Enhanced Performance of Inverted Perovskite Quantum Dot Light-Emitting Diode Using Electron Suppression Layer and Surface Morphology Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CsPbBr3 Quantum Dots and ZnO
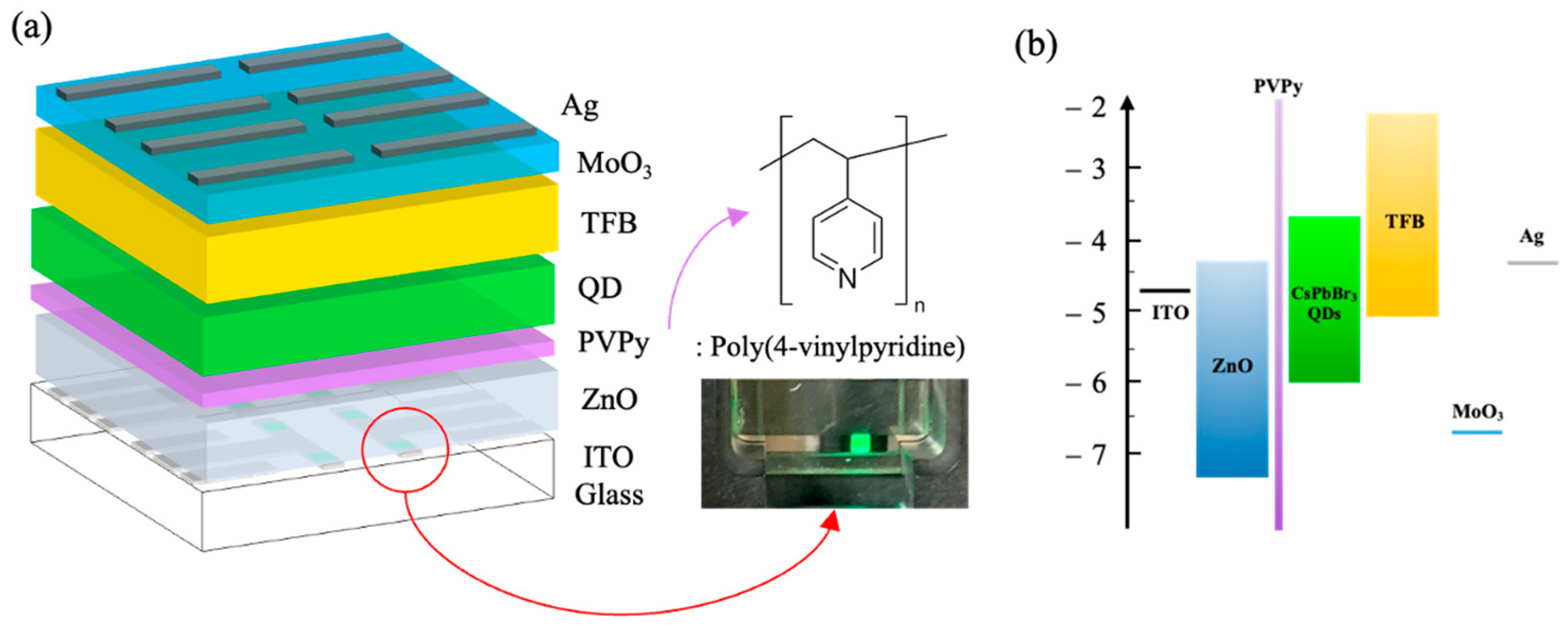
2.3. PeQLED Device Fabrication
2.4. PeQLED Device Characterization
3. Results and Discussion
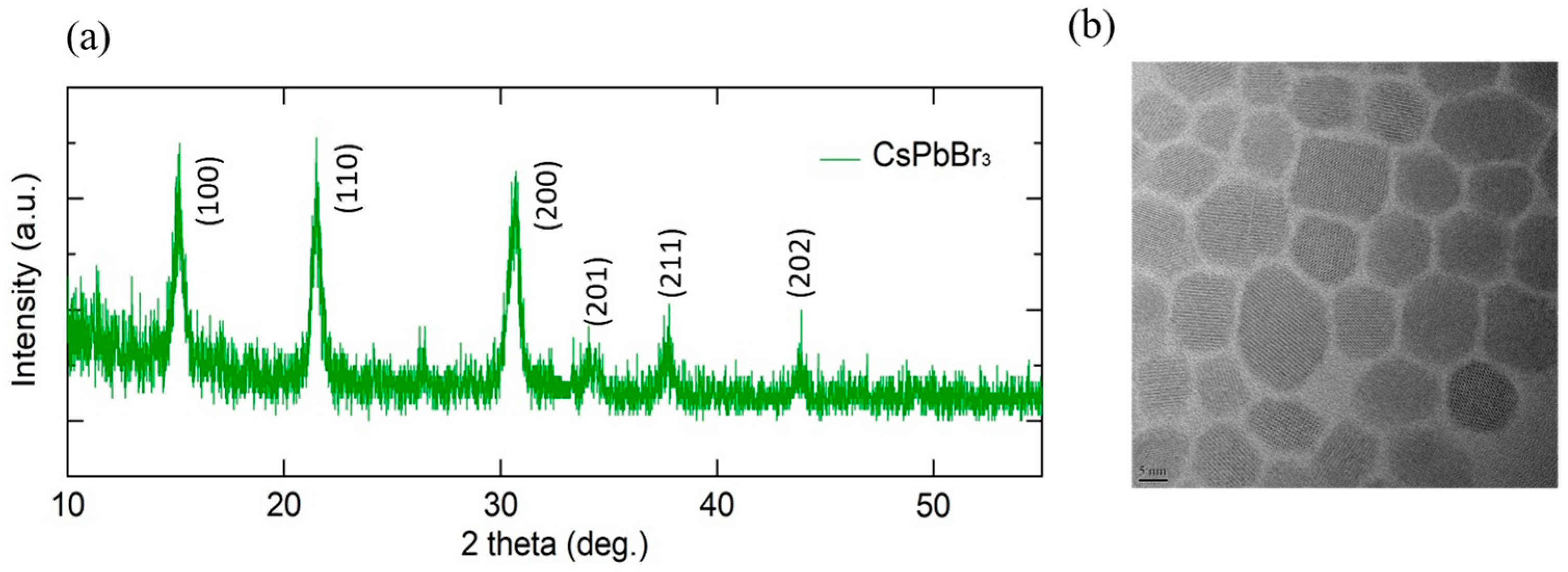
3.1. Characterization of CsPbBr3 QDs
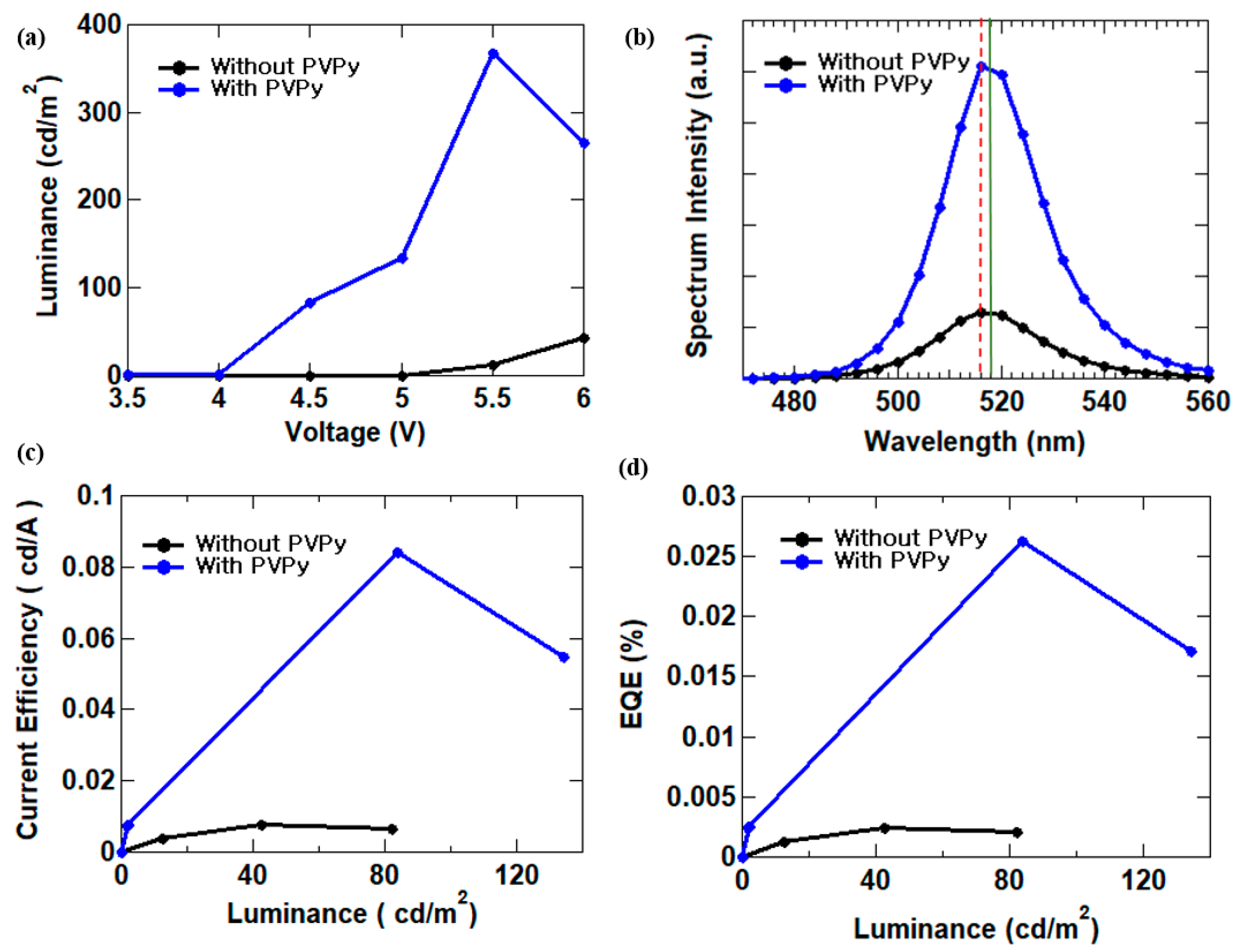
3.2. Performance of Perovskite Inverted QLEDs
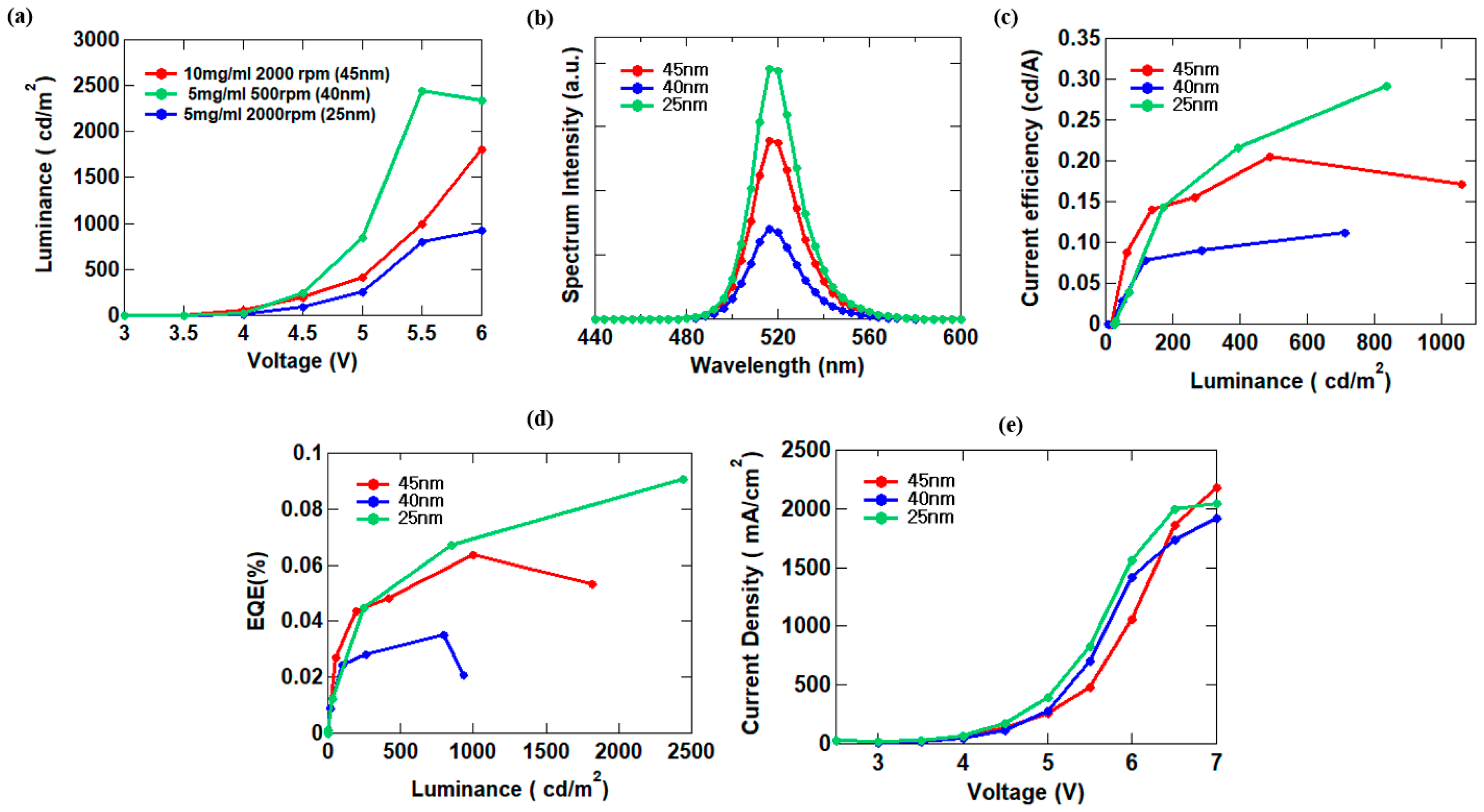
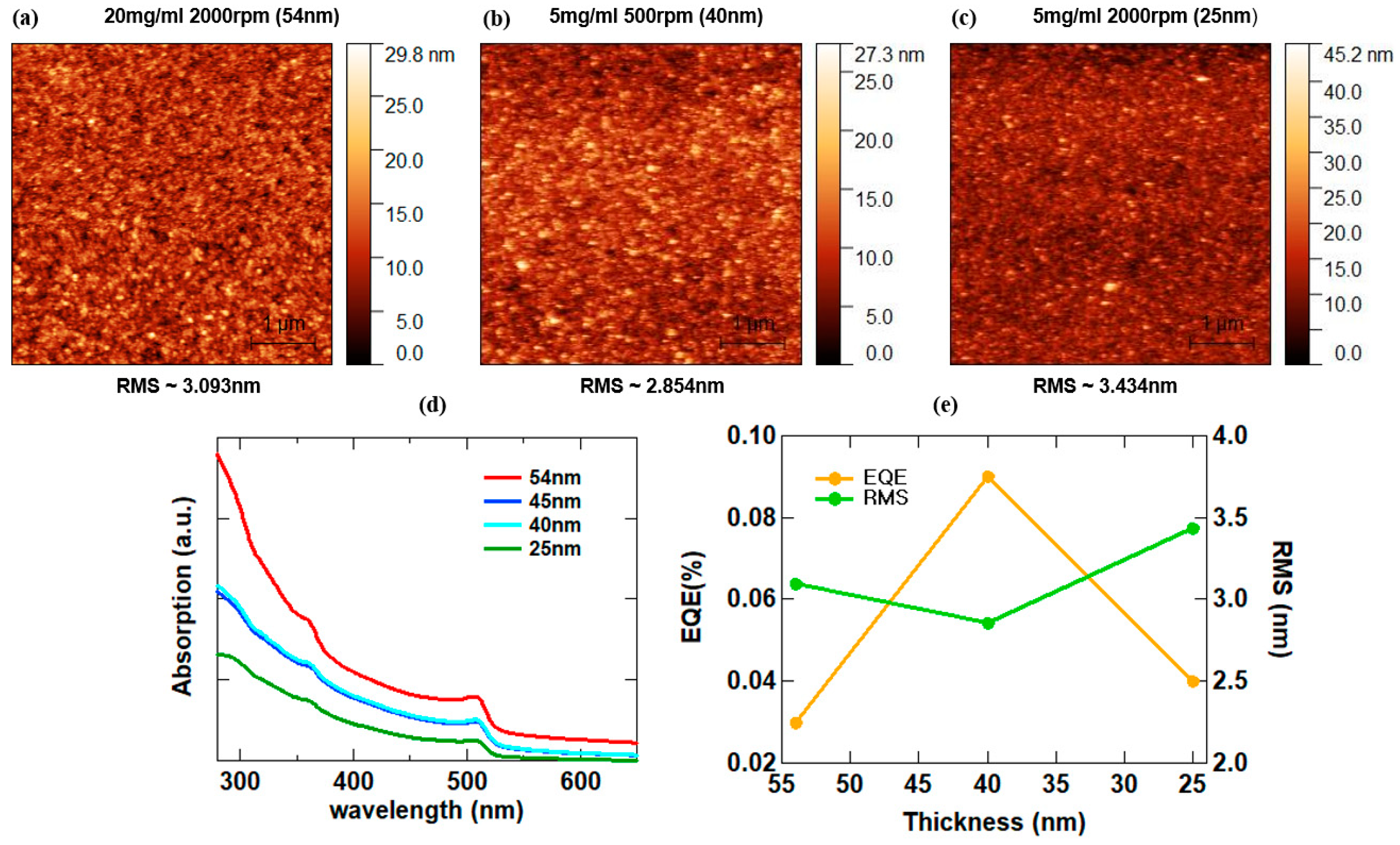
3.3. CsPbBr3 QD Morphology Control
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colvin, V.L.; Schlamp, M.C.; Alivisatos, A.P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 1994, 370, 354–357. [Google Scholar] [CrossRef]
