Dental Implants: Modern Materials and Methods of Their Surface Modification
Abstract
1. Introduction

2. Dental Implant Materials
- To be strong enough to withstand chewing pressure (sometimes exceeding 100 kgf/cm2) and not to destroy the bone with its weight;
- To be well machinable at the manufacturing stage in order to retain its shape throughout its entire service life;
- To not be destroyed (not corroded) by the action of the biological environment (saliva, blood, etc.);
- To not show toxic, allergenic, and carcinogenic effects on the body;
- To not provoke an increase in galvanic currents when interacting with metal structures installed in the patient’s mouth.
2.1. Titanium and Its Alloys
2.2. Zirconium and Its Alloys
- Ceramic materials based on zirconium dioxide:
- ◦
- ◦
- ◦
- ◦
2.3. Tantalum and Its Alloys
2.4. Other Materials for Dental Implants
3. Implant Surface Modification
- (1)
- An absence of rejection reactions expressed in the development of inflammation in the adjacent tissues, local necrotic changes, and systemic manifestations such as allergic and immune reactions;
- (2)
- The formation of morphofunctional determinants of the integration process in the area of contact, i.e., the “implant–tissue medium”: bone or bone-like substance (in the case of osteointegration);
- (3)
- The relative stability (including mechanical stability) of the above-mentioned morphofunctional determinants for a certain period of time as a reflection of the dynamic equilibrium occurring in the system, i.e., the “implant–tissue substrate”.
- (1)
- Methods aimed at changing the surface roughness of implants to improve their integration;
- (2)
- Methods forming protective and/or bioactive coatings on the implants to improve their corrosion resistance, biocompatibility, biomechanical stability, and antibacterial properties and to promote bone tissue regeneration.
4. Conclusions
- There is a great variety of biomaterials that are promising for application in dental implantology; however, unfortunately, to date, there has been no clearly structured methodology for implant material selection for specific operating conditions (specific clinical cases) or rational technology of implant manufacturing from the selected material;
- There is a need for a complex approach to improve the quality of dental implants, including the choice of the optimal material, implant manufacturing technology, and the method of its surface modification;
- The modification of the dental implant surface should combine different methods aimed at creating the surface texture and formation of bioactive coatings;
- A comparison of the aesthetic indicators and durability of metal and ceramic implants indicates the following for implants of bearing structures: for masticatory teeth, more durable metal alloys based on titanium and/or zirconium are preferable, and in the smile zone, more aesthetic white ceramic materials are preferable (based on stabilized zirconium dioxide or silicon nitride (to a lesser extent, it has a gray shade));
- Among metal alloys, the most promising are alloys of the Ti-Nb-Zr system alloyed additionally with Ga and/or Sn, but the elasticity modulus of these alloys (dense structure) exceeds the elasticity modulus of bone tissue; to reduce the elasticity modulus and increase the bioactivity and corrosion resistance of metal alloys, it is promising to develop composite meta-materials from these alloys (i.e., manufacturing of alloys of the specified composition of the lattice structure using additive technologies, with further “impregnation” of them with polymeric bioactive materials);
- A promising direction is the creation of biomedical nanomaterials; nanotextured surfaces have a positive effect on bone tissue cells and have an antibacterial effect. Promising in the production of dental implants is the use of nanostructured titanium, the advantage of which is the absence of toxic elements (aluminum and vanadium) and the higher strength and corrosion resistance inherent in unalloyed titanium;
- Due to the development of additive technologies, it has become possible to manufacture products with a “controlled” gradient of properties by volume; a one-piece implant consisting of two layers has been developed: zirconium ceramic, used for the crown, and titanium alloy, used for the body of the implant. This has avoided the problems of both the mobility of the components of a prefabricated dental implant (between the implant and abutment and the abutment and crown) as well as the colonization of bacteria in the gaps of mechanical connections, thus preventing the fusion of the gingiva or bone tissue with the implant, which can lead to the rejection of the dental implant; however, it should be taken into account that the heterogeneity of implant and abutment materials may increase corrosion processes due to galvanic processes, so further studies both in vitro and in vivo are required.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030. Available online: https://www.who.int/publications/i/item/9789240061484 (accessed on 15 August 2023).
- Follow-Up to the Political Declaration of the Third High-Level Meeting of the General Assembly on the Prevention and Control of Noncommunicable Diseases. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA75/A75_10Add1-ru.pdf (accessed on 15 August 2023). (In Russian).
- Russian Nanotitanium for Bioimplants Has No Analogues in the World. Available online: https://new.ras.ru/activities/news/rossiyskiy-nanotitan-dlya-bioimplantatov-ne-imeet-analogov-v-mire (accessed on 15 August 2023). (In Russian).
- Global Dental Implants Market by Design, Type, Price, Procedure, Material, Component, End User—Cumulative Impact of COVID-19, Russia Ukraine Conflict, and High Inflation—Forecast 2023–2030. Available online: https://www.researchandmarkets.com/report/dental-implant#reld0-4620505 (accessed on 15 August 2023).
- Dental Implants Market—Growth, Trends, COVID-19 Impact and Forecasts (2023–2028). Available online: https://www.mordorintelligence.com/ru/industry-reports/dental-implants-market (accessed on 24 August 2023).
- Thalji, G.; Cooper, L.F. Molecular assessment of osseointegration in vitro: A review of current literature. Int. J. Oral Maxillofac. Implant. 2014, 29, 171–199. [Google Scholar] [CrossRef]
- Yuan, K.; Chan, Y.-J.; Kung, K.-C.; Lee, T.-M. Comparison of Osseointegration on Various Implant Surfaces After Bacterial Contamination and Cleaning: A Rabbit Study. Int. J. Oral Maxillofac. Implant. 2014, 29, 32–40. [Google Scholar] [CrossRef]
- Yao, L.; Al-Bishari, A.M.; Shen, J.; Wang, Z.; Liu, T.; Sheng, L.; Wu, G.; Lu, L.; Xu, L.; Liu, J. Osseointegration and anti-infection of dental implant under osteoporotic conditions promoted by gallium oxide nano-layer coated titanium dioxide nanotube arrays. Ceram. Int. 2023, 49, 22961–22969. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Juaim, A.N.; Chen, X.; Lu, C.; Zou, L.; Wang, Y.; Zhou, X. Biocompatibility and osteogenic activity of Zr–30Ta and Zr–25Ta–5Ti sintered alloys for dental and orthopedic implants. Trans. Nonferrous Met. Soc. China 2023, 33, 851–864. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Hsu, Y.; He, Y.; Wang, F.; Yang, F.; Yan, F.; Xia, D.; Liu, Y. Surface modification of titanium implants with Mg-containing coatings to promote osseointegration. Acta Biomater. 2023, 169, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Chugh, A.; Chaudhry, K.; Bajpayee, A.; Jain, G.; Chugh, V.K.; Kumar, P.; Singh, S. Efficacy and Safety of Concentrated Growth Factors and Platelet- Rich Fibrin on Stability and Bone Regeneration in Patients with Immediate Dental Implants: A Randomized Controlled Trial. Int. J. Oral Maxillofac. Implant. 2022, 37, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-H.; Kim, S.; Chung, M.-K.; Heo, S.-J.; Rhyu, I.-C.; Kwon, Y.; Chang, J.-S. Regenerated Bone Pattern Around Exposed Implants with Various Designs. Int. J. Oral Maxillofac. Implant. 2019, 34, 61–67. [Google Scholar] [CrossRef]
- Bang, S.-M.; Moon, H.-J.; Kwon, Y.-D.; Yoo, J.-Y.; Pae, A.; Kwon, I.K. Osteoblastic and osteoclastic differentiation on SLA and hydrophilic modified SLA titanium surfaces. Clin. Oral. Implant. Res. 2014, 25, 831–837. [Google Scholar] [CrossRef]
- Alavi, S.E.; Alavi, S.Z.; Gholami, M.; Sharma, A.; Sharma, L.A.; Shahmabadi, H.E. Biocomposite-based strategies for dental bone regeneration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, in press. [CrossRef] [PubMed]
- Tangri, S.; Hasan, N.; Kaur, J.; Mohammad, F.; Maan, S.; Kesharwani, P.; Ahmad, F.J. Drug loaded bioglass nanoparticles and their coating for efficient tissue and bone regeneration. J. Non-Cryst. Solids 2023, 616, 122469. [Google Scholar] [CrossRef]
- Shi, C.; Gao, J.; Wang, M.; Fu, J.; Wang, D.; Zhu, Y. Ultra-trace silver-doped hydroxyapatite with non-cytotoxicity and effective antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Construction of N-halamine labeled silica/zinc oxide hybrid nanoparticles for enhancing antibacterial ability of Ti implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 50–58. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y. Polydopamine-induced nanocomposite Ag/CaP coatings on the surface of titania nanotubes for antibacterial and osteointegration functions. J. Mater. Chem. B 2015, 45, 8796–8805. [Google Scholar] [CrossRef] [PubMed]
- Lung, C.Y.K.; Khan, A.S.; Zeeshan, R.; Akhtar, S.; Chaudhry, A.A.; Matinlinna, J.P. An antibacterial porous calcium phosphate bilayer functional coatings on titanium dental implants. Ceram. Int. 2023, 49, 2401–2409. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Pérez-Álvarez, L.; Sáez Martínez, V.; Benito Cid, S.; Ruiz-Rubio, L.; Pérez González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Multifunctional antibacterial chitosan-based hydrogel coatings on Ti6Al4V biomaterial for biomedical implant applications. Int. J. Biol. Macromol. 2023, 231, 123328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wan, Y.; Yu, M.; Wang, H.; Cai, Y.; Liu, C.; Zhang, D. Biocompability evaluation of micro textures coated with zinc oxide on Ti-6Al-4V treated by nanosecond laser. Surf. Coat. Technol. 2021, 422, 127453. [Google Scholar] [CrossRef]
- Hashim, N.C.; Nordin, D. Nano-Hydroxyapatite Powder for Biomedical Implant Coating. Mater. Today 2019, 19, 1562–1571. [Google Scholar] [CrossRef]
- Mishra, S.; Chowdhary, R. PEEK materials as an alternative to titanium in dental implants: A systematic review. Clin. Implant Dent. Relat. Res. 2019, 21, 208–222. [Google Scholar] [CrossRef]
- Zou, R.; Bi, L.; Huang, Y.; Wang, Y.; Wang, Y.; Li, L.; Liu, J.; Feng, L.; Jiang, X.; Deng, B. A biocompatible silicon nitride dental implant material prepared by digital light processing technology. J. Mech. Behav. Biomed. 2023, 141, 105756. [Google Scholar] [CrossRef]
- Jayasree, R.; Raghava, K.; Sadhasivam, M.; Srinivas, P.V.V.; Vijay, R.; Pradeep, K.G.; Rao, T.N.; Chakravarty, D. Bi-layered metal-ceramic component for dental implants by spark plasma sintering. Mater. Lett. 2023, 344, 134403. [Google Scholar] [CrossRef]
- Zagorsky, V.A. Dental implantation. Materials and components. Symb. Sci. 2016, 9, 132–136. (In Russian) [Google Scholar]
- Jae-Hyun, L.; Kim, J.C.; Kim, H.-Y.; Yeo, I.-S.L. Influence of Connections and Surfaces of Dental Implants on Marginal Bone Loss: A Retrospective Study Over 7 to 19 Years. Int. J. Oral Maxillofac. Implant. 2020, 35, 1195–1202. [Google Scholar]
- Canullo, L.; Peñarrocha, D.; Clementini, M.; Iannello, G.; Micarelli, C. Impact of plasma of argon cleaning treatment on implant abutments in patients with a history of periodontal disease and thin biotype: Radiographic results at 24-month follow-up of a RCT. Clin. Oral. Implant. Res. 2015, 26, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhang, P.; Wang, X.; Kasugai, S. A doxycycline-treated hydroxyapatite implant surface attenuates the progression of peri-implantitis: A radiographic and histological study in mice. Clin. Implant Dent. Relat. Res. 2019, 21, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Mendonca, G.; Sartori, E.; Funkenbusch, P.; Ercoli, C.; Meirelles, L. Bone response to porous tantalum implants in a gap-healing model. Clin. Oral. Implant. Res. 2019, 30, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Anchieta, R.B.; Baldassarri, M.; Guastaldi, F.; Tovar, N.; Janal, M.N.; Gottlow, J.; Dard, M.; Jimbo, R.; Coelho, P.G. Mechanical property assessment of bone healing around a titanium-zirconium alloy dental implant. Clin. Implant Dent. Relat. Res. 2014, 16, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.A.; Rossi, A.L.; Ribeiro, A.R.; Toptan, F.; Pinto, A.M.; Shokuhfar, T.; Celis, J.-P.; Rocha, L.A. Improved tribocorrosion performance of bio-functionalized TiO2 nanotubes under two-cycle sliding actions in artificial saliva. J. Mech. Behav. Biomed. 2018, 80, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-S.; Seo, Y.-S.; Lee, G.-J.; You, J.-S.; Kim, S.-G. A comparative study with biphasic calcium phosphate and deproteinized bovine bone in maxillary sinus augmentation: A prospective randomized controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2019, 34, 233–242. [Google Scholar] [CrossRef]
- Jiang, Q.-H.; Gong, X.; Wang, X.-X.; He, F.-M. Osteogenesis of rat mesenchymal stem cells and osteoblastic cells on strontium-doped nanohydroxyapatite-coated titanium surfaces. Int. J. Oral Maxillofac. Implant. 2015, 30, 461–471. [Google Scholar] [CrossRef][Green Version]
- Hunziker, E.B.; Spiegl-Habegger, M.; Rudolf, S.; Liu, Y.; Gu, Z.; Lippuner, K.; Shintani, N.; Enggist, L. A novel experimental dental implant permits quantitative grading of surface-property effects on osseointegration. Int. J. Oral Maxillofac. Implant. 2018, 33, 967–978. [Google Scholar] [CrossRef]
- Fardjahromi, M.A.; Ejeian, F.; Razmjou, A.; Vesey, G.; Mukhopadhyay, S.C.; Derakhshan, A.; Warkiani, M.E. Enhancing osteoregenerative potential of biphasic calcium phosphates by using bioinspired ZIF8 coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111972. [Google Scholar] [CrossRef] [PubMed]
- Bryington, M.; Mendonça, G.; Nares, S.; Cooper, L.F. Osteoblastic and cytokine gene expression of implant-adherent cells in humans. Clin. Oral. Implant. Res. 2014, 25, 52–58. [Google Scholar] [CrossRef]
- Zhou, C.; Ge, Z.; Song, L.; Yan, J.; Lang, X.; Zhang, Y.; He, F. Strontium-modified titanium substrate promotes osteogenic differentiation of MSCs and implant osseointegration via upregulating CDH2. Clin. Oral. Implant. Res. 2023, 34, 297–311. [Google Scholar] [CrossRef]
- Mehl, C.; Gaßling, V.; Schultz-Langerhans, S.; Açil, Y.; Bähr, T.; Wiltfang, J.; Kern, M. Influence of four different abutment materials and the adhesive joint of two-piece abutments on cervical implant bone and soft tissue. Int. J. Oral Maxillofac. Implant. 2016, 31, 1264–1272. [Google Scholar] [CrossRef]
- Chappuis, V.; Cavusoglu, Y.; Gruber, R.; Kuchler, U.; Buser, D.; Bosshardt, D.D. Osseointegration of Zirconia in the Presence of Multinucleated Giant Cells. Clin. Implant Dent. Relat. Res. 2016, 18, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Ferroni, L.; Gardin, C.; Sivolella, S.; Piattelli, A.; Mijiritsky, E. Release of VEGF from dental implant improves osteogenetic process: Preliminary in vitro tests. Materials 2017, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.D. Occlusion in implant dentistry. A review of the literature of prosthetic determinants and current concepts. Aust. Dent. J. 2008, 53 (Suppl. 1), S60–S68. [Google Scholar] [CrossRef]
- Mishra, S.K.; Chowdhary, R.; Chrcanovic, B.R.; Brånemark, P.-I. Osseoperception in Dental Implants: A Systematic Review. J. Prosthodont. 2016, 25, 185–195. [Google Scholar] [CrossRef]
- González-Gil, D.; Dib-Zaitun, I.; Flores-Fraile, J.; López-Marcos, J. Active Tactile Sensibility in Implant Prosthesis vs. Complete Dentures: A Psychophysical Study. J. Clin. Med. 2022, 11, 6819. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.Á.; Gay-Escoda, C.; Valmaseda-Castellón, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical properties and corrosion behavior of Ti6Al4V particles obtained by implantoplasty: An in vitro study. Part II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef]
- Sikora, C.L.; Alfaro, M.F.; Yuan, J.C.-C.; Barao, V.A.; Sukotjo, C.; Mathew, M.T. Wear and Corrosion Interactions at the Titanium/Zirconia Interface: Dental Implant Application. J. Prosthodont. 2018, 27, 842–852. [Google Scholar] [CrossRef]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1354–1368. [Google Scholar] [CrossRef]
- Weller, J.; Vasudevan, P.; Kreikemeyer, B.; Ekat, K.; Jackszis, M.; Springer, A.; Chatzivasileiou, K.; Lang, H. The role of bacterial corrosion on recolonization of titanium implant surfaces: An in vitro study. Clin. Implant Dent. Relat. Res. 2022, 24, 664–675. [Google Scholar] [CrossRef]
- Silva, M.D.; Walton, T.R.; Alrabeah, G.O.; Layton, D.M.; Petridis, H. Comparison of corrosion products from implant and various golad-based abutment couplings: The effect of gold plating. J. Oral. Implant. 2021, 47, 370–379. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Trezubov, V.N.; Mishnev, L.M.; Zhulev, E.N.; Trezubov, V.V. Orthopedic Dentistry. Applied Materials Science, 7th ed.; MED Press-inform: Moscow, Russia, 2017; 328p. [Google Scholar]
- Grigoriev, S.; Sotova, C.; Vereschaka, A.; Uglov, V.; Cherenda, N. Modifying Coatings for Medical Implants Made of Titanium Alloys. Metals 2023, 13, 718. [Google Scholar] [CrossRef]
- Falanga, A.; Laheurte, P.; Vahabi, H.; Tran, N.; Khamseh, S.; Saeidi, H.; Khodadadi, M.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Niobium-treated titanium implants with improved cellular and molecular activities at the tissue-implant interface. Materials 2019, 12, 3861. [Google Scholar] [CrossRef] [PubMed]
- Fintová, S.; Dlhý, P.; Mertová, K.; Chlup, Z.; Duchek, M.; Procházka, R.; Hutař, P. Fatigue properties of UFG Ti grade 2 dental implant vs. conventionally tested smooth specimens. J. Mech. Behav. Biomed. 2021, 123, 104715. [Google Scholar] [CrossRef] [PubMed]
- Monetta, T.; Acquesta, A.; Bellucci, F. Evaluation of roughness and electrochemical behavior of titanium in biological environment. Metall. Ital. 2014, 106, 13–21. [Google Scholar]
- Laurindo, C.A.H.; Torres, R.D.; Mali, S.A.; Gilbert, J.L.; Soares, P. Incorporation of Ca and P on anodized titanium surface: Effect of high current density. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Babuska, V.; Palan, J.; Dobra, J.K.; Kulda, V.; Duchek, M.; Cerny, J.; Hrusak, D. Proliferation of osteoblasts on laser-modified nanostructured titanium surfaces. Materials 2018, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Fintová, S.; Kuběna, I.; Palán, J.; Mertová, K.; Duchek, M.; Hutař, P.; Pastorek, F.; Kunz, L. Influence of sandblasting and acid etching on fatigue properties of ultra-fine grained Ti grade 4 for dental implants. J. Mech. Behav. Biomed. 2020, 111, 104016. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Jimbo, R.; Stübinger, S.; Obrecht, M.; Dard, M.; Berner, S. Nanostructures and hydrophilicity influence osseointegration: A biomechanical study in the rabbit tibia. Clin. Oral. Implant. Res. 2014, 25, 1041–1050. [Google Scholar] [CrossRef]
- Jacobs, N.; Seghi, R.; Johnston, W.M.; Yilmaz, B. Displacement and performance of abutments in narrow-diameter implants with different internal connections. J. Prosthet. Dent. 2022, 127, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Pacifico, S. Modulation of indomethacin release from ZrO2/PCL hybrid multilayers synthesized via solegel dip coating. J. Drug Deliv. Sci. Technol. 2015, 26, 10–16. [Google Scholar] [CrossRef]
- Moghaddam, N.S.; Skoracki, R.; Miller, M.; Elahinia, M.; Dean, D. Three Dimensional Printing of Stiffness-tuned, Nitinol Skeletal Fixation Hardware with an Example of Mandibular Segmental Defect Repair. Procedia CIRP 2016, 49, 45–50. [Google Scholar] [CrossRef]
- Mastrangelo, F.; Quaresima, R.; Abundo, R.; Spagnuolo, G.; Marenzi, G. Esthetic and physical changes of innovative titanium surface properties obtained with laser technology. Materials 2020, 13, 1066. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Blus, C.; Schwarz, D.M.; Orrù, G. Characterization of a macro-and micro-textured titanium grade 5 alloy surface obtained by etching only without sandblasting. Materials 2020, 13, 5074. [Google Scholar] [CrossRef]
- Ghensi, P.; Bettio, E.; Maniglio, D.; Bonomi, E.; Piccoli, F.; Gross, S.; Caciagli, P.; Segata, N.; Nollo, G.; Tessarolo, F. Dental implants with anti-biofilm properties: A pilot study for developing a new sericin-based coating. Materials 2019, 12, 2429. [Google Scholar] [CrossRef]
- Di Giulio, M.; Traini, T.; Sinjari, B.; Nostro, A.; Caputi, S.; Cellini, L. Porphyromonas gingivalis biofilm formation in different titanium surfaces, an in vitro study. Clin. Oral. Implant. Res. 2016, 27, 918–925. [Google Scholar] [CrossRef]
- Sancar, B.; Dayı, E. Evaluation of metal concentrations in hair and nails after dental implant placement. J. Prosthet. Dent. 2022, 128, 625–631. [Google Scholar] [CrossRef]
- More than Solid—Roxolid®. Reducing Invasiveness. Straumann® Roxolid®. Available online: https://www.straumann.com/content/dam/media-center/straumann/en/documents/interactive-sales-presentation/490.137-en_low.pdf (accessed on 20 August 2023).
