Organic-Solvent-Resistant Polyimide/Hydroxyapatite Mixed Matrix Membranes for Lysozyme Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.2.1. Preparation of PI/HAP MMMs
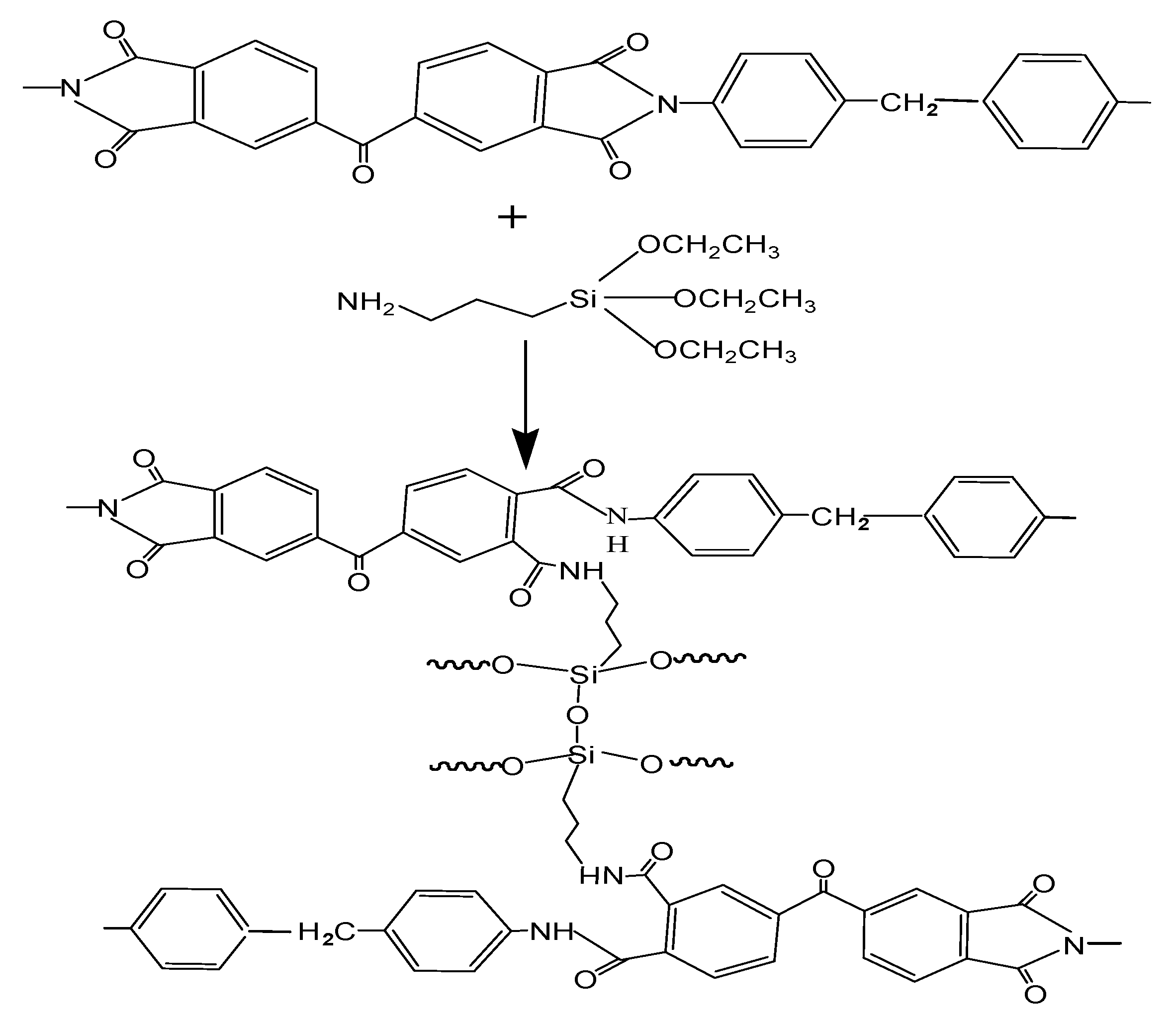
2.2.2. Crosslinking of PI/HAP MMMs
2.3. Membrane Characterization
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Effects of Crosslink Conditions on Pure Water Flux, Contact Angle, Mechanical Properties of MMMs
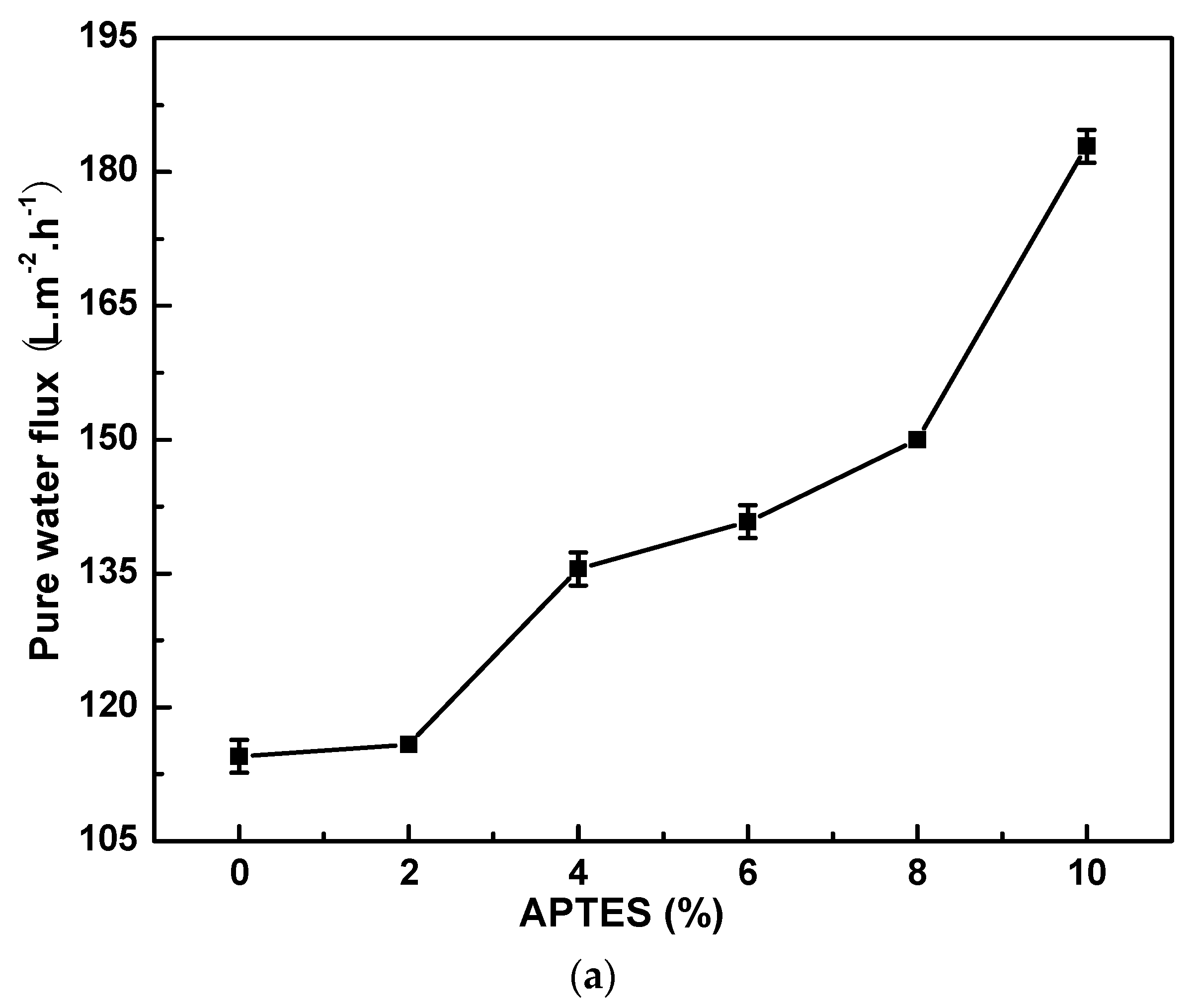
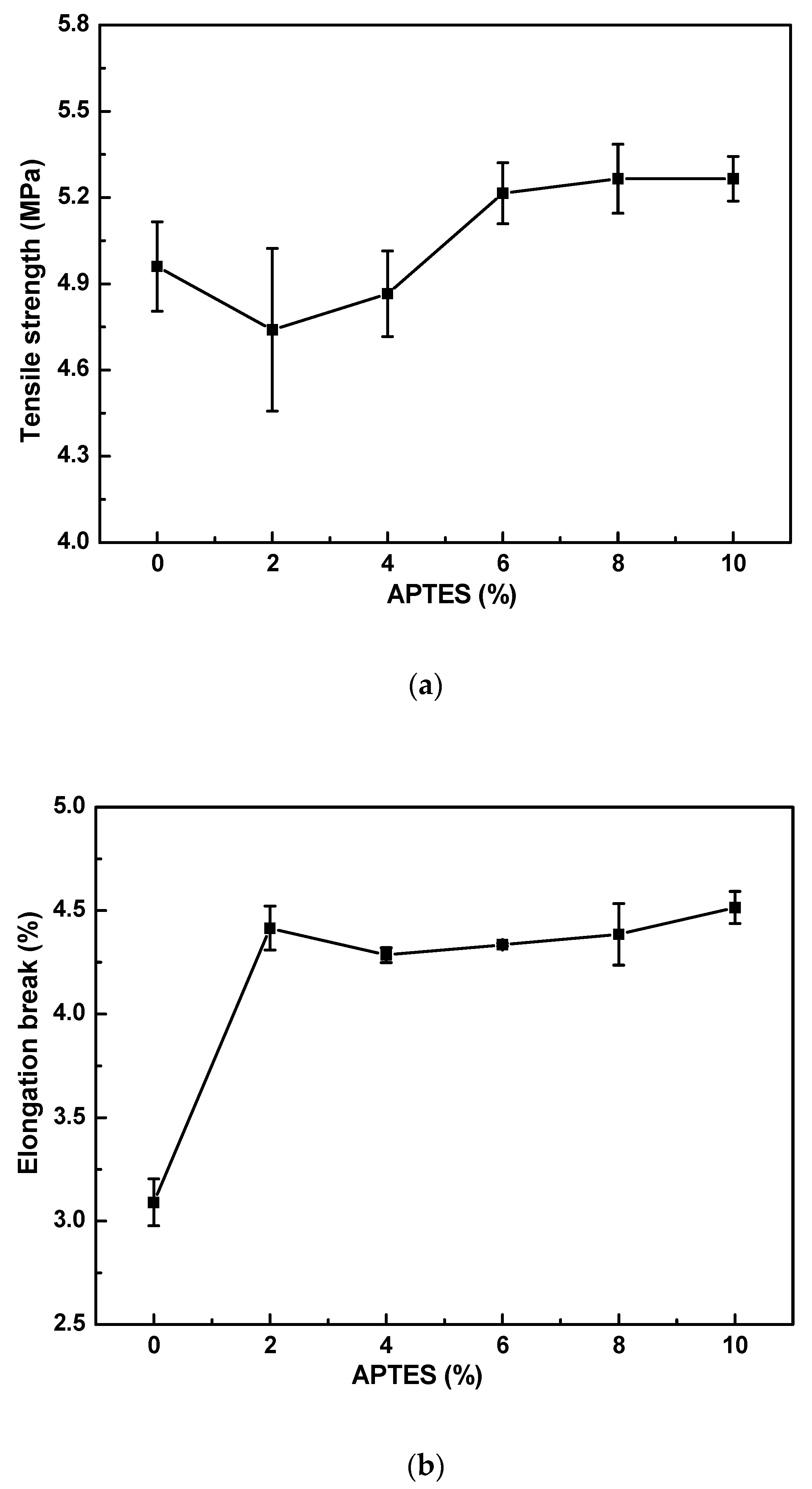
3.1.1. Effect of APTES Concentration
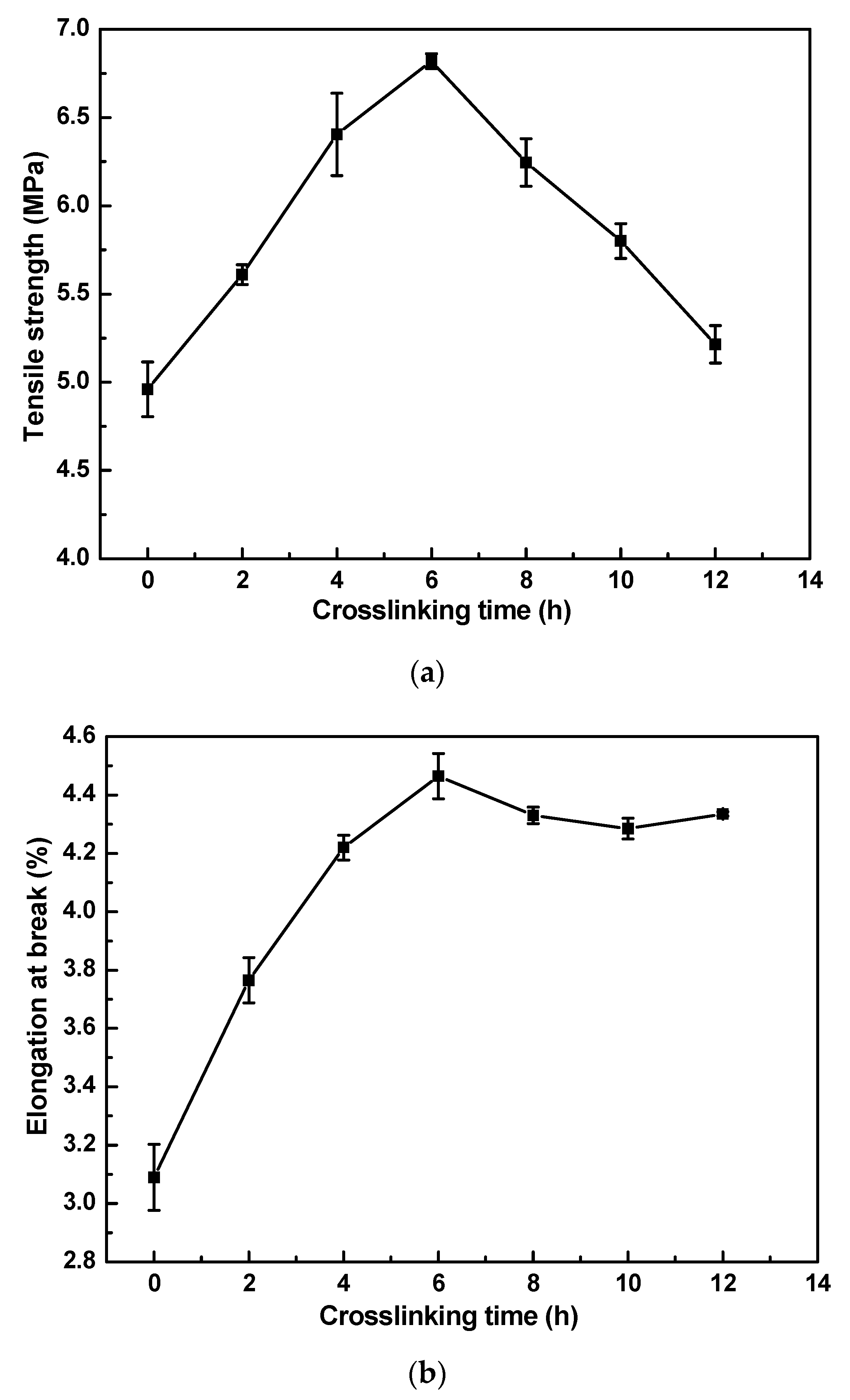
3.1.2. Effect of Crosslinking Time
3.2. Characterization of PI/HAP MMMs
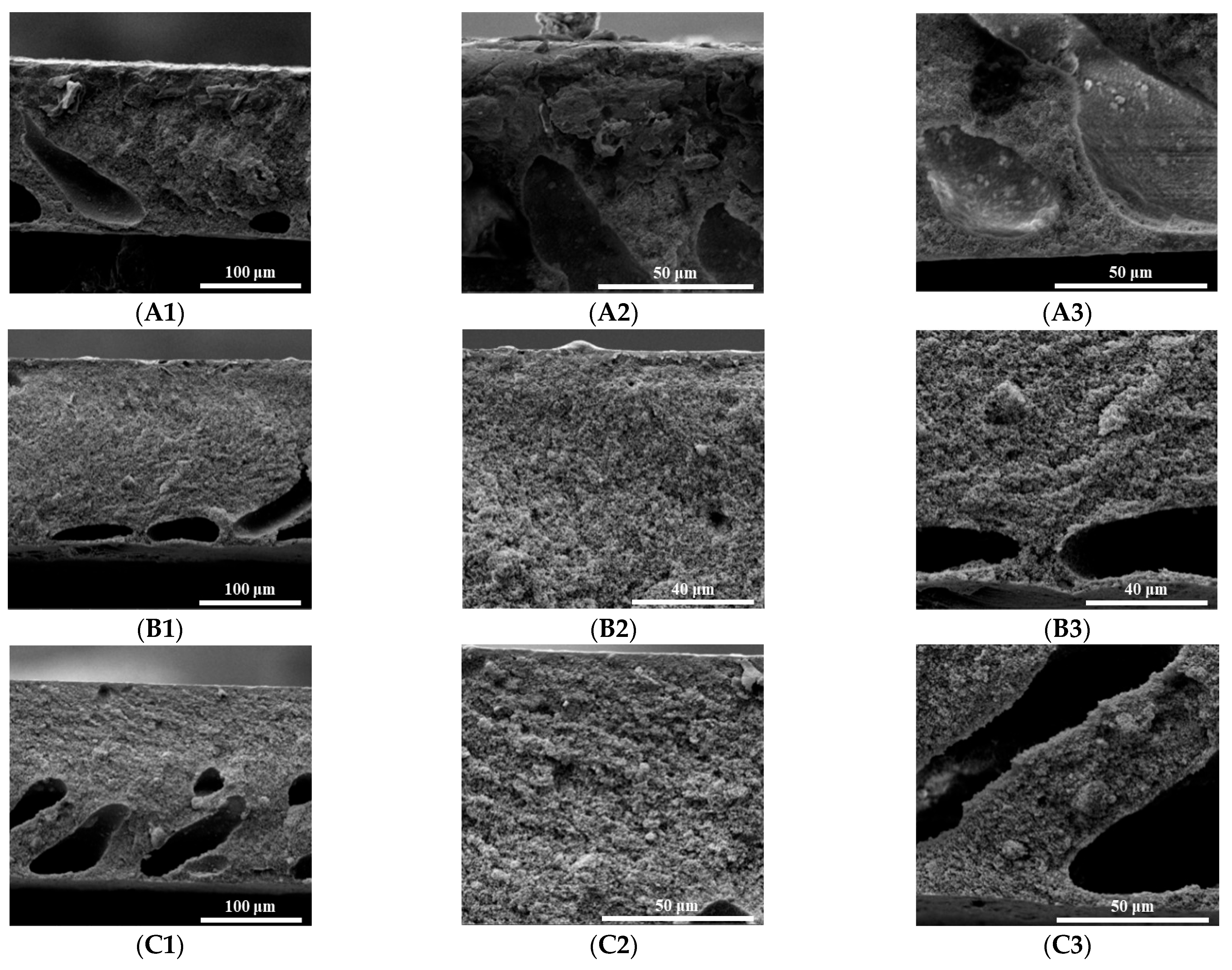
3.3. Morphology of PI/HAP MMMs
3.4. LZ Adsorption of PI/HAP MMMs
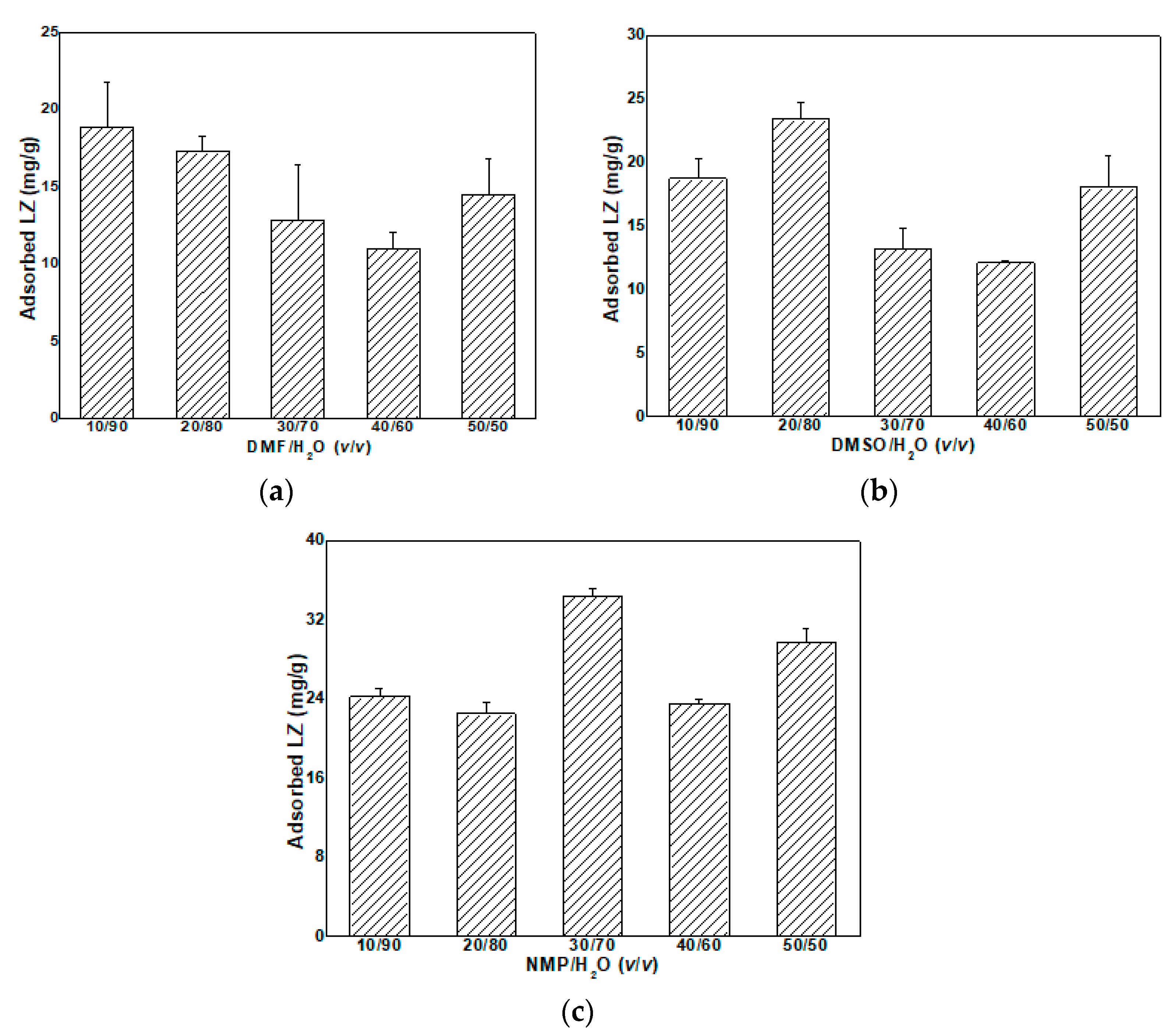
3.4.1. LZ Adsorption of MMMs in Organic-Solvent-Aqueous Solutions
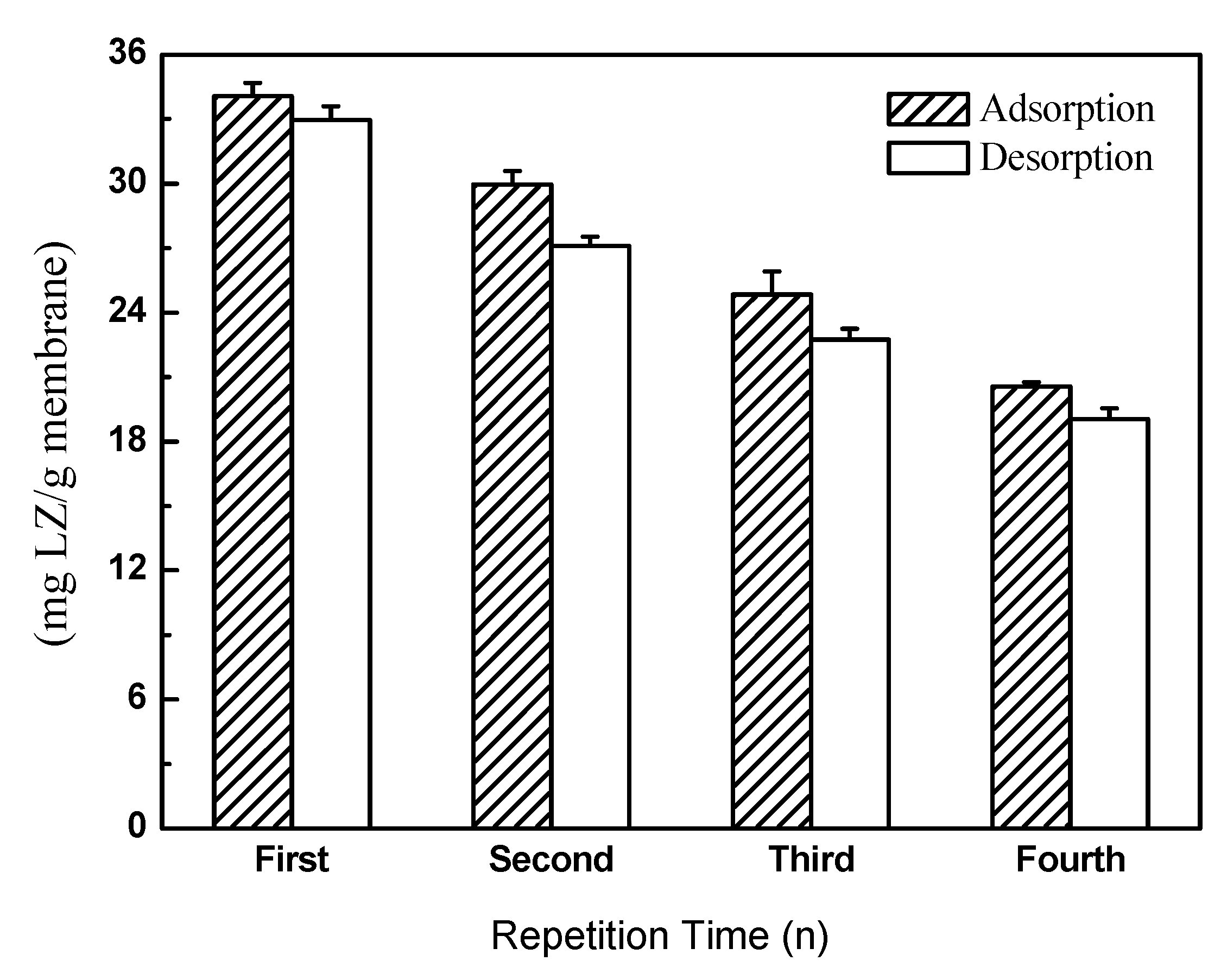
3.4.2. LZ Recovery of MMMs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Junceda, E.; García-García, J.F.; Bastida, A.; Fernández-Mayoralas, A. Enzymes in the synthesis of bioactive compounds: The prodigious decades. Bioorganic Med. Chem. 2004, 12, 1817–1834. [Google Scholar] [CrossRef] [PubMed]
- Nóbile, M.L.; Stricker, A.M.; Iribarren, A.M.; Lewkowicz, E.S. Streptomyces griseus: A new biocatalyst with N-oxygenase activity. J. Biotechnol. 2021, 327, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Herbst, D.; Peper, S.; Niemeyer, B. Enzyme catalysis in organic solvents: Influence of water content, solvent composition and temperature on Candida rugosa lipase catalyzed transesterification. J. Biotechnol. 