A Simple Approach for Regenerating Electrolyzed Hydrogen Production Using Non-De-Ionized Water Sources
Abstract
1. Introduction
2. Research Methods
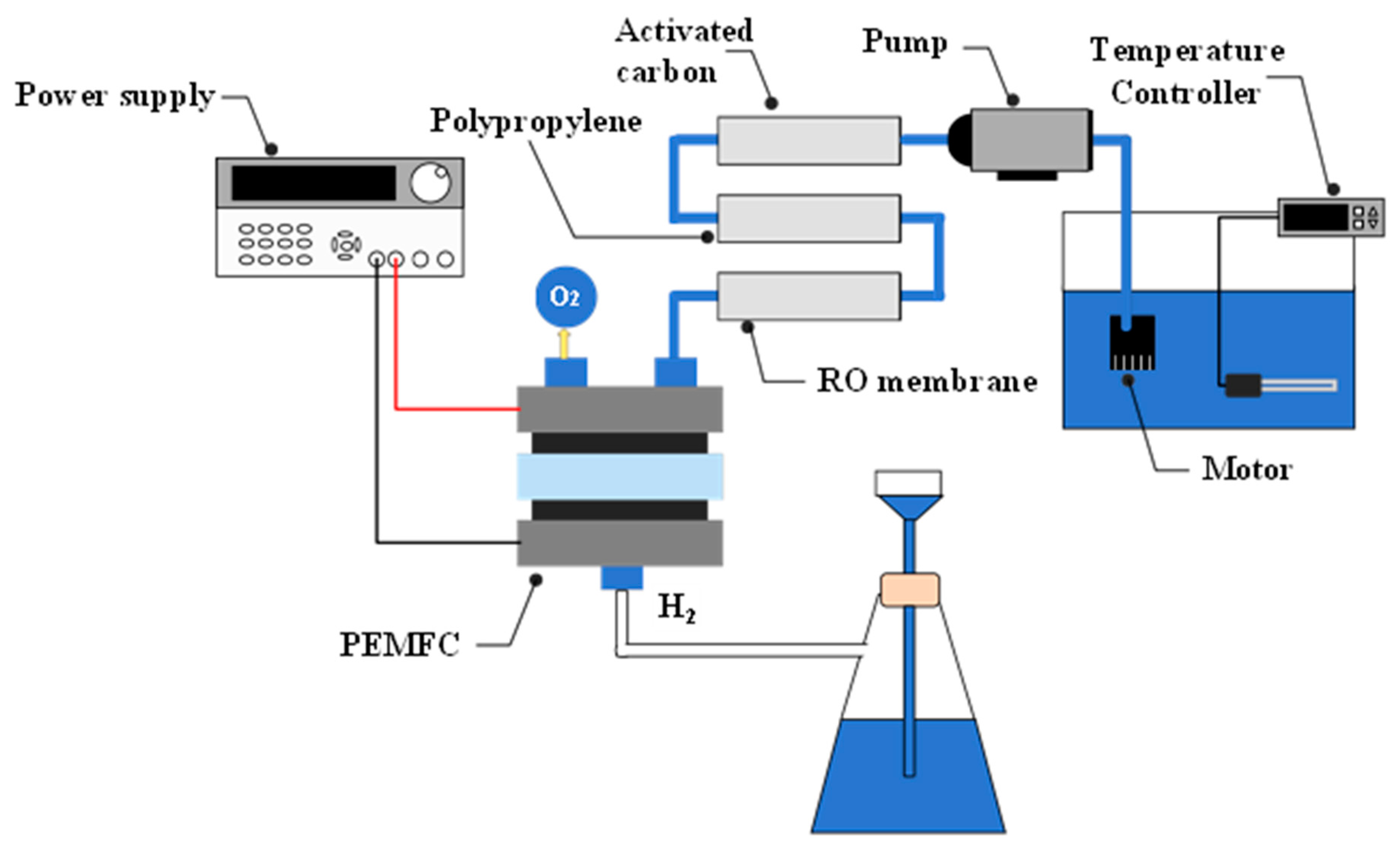
2.1. Overview
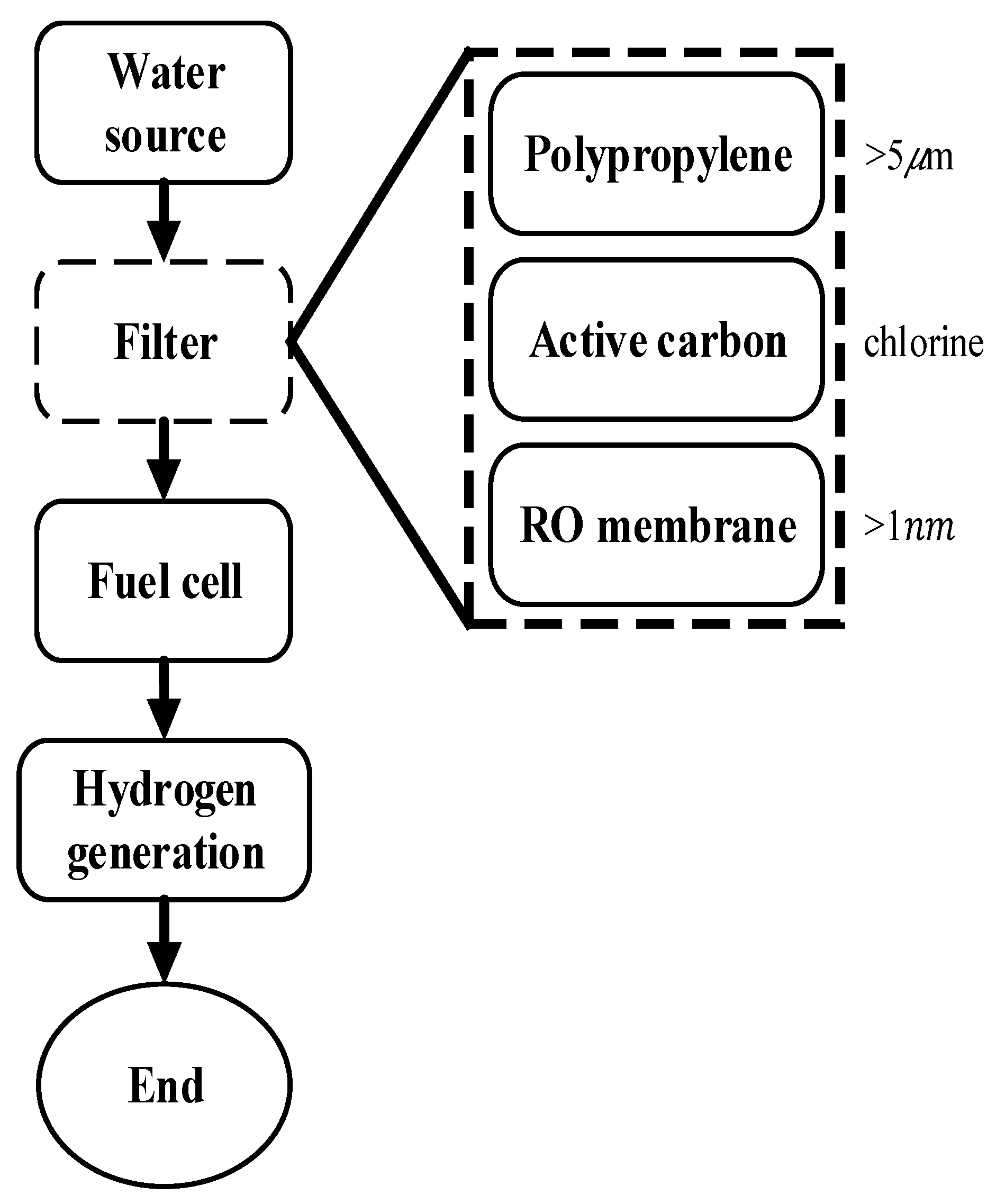
2.2. Water Filtering and Filtration Equipment
2.3. Hydrogen Production Experiment
2.4. Performance Recovery Experiment
2.5. Water Sample Analysis Using ICP-MS