- El Chaar, E.; Zhang, L.; Zhou, Y.; Sandgren, R.; Fricain, J.-C.; Dard, M.; Pippenger, B.; Catros, S. Osseointegration of superhydrophilic implants placed in defect grafted bones. Int. J. Oral Maxillofac. Implant. 2019, 34, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Lotz, E.M.; Olivares-Navarrete, R.; Hyzy, S.L.; Berner, S.; Schwartz, Z.; Boyan, B.D. Comparable responses of osteoblast lineage cells to microstructured hydrophilic titanium–zirconium and microstructured hydrophilic titanium. Clin. Oral. Implant. Res. 2017, 28, e51–e59. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, Y.; Chu, C.; Dard, M.M.; Man, Y. Influence of implant location on titanium-zirconium alloy narrow-diameter implants: A 1-year prospective study in smoking and nonsmoking populations. J. Prosthet. Dent. 2022, 128, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Chowdhary, R. Evolution of dental implants through the work of per-ingvar branemark: A systematic review. Indian J. Dent. Res. 2020, 31, 930–956. [Google Scholar] [PubMed]
- Caparrós, C.; Ortiz-Hernandez, M.; Molmeneu, M.; Punset, M.; Calero, J.A.; Aparicio, C.; Fernández-Fairén, M.; Perez, R.; Gil, F.J. Bioactive macroporous titanium implants highly interconnected. J. Mater. Sci. Mater. Med. 2016, 27, 151. [Google Scholar] [CrossRef] [PubMed]
- Liens, A.; Etiemble, A.; Rivory, P.; Balvay, S.; Pelletier, J.-M.; Cardinal, S.; Fabrègue, D.; Kato, H.; Steyer, P.; Munhoz, T.; et al. On the potential of Bulk Metallic Glasses for dental implantology: Case study on Ti40Zr10Cu36Pd14. Materials 2018, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, X.; Tong, T.; Wang, Y. Microstructural and mechanical properties of β-type Ti–Mo–Nb biomedical alloys with low elastic modulus. J. Alloys Compd. 2020, 815, 152412. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.; Li, Y.; Ipek, R.; Wen, C. Development of Ti–Nb–Zr alloys with high elastic admissible strain for temporary orthopedic devices. Acta Biomater. 2015, 20, 176–187. [Google Scholar] [CrossRef]
- Boehlert, C.J.; Cowen, C.J.; Quast, J.P.; Akahori, T.; Niinomi, M. Fatigue and wear evaluation of Ti-Al-Nb alloys for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2008, 28, 323–330. [Google Scholar] [CrossRef]
- Boehlert, C.J.; Cowen, C.J.; Jaeger, C.R.; Niinomi, M.; Akahori, T. Tensile and fatigue evaluation of Ti–15Al–33Nb (at.%) and Ti–21Al–29Nb (at.%) alloys for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2005, 25, 263–275. [Google Scholar] [CrossRef]
- Hussain, S.A.; Panchal, M.; Allamraju, K.V.; Rajak, U.; Verma, T.N.; Brindhadevi, K. Optimization of wear behavior of heat-treated Ti-6Al-7Nb biomedical alloy by response surface methodology. Environ. Res. 2023, 231, 116193. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, S.; Bramowicz, M.; Czaja, P.; Jabłoński, R.; Kropiwnicki, J.; Charkiewicz, M. Application of atomic force microscopy for studies of fractal and functional properties of biomaterials. Acta Phys. Pol. A 2016, 130, 1013–1015. [Google Scholar] [CrossRef]
- Msweli, N.P.; Akinwamide, S.O.; Olubambi, P.A.; Obadele, B.A. Microstructure and biocorrosion studies of spark plasma sintered yttria stabilized zirconia reinforced Ti6Al7Nb alloy in Hanks’ solution. Mater. Chem. Phys. 2023, 293, 126940. [Google Scholar] [CrossRef]
- Schulze, C.; Weinmann, M.; Schweigel, C.; Keßler, O.; Bader, R. Mechanical properties of a newly additive manufactured implant material based on Ti-42Nb. Materials 2018, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-H.; Tung, K.-L.; Lin, Y.-Y. Treating Ti containing dental orthodontic wires with nitrogen plasma immersion ion implantation to reduce the metal ions release and bacterial adhesion. Mater. Technol. 2015, 30, B73–B79. [Google Scholar] [CrossRef]
- Kalita, D.; Rogal, Ł.; Czeppe, T.; Wójcik, A.; Kolano-Burian, A.; Zackiewicz, P.; Kania, B.; Dutkiewicz, J. Microstructure and Mechanical Properties of Ti-Nb Alloys Prepared by Mechanical Alloying and Spark Plasma Sintering. J. Mater. Eng. Perform. 2020, 29, 1445–1452. [Google Scholar] [CrossRef]
- Hoppe, V.; Szymczyk-Ziółkowska, P.; Rusińska, M.; Dybała, B.; Poradowski, D.; Janeczek, M. Assessment of mechanical, chemical, and biological properties of Ti-Nb-Zr alloy for medical applications. Materials 2021, 14, 126. [Google Scholar] [CrossRef]
- Pérez-Pevida, E.; Brizuela-Velasco, A.; Chávarri-Prado, D.; Jiménez-Garrudo, A.; Sánchez-Lasheras, F.; Solaberrieta-Méndez, E.; Diéguez-Pereira, M.; Fernández-González, F.J.; Dehesa-Ibarra, B.; Monticelli, F. Biomechanical Consequences of the Elastic Properties of Dental Implant Alloys on the Supporting Bone: Finite Element Analysis. BioMed Res. Int. 2016, 2016, 1850401. [Google Scholar] [CrossRef]
- Hussein, M.A.; Suryanarayana, C.; Al-Aqeeli, N. Fabrication of nano-grained Ti-Nb-Zr biomaterials using spark plasma sintering. Mater. Design 2015, 87, 693–700. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Nagay, B.E.; Ribeiro, A.L.R.; Cruz, N.C.; Rangel, E.C.; Fais, L.M.G.; Vaz, L.G.; Barão, V.A.R. Functionalization of an experimental Ti-Nb-Zr-Ta alloy with a biomimetic coating produced by plasma electrolytic oxidation. J. Alloys Compd. 2019, 770, 1038–1048. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, L.; Xue, X.; Lu, W.; Qin, J.; Zhang, D. Microstructure evolution and mechanical properties of a Ti–35Nb–3Zr–2Ta biomedical alloy processed by equal channel angular pressing (ECAP). Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4551–4561. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Guo, Y.; Ma, S.; Luo, W.; Xiang, X.; Zhao, J.; Zhou, Y. Biocompatibility evaluation of a newly developed Ti-Nb-Zr-Ta-Si alloy implant. J. Biomater. Tissue Eng. 2016, 6, 861–869. [Google Scholar] [CrossRef]
- Angelescu, R.M.; Rəducanu, D.; Cojocaru, V.D.; Angelescu, M.L.; Buţu, M.; Cincə, I.; Dan, I. Microstructural and mechanical evaluation of a Ti-Nb-Ta alloy. Univ. Politeh. Buchar. Sci. Bull. Ser. B 2015, 77, 221–228. [Google Scholar]
- Mutlu, I.; Yeniyol, S.; Oktay, E. Production and Precipitation Hardening of Beta-Type Ti-35Nb-10Cu Alloy Foam for Implant Applications. J. Mater. Eng. Perform. 2016, 25, 1586–1593. [Google Scholar] [CrossRef]
- Alberta, L.A.; Vishnu, J.; Hariharan, A.; Pilz, S.; Gebert, A.; Calin, M. Novel low modulus beta-type Ti–Nb alloys by gallium and copper minor additions for antibacterial implant applications. J. Mater. Res. Technol. 2022, 20, 3306–3322. [Google Scholar] [CrossRef]
- Alberta, L.A.; Vishnu, J.; Douest, Y.; Perrin, K.; Trunfio-Sfarghiu, A.-M.; Courtois, N.; Gebert, A.; Ter-Ovanessian, B.; Calin, M. Tribocorrosion behavior of β-type Ti-Nb-Ga alloys in a physiological solution. Tribol. Int. 2023, 181, 108325. [Google Scholar] [CrossRef]
- Alberta, L.A.; Fortouna, Y.; Vishnu, J.; Pilz, S.; Gebert, A.; Lekka, C.; Nielsch, K.; Calin, M. Effects of Ga on the structural, mechanical and electronic properties of β-Ti-45Nb alloy by experiments and ab initio calculations. J. Mech. Behav. Biomed. 2023, 140, 105728. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.C.; Kuroda, P.A.B.; Grandini, C.R. Influence of Nb addition on the structure, microstructure, Vickers microhardness, and Young’s modulus of new β Ti-xNb-5Mo alloys system. J. Mater. Res. Technol. 2023, 25, 3061–3070. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Huang, W.; Chen, X.; He, H. Micro-abrasion–corrosion behaviour of a biomedical Ti–25Nb–3Mo–3Zr–2Sn alloy in simulated physiological fluid. J. Mech. Behav. Biomed. 2016, 63, 361–374. [Google Scholar] [CrossRef]
- Li, H.; Cai, Q.; Li, S.; Xu, H. Effects of Mo equivalent on the phase constituent, microstructure and compressive mechanical properties of Ti–Nb–Mo–Ta alloys prepared by powder metallurgy. J. Mater. Res. Technol. 2022, 16, 588–598. [Google Scholar] [CrossRef]
- Koch, M.; Burkovski, A.; Zulla, M.; Rosiwal, S.; Geißdörfer, W.; Dittmar, R.; Grobecker-Karl, T. Pilot study on the use of a laser-structured double diamond electrode (Dde) for biofilm removal from dental implant surfaces. J. Clin. Med. 2020, 9, 3036. [Google Scholar] [CrossRef]
- Latimer, J.M.; Roll, K.L.; Daubert, D.M.; Zhang, H.; Shalev, T.; Wolff, L.F.; Kotsakis, G.A. Clinical performance of hydrophilic, titanium-zirconium dental implants in patients with well-controlled and poorly controlled type 2 diabetes: One-year results of a dual-center cohort study. J. Periodontol. 2022, 93, 745–757. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Molotnikov, A.; Lapovok, R.; Zeller, R.; Berner, S.; Habersetzer, P.; Dalla Torre, F. Microstructure and mechanical properties of Ti-15Zr alloy used as dental implant material. J. Mech. Behav. Biomed. 2016, 62, 384–398. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Faverani, L.P.; Grandini, C.R.; Rangel, E.C.; da Cruz, N.C.; Nociti, F.H., Jr.; Almeida, A.B.; Vicente, F.B.; Morais, B.R.G.; Barão, V.A.R.; et al. Characterization of chemically treated Ti-Zr system alloys for dental implant application. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Brizuela-Velasco, A.; Pérez-Pevida, E.; Jiménez-Garrudo, A.; Gil-Mur, F.J.; Manero, J.M.; Punset-Fuste, M.; Chávarri-Prado, D.; Diéguez-Pereira, M.; Monticelli, F. Mechanical characterisation and biomechanical and biological behaviours of Ti-Zr binary-Alloy dental implants. BioMed Res. Int. 2017, 2017, 2785863. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Katsuda, S.-I. Biological safety evaluation and surface modification of biocompatible ti–15Zr–4Nb alloy. Materials 2021, 14, 731. [Google Scholar] [CrossRef] [PubMed]
- Eldabah, N.M.; Shoukry, A.; Khair-Eldeen, W.; Kobayashi, S.; Gepreel, M.A.-H. Design and characterization of low Young’s modulus Ti-Zr-Nb-based medium entropy alloys assisted by extreme learning machine for biomedical applications. J. Alloys Compd. 2023, 968, 171755. [Google Scholar] [CrossRef]
- Qi, P.; Li, B.; Wang, T.; Zhou, L.; Nie, Z. Microstructure and properties of a novel ternary Ti–6Zr–xFe alloy for biomedical applications. J. Alloys Compd. 2021, 854, 157119. [Google Scholar] [CrossRef]
- Bai, L.; Cui, C.; Wang, Q.; Bu, S.; Qi, Y. Ti–Zr–Fe–Si system amorphous alloys with excellent biocompatibility. J. Non-Cryst. Solids 2008, 354, 3935–3938. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, S.; Ouyang, Z.; Yang, Y.; Liu, Y. Multi-scale nacre-inspired lamella-structured Ti-Ta composites with high strength and low modulus for load-bearing orthopedic and dental applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111458. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Pradies, G.; Mehmke, W.-U.; Beneytout, A.; Stamm, M.; Meda, R.G.; Kamm, T.; Poiroux, F.; Weinlich, F.; del Canto Pingarron, M.; et al. Prospective, Multicenter Evaluation of Trabecular Metal-Enhanced Titanium Dental Implants Placed in Routine Dental Practices: 1-Year Interim Report from the Development Period (2010 to 2011). Clin. Implant Dent. Relat. Res. 2015, 17, 1141–1153. [Google Scholar] [CrossRef]