2012, 162, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Greifenstein, R.; Ballweg, T.; Hashem, T.; Gottwald, E.; Achauer, D.; Kirschhöfer, F.; Nusser, M.; Brenner-Weiß, G.; Sedghamiz, E.; Wenzel, W.; et al. MOF-Hosted Enzymes for Continuous Flow Catalysis in Aqueous and Organic Solvents. Angew. Chem. Int. Ed. 2022, 61, e202117144. [Google Scholar] [CrossRef]
- Rajesh, S.; Schneiderman, S.; Crandall, C.; Fong, H.; Menkhaus, T.J. Synthesis of Cellulose-graft-Polypropionic Acid Nanofiber Cation-Exchange Membrane Adsorbers for High-Efficiency Separations. ACS Appl. Mater. Interfaces 2017, 9, 41055–41065. [Google Scholar] [CrossRef]
- Kopeć, K.K.; Dutczak, S.M.; Bolhuis-Versteeg, L.; Wessling, M.; Stamatialis, D.F. Solvent-resistant P84-based mixed matrix membrane adsorbers. Sep. Purif. Technol. 2011, 80, 306–314. [Google Scholar] [CrossRef]
- Saufi, S.M.; Fee, C.J. Simultaneous anion and cation exchange chromatography of whey proteins using a customizable mixed matrix membrane. J. Chromatogr. A 2011, 1218, 9003–9009. [Google Scholar] [CrossRef]
- Beck, J.; Hochdaninger, G.; Carta, G.; Hahn, R. Resin structure impacts two-component protein adsorption and separation in anion exchange chromatography. J. Chromatogr. A 2023, 1705, 464208. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Lv, Z.; Chen, Y. In-Situ preparation of poly(ether imide)/amino functionalized silica mixed matrix membranes for application in enzyme separation. Mater. Des. 2016, 92, 610–620. [Google Scholar] [CrossRef]