3. Results and Discussion
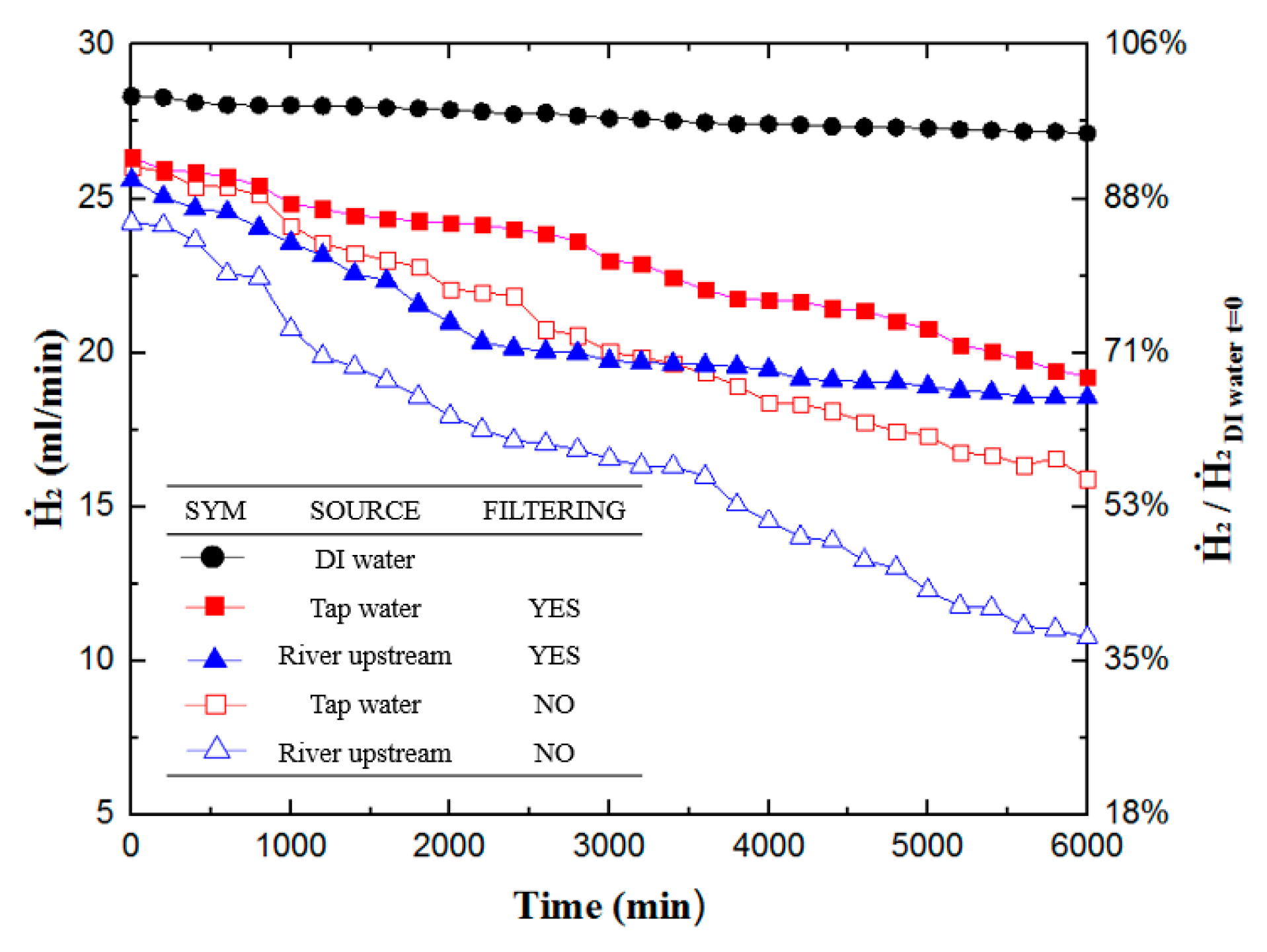
3.1. Hydrogen Production Experiment
3.2. Performance Recovery Experiment
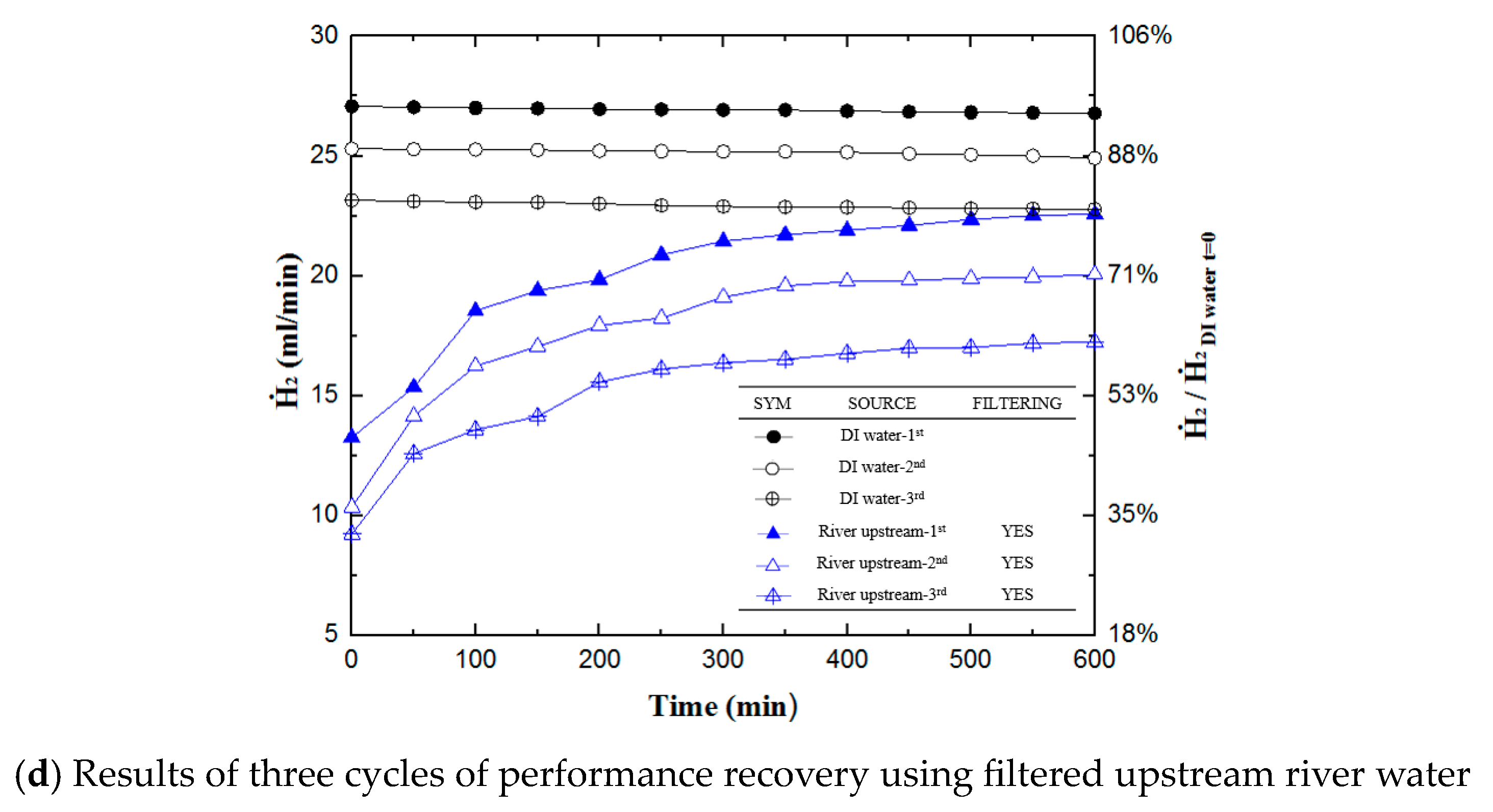
3.3. Results after Three Cycles of Hydrogen Production and Performance
Recovery Experiment
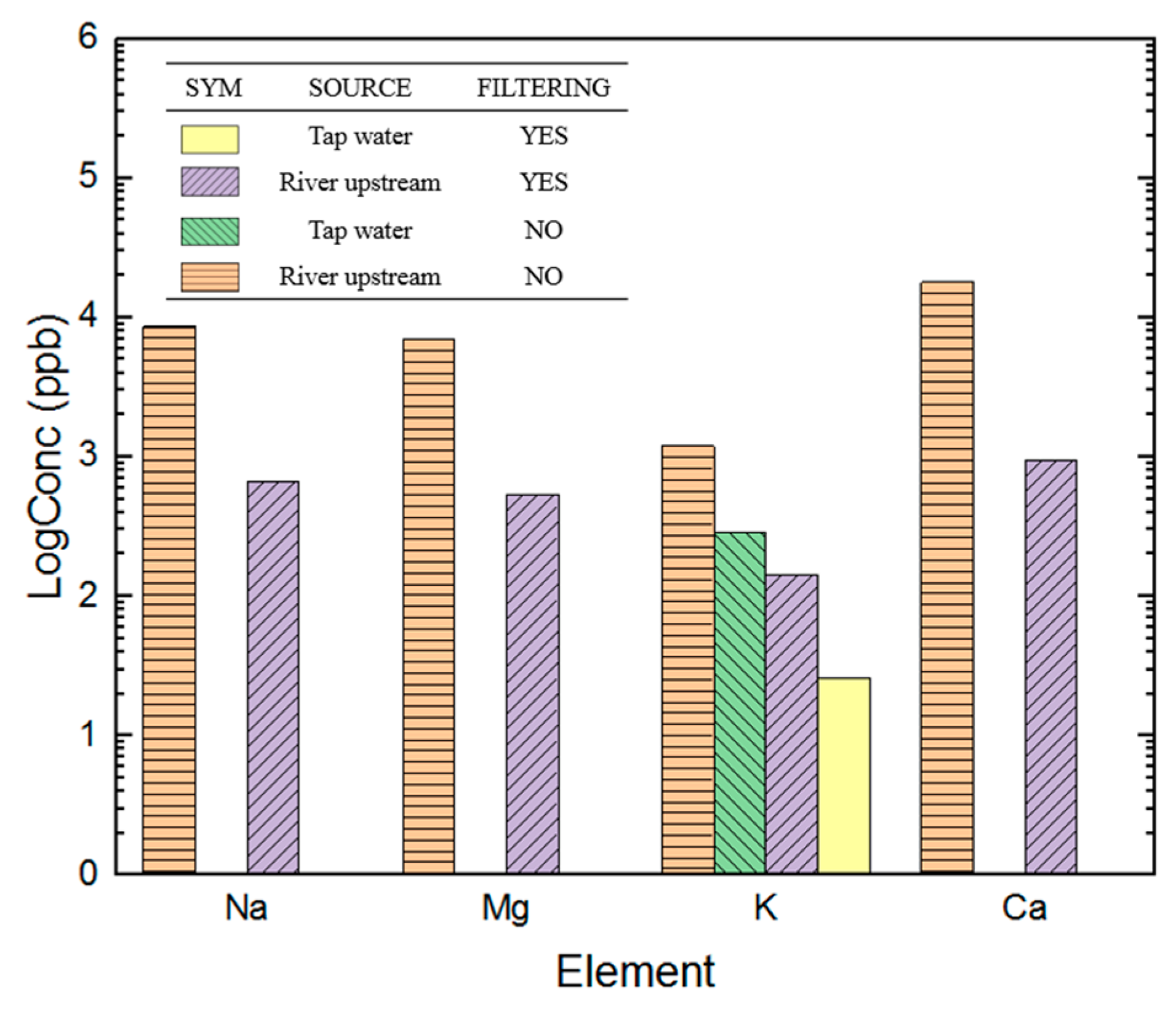
3.4. Water Sample Analysis Using ICP-MS
3.5. Cost-Effectiveness Analysis
4. Conclusions
- (1)
- The experiment utilizing low-cost water sources, such as upstream river water and tap water, to generate hydrogen using PEMFCs shows that filtered water sources have a higher hydrogen generation output than unfiltered water sources. From the hydrogen output data towards the end of the experiment, the hydrogen production of filtered tap water increased by 13.6% and that of filtered upstream river water improved by 30%.
- (2)
- After the first 6000 min hydrogen production experiment, performance recovery for 600 min using water showed that the filtered tap water sample recovered 22.8% of its efficiency, while the filtered upstream river water recovered 14.3%.
- (3)
- The method proposed in this research allows for repeated usage of the fuel cell, and even non-DI water sources are used. This research conducted three cycles of hydrogen generation and performance recovery experiments. After the final cycle of experimentation, filtered tap water yielded a hydrogen generation rate of 73.2%, while the filtered upstream river water produced a rate of 60.9%. Note that the hydrogen production rate of DI water decreased to 80%.