- Aguilar, C.; San Martín, F.; Martínez, C.; Cámara, B.; Claverías, F.; Undabarrena, A.; Sancy, M.; Salinas, V.; Muñoz, L. Improving mechanical properties and antibacterial response of α/β ternary Ti–Ta alloy foams for biomedical uses. J. Mater. Res. Technol. 2023, 24, 8735–8753. [Google Scholar] [CrossRef]
- Mareci, D.; Chelariu, R.; Gordin, D.-M.; Ungureanu, G.; Gloriant, T. Comparative corrosion study of Ti–Ta alloys for dental applications. Acta Biomater. 2009, 5, 3625–3639. [Google Scholar] [CrossRef]
- Mendis, S.; Xu, W.; Tang, H.P.; Jones, L.A.; Liang, D.; Thompson, R.; Choong, P.; Brandt, M.; Qian, M. Characteristics of oxide films on Ti-(10–75)Ta alloys and their corrosion performance in an aerated Hank’s balanced salt solution. Appl. Surf. Sci. 2020, 506, 145013. [Google Scholar] [CrossRef]
- Kim, W.-G.; Choe, H.-C. Effects of TiN coating on the corrosion of nanostructured Ti–30Ta–xZr alloys for dental implants. Appl. Surf. Sci. 2012, 258, 1929–1934. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jeong, Y.-H.; Choe, H.-C.; Brantley, W.A. Surface morphology of TiN-coated nanotubular Ti–25Ta–xZr alloys for dental implants prepared by RF sputtering. Thin Solid Films 2013, 549, 131–134. [Google Scholar] [CrossRef]
- Stenlund, P.; Omar, O.; Brohede, U.; Norgren, S.; Norlindh, B.; Johansson, A.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Bone response to a novel Ti–Ta–Nb–Zr alloy. Acta Biomater. 2015, 20, 165–175. [Google Scholar] [CrossRef]
- Nguyen, P.M.H.; Won, D.-H.; Kim, B.-S.; Jang, Y.-S.; Nguyen, T.-D.T.; Lee, M.-H.; Bae, T.-S. The effect of two-step surface modification for Ti-Ta-Mo-Zr alloys on bone regeneration: An evaluation using calvarial defect on rat model. Appl. Surf. Sci. 2018, 442, 630–639. [Google Scholar] [CrossRef]
- Fowler, L.; Masia, N.; Cornish, L.A.; Chown, L.H.; Engqvist, H.; Norgren, S.; Öhman-Mägi, C. Development of antibacterial Ti-Cux alloys for dental applications: Effects of ageing for alloys with up to 10 wt% Cu. Materials 2019, 12, 4017. [Google Scholar] [CrossRef]
- Wang, X.; Dong, H.; Liu, J.; Qin, G.; Chen, D.; Zhang, E. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 38–47. [Google Scholar] [CrossRef]
- Pina, V.G.; Amigó, V.; Muñoz, A.I. Microstructural, electrochemical and tribo-electrochemical characterisation of titanium-copper biomedical alloys. Corros. Sci. 2016, 109, 115–125. [Google Scholar] [CrossRef]
- Alshammari, Y.; Yang, F.; Bolzoni, L. Fabrication and characterisation of low-cost powder metallurgy Ti-xCu-2.5Al alloys produced for biomedical applications. J. Mech. Behav. Biomed. 2022, 126, 105022. [Google Scholar] [CrossRef]
- Pandey, A.K.; Gautam, R.K.; Behera, C.K. Corrosion and wear behavior of Ti–5Cu-xNb biomedical alloy in simulated body fluid for dental implant applications. J. Mech. Behav. Biomed. 2023, 137, 105533. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lu, X.; Wang, L.N.; Shi, Z.M.; Lv, S.M.; Qian, M.; Qu, X.H. Mechanical properties, in vitro corrosion resistance and biocompatibility of metal injection molded Ti-12Mo alloy for dental applications. J. Mech. Behav. Biomed. 2018, 88, 534–547. [Google Scholar] [CrossRef]
- Mohan, P.; Rajak, D.K.; Pruncu, C.I.; Behera, A.; Amigó-Borrás, V.; Elshalakany, A.B. Influence of β-phase stability in elemental blended Ti-Mo and Ti-Mo-Zr alloys. Micron 2021, 142, 102992. [Google Scholar] [CrossRef] [PubMed]
- Asl, M.S.; Delbari, S.A.; Azadbeh, M.; Namini, A.S.; Mehrabian, M.; Nguyen, V.-H.; Van Le, Q.; Shokouhimehr, M.; Mohammadi, M. Nanoindentational and conventional mechanical properties of spark plasma sintered Ti–Mo alloys. J. Mater. Res. Technol. 2020, 9, 10647–10658. [Google Scholar]
- Mohan, P.; Elshalakany, A.B.; Osman, T.A.; Amigo, V.; Mohamed, A. Effect of Fe content, sintering temperature and powder processing on the microstructure, fracture and mechanical behaviours of Ti-Mo-Zr-Fe alloys. J. Alloys Compd. 2017, 729, 1215–1225. [Google Scholar] [CrossRef]
- Tejeda-Ochoa, A.; Kametani, N.; Carreño-Gallardo, C.; Ledezma-Sillas, J.E.; Adachi, N.; Todaka, Y.; Herrera-Ramirez, J.M. Formation of a metastable fcc phase and high Mg solubility in the Ti-Mg system by mechanical alloying. Powder Technol. 2020, 374, 348–352. [Google Scholar] [CrossRef]
- Cai, X.; Ding, S.; Li, Z.; Zhang, X.; Wen, K.; Xu, L.; Zhang, Y.; Peng, Y.; Shen, T. Simultaneous sintering of low-melting-point Mg with high-melting-point Ti via a novel one-step high-pressure solid-phase sintering strategy. J. Alloys Compd. 2021, 858, 158344. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Luo, T.; Song, M.; Wu, H.; Xiao, J.; Tan, Y.; Cheng, M.; Chen, B.; Niu, X.; et al. Powder metallurgical low-modulus Ti–Mg alloys for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Balog, M.; Hassan Ibrahim, A.M.; Krizik, P.; Bajana, O.; Klimova, A.; Catic, A.; Schauperl, Z. Bioactive Ti + Mg composites fabricated by powder metallurgy: The relation between the microstructure and mechanical properties. J. Mech. Behav. Biomed. 2019, 90, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, N.B.; Rajath Hegde, M.M.; Rajendrachari, S.; Surendranathan, A.O. Investigation of microstructure and mechanical properties of microwave consolidated TiMgSr alloy prepared by high energy ball milling. Powder Technol. 2022, 408, 117715. [Google Scholar] [CrossRef]
- Niu, J.; Guo, Y.; Li, K.; Liu, W.; Dan, Z.; Sun, Z.; Chang, H.; Zhou, L. Improved mechanical, bio-corrosion properties and in vitro cell responses of Ti-Fe alloys as candidate dental implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111917. [Google Scholar] [CrossRef]
- Sjafrizal, T.; Kenta, D.; Dehghan-Manshadi, A.; Xiao, W.; Dargusch, M.S. Metastable Ti-Fe-Ge alloys with high elastic admissible strain. Materialia 2022, 21, 101304. [Google Scholar] [CrossRef]
- Haruna, T. Development of Ti alloys for dental devices with corrosion resistance to fluoride solutions. Zairyo-to-Kankyo/Corros. Eng. 2014, 63, 309–315. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.B.; Hu, H.X.; Zheng, Y.G.; Ke, W.; Qiao, Y.X. Comparison of the corrosion behavior of pure titanium and its alloys in fluoride-containing sulfuric acid. Corros. Sci. 2016, 103, 50–65. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhou, Q.; Yang, K.; Zou, C.-H.; Wang, L. Performance of surface on ultrafine grained Ti-0.2Pd in simulated body fluid. Appl. Surf. Sci. 2018, 434, 957–966. [Google Scholar] [CrossRef]
- Hua, F.; Mon, K.; Pasupathi, P.; Gordon, G.; Shoesmith, D. A review of corrosion of titanium grade 7 and other titanium alloys in nuclear waste repository environments. Corrosion 2005, 61, 987–1003. [Google Scholar] [CrossRef]
- Steinemann, S.G.; Perren, S.M. Titanium alloys as metallic biomaterials. In Proceedings of the Fifth World Conference On Titanium, Munich, Germany, 10–14 September 1984; Volume 2, pp. 1327–1334. [Google Scholar]
- Sidelnikov, A.I. Comparative characteristics of materials of the titanium group used in the production of modern dental implants. InfoDENT 2000, 5, 10–12. [Google Scholar]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 2017, 40, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Cremasco, A.; Messias, A.D.; Esposito, A.R.; Duek, E.A.R.; Caram, R. Effects of alloying elements on the cytotoxic response of titanium alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 833–839. [Google Scholar] [CrossRef]
- Lin, C.-W.; Ju, C.-P.; Lin, J.-H.C. A comparison of the fatigue behavior of cast Ti–7.5Mo with c.p. titanium, Ti–6Al–4V and Ti–13Nb–13Zr alloys. Biomaterials 2005, 26, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.C.; de Almeida, G.S.; Corrêa, D.O.G.; Zambuzzi, W.F.; Buzalaf, M.A.R.; Correa, D.R.N.; Grandini, C.R. Preparation and characterization of novel as-cast Ti-Mo-Nb alloys for biomedical applications. Sci. Rep. 2022, 12, 11874. [Google Scholar] [CrossRef]
- Albrektsson, T.; Hansson, H.A.; Ivarsson, B. Interface analysis of titanium and zirconium bone implants. Biomaterials 1985, 6, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef]
- Fikeni, L.; Annan, K.A.; Mutombo, K.; Machaka, R. Effect of Nb content on the microstructure and mechanical properties of binary Ti-Nb alloys. Mater. Today 2021, 38, 913–917. [Google Scholar] [CrossRef]
- Cremasco, A.; Osório, W.R.; Freire, C.M.A.; Garcia, A.; Caram, R. Electrochemical corrosion behavior of a Ti–35Nb alloy for medical prostheses. Electrochim. Acta 2008, 53, 4867–4874. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.M.; Costa, R.C.; Nagay, B.E.; Dongari-Bagtzoglou, A.; Barão, V.A.R. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. iScience 2021, 24, 102008. [Google Scholar] [CrossRef]
- Sarraf, M.; Ghomi, E.R.; Alipour, S.; Ramakrishna, S.; Sukiman, N.L. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Design Manuf. 2022, 5, 371–395. [Google Scholar] [CrossRef]
- Lemire, J.; Harrison, J.; Turner, R. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ueno, T.; Wakabayashi, N. A review in titanium-zirconium binary alloy for use in dental implants: Is there an ideal Ti-Zr composing ratio? Jpn. Dent. Sci. Rev. 2023, 59, 28–37. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Vicente, F.B.; Donato, T.A.G.; Arana-Chavez, V.E.; Buzalaf, M.A.R.; Grandini, C.R. The effect of the solute on the structure, selected mechanical properties, and biocompatibility of Ti–Zr system alloys for dental applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 354–359. [Google Scholar] [CrossRef]
- Ho, W.-F.; Chen, W.-K.; Wu, S.-C.; Hsu, H.-C. Structure, mechanical properties, and grindability of dental Ti–Zr alloys. J. Mater. Sci. Mater. Med. 2008, 19, 3179–3186. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Wu, S.-C.; Sung, Y.-C.; Ho, W.-F. The structure and mechanical properties of as-cast Zr–Ti alloys. J. Alloys Compd. 2009, 488, 279–283. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Beline, T.; Ribeiro, A.L.R.; Rangel, E.C.; da Cruz, N.C.; Landers, R.; Faverani, L.P.; Vaz, L.G.; Fais, L.M.G.; Vicentei, F.B.; et al. Development of binary and ternary titanium alloys for dental implants. Dent. Mater. 2017, 33, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Grandin, H.M.; Berner, S.; Dard, M. A Review of Titanium Zirconium (TiZr) Alloys for Use in Endosseous Dental Implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Hacisalihoglu, I.; Samancioglu, A.; Yildiz, F.; Purcek, G.; Alsaran, A. Tribocorrosion properties of different type titanium alloys in simulated body fluid. Wear 2015, 332–333, 679–686. [Google Scholar] [CrossRef]
- Froes, F.H. Titanium Alloys: Thermal Treatment and Thermomechanical Processing. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier Ltd.: Exeter Devon, UK, 2001; pp. 9369–9373. [Google Scholar]
- Hon, Y.-H.; Wang, J.-Y.; Pan, Y.-N. Composition/Phase Structure and Properties of Titanium-Niobium Alloys. Mater. Trans. 2003, 44, 2384–2390. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical Devices. The International Organization for Standard (ISO): Geneva, Switzerland, 2010.