- Tijink, M.S.L.; Wester, M.; Glorieux, G.; Gerritsen, K.G.; Sun, J.; Swart, P.C.; Borneman, Z.; Wessling, M.; Vanholder, R.; Joles, J.A.; et al. Mixed matrix hollow fiber membranes for removal of protein-bound toxins from human plasma. Biomaterials 2013, 34, 7819–7828. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, L. Adsorption of protein onto double layer mixed matrix membranes. Colloid. Surface. B 2014, 123, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Bystrova, A.V.; Dekhtyar, Y.D.; Popov, A.I.; Coutinho, J.; Bystrov, V.S. Modified Hydroxyapatite Structure and Properties: Modeling and Synchrotron Data Analysis of Modified Hydroxyapatite Structure. Ferroelectrics 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Limlawan, P.; Marger, L.; Durual, S.; Vacharaksa, A. Delivery of microRNA-302a-3p by APTES modified hydroxyapatite nanoparticles to promote osteogenic differentiation in vitro. BDJ Open 2023, 9, 8. [Google Scholar] [CrossRef]
- Heidari, B.S.; Lopez, E.M.; Chen, P.; Ruan, R.; Vahabli, E.; Davachi, S.M.; Granero-Moltó, F.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Silane-modified hydroxyapatite nanoparticles incorporated into polydioxanone/poly(lactide-co-caprolactone) creates a novel toughened nanocomposite with improved material properties and in vivo inflammatory responses. Mater. Today Bio. 2023, 22, 100778. [Google Scholar] [CrossRef]