- Dai, X.; Deng, Y.; Peng, X.; Jin, Y. Quantum-Dot Light emitting diodes for large-Area Displays: Towards the Dawn of commercialization. Adv. Mater. 2017, 29, 1607022. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Zheng, Y.; Cao, W.R.; Titov, A.; Hyvonen, J.; Manders, J.R.; Xue, J.G.; Holloway, P.H.; Qian, L. High-efficiency light emitting devices based on quantum dots with tailored nanostructures. Nat. Photonics 2015, 9, 259–266. [Google Scholar] [CrossRef]
- Kim, J.Y.; Voznyy, O.; Zhitomirsky, D.; Sargent, E.H. 25th Anniversary Article: Colloidal Quantum Dot Materials and Devices: A Quarter-Century of Advances. Adv. Mater. 2013, 25, 4986–5010. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, A.Y.; Supran, G.J.; Bawendi, M.G.; Bulovic, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.-G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.; Shao, J.; Yao, D.; Gao, D.H.; Liu, Y.; Yu, W.; Zhang, H.; Yang, B. CsPbxMn1−xCl3 Perovskite Quantum Dots with High Mn Substitution Ratio. ACS Nano 2017, 11, 2239–2247. [Google Scholar] [CrossRef]
- Moyen, E.; Kim, J.H.; Kim, J.; Jang, J. ZnO Nanoparticles for Quantum-Dot Light-Emitting Diodes. ACS Appl. Nano Mater. 2020, 3, 5203–5211. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Qi, S.; Sun, M. Optimized TFB-based perovskite quantum dot light emitting diode. Solid State Commun. 2023, 363, 115102. [Google Scholar] [CrossRef]
- Moon, H.; Lee, C.; Lee, W.; Kim, J.; Chae, H. Stability of Quantum Dots, Quantum Dot Films, and QLED for Display application. Adv. Mater. 2019, 31, 1804294. [Google Scholar] [CrossRef]
- Bae, W.K.; Park, Y.S.; Lim, J.; Lee, D.; Padilha, L.A.; McDaniel, H.; Robel, I.; Lee, C.; Pietryga, J.M.; Klimov, V.I. Controlling the influence of Auger recombination on the performance of quantum-dot light-emitting diodes. Nat. Commun. 2013, 4, 2661. [Google Scholar]
- Qian, L.; Zheng, Y.; Xue, J.; Holloway, P.H. Stable and efficient Quantum-dot Light-emitting Diodes Based on Solution-processed Multilayer Structures. Nat. Photonics 2011, 5, 543–548. [Google Scholar] [CrossRef]