- Trillo, E.A.; Ortiz, C.; Dickerson, P.; Villa, R.; Stafford, S.W.; Murr, L.E. Evaluation of mechanical and corrosion biocompatibility of TiTa alloys. J. Mater. Sci. Mater. Med. 2001, 12, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A-Struct. 2004, 371, 283–290. [Google Scholar] [CrossRef]
- Schildhauer, T.; Robie, B.; Muhr, G.; Köller, M. Bacterial Adherence to Tantalum Versus Commonly Used Orthopedic Metallic Implant Materials. J. Orthop. Trauma 2006, 20, 476–484. [Google Scholar] [CrossRef]
- Wang, X.; Ning, B.; Pei, X. Tantalum and its derivatives in orthopedic and dental implants: Osteogenesis and antibacterial properties. Colloid. Surf. B 2021, 208, 112055. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Wu, H.; Song, M.; Zhang, T.; Lan, X.; Yao, T. Elastic modulus of phases in Ti–Mo alloys. Mater. Charact. 2015, 106, 302–307. [Google Scholar] [CrossRef]
- Issariyapat, A.; Huang, J.; Teramae, T.; Kariya, S.; Bahador, A.; Visuttipitukul, P.; Umeda, J.; Alhazaa, A.; Kondoh, K. Microstructure refinement and strengthening mechanisms of additively manufactured Ti-Zr alloys prepared from pre-mixed feedstock. Addit. Manuf. 2023, 73, 103649. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Z.; Shao, H.; Liu, X.; Ji, Y. Mechanical Properties of Porous Ti-Mo and Ti-Nb Alloys for Biomedical Application by Gelcasting. Procedia Eng. 2012, 36, 160–167. [Google Scholar] [CrossRef]
- Wei, C.; Luo, L.; Wu, Z.; Zhang, J.; Su, S.; Zhan, Y. New Zr–25Ti–xMo alloys for dental implant application: Properties characterization and surface analysis. J. Mech. Behav. Biomed. 2020, 111, 104017. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Henao, P.A.; Caneiro Queija, L.; Mareque, S.; Tasende Pereira, A.; Liñares González, A.; Blanco Carrión, J. Titanium vs. ceramic single dental implants in the anterior maxilla: A 12-month randomized clinical trial. Clin. Implant Dent. Relat. Res. 2021, 32, 951–961. [Google Scholar] [CrossRef]
- Siddiqi, A.; Khan, A.S.; Zafar, S. Thirty years of translational research in zirconia dental implants: A systematic review of the literature. J. Oral. Implant. 2017, 43, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Kieser, J.A.; De Silva, R.K.; Thomson, W.M.; Duncan, W.J. Soft and Hard Tissue Response to Zirconia versus Titanium One-Piece Implants Placed in Alveolar and Palatal Sites: A Randomized Control Trial. Clin. Implant Dent. Relat. Res. 2015, 17, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Ioannidis, A.; Cathomen, E.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Discoloration of the peri-implant mucosa caused by zirconia and titanium implants. Int. J. Periodontics Restor. 2016, 36, 38–45. [Google Scholar] [CrossRef]
- Chiou, L.-L.; Panariello, B.H.D.; Hamada, Y.; Gregory, R.L.; Blanchard, S.; Duarte, S. Comparison of In Vitro Biofilm Formation on Titanium and Zirconia Implants. BioMed Res. Int. 2023, 2023, 8728499. [Google Scholar] [CrossRef]
- Yegorov, A.A.; Drovosekov, M.N.; Aronov, A.M.; Rozhnova, O.M.; Yegorova, O.P. Comparative characteristics of materials used in dental implantation. Bull. Sib. Med. 2014, 13, 41–47. (In Russian) [Google Scholar] [CrossRef]
- Paraskevich, V.L. Dental Implantology: Fundamentals of Theory and Practice, 3rd ed.; Medical Information Agency LLC: Moscow, Russia, 2011; 400p. (In Russian) [Google Scholar]
- Korniienko, V.; Oleshko, O.; Husak, Y.; Deineka, V.; Holubnycha, V.; Mishchenko, O.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; Pshenychnyi, R.; Leśniak-Ziółkowska, K.; et al. Formation of a bacteriostatic surface on ZrNb alloy via anodization in a solution containing Cu nanoparticles. Materials 2020, 13, 3913. [Google Scholar] [CrossRef] [PubMed]
- Oleshko, O.; Deineka, V.V.; Husak, Y.; Korniienko, V.; Mishchenko, O.; Holubnycha, V.; Pisarek, M.; Michalska, J.; Kazek-Kesik, A.; Jakóbik-Kolon, A.; et al. Ag nanoparticle-decorated oxide coatings formed via plasma electrolytic oxidation on ZrNb alloy. Materials 2019, 12, 3742. [Google Scholar] [CrossRef] [PubMed]
- Kondo, R.; Nomura, N.; Suyalatu; Tsutsumi, Y.; Doi, H.; Hanawa, T. Microstructure and mechanical properties of as-cast Zr–Nb alloys. Acta Biomater. 2011, 7, 4278–4284. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.F.; Liu, N.; Qin, G.W. Microstructures, mechanical properties and corrosion resistance of the Zr-xTi (Ag) alloys for dental implant application. Mater. Chem. Phys. 2016, 176, 161–166. [Google Scholar] [CrossRef]
- Akimoto, T.; Ueno, T.; Tsutsumi, Y.; Doi, H.; Hanawa, T.; Wakabayashi, N. Evaluation of corrosion resistance of implant-use Ti-Zr binary alloys with a range of compositions. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bolat, G.; Izquierdo, J.; Santana, J.; Mareci, D.; Souto, R.M. Electrochemical characterization of ZrTi alloys for biomedical applications. Electrochim. Acta 2013, 88, 447–456. [Google Scholar] [CrossRef]
- Bolat, G.; Izquierdo, J.; Mareci, D.; Sutiman, D.; Souto, R.M. Electrochemical characterization of ZrTi alloys for biomedical applications. Part 2: The effect of thermal oxidation. Electrochim. Acta 2013, 106, 432–439. [Google Scholar] [CrossRef]
- Mishchenko, O.; Ovchynnykov, O.; Kapustian, O.; Pogorielov, M. New Zr-Ti-Nb alloy for medical application: Development, chemical and mechanical properties, and biocompatibility. Materials 2020, 13, 1306. [Google Scholar] [CrossRef]
- Michalska, J.; Sowa, M.; Stolarczyk, A.; Warchoł, F.; Nikiforow, K.; Pisarek, M.; Dercz, G.; Pogorielov, M.; Mishchenko, O.; Simka, W. Plasma electrolytic oxidation of Zr-Ti-Nb alloy in phosphate-formate-EDTA electrolyte. Electrochim. Acta 2022, 419, 140375. [Google Scholar] [CrossRef]
- Roehling, S.; Gahlert, M.; Janner, S.; Meng, B.; Woelfler, H.; Cochran, D.L. Ligature-induced peri-implant bone loss around loaded zirconia and titanium implants. Int. J. Oral Maxillofac. Implant. 2019, 34, 357–365. [Google Scholar] [CrossRef]
- Bahadirli, G.; Yilmaz, S.; Jones, T.; Sen, D. Influences of implant and framework materials on stress distribution: A three-dimensional finite element analysis study. Int. J. Oral Maxillofac. Implant. 2018, 33, e117–e126. [Google Scholar] [CrossRef]
- Sancho-Puchades, M.; Hämmerle, C.H.F.; Benic, G.I. In vitro assessment of artifacts induced by titanium, titanium-zirconium and zirconium dioxide implants in cone-beam computed tomography. Clin. Oral. Implant. Res. 2015, 26, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Steiger-Ronay, V.; Krcmaric, Z.; Schmidlin, P.R.; Sahrmann, P.; Wiedemeier, D.B.; Benic, G.I. Assessment of peri-implant defects at titanium and zirconium dioxide implants by means of periapical radiographs and cone beam computed tomography: An in-vitro examination. Clin. Oral. Implant. Res. 2018, 29, 1195–1201. [Google Scholar] [CrossRef]
- Benic, G.I.; Thoma, D.S.; Sanz-Martin, I.; Munoz, F.; Hämmerle, C.H.F.; Cantalapiedra, A.; Fischer, J.; Jung, R.E. Guided bone regeneration at zirconia and titanium dental implants: A pilot histological investigation. Clin. Oral. Implant. Res. 2017, 28, 1592–1599. [Google Scholar] [CrossRef]
- Schriber, M.; Yeung, A.W.K.; Suter, V.G.A.; Buser, D.; Leung, Y.Y.; Bornstein, M.M. Cone beam computed tomography artefacts around dental implants with different materials influencing the detection of peri-implant bone defects. Clin. Oral. Implant. Res. 2020, 31, 595–606. [Google Scholar] [CrossRef]
- Saito, M.M.; Onuma, K.; Yamamoto, R.; Yamakoshi, Y. New insights into bioactivity of ceria-stabilized zirconia: Direct bonding to bone-like hydroxyapatite at nanoscale. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111665. [Google Scholar] [CrossRef]
- Palmero, P.; Fornabaio, M.; Montanaro, L.; Reveron, H.; Esnouf, C.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants: Part I: Innovative synthesis, microstructural characterization and invitro stability. Biomaterials 2015, 50, 38–46. [Google Scholar] [CrossRef]
- Schienle, S.; Al-Ahmad, A.; Kohal, R.J.; Bernsmann, F.; Adolfsson, E.; Montanaro, L.; Palmero, P.; Fürderer, T.; Chevalier, J.; Hellwig, E.; et al. Microbial adhesion on novel yttria-stabilized tetragonal zirconia (Y-TZP) implant surfaces with nitrogen-doped hydrogenated amorphous carbon (a-C:H:N) coatings. Clin. Oral. Investig. 2016, 20, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Brüll, F.C.W.E.; van Winkelhoff, A.J.; Cune, M.S. Zirconia dental implants: A clinical, radiographic, and microbiologic evaluation up to 3 years. Int. J. Oral Maxillofac. Implant. 2014, 29, 914–920. [Google Scholar]
- da Cruz, M.B.; Marques, J.F.; Peñarrieta-Juanito, G.M.; Costa, M.; Souza, J.C.M.; Magini, R.S.; Miranda, G.; Silva, F.S.; da Mata, A.D.S.P.; Caramês, J.M.M. Hard and soft tissue cell behavior on PEEK, Zirconia, and Titanium implant materials. Int. J. Oral Maxillofac. Implant. 2019, 34, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Payer, M.; Heschl, A.; Koller, M.; Arnetzl, G.; Lorenzoni, M.; Jakse, N. All-ceramic restoration of zirconia two-piece implants—A randomized controlled clinical trial. Clin. Oral. Implant. Res. 2015, 26, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Iwasa, F.; Tachi, K.; Baba, K. Effect of nanofeatured topography on ceria-stabilized zirconia/alumina nanocomposite on osteogenesis and osseointegration. Int. J. Oral Maxillofac. Implant. 2017, 32, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Nakabayashi, S.; Ikeda, T.; Ito, R. Ceria-Stabilized Zirconia/Alumina nanocomposite for fabricating the framework of removable dental prostheses: Preliminary results from a 4-Year Follow-up. Int. J. Prosthodont. 2019, 32, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Nakajima, K. Use of ceria-stabilized zirconia/alumina nanocomposite for fabricating the frameworks of removable dental prostheses: A clinical report. J. Prosthet. Dent. 2016, 116, 166–171. [Google Scholar] [CrossRef]
- Lopez-Píriz, R.; Fernández, A.; Goyos-Ball, L.; Rivera, S.; Díaz, L.A.; Fernández-Domínguez, M.; Prado, C.; Moya, J.S.; Torrecillas, R. Performance of a new Al2O3/Ce-TZP ceramic nanocomposite dental implant: A pilot study in dogs. Materials 2017, 10, 614. [Google Scholar] [CrossRef]
- Molaei, M.; Attarzadeh, N.; Fattah-Alhosseini, A. Tailoring the biological response of zirconium implants using zirconia bioceramic coatings: A systematic review. J. Trace Elem. Med. Biol. 2021, 66, 126756. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, Y.H.; Brantley, W.A.; Choe, H.C. Nanotube nucleation phenomena on Ti−25Ta−xZr alloys for implants using ATO technique. J. Nanosci. Nanotechnol. 2014, 14, 7569–7573. [Google Scholar] [CrossRef] [PubMed]
- Holländer, J.; Lorenz, J.; Stübinger, S.; Hölscher, W.; Heidemann, D.; Ghanaati, S.; Sader, R. Zirconia dental implants: Investigation of clinical parameters, patient satisfaction, and microbial contamination. Int. J. Oral Maxillofac. Implant. 2016, 31, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.D.; Pita, M.S.; Fernandes, F.H.N.C.; Pedrazzi, V.; de Albuquerque, R.F., Jr.; Ribeiro, R.F. Bacterial adhesion on the titanium and zirconia abutment surfaces. Clin. Oral. Implant. Res. 2014, 25, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, C.; Çelik, E.; Gönüldaş, F. Effect of different surface treatments on the biaxial flexural strength of zirconia ceramics. J. Prosthet. Dent. 2023, 129, 220.e1–220.e5. [Google Scholar] [CrossRef] [PubMed]
- Chatchai, D. The Structure of the Mechanical Properties of Composites Based on Zirconium Dioxide and Wollastonite. Dissertation for the Degree of Candidate of Technical Sciences, Tomsk Polytechnic University, Tomsk, Russia, 2021. [Google Scholar]
- ISO 13356:2015; Implants for Surgery—Ceramic Materials Based on Yttria-Stabilized Tetragonal Zirconia (Y-TZP). ISO: Geneva, Switzerland, 2015.
- Hasiak, M.; Sobieszczańska, B.; Łaszcz, A.; Biały, M.; Chęcmanowski, J.; Zatoński, T.; Bożemska, E.; Wawrzyńska, M. Production, mechanical properties and biomedical characterization of zrti-based bulk metallic glasses in comparison with 316L stainless steel and Ti6Al4V alloy. Materials 2022, 15, 252. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, B.; Baptista, A.A.; Patoor, E.; Bravetti, P.; Eberhardt, A.; Laheurte, P. Interaction of bone-dental implant with new ultra low modulus alloy using a numerical approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Dmitrievskii, A.A.; Zhigacheva, D.G.; Zhigachev, A.O.; Ovchinnikov, P.N. Strength Properties of Zirconium Ceramics with Silica Additives Hardened with Alumina. Phys. Solid State 2021, 63, 295–299. [Google Scholar] [CrossRef]
- Dovgerd, A.; Sivolapov, K. Ceramic implants are the future of dental implantology. Actual. Probl. Dent. 2022, 18, 23–31. [Google Scholar] [CrossRef]
- Dovgerd, A.; Sivolapov, K. Features and differences of biofilm formation in the field of ceramic and titanium implants. Actual. Probl. Dent. 2023, 19, 5–11. [Google Scholar] [CrossRef]
- Kulakov, O.B.; Doktorov, A.A.; D’iakova, S.V.; Denisov-Nikol’skiĭ, I.; Grötz, K.A. Experimental study of osseointegration of zirconium and titanium dental implants. Morfologiia 2005, 127, 52–55. [Google Scholar]
- Reveron, H.; Fornabaio, M.; Palmero, P.; Fürderer, T.; Adolfsson, E.; Lughi, V.; Bonifacio, A.; Sergo, V.; Montanaro, L.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants: Transformation induced plasticity and its consequence on ceramic reliability. Acta Biomater. 2017, 48, 423–432. [Google Scholar] [CrossRef]
- Tanaka, S.; Takaba, M.; Ishiura, Y.; Kamimura, E.; Baba, K. A 3-year follow-up of ceria-stabilized zirconia/alumina nanocomposite (Ce-TZP/A) frameworks for fixed dental prostheses. J. Prosthodont. Res. 2015, 59, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Saulacic, N.; Erdosi, R.; Bosshardt, D.D.; Gruber, R.; Buser, D. Acid and alkaline etching of sandblasted zirconia implants: A histomorphometric study in miniature pigs. Clin. Implant. Dent. Res. 2014, 16, 313–322. [Google Scholar] [CrossRef]
- Fontoura, D.C.; Barros, V.M.; de Magalhães, C.S.; Vaz, R.R.; Moreira, A.N. Evaluation of vertical misfit of CAD/CAM implant-supported titanium and zirconia frameworks. Int. J. Oral Maxillofac. Implant. 2018, 33, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Tsymbalov, O.V. Dental Implantation for Periodontal Diseases (Monograph); EDVI: Krasnodar, Russia, 2014. [Google Scholar]
- Marin, E. Forged to heal: The role of metallic cellular solids in bone tissue engineering. Mater. Today Bio 2023, 23, 100777. [Google Scholar] [CrossRef] [PubMed]
- Barrack, R.L.; Burak, C.; Skinner, H.B. Concerns about ceramics in THA. Clin. Orthop. Relat. R. 2004, 429, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, S.N.; Volosova, M.A.; Okunkova, A.A. Advances in Electrical Discharge Machining of Insulating Ceramics. Materials 2023, 16, 5959. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, S.N.; Volosova, M.A.; Peretyagin, P.Y.; Seleznev, A.E.; Okunkova, A.A.; Smirnov, A. The effect of TiC additives on mechanical and electrical properties of Al2O3 ceramic. Appl. Sci. 2018, 8, 2385. [Google Scholar] [CrossRef]
- Vereschaka, A.S.; Grigoriev, S.N.; Sotova, E.S.; Vereschaka, A.A. Improving the efficiency of the cutting tools made of mixed ceramics by applying modifying nano-scale multilayered coatings. Adv. Mater. Res. 2013, 712–715, 391–394. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Vereschaka, A.A.; Vereschaka, A.S.; Kutin, A.A. Cutting tools made of layered composite ceramics with nano-scale multilayered coatings. Procedia CIRP 2012, 1, 301–306. [Google Scholar] [CrossRef]
- Solís, N.W.; Peretyagin, P.; Torrecillas, R.; Fernández, A.; Menéndez, J.L.; Mallada, C.; Díaz, L.A.; Moya, J.S. Electrically conductor black zirconia ceramic by SPS using graphene oxide. J. Electroceram. 2017, 38, 119–124. [Google Scholar] [CrossRef]
- Chopra, D.; Jayasree, A.; Guo, T.; Gulati, K.; Ivanovski, S. Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioact. Mater. 2022, 13, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Titanium. Available online: https://www.allmetals.ru/metals/titanium/ (accessed on 10 September 2023).
- Tantalum. Available online: https://www.allmetals.ru/metals/tantalum/ (accessed on 10 September 2023).