- Hübner, W.; Blume, A.; Pushnjakova, R.; Dekhtyar, Y.; Hein, H.-J. The Influence of X-ray Radiation on the Mineral/Organic Matrix Interaction of Bone Tissue: An FT-IR Microscopic Investigation. Int. J. Artif. Organs 2005, 28, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Sinusaite, L.; Antuzevics, A.; Popov, A.I.; Rogulis, U.; Misevicius, M.; Katelnikovas, A.; Kareiva, A.; Zarkov, A. Synthesis and luminescent properties of Mn-doped alpha-tricalcium phosphate. Ceram. Int. 2021, 47, 5335–5340. [Google Scholar] [CrossRef]
- Chaiariyakul, D.; Hamai, R.; Shiwaku, Y.; Tsuchiya, K.; Suzuki, O. Adsorption behavior and osteoblastic cellular activity of metronidazole molecule with octacalcium phosphate and hydroxyapatite materials. J. Drug Deliv. Sci. Technol. 2023, 89, 105046. [Google Scholar] [CrossRef]
- Sun, J.; Wu, L. Polyether sulfone/hydroxyapatite mixed matrix membranes for protein purification. Appl. Surf. Sci. 2014, 308, 155–160. [Google Scholar] [CrossRef]
- Sun, J.; Wu, L.; Chen, J. Efficient lysozyme adsorption on chitosan/hydroxyapatite hybrid membrane via in situ synthesis. Cellulose 2016, 23, 3861–3874. [Google Scholar] [CrossRef]
- Sun, J.; Cao, Z.; Wu, L. Polyvinylidene fluoride/silane-treated hydroxyapatite mixed matrix membrane for enzyme capturing. Colloid. Surface. B 2015, 126, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Saiful, B.Z.; Wessling, M. Enzyme capturing and concentration with mixed matrix membrane adsorbers. J. Membr. Sci. 2006, 280, 406–417. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, G.; Wang, L.; Li, J.; An, Q.; Ji, S. Pervaporation dehydration of acetic acid using NH2-UiO-66/PEI mixed matrix membranes. Sep. Purif. Technol. 2017, 186, 20–27. [Google Scholar] [CrossRef]
- Davood Abadi Farahani, M.H.; Hua, D.; Chung, T.S. Cross-linked mixed matrix membranes (MMMs) consisting of amine-functionalized multi-walled carbon nanotubes and P84 polyimide for organic solvent nanofiltration (OSN) with enhanced flux. J. Membr. Sci. 2018, 548, 319–331. [Google Scholar] [CrossRef]
- Xiao, Y.C.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyi-mide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhang, X.; Li, J. Co@NC@ZIF-8-hybridized carbon molecular sieve membranes for highly efficient gas separation. J. Membr. Sci. 2023, 682, 121781. [Google Scholar] [CrossRef]
- Moghadam, F.; Lee, T.H.; Park, I.; Park, H.B. Thermally annealed polyimide-based mixed matrix membrane containing ZIF-67 decorated porous graphene oxide nanosheets with enhanced propylene/propane selectivity. J. Membr. Sci. 2020, 603, 118019. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Lee, J.; Song, J.; Bae, T.H. Co2/N2 separation properties of polyimide-based mixed-matrix membranes comprising UiO-66 with various functionalities. Membranes 2020, 10, 154. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, X.; Lin, L.; Qiang, R.; Wang, Q.; Cheng, Q.; Yang, J.; Yang, X.; Ma, W.; Li, X.; et al. A tris(hydroxymethyl)aminomethane-modified polyimide membrane with efficient organic solvent resistant performance and high separation selectivity for dye/salt separation. Desalination 2023, 549, 116325. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Xiao, Y.-C.; Wu, C.; Chung, T.-S. Chemical modification of P84 polyimide as anion-exchange membranes in a free-flow isoelectric focusing system for protein separation. Chem. Eng. J. 2010, 160, 340–350. [Google Scholar] [CrossRef]
- Shao, L.; Chung, T.-S.; Goh, S.H.; Pramoda, K.P. Polyimide modification by a linear aliphatic diamine to enhance transport performance and plasticization resistance. J. Membr. Sci. 2005, 256, 46–56. [Google Scholar] [CrossRef]
- Arai, T.; Kawakami, H. Ultrafine electrospun nanofiber created from cross-linked polyimide solution. Polymer 2012, 53, 2217–2222. [Google Scholar] [CrossRef]
- Kim, S.; Ando, S.; Wang, X. Highly dispersible ternary composites with high transparency and ultra low dielectric constants based on hyperbranched polyimide with organosilane termini and cross-linked polyimide with silica. RSC Adv. 2015, 5, 98419–98428. [Google Scholar] [CrossRef]
- Coombs, S.G.; Khodjaniyazova, S.; Bright, F.V. Exploiting the 3-Aminopropyltriethoxysilane (APTES) autocatalytic nature to create bioconjugated microarrays on hydrogen-passivated porous silicon. Talanta 2018, 177, 26–33. [Google Scholar] [CrossRef]
- Hijazi, M.; Rieu, M.; Stambouli, V.; Tournier, G.; Viricelle, J.-P.; Pijolat, C. Ambient temperature selective ammonia gas sensor based on SnO2-APTES modifications. Sens. Actuators B Chem. 2018, 256, 440–447. [Google Scholar] [CrossRef]