- (4)
- The method proposed by this research shows significant potential in promoting the usage of PEMFCs. Compared to the usage of DI water, using filtered water and performance recovery significantly reduces the cost of production. Under the exact production rate requirement (600 kg/year), the total cost of producing hydrogen with DI water after five years is 1.8-times that of the method proposed in this research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonisation. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Navarro, R.M.; Pena, M.A.; Fierro, J.L.G. Hydrogen production reactions from carbon feedstocks: Fossil fuels and biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef] [PubMed]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2011, 100, 410–426. [Google Scholar] [CrossRef]
- Rashid, M.D.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 80–93. [Google Scholar]
- Grove, W.R. XXIV. On voltaic series and the combination of gases by platinum. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1839, 14, 127–130. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)–A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Porembsky, V.I.; Fateev, V.N. Pure hydrogen production by PEM electrolysis for hydrogen energy. Int. J. Hydrogen Energy 2006, 31, 171–175. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Yuan, X.Z.; Li, H.; Zhang, S.; Martin, J.; Wang, H. A review of polymer electrolyte membrane fuel cell durability test protocols. J. Power Sources 2011, 196, 9107–9116. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Dam, V.A.T.; De Bruijn, F.A. The stability of PEMFC electrodes: Platinum dissolution vs. potential and temperature investigated by quartz crystal microbalance. J. Electrochem. Soc. 2007, 154, B494. [Google Scholar] [CrossRef][Green Version]
- Cindrella, L.; Kannan, A.M.; Lin, J.F.; Saminathan, K.; Ho, Y.; Lin, C.W.; Wertz, J. Gas diffusion layer for proton exchange membrane fuel cells—A review. J. Power Sources 2009, 194, 146–160. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Dhathathreyan, K.S. Catalyst layer in PEMFC electrodes—Fabrication, characterisation and analysis. Chem. Eng. J. 2007, 129, 31–40. [Google Scholar] [CrossRef]
- Wang, H.; Turner, J.A. Reviewing metallic PEMFC bipolar plates. Fuel Cells 2010, 10, 510–519. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Pafford, J. A new approach to the problem of carbon monoxide poisoning in fuel cells operating at low temperatures. J. Electrochem. Soc 1988, 135, 2651–2652. [Google Scholar] [CrossRef]
- Dhar, H.P.; Christner, L.G.; Kush, A.K. Nature of CO adsorption during H2 oxidation in relation to modeling for CO poisoning of a fuel cell anode. J. Electrochem. Soc. 1987, 134, 3021. [Google Scholar] [CrossRef]
- US DRIVE. Fuel Cell Technical Team Roadmap, 45–59; Department of Energy: Washington, DC, USA, 2017. [Google Scholar]
- Myers, D.; Kariuki, N.; Ahluwalia, R.; Wang, X.; Peng, J.K. Rationally designed catalyst layers for PEMFC performance optimization. In Annual Merit Review Evaluation Meeting; Department of Energy: Washington, DC, USA, 2015. [Google Scholar]



| Method | DI Water (5 Year) | DI Water (1 Year) | Tap Water (5 Year) | Tap Water (1 Year) | |
|---|---|---|---|---|---|
| Cost | |||||
| NRE | |||||
| Construction * | 7765 | 1553 | 67 | 13 | |
| Fuel Cell ** | 2400 | 2400 | 4140 | 4140 | |
| RE | |||||
| Maintenance *** | 1017 | 395 | 421 | 415 | |
| Utility bill # | 760 | 152 | 179 | 36 | |
| PEM ## | 1553 | 307 | 2645 | 529 | |
| Sum | 13,495 | 4807 | 7452 | 5133 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, W.-H.; Lin, S.-J.; Wu, J.-S. A Simple Approach for Regenerating Electrolyzed Hydrogen Production Using Non-De-Ionized Water Sources. Materials 2023, 16, 7382. https://doi.org/10.3390/ma16237382
Chiang W-H, Lin S-J, Wu J-S. A Simple Approach for Regenerating Electrolyzed Hydrogen Production Using Non-De-Ionized Water Sources. Materials. 2023; 16(23):7382. https://doi.org/10.3390/ma16237382
Chicago/Turabian StyleChiang, Wei-Hsiang, Shiow-Jyu Lin, and Jong-Shinn Wu. 2023. "A Simple Approach for Regenerating Electrolyzed Hydrogen Production Using Non-De-Ionized Water Sources" Materials 16, no. 23: 7382. https://doi.org/10.3390/ma16237382
APA StyleChiang, W.-H., Lin, S.-J., & Wu, J.-S. (2023). A Simple Approach for Regenerating Electrolyzed Hydrogen Production Using Non-De-Ionized Water Sources. Materials, 16(23), 7382. https://doi.org/10.3390/ma16237382