- Liu, Y.; Bao, C.; Wismeijer, D.; Wu, G. The physicochemical/biological properties of porous tantalum and the potential surface modification techniques to improve its clinical application in dental implantology. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Stiehler, M.; Lind, M.; Mygind, T.; Baatrup, A.; Dolatshahi-Pirouz, A.; Li, H.; Foss, M.; Besenbacher, F.; Kassem, M.; Bünger, C. Morphology, proliferation, and osteogenic differentiation of mesenchymal stem cells cultured on titanium, tantalum, and chromium surfaces. J. Biomed. Mater. Res. A 2008, 86, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, X.; Zhang, P.; Zhu, Z.; Xu, X. Effects of nano tantalum implants on inducing osteoblast proliferation and differentiation. Exp. Ther. Med. 2016, 12, 3541–3544. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gu, Y.; Qiao, S.; Zhou, L.; Shi, J.; Lai, H. Bacterial and mammalian cells adhesion to tantalum-decorated micro-/nano-structured titanium. J. Biomed. Mater. Res. A 2017, 105, 871–878. [Google Scholar] [CrossRef]
- Piglionico, S.; Bousquet, J.; Fatima, N.; Renaud, M.; Collart-dutilleul, P.-Y.; Bousquet, P. Porous tantalum vs. titanium implants: Enhanced mineralized matrix formation after stem cells proliferation and differentiation. J. Clin. Med. 2020, 9, 3657. [Google Scholar] [CrossRef]
- Magic, M.; Zupanek, G.; Lazic, Z.; El Chaar, E. Primary Stability of Trabecular Metal Implant in Comparison to Fully Threaded Implants: In Vitro Study Simulating Immediate Implant Placement. J. Oral. Implant. 2022, 48, 584–589. [Google Scholar] [CrossRef]
- Fraser, D.; Funkenbusch, P.; Ercoli, C.; Meirelles, L. Biomechanical analysis of the osseointegration of porous tantalum implants. J. Prosthet. Dent. 2020, 123, 811–820. [Google Scholar] [CrossRef]
- Bencharit, S.; Morelli, T.; Barros, S.; Seagroves, J.T.; Kim, S.; Yu, N.; Byrd, K.; Brenes, C.; Offenbacher, S. Comparing Initial Wound Healing and Osteogenesis of Porous Tantalum Trabecular Metal and Titanium Alloy Materials. J. Oral. Implant. 2019, 45, 173–180. [Google Scholar] [CrossRef]
- Benoit, B.; Fouad, Z.; Laflamme, G.H.; Rouleau, D.; Laflamme, G.Y. Augmentation of tibial plateau fractures with Trabecular Metal™: A biomechanical study. J. Orthop. Surg. Res. 2009, 4, 37. [Google Scholar] [CrossRef]
- Gorbatyuk, D.S.; Kolesov, S.V.; Sazhnev, M.L.; Pereverzev, V.S.; Kazmin, A.I. Tantalum based implants experimental and clinical aspects of application. Bull. Traumatol. Orthop. After N. N. Priorova 2018, 25, 71–83. [Google Scholar] [CrossRef]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. A 2020, 108, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, R.M.; Meyer, C.; Buckley, C.A.; Hanssen, A.D.; Lewallen, D.G. Mechanical Stability of Novel Highly Porous Metal Acetabular Components in Revision Total Hip Arthroplasty. J. Arthroplast. 2010, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Cachinho, S.C.P.; Correia, R.N. Titanium scaffolds for osteointegration: Mechanical, in vitro and corrosion behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Zardiackas, L.D.; Parsell, D.E.; Dillon, L.D.; Mitchell, D.W.; Nunnery, L.A.; Poggie, R. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J. Biomed. Mater. Res. B Appl. Biomater. 2001, 58, 180–187. [Google Scholar] [CrossRef]
- Peron, C.; Romanos, G. Immediate placement and occlusal loading of single-tooth restorations on partially threaded, titanium-tantalum combined dental implants: 1-year results. Int. J. Periodontics Restor. 2016, 36, 392–399. [Google Scholar] [CrossRef][Green Version]
- Brauner, E.; Di Carlo, S.; Ciolfi, A.; Pompa, G.; Jamshir, S.; De Angelis, F.; Della Monaca, M.; Valentini, V. Use of porous implants for the prosthetic rehabilitation of fibula free flap reconstructed patients. J. Craniofac. Surg. 2019, 30, 1163–1169. [Google Scholar] [CrossRef]
- Schlee, M.; van der Schoor, W.P.; van der Schoor, A.R.M. Immediate loading of trabecular metal-enhanced titanium dental implants: Interim results from an international proof-of-principle study. Clin. Implant Dent. Relat. Res. 2015, 17, e308–e320. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, X.; Lin, Y. Coating of Sandblasted and Acid-Etched Dental Implants with Tantalum Using Vacuum Plasma Spraying. Implant Dent. 2018, 27, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Almeida Alves, C.F.; Cavaleiro, A.; Carvalho, S. Bioactivity response of Ta1-xOx coatings deposited by reactive DC magnetron sputtering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Beline, T.; da Silva, J.H.D.; Matos, A.O.; Azevedo Neto, N.F.; de Almeida, A.B.; Nociti, F.H., Jr.; Leite, D.M.G.; Rangel, E.C.; Barão, V.A.R. Tailoring the synthesis of tantalum-based thin films for biomedical application: Characterization and biological response. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Wen, J.; Qian, S.; Cao, H.; Ning, C.; Pan, X.; Jiang, X.; Liu, X.; Chu, P.K. Enhanced osteointegration on tantalum-implanted polyetheretherketone surface with bone-like elastic modulus. Biomaterials 2015, 51, 173–183. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, L.; Zhu, W.; Wang, B.; Zhao, C.; Fang, L.; Ren, F. Antibacterial activity, corrosion resistance and wear behavior of spark plasma sintered Ta-5Cu alloy for biomedical applications. J. Mech. Behav. Biomed. 2017, 74, 315–323. [Google Scholar] [CrossRef]
- Hlinka, J.; Kraus, M.; Hajnys, J.; Pagac, M.; Petru, J.; Brytan, Z.; Tanski, T. Complex corrosion properties of aisi 316L steel prepared by 3D printing technology for possible implant applications. Materials 2020, 13, 1527. [Google Scholar] [CrossRef]
- Kayali, Y.; Aslan, O.; Karabaş, M.; Talaş, Ş. Corrosion behaviour of single and double layer hydroxyapatite coatings on 316L stainless steel by plasma spray. Prot. Met. Phys. Chem. Surf. 2016, 52, 1079–1085. [Google Scholar] [CrossRef]
- Pavlic, A.; Perissinotto, F.; Turco, G.; Contardo, L.; Stjepan, S. Do chlorhexidine and probiotics solutions provoke corrosion of orthodontic mini-implants? An in vitro study. Int. J. Oral Maxillofac. Implant. 2019, 34, 1379–1388. [Google Scholar] [CrossRef]
- Marques, R.A.; Rogero, S.O.; Terada, M.; Pieretti, E.F.; Costa, I. Localized Corrosion Resistance and Cytotoxicity Evaluation of Ferritic Stainless Steels for Use in Implantable Dental Devices with Magnetic Connections. Int. J. Electrochem. Sci. 2014, 9, 1340–1354. [Google Scholar] [CrossRef]
- Dos Santos, L.C.P.; Malheiros, F.C.; Guarato, A.Z. Surface parameters of as-built additive manufactured metal for intraosseous dental implants. J. Prosthet. Dent. 2020, 124, 217–222. [Google Scholar] [CrossRef] [PubMed]
- ISO 5832-1; Implants for Surgery—Metallic Materials—Part 1: Wrought Stainless Steel. The International Organization for Standard (ISO): Geneva, Switzerland, 2007.
- Aminian, A.; Shirzadi, B.; Azizi, Z.; Maedler, K.; Volkmann, E.; Hildebrand, N.; Maas, M.; Treccani, L.; Rezwan, K. Enhanced cell adhesion on bioinert ceramics mediated by the osteogenic cell membrane enzyme alkaline phosphatase. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Bulut, B.; Erkmen, Z.E.; Kayali, E.S. Biocompatibility of hydroxyapatite-alumina and hydroxyapatite-zirconia composites including commercial inert glass (CIG) as a ternary component. J. Ceram. Sci. Technol. 2016, 7, 263–275. [Google Scholar]
- Badran, Z.; Struillou, X.; Hughes, F.J.; Soueidan, A.; Hoornaert, A.; Ide, M. Silicon nitride (Si3N4) implants: The future of dental implantology? J. Oral. Implant. 2017, 43, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Purcel, G.; Surdu, V.-A.; Stoleriu, S. Influence of spark plasma sintering conditions on Si3N4 ceramics used for bone implants. Rev. Rom. Mater. 2015, 45, 160–170. [Google Scholar]
- Da Cruz, M.B.; Marques, J.F.; Peñarrieta-Juanito, G.M.; Costa, M.; Souza, J.C.M.; Magini, R.S.; Miranda, G.; Silva, F.S.; Caramês, J.M.M.; Da Mata, A.S.P. Bioactive-enhanced polyetheretherketone dental implant materials: Mechanical characterization and cellular responses. J. Oral. Implant. 2021, 47, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Abyzov, A.M. Aluminum oxide and aluminum oxide ceramics (review). Part 2. Foreign manufacturers of aluminum oxide ceramics. Technologies and research in the field of alumina ceramics. Novye Ogneupory 2019, 2, 13–22. (In Russian) [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Volosova, M.A.; Maslov, A.R.; Okunkova, A.A.; Smurov, I.Y. Highly Efficient Processing Technologies (Monograph); Mashinostroenie: Moscow, Russia, 2014; 455p. (In Russian) [Google Scholar]
- Sotova, E.S.; Vereshchaka, A.S.; Vereshchaka, A.A. Ceramic Cutting Tools (Monograph); MSUT “STANKIN”: Moscow, Russia, 2013; 149p. (In Russian) [Google Scholar]
- Grigoriev, S.N.; Hamdy, K.; Volosova, M.A.; Okunkova, A.A.; Fedorov, S.V. Electrical discharge machining of oxide and nitride ceramics: A review. Mater. Design 2021, 209, 109965. [Google Scholar] [CrossRef]
- Liu, X.-J.; Huang, Z.-Y.; Ge, Q.-M.; Sun, X.-W.; Huang, L.-P. Microstructure and mechanical properties of silicon nitride ceramics prepared by pressureless sintering with MgO–Al2O3–SiO2 as sintering additive. J. Eur. Ceram. Soc. 2005, 25, 3353–3359. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, Y.; Sun, C.; He, Z.; Li, Y.; Qiu, H.; Yuan, D.; Zhang, J.; Chu, C.; Shen, B. Preparation and characterization of metastable β-type titanium alloys with favorable properties for orthopedic applications. J. Alloys Compds 2023, 949, 169839. [Google Scholar] [CrossRef]
- Skinner, H.B. Composite technology for total hip arthroplasty. Clin. Orthop. Relat. Res. 1988, 235, 224–236. [Google Scholar] [CrossRef]
- Schwitalla, A.; Müller, W.-D. PEEK Dental Implants: A Review of the Literature. J. Oral. Implant. 2013, 39, 743–749. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z.; Zhang, Z.; Wu, G.; Sang, L. Biomimetic design and fabrication of PEEK and PEEK/CF cage with minimal surface structures by fused filament fabrication. J. Mater. Res. Technol. 2023, 26, 5001–5015. [Google Scholar] [CrossRef]
- Rakotoaridina, K.; Delrieu, J.; Pages, P.; Vergé, T.; Nasr, K.; Canceill, T. Evaluation of Poly(etheretherketone) Post’s Mechanical Strength in Comparison with Three Metal-Free Biomaterials: An In Vitro Study. Polymers 2023, 15, 3583. [Google Scholar] [CrossRef]
- Polyaryletherketone. Available online: https://rusplast.com/catalog/polyaryletherketone/ (accessed on 10 November 2023).
- Bilyalov, A.R.; Minasov, B.S.; Yakupov, R.R.; Akbashev, V.N.; Rafikova, G.A.; Bikmeev, A.T.; Chugunov, S.S.; Kireev, V.N.; Pavlov, V.N.; Krzyszkowska, Y.G. Using ceramic 3D printing for tissue engineering problems: A review. Polytrauma 2023, 1, 89–109. [Google Scholar]
- Webster, T.J.; Patel, A.A.; Rahaman, M.N.; Bal, B.S. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- El Awadly, T.A.; Wu, G.; Ayad, M.; Radi, I.A.W.; Wismeijer, D.; Abo El Fetouh, H.; Osman, R.B. A histomorphometric study on treated and untreated ceramic filled PEEK implants versus titanium implants: Preclinical in vivo study. Clin. Oral. Implant. Res. 2020, 31, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, I.; Takao, M.; Goto, T.; Ohtsuki, C.; Hibino, S.; Sugano, N. Interfacial shear strength of bioactive-coated carbon fiber reinforced polyetheretherketone after in vivo implantation. J. Orthop. Res. 2012, 30, 1618–1625. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zohaib, S.; Zafar, M.S. Bioactivity and osseointegration of PEEK are inferior to those of titanium: A systematic review. J. Oral. Implant. 2016, 42, 512–516. [Google Scholar] [CrossRef]
- Gheisarifar, M.; Thompson, G.A.; Drago, C.; Tabatabaei, F.; Rasoulianboroujeni, M. In vitro study of surface alterations to polyetheretherketone and titanium and their effect upon human gingival fibroblasts. J. Prosthet. Dent. 2021, 125, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Elawadly, T.; Radi, W.I.A.; El Khadem, A.; Osman, R.B. Can PEEK be an implant material? Evaluation of surface topography and wettability of filled versus unfilled peek with different surface roughness. J. Oral. Implant. 2017, 43, 456–461. [Google Scholar] [CrossRef]
- Sirandoni, D.; Leal, E.; Weber, B.; Noritomi, P.Y.; Fuentes, R.; Borie, E. Effect of different framework materials in implant-supported fixed mandibular prostheses: A finite element analysis. Int. J. Oral Maxillofac. Implant. 2019, 34, e107–e114. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.; Santos, L.A.; Baptista, C.A.R.P.; Bicalho, L.A.; Strecker, K.; Barboza, M.J.R.; Santos, C. Subcritical crack growth and fatigue life prediction of ZrO2-Al2O3 composite ceramic. Ceramica 2014, 60, 41–51. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Costa, M.M.; Alves, N.; Gasik, M.; Silva, F.S.; Miranda, G. Multi-material Ti6Al4V & PEEK cellular structures produced by Selective Laser Melting and Hot Pressing: A tribocorrosion study targeting orthopedic applications. J. Mech. Behav. Biomed. 2019, 89, 54–64. [Google Scholar]
- Warreth, A.; Ibieyou, N.; O’Leary, R.B.; Cremonese, M.; Abdulrahim, M. Dental implants: An overview. Dent. Update 2017, 44, 596–620. [Google Scholar] [CrossRef]
- Demiyanova, A.V.; Mitrofanov, E.A.; Simakin, S.B.; Sipkin, A.M. Analysis of ion-plasma technology application in medical products proceedings to be used for maxillofacial surgery (Review). IOP Conf. Ser-Mater. Sci. 2020, 781, 012015. [Google Scholar] [CrossRef]
- Kulakov, A.A.; Grigoryan, A.S.; Arkhipov, A.V. Impact of surface modifications of dental implants on their integration potential. Stomatologiya 2012, 91, 75–77. (In Russian) [Google Scholar]
