of densely-filled resin composites. Oper. Dent. 2001, 26, 498–504. [Google Scholar]
- Shah, P.K.; Stansbury, J.W.; Bowman, C.N. Application of an addition–fragmentation-chain transfer monomer in di(meth)acrylate network formation to reduce polymerization shrinkage stress. Polym. Chem. 2017, 8, 4339–4351. [Google Scholar] [CrossRef]
- Gonçalves, L.; Noronha-Filho, J.D.; Guimarães, J.G.A.; Poskus, L.T.; Silva, E.M. Solubility, salivary sorption and degree of conversion of dimethacrylate-based polymeric matrixes. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2008, 85B, 320–325. [Google Scholar] [CrossRef]
- Irie, M.; Hatanaka, K.; Suzuki, K.; Watts, D.C. Immediate versus water-storage performance of Class V flowable composite restorative. Dent. Mater. 2006, 22, 875–883. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suiter, E.A.; Watson, L.E.; Tantbirojn, D.; Lou, J.S.B.; Versluis, A. Effective Expansion: Balance between Shrinkage and Hygroscopic Expansion. J. Dent. Res. 2016, 5, 543–549. [Google Scholar] [CrossRef] [PubMed]


| Material (Manufacturer) | Type | Shade | *Composition |
|---|---|---|---|
| Filtek™ Z350 XT (3M ESPE, St Paul, MN, USA) | Nanofilled dental composite | A3 | Zirconia/silica (78.5% wt%, 63.3 vol%) Bis-GMA, UDMA, TEGDMA, and Bis-EMA, initiators, and stabilizers. |
| Filtek™ One Bulk Fill (3M ESPE, St Paul, MN, USA) | Bulk-fill dental composite | A3 | Zirconia/silica and an ytterbium trifluoride filler consisting of agglomerate 100 nm particles (76.5% wt%, 58.5 vol%), AFM (dynamic stress-relieving monomer), AUDMA, UDMA and 1, 12-dodecane-dimethacrylate, initiators, and stabilizers. |
| Single Bond Universal (3M ESPE, St Paul, MN, USA) | Universal adhesive | --- | MDP phosphate monomer, dimethacrylate resins, HEMA, 3M™ Vitrebond™ copolymer, filler, ethanol, water, initiators, and silane. |
| Material (Manufacturer) | Etch | Adhesive | Light Cure |
|---|---|---|---|
| Single Bond Universal (3M ESPE, St Paul, MN, USA) | --- | Rub the adhesive in for 20 s; 5 s of gentle air. | 10 s light cure. |
| Single Bond Universal (3M ESPE, St Paul, MN, USA) | 15 s selective enamel etching; 30 s rinse; blot excess water using absorbent paper. | Rub the adhesive in for 20 s; 5 s of gentle air. | 10 s light cure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, M.P.; Rabello, T.B.; Silva, E.M.d. The Influence of Adhesive Strategy, Type of Dental Composite, and Polishing Time on Marginal Gap Formation in Class I-like Cavities. Materials 2023, 16, 7411. https://doi.org/10.3390/ma16237411
Barbosa MP, Rabello TB, Silva EMd. The Influence of Adhesive Strategy, Type of Dental Composite, and Polishing Time on Marginal Gap Formation in Class I-like Cavities. Materials. 2023; 16(23):7411. https://doi.org/10.3390/ma16237411
Chicago/Turabian StyleBarbosa, Marianna Pires, Tiago Braga Rabello, and Eduardo Moreira da Silva. 2023. "The Influence of Adhesive Strategy, Type of Dental Composite, and Polishing Time on Marginal Gap Formation in Class I-like Cavities" Materials 16, no. 23: 7411. https://doi.org/10.3390/ma16237411
APA StyleBarbosa, M. P., Rabello, T. B., & Silva, E. M. d. (2023). The Influence of Adhesive Strategy, Type of Dental Composite, and Polishing Time on Marginal Gap Formation in Class I-like Cavities. Materials, 16(23), 7411. https://doi.org/10.3390/ma16237411