- See Toh, Y.H.; Lim, F.W.; Livingston, A.G. Polymeric membranes for nanofiltration in polar aprotic solvents. J. Membr. Sci. 2007, 301, 3–10. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, X.; Zhao, J.; Liang, H. Preparation and characterization of a novel magnetic composite particles. Russ. J. Appl. Chem. 2015, 88, 529–532. [Google Scholar] [CrossRef]
- Wang, S.; Liang, S.; Liang, P.; Zhang, X.; Sun, J.; Wu, S. In-situ combined dual-layer CNT/PVDF membrane for electrical-ly-enhanced fouling resistance. J. Membr. Sci. 2015, 491, 37–44. [Google Scholar] [CrossRef]
- Bottino, A.; Camera-Roda, G.; Capannelli, G.; Munari, S. The formation of microporous polyvinylidene difluoride membranes by phase separation. J. Membr. Sci. 1991, 57, 1–20. [Google Scholar] [CrossRef]
- Ro, J.; Park, C.; Kim, J.T.; Kim, H.; Lee, Y.-E.; Yoo, S.-Y.; Hong, S.-C.; Lee, K.B.; Lee, J. Enhancing lysozyme loading in powderized liposomes by controlling encapsulation processes. Bull. Korean Chem. Soc. 2017, 38, 744–750. [Google Scholar] [CrossRef]


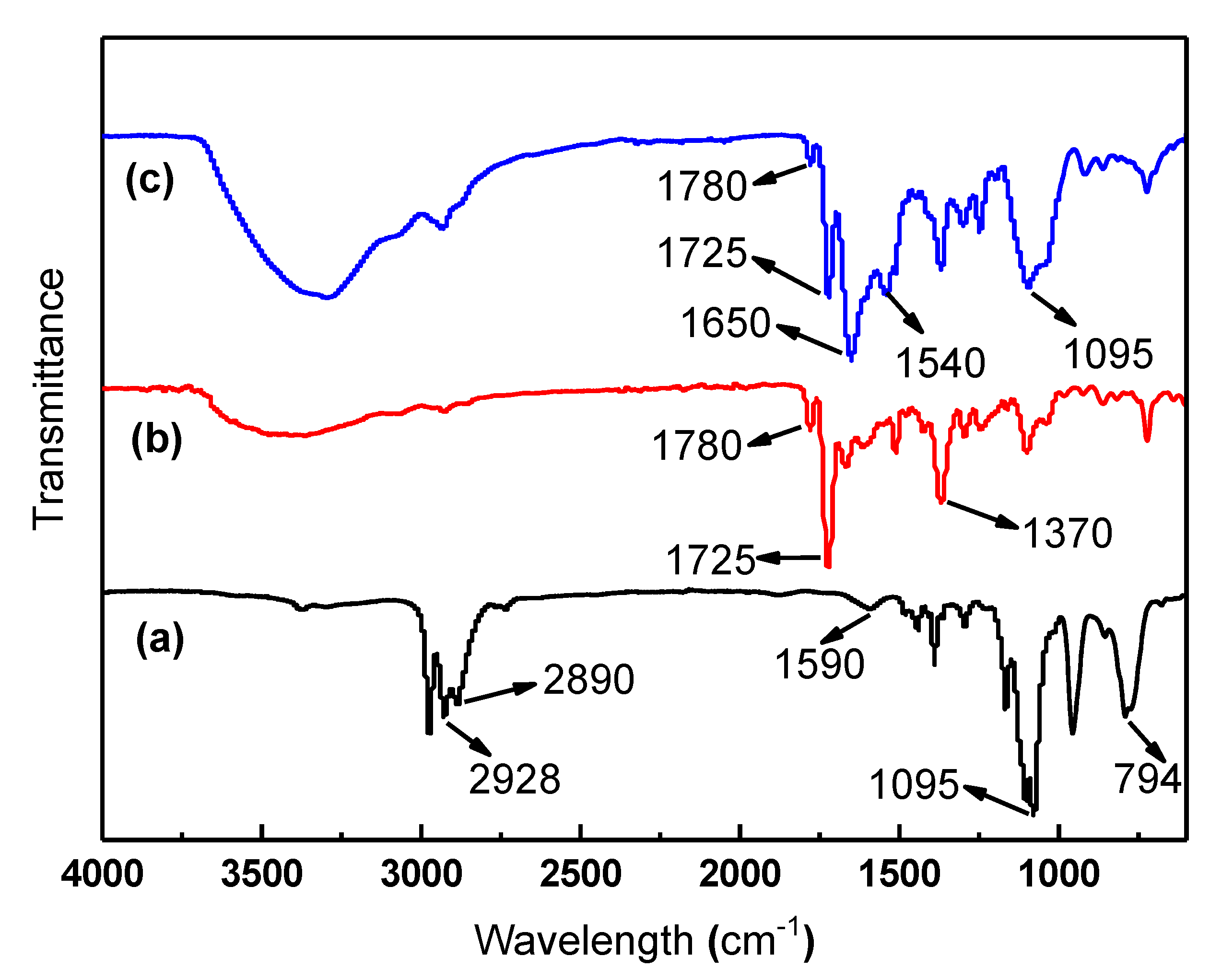
| APTES | PI/HAP MMMs | Crosslinked PI/HAP MMMs | |||
|---|---|---|---|---|---|
| Wavelength (cm−1) | Group | Wavelength (cm−1) | Group | Wavelength (cm−1) | Group |
| 794 | bending vibration band of Si–O–Si | 1370 | C–N stretching | 1095 | stretching vibration band of Si–O–Si |