- Lang, M.S.; Roselyn Cerutis, D.; Miyamoto, T.; Nunn, M.E. Cell attachment following instrumentation with titanium and plastic instruments, diode laser, and titanium brush on titanium, titanium-zirconium, and zirconia surfaces. Int. J. Oral Maxillofac. Implant. 2016, 31, 799–806. [Google Scholar] [CrossRef]
- Guarnieri, R.; Rappelli, G.; Piemontese, M.; Procaccini, M.; Quaranta, A. A double-blind randomized trial comparing implants with laser-microtextured and machined collar surfaces: Microbiologic and clinical results. Int. J. Oral Maxillofac. Implant. 2016, 31, 1117–1125. [Google Scholar] [CrossRef]
- Xing, R.; Lyngstadaas, S.P.; Ellingsen, J.E.; Taxt-Lamolle, S.; Haugen, H.J. The influence of surface nanoroughness, texture and chemistry of TiZr implant abutment on oral biofilm accumulation. Clin. Oral. Implant. Res. 2015, 26, 649–656. [Google Scholar] [CrossRef]
- Guarnieri, R.; Miccoli, G.; Reda, R.; Mazzoni, A.; Di Nardo, D.; Testarelli, L. Sulcus fluid volume, IL-6, and Il-1b concentrations in periodontal and peri-implant tissues comparing machined and laser-microtextured collar/abutment surfaces during 12 weeks of healing: A split-mouth RCT. Clin. Oral. Implant. Res. 2022, 33, 94–104. [Google Scholar] [CrossRef]
- Camarda, A.J.; Durand, R.; Benkarim, M.; Rompré, P.H.; Guertin, G.; Ciaburro, H. Prospective randomized clinical trial evaluating the effects of two different implant collar designs on peri-implant healing and functional osseointegration after 25 years. Clin. Oral. Implant. Res. 2021, 32, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Ko, Y.; Kye, S.-B.; Yang, S.-M. Human gingival fibroblast (HGF-1) attachment and proliferation on several abutment materials with various colors. Int. J. Oral Maxillofac. Implant. 2014, 29, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Zamparini, F.; Scialabba, V.S.; Gatto, M.R.; Piattelli, A.; Montebugnoli, L.; Gandolfi, M.G. A 3-year prospective cohort study on 132 calcium phosphate-blasted implants: Flap vs. flapless technique. Int. J. Oral Maxillofac. Implant. 2016, 31, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Corvino, E.; Pesce, P.; Mura, R.; Marcano, E.; Canullo, L. Influence of modified titanium abutment surface on peri-implant soft tissue behavior: A systematic review of in vitro studies. Int. J. Oral Maxillofac. Implant. 2020, 35, 503–519. [Google Scholar] [CrossRef]
- El-Din El-Helbawy, N.G.; El-Wahab El-Hatery, A.A.; Ahmed, M.H. Comparison of oxygen plasma treatment and sandblasting of titanium implant-abutment surface on bond strength and surface topography. Int. J. Oral Maxillofac. Implant. 2016, 31, 555–562. [Google Scholar] [CrossRef][Green Version]
- Turker, N.T.; Özarslan, M.M.; Buyukkaplan, U.S.; Başar, E.K. Effect of Different Surface Treatments Applied to Short Zirconia and Titanium Abutments. Int. J. Oral Maxillofac. Implant. 2020, 35, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhang, R.; Zhang, L.; Sun, Y.; Xie, Q. Effects of Different Microstructured Surfaces on the Osseointegration of CAD/CAM Zirconia Dental Implants: An Experimental Study in Rabbits. Int. J. Oral Maxillofac. Implant. 2020, 35, 1113–1121. [Google Scholar] [CrossRef]
- Lee, J.W.; Wen, H.B.; Gubbi, P.; Romanos, G.E. New bone formation and trabecular bone microarchitecture of highly porous tantalum compared to titanium implant threads: A pilot canine study. Clin. Oral. Implant. Res. 2018, 29, 164–174. [Google Scholar] [CrossRef]
- Hariharan, K.; Arumaikkannu, G. Influence of Hydroxyapatite coated Additive Manufactured polyamide substrate on Biocompatibility. Biomed. Res. 2015, 26, S15–S21. [Google Scholar]
- Chen, J.; Zhang, Z.; Chen, X.; Zhang, C.; Zhang, G.; Xu, Z. Design and manufacture of customized dental implants by using reverse engineering and selective laser melting technology. J. Prosthet. Dent. 2014, 112, 1088–1095. [Google Scholar] [CrossRef]
- Revilla-León, M.; Ceballos, L.; Martínez-Klemm, I.; Özcan, M. Discrepancy of complete-arch titanium frameworks manufactured using selective laser melting and electron beam melting additive manufacturing technologies. J. Prosthet. Dent. 2018, 120, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, S.; Peretyagin, N.; Apelfeld, A.; Smirnov, A.; Rybkina, A.; Kameneva, E.; Zheltukhin, A.; Gerasimov, M.; Volosova, M.; Yanushevich, O.; et al. Investigation of the Characteristics of MAO Coatings Formed on Ti6Al4V Titanium Alloy in Electrolytes with Graphene Oxide Additives. J. Compos. Sci. 2023, 7, 142. [Google Scholar] [CrossRef]
- Solís Pinargote, N.W.; Smirnov, A.; Peretyagin, N.; Seleznev, A.; Peretyagin, P. Direct Ink Writing Technology (3D Printing) of Graphene-Based Ceramic Nanocomposites: A Review. Nanomaterials 2020, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, S.; Smirnov, A.; Solis Pinargote, N.W.; Yanushevich, O.; Kriheli, N.; Kramar, O.; Pristinskiy, Y.; Peretyagin, P. Evaluation of Mechanical and Electrical Performance of Aging Resistance ZTA Composites Reinforced with Graphene Oxide Consolidated by SPS. Materials 2022, 15, 2419. [Google Scholar] [CrossRef]
- Smirnov, A.; Peretyagin, P.; Bartolomé, J.F. Processing and mechanical properties of new hierarchical metal-graphene flakes reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 3491–3497. [Google Scholar] [CrossRef]
- Peretyagin, N.Y.; Suminov, I.V.; Smirnov, A.; Krikheli, N.I.; Kramar, O.V.; Peretyagin, P.Y. Electrochemical deposition of ceramic-like coatings on Ti-6Al-4V parts fabricated by electron beam melting. J. Phys. Conf. Ser. 2021, 2144, 012017. [Google Scholar] [CrossRef]
- Smirnov, A.; Volosova, M.; Peretyagin, P.; Bartolomé, J.F. Tribological behaviour of a 3Y-TZP/Ta ceramic-metal biocomposite against ultrahigh molecular weight polyethylene (UHMWPE). Ceram. Int. 2018, 44, 1404–1410. [Google Scholar] [CrossRef]
- Guarnieri, R.; Serra, M.; Bava, L.; Grande, M.; Farronato, D.; Iorio-Siciliano, V. The impact of a laser-microtextured collar on crestal bone level and clinical parameters under various placement and loading protocols. Int. J. Oral Maxillofac. Implant. 2014, 29, 354–363. [Google Scholar] [CrossRef]
- Mastrangelo, F.; Quaresima, R.; Canullo, L.; Scarano, A.; Muzio, L.L.; Piattelli, A. Effects of novel laser dental implant microtopography on human osteoblast proliferation and bone deposition. Int. J. Oral Maxillofac. Implant. 2020, 35, 320–329. [Google Scholar] [CrossRef]
- Ferraris, S.; Warchomicka, F.; Iranshahi, F.; Rimondini, L.; Cochis, A.; Spriano, S. Electron beam structuring of Ti6Al4V: New insights on the metal surface properties influencing the bacterial adhesion. Materials 2020, 13, 409. [Google Scholar] [CrossRef]
- Hu, F.; Fan, X.; Peng, F.; Yan, X.; Song, J.; Deng, C.; Liu, M.; Zeng, D.; Ning, C. Characterization of Porous Titanium-Hydroxyapatite Composite Biological Coating on Polyetheretherketone (PEEK) by Vacuum Plasma Spraying. Coatings 2022, 12, 433. [Google Scholar] [CrossRef]
- Brunello, G.; Brun, P.; Gardin, C.; Ferroni, L.; Bressan, E.; Meneghello, R.; Zavan, B.; Sivolella, S. Biocompatibility and antibacterial properties of zirconium nitride coating on titanium abutments: An in vitro study. PLoS ONE 2018, 13, e0199591. [Google Scholar] [CrossRef] [PubMed]
- Prachar, P.; Bartakova, S.; Brezina, V.; Cvrcek, L.; Vanek, J. Cytocompatibility of implants coated with titanium nitride and zirconium nitride. Bratisl. Med. J. 2015, 116, 154–156. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Volosova, M.A.; Migranov, M.S.; Minin, I.V.; Shekhtman, S.R.; Suhova, N.A.; Gurin, V.D.; Pivkin, P.M. Nanostructured biocompatible Ti-TiN coating for implants with improved functional properties. Proc. SPIE 2021, 11867, 1186708. [Google Scholar]
- Tao, H.; Zhylinski, V.; Vereschaka, A.; Chayeuski, V.; Yuanming, H.; Milovich, F.; Sotova, C.; Seleznev, A.; Salychits, O. Comparison of the Mechanical Properties and Corrosion Resistance of the Cr-CrN, Ti-TiN, Zr-ZrN, and Mo-MoN Coatings. Coatings 2023, 13, 750. [Google Scholar] [CrossRef]
- Vereschaka, A.; Grigoriev, S.; Chigarev, A.; Milovich, F.; Sitnikov, N.; Andreev, N.; Sotova, C.; Bublikov, J. Development of a Model of Crack Propagation in Multilayer Hard Coatings under Conditions of Stochastic Force Impact. Materials 2021, 14, 260. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Tabakov, V.; Sitnikov, N.; Andreev, N.; Sviridova, T.; Bublikov, J. Investigation of multicomponent nanolayer coatings based on nitrides of Cr, Mo, Zr, Nb, and Al. Surf. Coat. Technol. 2020, 401, 126258. [Google Scholar] [CrossRef]
- Alves, C.F.A.; Calderón, S.V.; Dias, D.; Carvalho, S. Influence of Oxygen content on the electrochemical behavior of Ta1-xOx coatings. Electrochim. Acta 2016, 211, 385–394. [Google Scholar] [CrossRef]
- Liu, F.; Li, Y.; Liang, J.; Sui, W.; Bellare, A.; Kong, L. Effects of micro/nano strontium-loaded surface implants on osseointegration in ovariectomized sheep. Clin. Implant Dent. Relat. Res. 2019, 21, 377–385. [Google Scholar] [CrossRef]
- Yokota, S.; Nishiwaki, N.; Ueda, K.; Narushima, T.; Kawamura, H.; Takahashi, T. Evaluation of thin amorphous calcium phosphate coatings on titanium dental implants deposited using magnetron sputtering. Implant Dent. 2014, 23, 343–350. [Google Scholar] [CrossRef]
- Pan, Y.-H.; Yi Lin, J.C.; Chen, M.K.; Salamanca, E.; Choy, C.S.; Tsai, P.-Y.; Leu, S.-J.; Yang, K.-C.; Huang, H.-M.; Yao, W.-L.; et al. Glow discharge plasma treatment on zirconia surface to enhance osteoblastic-like cell differentiation and antimicrobial effects. Materials 2020, 13, 3771. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.; Riahinezhad, S.; Li, Y.; Vaughan, M.B.; Sultana, F.; Morris, T.L.; Phinney, L.; Hossain, K. Plasma nitriding of titanium alloy: Effect of roughness, hardness, biocompatibility, and bonding with bone cement. Bio-Med. Mater. Eng. 2016, 27, 461–474. [Google Scholar] [CrossRef]
- Canullo, L.; Genova, T.; Wang, H.-L.; Carossa, S.; Mussano, F. Plasma of argon increases cell attachment and bacterial decontamination on different implant surfaces. Int. J. Oral Maxillofac. Implant. 2017, 32, 1315–1323. [Google Scholar] [CrossRef]
- Ogawa, T. Ultraviolet Photofunctionalization of Titanium Implant. Int. J. Oral Maxillofac. Implant. 2014, 29, 95–102. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.I.; Yang, S.S.; Kim, B.S.; Kim, B.C.; Lee, J. The effect of alendronate soaking and ultraviolet treatment on bone–implant interface. Clin. Oral. Implant. Res. 2017, 28, 1164–1172. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, T.; Wen, J.; Xu, L.; Zeng, D.; Wu, Q.; Cao, L.; Lin, S.; Liu, X.; Jiang, X. Selective responses of human gingival fibroblasts and bacteria on carbon fiber reinforced polyetheretherketone with multilevel nanostructured TiO2. Biomaterials 2016, 83, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Meng, F.; Liu, X. Antimicrobial activity of tantalum oxide coatings decorated with Ag nanoparticles. J. Vac. Sci. Technol. A 2016, 34, 04C102. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; You, J. Surface modification of TiO2 coatings by Zn ion implantation for improving antibacterial activities. Bull. Mater. Sci. 2016, 39, 285–291. [Google Scholar] [CrossRef]
- Benalcázar Jalkh, E.B.; Parra, M.; Torroni, A.; Nayak, V.V.; Tovar, N.; Castellano, A.; Badalov, R.M.; Bonfante, E.A.; Coelho, P.G.; Witek, L. Effect of supplemental acid-etching on the early stages of osseointegration: A preclinical model. J. Mech. Behav. Biomed. 2021, 122, 104682. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, Y.W.; Soare, R.V.; Leite Assis, M.A.; Zenóbio, E.G.; Girundi, F.M. Titanium Surface Roughing Treatments contribute to Higher Interaction with Salivary Proteins MG2 and Lactoferrin. J. Contemp. Dent. Pract. 2015, 16, 141–146. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111505. [Google Scholar] [CrossRef] [PubMed]
- Al Mustafa, M.; Agis, H.; Müller, H.-D.; Watzek, G.; Gruber, R. In vitro adhesion of fibroblastic cells to titanium alloy discs treated with sodium hydroxide. Clin. Oral. Implant. Res. 2015, 26, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Song, I.-H.; Kim, S. Water glass coating on a Ti substrate to form Si-OH groups for improving cell behaviors of dental implants. Korean J. Met. Mater. 2014, 52, 123–128. [Google Scholar] [CrossRef]
- Lee, K.; Shin, G. RF magnetron sputtering coating of hydroxyapatite on alkali solution treated titanate nanorods. Arch. Metall. Mater. 2015, 60, 1319–1322. [Google Scholar] [CrossRef]
- Karl, M.; Albrektsson, T. Clinical performance of dental implants with a moderately rough (TiUnite) surface: A meta-analysis of prospective clinical studies. Int. J. Oral Maxillofac. Implant. 2017, 32, 717–734. [Google Scholar] [CrossRef]
- Martínez-Rus, F.; Prieto, M.; Salido, M.P.; Madrigal, C.; Özcan, M.; Pradíes, G. A clinical study assessing the influence of anodized titanium and zirconium dioxide abutments and periimplant soft tissue thickness on the optical outcome of implant-supported lithium disilicate single crowns. Int. J. Oral Maxillofac. Implant. 2017, 32, 156–163. [Google Scholar] [CrossRef]
- Kasatkin, V.E.; Kasatkina, I.V.; Bogdashkina, N.L.; Gerasimov, M.V.; Krit, B.L.; Grigoriev, S.N.; Suminov, I.V.; Kozlov, I.A. Influence of different modes of microarc oxidation of titanium on the electrochemical properties and surface morphology of the obtained coatings. Surf. Eng. 2023, 39, 295–306. [Google Scholar] [CrossRef]
- Djošić, M.; Janković, A.; Mišković-Stanković, V. Electrophoretic deposition of biocompatible and bioactive hydroxyapatite-based coatings on titanium. Materials 2021, 14, 5391. [Google Scholar] [CrossRef]
- Prem Ananth, K.; Joseph Nathanael, A.; Jose, S.P.; Oh, T.H.; Mangalaraj, D. A novel silica nanotube reinforced ionic incorporated hydroxyapatite composite coating on polypyrrole coated 316L SS for implant application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Mehboob, H.; Awais, M.; Khalid, H.; Ch, A.A.; Siddiqi, S.A.; Rehman, I. Polymer-assisted deposition of hydroxyapatite coatings using electrophoretic technique. Biomed. Eng. Appl. Basis Commun. 2014, 26, 1450073. [Google Scholar] [CrossRef]
- Zhenga, C.Y.; Niea, F.L.; Zhenga, Y.F.; Cheng, Y.; Wei, S.C.; Ruan, L.; Valiev, R.Z. Enhanced corrosion resistance and cellular behavior of ultrafine-grained bio-medical NiTi alloy with a novel SrO-SiO2-TiO2 sol-gel coating. Appl. Surf. Sci. 2011, 257, 5913–5918. [Google Scholar] [CrossRef]
- Mohammad, N.F.; Ahmad, R.N.; Mohd Rosli, N.L.; Abdul Manan, M.S.; Marzuki, M.; Wahi, A. Sol gel deposited hydroxyap-atite-based coating technique on porous titanium niobium for biomedical applications: A mini review. Mater. Today 2021, 41, 127–135. [Google Scholar]
- Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol–gel films on titanium alloy fracture plate material. Biomaterials 2007, 28, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Panah, N.; Page, F.; Gholami, M.; Dastfal, A.; Sharma, L.A.; Shahmabadi, H.E. Hydrogel-based therapeutic coatings for dental implants. Eur. Polym. J. 2022, 181, 111652. [Google Scholar] [CrossRef]
- Ali, M.; Ali, F.; Yang, B.; Abbas, A. A comprehensive account of biomedical applications of CVD diamond coatings. J. Phys. D Appl. Phys. 2021, 54, 443001. [Google Scholar] [CrossRef]
- Gu, M.; Lv, L.; Du, F.; Niu, T.; Chen, T.; Xia, D.; Wang, S.; Zhao, X.; Liu, J.; Liu, Y.; et al. Effects of thermal treatment on the adhesion strength and osteoinductive activity of single-layer graphene sheets on titanium substrates. Sci. Rep. 2018, 8, 8141. [Google Scholar] [CrossRef]
- Strąkowska, P.; Beutner, R.; Gnyba, M.; Zielinski, A.; Scharnweber, D. Electrochemically assisted deposition of hydroxyapatite on Ti6Al4V substrates covered by CVD diamond films—Coating characterization and first cell biological results. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 624–635. [Google Scholar] [CrossRef]
- Shubladze, G.K. Comparison of implant anodized surface advantage with other types of surfaces. Med. Alph. 2014, 3, 20–25. (In Russian) [Google Scholar]
- Rajan, A.; Sivarajan, S.; Vallabhan, C.G.; Nair, A.S.; Jayakumar, S.; Pillai, A.S. An in vitro study to evaluate and compare the hemocompatibility of titanium and zirconia implant materials after sandblasted and acid etched surface treatment. J. Contemp. Dent. Pract. 2018, 19, 1449–1455. [Google Scholar]
- Kim, H.-C.; Park, S.-Y.; Han, M.-S.; Lee, Y.-M.; Ku, Y.; Rhyu, I.-C.; Seol, Y.-J. Occurrence of progressive bone loss around anodized surface implants and resorbable blasting media implants: A retrospective cohort study. J. Periodontol. 2017, 88, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and acid etched titanium dental implant surfaces systematic review and confocal microscopy evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef] [PubMed]
- Gnedenkov, S.V.; Sharkeev, Y.P.; Sinebryukhov, S.L.; Khrisanfova, O.A.; Legostaeva, E.V.; Zavidnaya, A.G.; Puz’, A.V.; Khlusov, I.A.; Opra, D.P. Functional coatings formed on the titanium and magnesium alloys as implant materials by plasma electrolytic oxidation technology: Fundamental principles and synthesis conditions. Corros. Rev. 2016, 34, 65–83. [Google Scholar] [CrossRef]
- Momesso, G.A.C.; de Souza Santos, A.M.; Fonseca e Santos, J.M.; da Cruz, N.C.; Okamoto, R.; Ricardo Barão, V.A.; Siroma, R.S.; Shibli, J.A.; Faverani, L.P. Comparison between plasma electrolytic oxidation coating and sandblasted acid-etched surface treatment: Histometric, tomographic, and expression levels of osteoclastogenic factors in osteoporotic rats. Materials 2020, 13, 1604. [Google Scholar] [CrossRef] [PubMed]
- Apachiteia, I.; Leonia, A.; Riemslagb, A.C.; Fratila-Apachitei, L.E.; Duszczyk, J. Enhanced fatigue performance of porous coated Ti6Al4V biomedical alloy. Appl. Surf. Sci. 2011, 257, 6941–6944. [Google Scholar] [CrossRef]
- Apelfeld, A.; Grigoriev, S.; Krit, B.; Ludin, V.; Suminov, I.; Chudinov, D. Improving the stability of the coating properties for group plasma electrolytic oxidation. Manuf. Lett. 2022, 33, 54–59. [Google Scholar] [CrossRef]
- Kusmanov, S.; Mukhacheva, T.; Tambovskiy, I.; Kusmanova, I.; Shadrin, S.; Belov, R.; Nikiforov, R.; Suminov, I.; Karasev, M.; Grigoriev, S. Possibilities of Duplex Plasma Electrolytic Treatment for Increasing the Hardness and Wear Resistance of a Commercially Pure Titanium Surface. Coatings 2023, 13, 1363. [Google Scholar] [CrossRef]
- Krit, B.L.; Apelfeld, A.V.; Borisov, A.M.; Morozova, N.V.; Rakoch, A.G.; Suminov, I.V.; Grigoriev, S.N. Plasma Electrolytic Modification of Zirconium and Its Alloys: Brief Review. Materials 2023, 16, 5543. [Google Scholar] [CrossRef]
- Grigoriev, S.; Peretyagin, N.; Apelfeld, A.; Smirnov, A.; Morozov, A.; Torskaya, E.; Volosova, M.; Yanushevich, O.; Yarygin, N.; Krikheli, N.; et al. Investigation of Tribological Characteristics of PEO Coatings Formed on Ti6Al4V Titanium Alloy in Electrolytes with Graphene Oxide Additives. Materials 2023, 16, 3928. [Google Scholar] [CrossRef]
- Tverdokhlebov, S.I.; Shesterikov, E.V.; Malchikhina, A.I. Formation hybrid method of multilayer biocoatings based on PVD technology. Adv. Mater. Res. 2014, 872, 241–247. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, T.; Zhou, W.; Lin, Z.; Liu, Z. Effect of plasma oxidation-treated TiOx film on early osseointegration. Int. J. Oral Maxillofac. Implant. 2018, 33, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Velten, M.F.; Odatsu, T.; Aswath, P.B.; Kamiya, N.; Kim, H.; Varanasi, V.G. PeCVD SiOx accelerates hydroxyapatite surface formation for enhanced early osteogenic differentiation. Ceram. Trans. 2014, 251, 105–113. [Google Scholar]
- Novikov, S.V.; Tamazov, I.D.; Matveev, A.I.; Topoljanskij, P.A.; Topoljanskij, A.P. Optimization of the surface of titanium dental implants of grade 5 alloy by barrier glass ceramic coating. Clin. Dent. 2021, 24, 29–36. [Google Scholar] [CrossRef]
- Gao, F.; Li, G.; Xia, Y. Influence of hysteresis effect on properties of reactively sputtered TiAlSiN films. Appl. Surf. Sci. 2018, 431, 160–164. [Google Scholar] [CrossRef]
- Momozawa, A.; Katsui, H.; Ito, A.; Goto, T. Dental application of TiO2 films prepared by laser CVD. J. Jpn. Soc. Powder Metall. 2016, 63, 128–131. [Google Scholar] [CrossRef]
- Pou, J.; Lusquiños, F.; Comesaña, R.; Boutinguiza, M. Production of biomaterial coatings by laser-assisted processes. In Advances in Laser Materials Processing: Technology, Research and Application; Lawrence, J., Pou, J., Low, D.K.Y., Toyserkani, E., Eds.; Woodhead Publishing: Cambridge, UK, 2010; pp. 394–425. [Google Scholar]
- Al-Moraissi, E.A.; Oginni, F.O.; Holkom, M.A.M.; Mohamed, A.A.S.; Al-Sharani, H.M. Tissue-Engineered bone using mesenchymal stem cells versus conventional bone grafts in the regeneration of maxillary alveolar bone: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2020, 35, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Garcés, M.; Alvira-González, J.; Sánchez, C.M.; Cairó, J.R.B.; del Pozo, M.R.; Gay-Escoda, C. Bone regeneration using adipose-derived stem cells with fibronectin in dehiscence-type defects associated with dental implants: An experimental study in a dog model. Int. J. Oral Maxillofac. Implant. 2017, 32, e97–e106. [Google Scholar] [CrossRef]
- Zanicotti, D.G.; Duncan, W.J.; Seymour, G.J.; Coates, D.E. Effect of titanium surfaces on the osteogenic differentiation of human adipose-derived stem cells. Int. J. Oral Maxillofac. Implant. 2018, 33, e77–e87. [Google Scholar] [CrossRef]
- Yoo, S.-Y.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y.; Lee, J.-H.; Park, J.-M. Biochemical responses of anodized titanium implants with a poly(lactide-co-glycolide)/bone morphogenic protein-2 submicron particle coating. part 1: An in vitro study. Int. J. Oral Maxillofac. Implant. 2015, 30, 512–518. [Google Scholar] [CrossRef]
- Knopf-Marques, H.; Barthes, J.; Lachaal, S.; Mutschler, A.; Muller, C.; Dufour, F.; Rabineau, M.; Courtial, E.-J.; Bystroňová, J.; Marquette, C.; et al. Multifunctional polymeric implant coatings based on gelatin, hyaluronic acid derivative and chain length-controlled poly(arginine). Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109898. [Google Scholar] [CrossRef] [PubMed]
- Horasawa, N.; Yamashita, T.; Uehara, S.; Udagawa, N. High-performance scaffolds on titanium surfaces: Osteoblast differentiation and mineralization promoted by a globular fibrinogen layer through cell-autonomous BMP signaling. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 86–96. [Google Scholar] [CrossRef]
- de Oliveira Fernandes, G.V.; Santos, N.B.M.; de Sousa, M.F.C.; Fernandes, J.C.H. Liquid Platelet-Rich Fibrin Coating Implant Surface to Enhance Osseointegration: A Double-Blinded, Randomized Split-Mouth Trial with 1-Year Follow-up. Int. J. Oral Maxillofac. Implant. 2022, 37, 159–170. [Google Scholar] [CrossRef]
- Öncü, E.; Alaaddinoğlu, E.E. The effect of platelet-rich fibrin on implant stability. Int. J. Oral Maxillofac. Implant. 2015, 30, 578–582. [Google Scholar] [CrossRef]
- Shah, R.; Thomas, R.; Gowda, T.M.; Baron, T.K.A.; Vemanaradhya, G.G.; Bhagat, S. In Vitro Evaluation of Osteoblast Response to the Effect of Injectable Platelet-rich Fibrin Coating on Titanium Disks. J. Contemp. Dent. Pract. 2021, 22, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Kulakov, A.A.; Kasparov, A.S.; Porfenchuk, D.A. Factors affecting osseointegration and the use of early functional loading to reduce the treatment time for dental implantation. Stomatologiya 2019, 4, 107–115. [Google Scholar]
- Nakazawa, M.; Yamada, M.; Wakamura, M.; Egusa, H.; Sakurai, K. Activation of osteoblastic function on titanium surface with titanium-doped hydroxyapatite nanoparticle coating: An in vitro study. Int. J. Oral Maxillofac. Implant. 2017, 32, 779–791. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Liao, Y.; Li, J.; Zhang, L.; Hu, J. The Effect of Magnesium-Incorporated Hydroxyapatite Coating on Titanium Implant Fixation in Ovariectomized Rats. Int. J. Oral Maxillofac. Implant. 2014, 29, 196–202. [Google Scholar] [CrossRef]
- Martinez, E.F.; Ishikawa, G.J.; de Lemos, A.B.; Bezerra, F.J.B.; Sperandio, M.; Napimoga, M.H. Evaluation of a Titanium surface treated with hydroxyapatite nanocrystals on osteoblastic cell behavior: An in vitro study. Int. J. Oral Maxillofac. Implant. 2018, 33, 597–602. [Google Scholar] [CrossRef]
- Thiem, D.G.E.; Adam, M.; Ganz, C.; Gerber, T.; Kämmerer, P.W. The implant surface and its role in affecting the dynamic processes of bone remodeling by means of distance osteogenesis: A comparative in vivo study. Int. J. Oral Maxillofac. Implant. 2019, 34, 133–140. [Google Scholar] [CrossRef]
- Thoma, D.S.; Jung, U.-W.; Park, J.-Y.; Bienz, S.P.; Hüsler, J.; Jung, R.E. Bone augmentation at peri-implant dehiscence defects comparing a synthetic polyethylene glycol hydrogel matrix vs. standard guided bone regeneration techniques. Clin. Oral. Implant. Res. 2017, 28, e76–e83. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Volchenkova, V.A.; Fomina, A.A.; Mamin, G.V.; Shurtakova, D.V.; Kalita, V.I. Plasma-sprayed manganese-containing tricalcium phosphate coatings on titanium. Inorg. Mater. 2021, 57, 967–972. [Google Scholar] [CrossRef]
- Mayborodin, I.V.; Toder, M.S.; Shevela, A.I.; Razumakhina, M.S.; Shevela, A.A.; Patrushev, A.Y.; Ragimova, T.M.; Kuznetsova, I.V. Histological results of implantation of metal products with rough and smooth surfaces into bone tissue in an experiment. Fund Res. 2014, 7, 114–118. [Google Scholar]
- Gadallah, M.A.; Darwish, R.M.; Alshimy, A.M.; Gepreel, M.A.; Marei, M.K. Biomimetic Coating of Titanium Surface Using Bioactive Glass Nanoparticles. Int. J. Oral Maxillofac. Implant. 2022, 37, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Climent, M.; Romero Ruiz, M.M.; Calvo, P.L.; Santos, J.V.R.; Perez, R.A.; Gil Mur, F.J. Effectiveness of a new dental implant bioactive surface: Histological and histomorphometric comparative study in minipigs. Clin. Oral. Investig. 2018, 22, 1423–1432. [Google Scholar] [CrossRef]
- Janson, O.; Gururaj, S.; Pujari-Palmer, S.; Karlsson Ott, M.; Strømme, M.; Engqvist, H.; Welch, K. Titanium surface modification to enhance antibacterial and bioactive properties while retaining biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 272–279. [Google Scholar] [CrossRef]
- Hao, J.; Chou, J.; Kuroda, S.; Otsuka, M.; Kasugai, S.; Lang, N.P. Strontium hydroxyapatite in situ gel-forming system—A new approach for minimally invasive bone augmentation. Clin. Oral. Implant. Res. 2015, 26, 581–585. [Google Scholar] [CrossRef]
- Badiadka, N. Studies on characteristics and corrosion behaviour of chitosan/eudragit RS100 bilayer film coated Ti-6Al-4V. Indian J. Chem. Technol. 2023, 30, 570–578. [Google Scholar]
- Fontelo, R.; da Costa, D.S.; Gomez-Florit, M.; Tiainen, H.; Reis, R.L.; Novoa-Carballal, R.; Pashkuleva, I. Antibacterial nanopatterned coatings for dental implants. J. Mater. Chem. B 2022, 10, 8710–8718. [Google Scholar] [CrossRef]
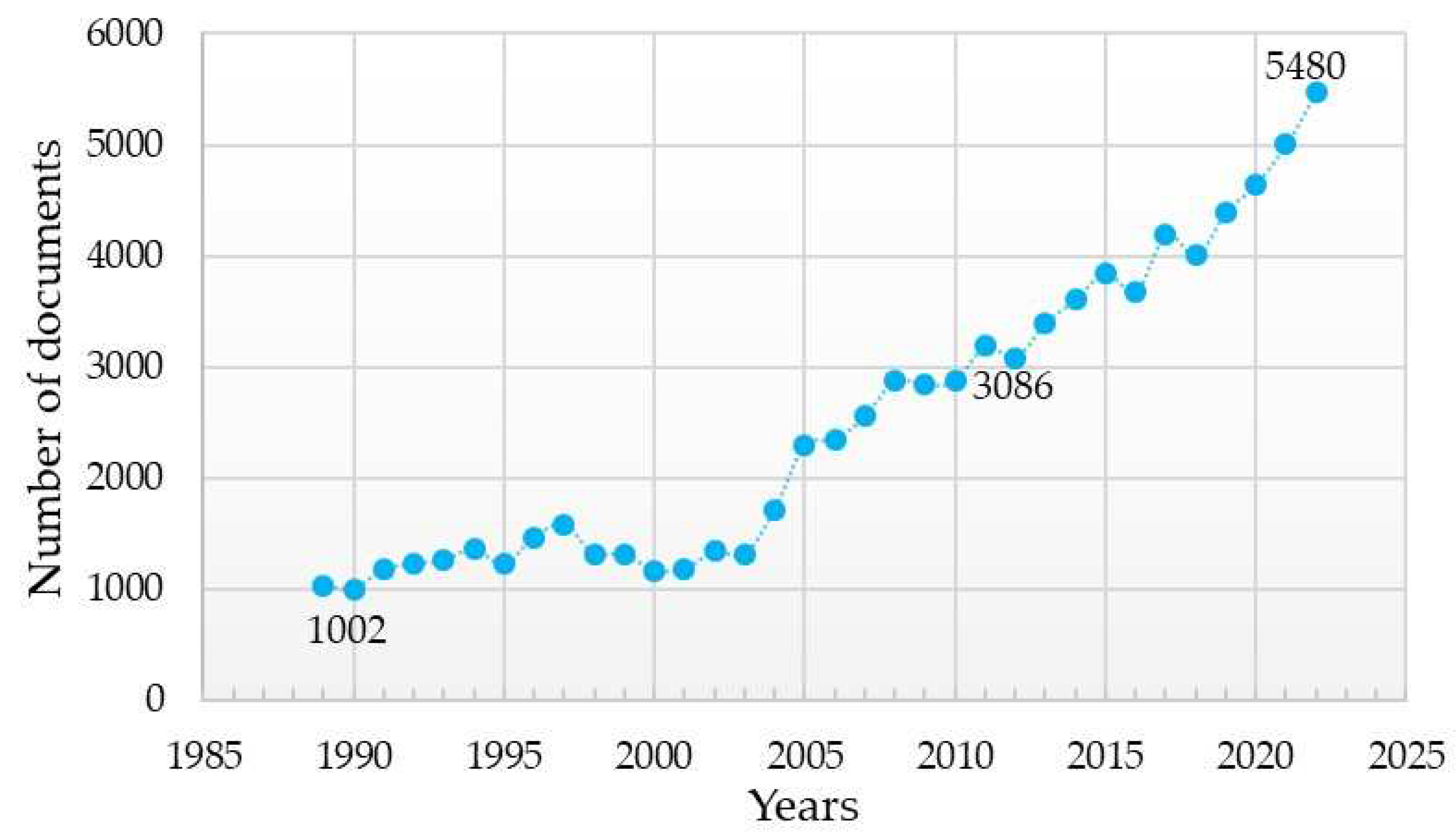

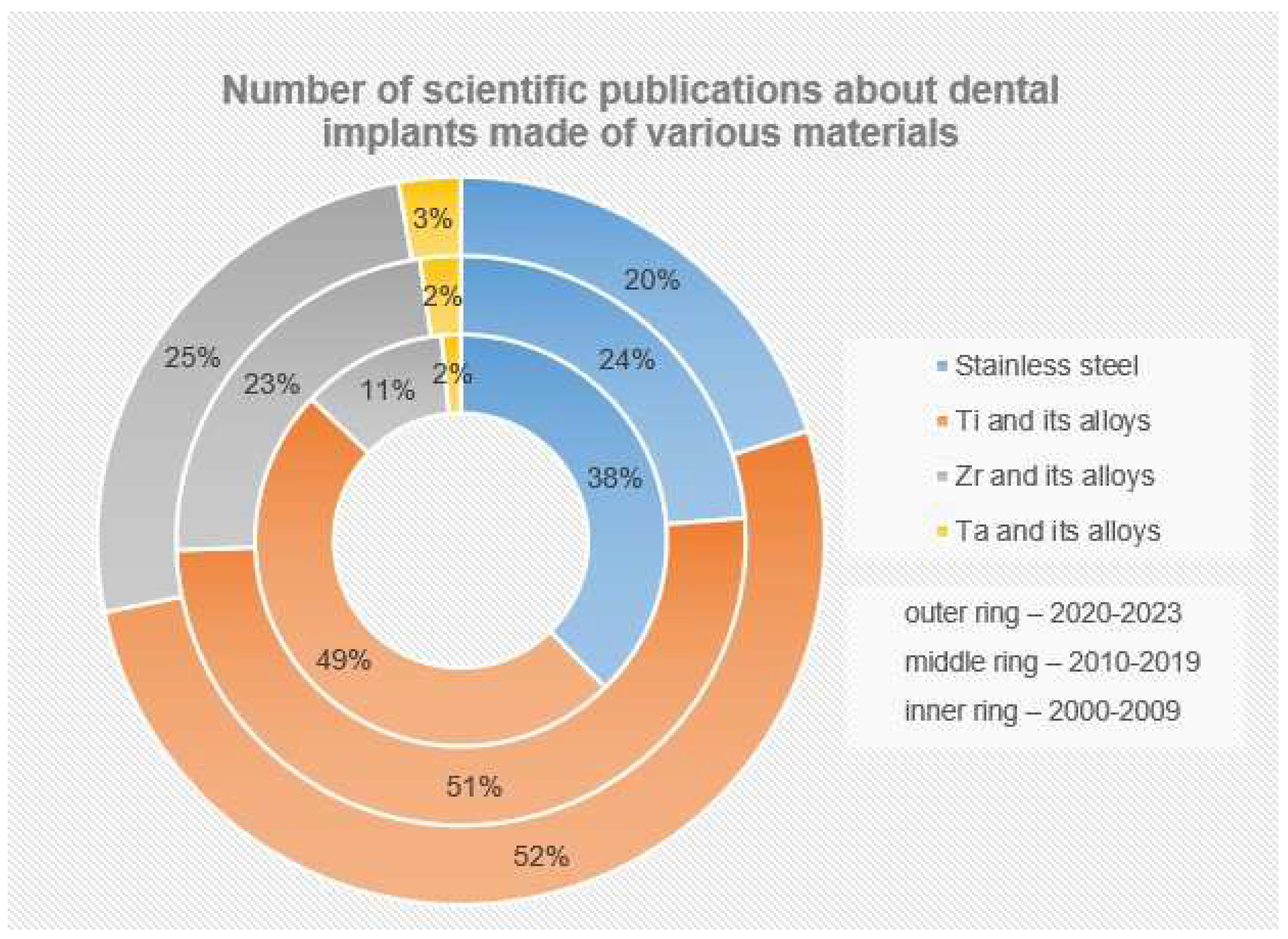
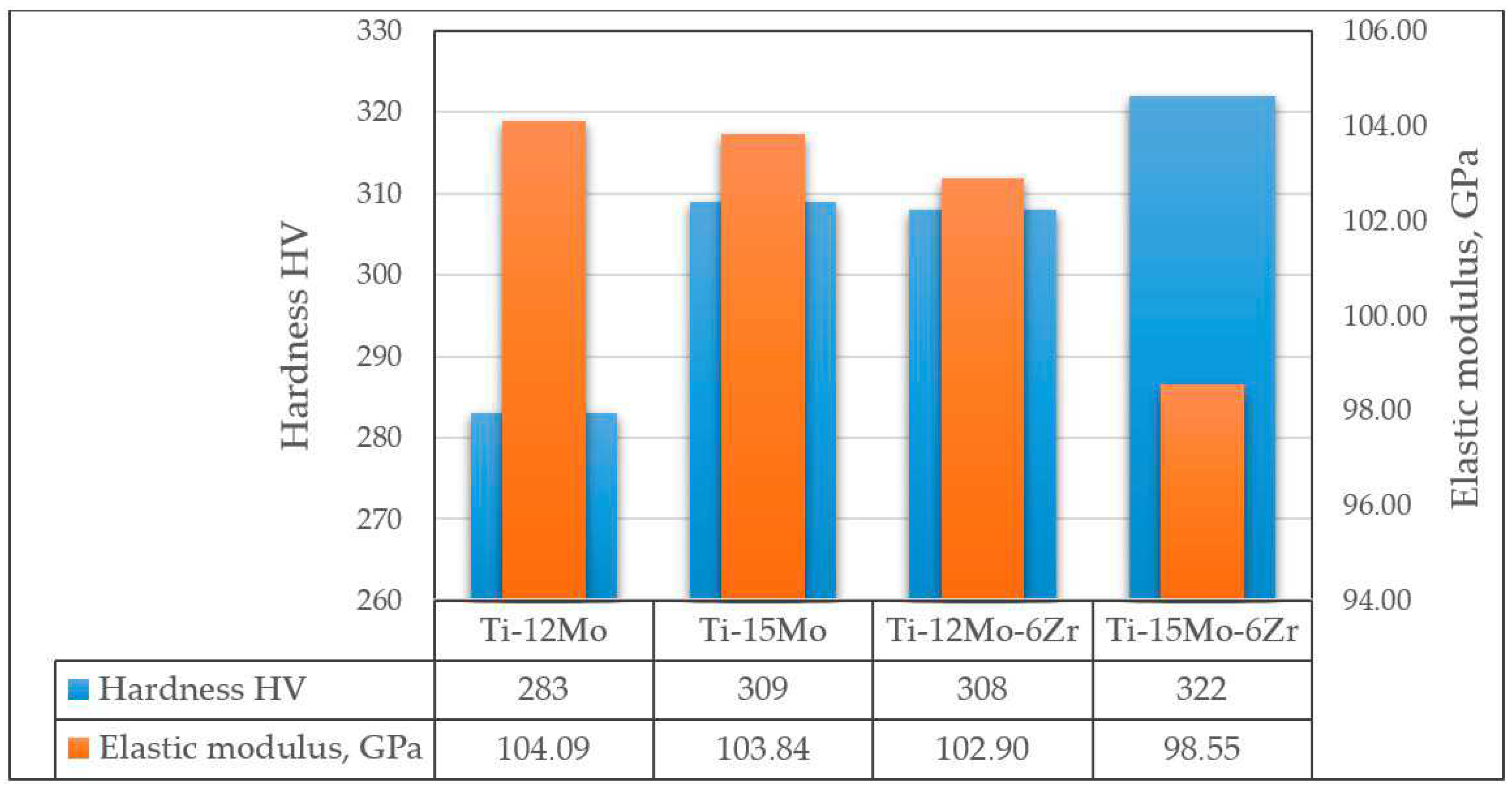
| Double Alloys | Triple Alloys | More Complex Alloys |
|---|---|---|
| Ti-Al | Ti-Al-V [62,63,64,65,66,67] | |
| Ti-Al-Nb [77,78,79,80] | Ti-Al-Nb + YSZ (ZrO2, stabilised Y) [81] | |
| Ti-Nb [53,82,83,84] | Ti-Nb-Zr (TNZ alloys) [76,85,86,87] | Ti-Nb-Zr-Ta [88,89] |
| Ti-Nb-Zr-Ta-Si [90] | ||
| Ti-Nb-Ta [91] | ||
| Ti-Nb-Cu [92,93] | Ti-Nb-Cu-Ga [93] | |
| Ti-Nb-Ga [93,94,95] | ||
| Ti-Nb-Mo [75,96] | Ti-Nb-Mo-Zr-Sn [97] | |
| Ti-Nb-Mo-Ta [98] | ||