| 1095 | stretching vibration band of Si–O–Si | 1725 | asymmetric C=O stretching | 1540 | C–N stretching of C–N– group |
| 1590 | bending vibration band of –NH2 | 1780 | symmetric C=O stretching | 1650 | C=O stretching |
| 2890 | asymmetric stretching bands of –CH2– | 1725 | asymmetric C=O stretching | ||
| 2928 | anti-symmetric asymmetric stretching bands of –CH2– | 1780 | symmetric C=O stretching | ||
| References [30,35] | Reference [36] | References [37,38] | |||
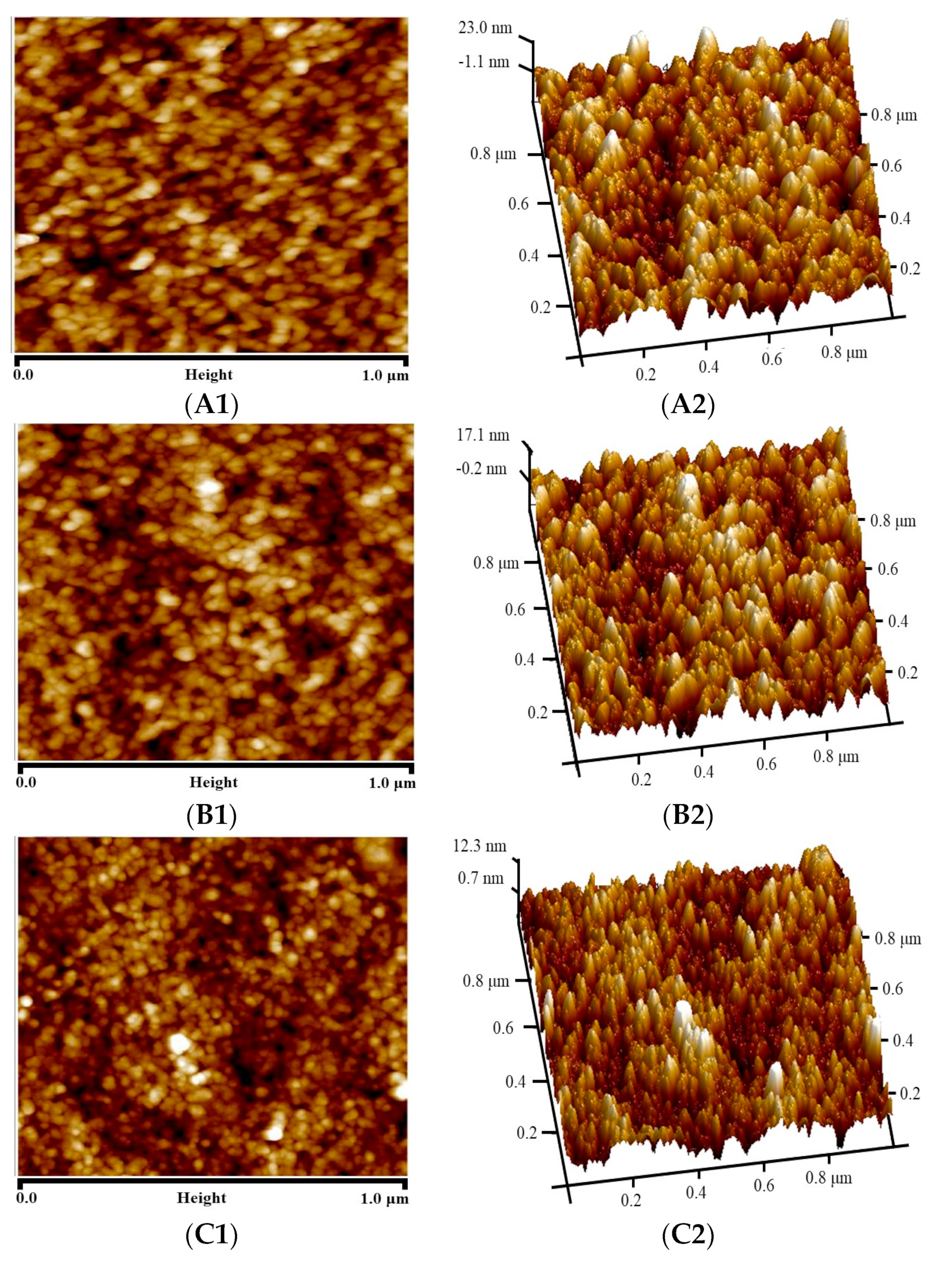
| Roughness Parameter | PI Membrane | MMMs | Crosslinked MMMs |
|---|---|---|---|
| Ra (nm) | 5.49 | 4.07 | 2.54 |
| Rq (nm) | 6.86 | 5.07 | 3.25 |
| Rmax (nm) | 51.10 | 43.20 | 32.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Pang, H.; Chen, L. Organic-Solvent-Resistant Polyimide/Hydroxyapatite Mixed Matrix Membranes for Lysozyme Adsorption. Materials 2023, 16, 7210. https://doi.org/10.3390/ma16227210
Sun J, Pang H, Chen L. Organic-Solvent-Resistant Polyimide/Hydroxyapatite Mixed Matrix Membranes for Lysozyme Adsorption. Materials. 2023; 16(22):7210. https://doi.org/10.3390/ma16227210
Chicago/Turabian StyleSun, Junfen, Hao Pang, and Long Chen. 2023. "Organic-Solvent-Resistant Polyimide/Hydroxyapatite Mixed Matrix Membranes for Lysozyme Adsorption" Materials 16, no. 22: 7210. https://doi.org/10.3390/ma16227210
APA StyleSun, J., Pang, H., & Chen, L. (2023). Organic-Solvent-Resistant Polyimide/Hydroxyapatite Mixed Matrix Membranes for Lysozyme Adsorption. Materials, 16(22), 7210. https://doi.org/10.3390/ma16227210