| Ti-Zr [68,69,70,71,99,100,101,102,103] | Ti-Zr-Nb [104,105] | Ti-Zr-Nb-Ta [104] |
| Ti-Zr-Fe [106] | Ti-Zr-Fe-Si [107] | |
| Ti-Ta [108,109,110,111,112] | Ti-Ta-Zr [113,114] | Ti-Ta-Zr-Nb [115] |
| Ti-Ta-Zr-Mo [116] | ||
| Ti-Cu [117,118,119] | Ti-Cu-Al [120] | |
| Ti-Cu-Nb [121] | ||
| Ti-Mo [122,123,124] | Ti-Mo-Zr [123] | Ti-Mo-Zr-Fe [125] |
| Ti-Mg [126,127,128,129] | Ti-Mg-Sr [130] | |
| Ti-Fe [131] | Te-Fe-Ge [132] | |
| Ti-Ca [133] | ||
| Ti-Pd [133,134,135] | ||
| Ti-Pt [133] |
| Material | Compressive Strength, MPa | Tensile Strength, MPa | Modulus of Elasticity, GPa | Hardness HV, GPa |
|---|---|---|---|---|
| ZrO2 | 1300–2000 | 150 | 190–210 | 10–13 |
| Al2O3 | 2100–2500 | 260 | 221–400 | 12.5–21 |
| Si3N4 | 2200–3000 | <350 | 210–300 | 15–22 |
| PEEK | 118–240 | 100–230 | 3.6–3.8 | 0.7 |
| PEEK with carbon fibres | 280–300 | 250–260 | 14–18 | 0.8 |
| cp-Ti | 235–353 | 860 | 100–105 | 1.8–2.2 |
| Ti-6Al-4V | 990–1565 | 689 | 110–115 | 3.1–3.5 |
| Group | Method | Features (Characteristics) of the Method |
|---|---|---|
| Mechanical treatment | Machining through cutting (machine stroking) [7,287,288,289,290,291,292] | It is performed in order to increase the surface roughness in order to increase the osteointegration of the implant. This method is inefficient, as the rough surface obtained by this method is much inferior to the porous one as per this indicator. The main materials of implants belong to the class of hard-to-machine materials. |
| Sandblasting [7,293,294,295,296,297] | A simple and inexpensive method. Cell adhesion, proliferation, and the differentiation of osteoblasts are improved. However, it is often necessary to carry out acid etching of the implant to homogenize the surface microprofile in order to remove the remaining sand particles. Otherwise, the inhomogeneity of the surface material reduces the corrosion resistance of the implant. | |
| Physical Techniques | Additive technologies [25,285,298,299,300,301,302,303,304,305,306,307] | It allows to create complex three-dimensional structures (e.g., trabecular, heroic), which increase the osteoconductivity of implants. Compared to traditional methods of manufacturing complex profile products, it is possible to save resources (materials, energy, labor costs). |
| Laser ablation (including laser surface texturing) [288,290,308,309] | Compared to machining, surface texturing reduces the operating costs of the production process (no consumables (e.g., cutting tools)). Provides an effective way to clean the surface of the product without the use of any chemicals. Ability to automate the texturing process with high precision. Allows the creation of micro-caverns—cavities with a diameter of a few micrometers—with an orderly programmed structure. Increases the osseointegration of the implant due to the creation of the precise “branched” macrostructure (topography) of the surface. Highly energetic and expensive processing method. | |
| Electron beam (EB) structuring/surface texturing [310] | It differs from laser texturing in that instead of a laser beam, a sharply focused beam of electrons moving at high speed is used for technological purposes. EL texturing shows a statistically significant reduction in bacterial adhesion in the absence of antibacterial agents (even after implant polishing). High-energy processing method. | |
| Plasma spraying [7,253,311] | Economical and safe method. Possibility of applying a wide variety of metal, ceramic, or plastic coatings at atmospheric pressure. Possibility of obtaining composite bioactive substances—in particular, osseointegration enhancing and coatings (e.g., porous hydroxyapatite reinforced with titanium). | |
| Vacuum arc coating (cathodic arc deposition; physical coating deposition (PVD)) [292,299,312,313,314,315,316,317] | The formation of coatings is carried out in vacuum. It is used for the deposition of metal, ceramic, and composite coatings on substrates made of various materials (including low-heat-resistant (up to 200 °C)). To improve the quality of coatings, equipment and technologies are used for the filtration of the microparticle phase from the plasma stream, ion bombardment and etching of the surface are used to clean and thermoactivate it, and ion implantation is used to increase adhesion and modify the outer layer of the substrate [a number of our publications]. By varying both the composition of cathodes and reaction gases as well as coating modes, it is possible to obtain multilayer functional coatings, thereby improving the operational properties of products—in particular, increasing the corrosion resistance of implants and giving the product antibacterial properties. | |
| Magnetron sputtering method [318,319,320] | High speed of coating formation at low vacuum of 0.1–1 Pa; no overheating of the substrate. Magnetron sputtering can be carried out both on direct and alternating currents. It is used for the formation of protective coatings in order to increase the corrosion resistance and osteointegration of implants. The coatings formed on the implants are, as a rule, single-layer and simple in composition films (oxide, carbide, nitride, or metallic). Complex coatings (e.g., calcium phosphate) can be deposited wherein the composition of each coating corresponds to the composition of the target atomized in argon. It is possible to combine the deposition of coatings with preliminary plasma-immersion ion implantation. The coatings are characterized by high uniformity, relatively low porosity, and high adhesion to the substrate. The compound formed after the chemical reaction is deposited not only on the substrate but also on the entire surface of the chamber (including the sputtering target, which reduces the efficiency and productivity of the process) | |
| Glow discharge plasma treatment [321,322,323] | It allows to reduce the roughness of the implant surface while increasing its wettability, which contributes to a reduction in bacterial adhesion. Surface treatment using the glow discharge plasma is carried out in vacuum, which makes it possible to combine this operation with the application of the functional coating in the working chamber of the device. By adjusting the plasma composition, it is possible to change the chemical composition of the surface, which leads to changes in the physical and mechanical properties of the implant (in particular, the hardness of the surface and the breaking strength of the “implant-bone” connection). | |
| Ultraviolet light treatment (UV photofunctionalization) [324,325] | Allows to restore the original surface qualities of “aged” implants and extend their service life by exposing them to ultraviolet light just before insertion into the bone. | |
| Ion implantation [326,327,328,329,330] | The introduction of functional metal ions (for example, bioactive ones: Ag, Zn) into the surface is carried out in vacuum. This operation can be performed as an independent method of surface modification (for example, to increase osteogenic activity and antibacterial activity of implants), or it can be combined with other technological operations (for example, to increase adhesion of vacuum–arc coatings). | |
| Chemical Techniques | Chemical (including electrochemical) etching:
| It is possible to obtain different surface textures (its roughness) by adjusting the composition of etching solutions (as a rule, acid concentration), temperature, and etching time. Acid etching significantly increases the roughness of the implant surface without changing its marginal wetting angle. Alkaline etching can increase the hydrophilicity of the dental implant surface. Acid etching is performed at a lower temperature and in a shorter period of time than the commonly used alkali etching treatment. |
| Electrochemical deposition of coatings, including oxidation (anodizing) [7,292,294,306,337,338,339] | A relatively simple inexpensive method of forming coatings (usually oxide films). Formed coatings have high porosity, which contributes to the improved osteogenesis of implants. Allows to obtain a hydrophilic surface, which contributes to the better osteointegration of implants. It is practically impossible to control the structure and properties of coatings in the process of their deposition. Due to the high level of environmental contamination and danger for personnel, it requires the use of complex treatment and protection facilities. | |
| Electrophoretic deposition of coatings [340,341,342] | A relatively simple low-temperature method for forming coatings on an electrically conductive substrate. Virtually indifferent to the shape of the coated surface, it has high productivity and is well adapted to mass production. It is possible to control the thickness and morphology of the coating by varying the deposition voltage and time. Possibility of producing polymer, metal–polymer, ceramic, and composite bioactive coatings (e.g., hydroxyapatite coatings with silver and lignin or chitosan coatings). | |
| Sol-gel method [343,344,345,346] | The synthesis process is relatively simple and allows excellent control over the composition of coatings and their application to surfaces of virtually any shape. Allows to achieve uniform distribution of elements of multicomponent systems on the surface of various solids. Enables the localized delivery of a wide range of drugs (in particular, antimicrobials) at a controlled rate. | |
| Chemical Deposition of Coatings (CDC) (CVD) [347,348,349] | The high-temperature process of formation of high-purity homogeneous solid films by means of a chemical reaction. Possibility of coating complex implants (including internal cavities of products). Coating is only possible on heat-resistant substrates. | |
| Combined Processing Techniques | Sandblasting of the surface followed by acid etching [70,309,350,351,352] | Combines the advantages of both types of surface modification.
|
| Plasma electrolytic treatment (e.g., Plasma electrolytic oxidation) [177,302,353,354,355,356,357,358,359,360] | Simple, inexpensive, and environmentally safe method. It allows to apply various (in particular, polymeric) coatings on metal and ceramic substrates with their simultaneous cleaning in order to increase the corrosion resistance of implants. It is possible to realize the microalloying of both the implant surface as well as of the formed coating (for example, the formation of Ca-P coatings with silver nanoparticles) | |
| Combined treatment with different PVD methods [361] | Allows for the formation of multilayer coatings, each layer of which is deposited in a layer-by-layer manner, such as in the deposition of a multilayer coating comprising an oxide sub-layer obtained via gas-thermal oxidation and a calcium phosphate layer obtained via magnetron sputtering. | |
| Cold atmospheric plasma assisted vapor phase CVD method (PECVD) [362,363,364,365] | The CVD process uses plasma to decompose the initial substances, activate the substrate surface, and achieve ion etching. Compared to the traditional method, it is low-temperature (due to plasma amplification), so it allows to obtain coatings on non-thermal substrates. Formed oxide coatings have superhydrophilic surfaces. Possibility to apply thin (up to 0.5 µm) glass–ceramic coatings. | |
| Laser chemical vapor deposition (LCVD) [366,367] | CVD process that utilizes a laser source to decompose starting substances and activate the substrate surface. Allows to obtain glass–ceramic coatings on heat-resistant substrates. By adjusting the laser power and pressure in the chamber, it is possible to obtain ceramic-like oxide films with different microstructures. | |
| Biochemical Techniques | Stem cell culturing [368,369,370] | Stem cells (mesenchymal (MSC), adipose-derived, bone marrow-derived, or embryonic (ESC)) can be successfully grown in culture media (e.g., serum-free and xenogeneic for adipose-derived MSC) for further culturing on various dental implant surfaces to enhance implant osseintegration and bone regeneration. |
| Protein-containing coating on implant surfaces [11,371,372,373,374,375,376] | It is possible to incorporate protein (e.g., bone morphogenetic protein (BMP-2) or extracellular matrix (ECM)) into polymeric biodegradable coatings applied to implants. It is possible to apply a liquid protein coating (e.g., platelet- or leukocyte-rich fibrin) to implant surfaces. Used to accelerate healing of soft and hard tissues of the body. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotova, C.; Yanushevich, O.; Kriheli, N.; Grigoriev, S.; Evdokimov, V.; Kramar, O.; Nozdrina, M.; Peretyagin, N.; Undritsova, N.; Popelyshkin, E.; et al. Dental Implants: Modern Materials and Methods of Their Surface Modification. Materials 2023, 16, 7383. https://doi.org/10.3390/ma16237383
Sotova C, Yanushevich O, Kriheli N, Grigoriev S, Evdokimov V, Kramar O, Nozdrina M, Peretyagin N, Undritsova N, Popelyshkin E, et al. Dental Implants: Modern Materials and Methods of Their Surface Modification. Materials. 2023; 16(23):7383. https://doi.org/10.3390/ma16237383
Chicago/Turabian StyleSotova, Catherine, Oleg Yanushevich, Natella Kriheli, Sergey Grigoriev, Vladimir Evdokimov, Olga Kramar, Margarita Nozdrina, Nikita Peretyagin, Nika Undritsova, Egor Popelyshkin, and et al. 2023. "Dental Implants: Modern Materials and Methods of Their Surface Modification" Materials 16, no. 23: 7383. https://doi.org/10.3390/ma16237383
APA StyleSotova, C., Yanushevich, O., Kriheli, N., Grigoriev, S., Evdokimov, V., Kramar, O., Nozdrina, M., Peretyagin, N., Undritsova, N., Popelyshkin, E., & Peretyagin, P. (2023). Dental Implants: Modern Materials and Methods of Their Surface Modification. Materials, 16(23), 7383. https://doi.org/10.3390/ma16237383