- Jing, J.; Lin, L.; Yang, K.; Hu, H.; Guo, T.; Li, F. Highly efficient inverted quantum dot light-emitting diodes employing sol-gel derived Li-doped ZnO as electron transport layer. Org. Electron. 2022, 103, 106466. [Google Scholar] [CrossRef]
- Kwak, J.; Bae, W.K.; Lee, D.; Park, I.; Lim, J.; Park, M.; Cho, H.; Woo, H.; Yoon, D.Y.; Char, K.; et al. Bright and Efficient Full-Color Colloidal Quantum Dot Light-Emitting Diodes Using an Inverted Device Structure. Nano Lett. 2012, 12, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Lee, E.K.; Joo, W.J.; Jang, E.; Kim, T.H.; Lee, S.J.; Kwon, S.J.; Han, J.Y.; Kim, B.-K.; Choi, B.L.; et al. High-performance crosslinked colloidal quantum-dot light-emitting diodes. Nat. Photonics 2009, 3, 341–345. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, Y.J.; Kim, J.; Han, C.J.; Lee, J.; Kim, Y.; Greco, T.; Ippen, C.; Wedel, A.; Ju, B.-K.; et al. Transparent InP Quantum Dot Light-Emitting Diodes with ZrO2 Electron Transport Layer and Indium Zinc Oxide Top Electrode. Adv. Funct. Mater. 2016, 26, 3454–3461. [Google Scholar] [CrossRef]
- Kiguye, C.; Jeong, W.J.; Jeong, G.H.; Park, J.H.; Kwak, H.J.; Kim, G.W.; Jang, S.H.; Kim, J.Y. Single, Double and ETL-Sandwiched PVPy Interlayer Effect on Charge Injection Balance and Performance of Inverted Quantum Dot Light-Emitting Diodes. Polymers 2023, 15, 3308. [Google Scholar] [CrossRef]
- Lim, J.; Park, M.; Bae, W.K.; Lee, D.; Lee, S.; Lee, C.; Char, K. High Efficiency cadmium free quantum dot light emitting diodes enabled by the direst formation of excitons within InP@ZnSeS quantum dots. ACS Nano 2013, 7, 9019–9026. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Kim, J.; Jang, H.-K.; Lee, K.J.; Seo, J.H.; Jung, B.J.; Kim, G.; Kwak, J. Controlling charge balance using non-conjugated polymer interlayer in quantum dot light emitting diodes. Org. Electron. 2017, 50, 82–86. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs. Adv. Mater. 2018, 30, 1800764. [Google Scholar] [CrossRef]
- Riel, H.; Karg, S.; Beierlein, T.; Ruhstaller, B.; Rieß, W. Phosphorescent top-emitting organic light emitting devices with improved light outcoupling. Appl. Phys. Lett. 2003, 82, 466–468. [Google Scholar] [CrossRef]
- Bae, W.K.; Kwak, J.; Park, J.W.; Char, K.; Lee, C.; Lee, S. Highly Efficient Green-Light-Emitting Diodes Based on CdSe@ZnS Quantum Dots with a Chemical-Composition Gradient. Adv. Mater. 2009, 21, 1690–1694. [Google Scholar] [CrossRef]
- Ambrose, A.M.; Vasil, D.S.; Jeff, K.; Dmitri, M.; Eugene, A.K.; Iris, V. Lead iodide as a buffer layer in UV-induced degradation of CH3NH3PbI3 films. Solar Energy 2018, 159, 794–799. [Google Scholar]
| Device | EL λmax [nm] | Max. Luminance [cd/m2] | Max. Current Efficiency [cd/A] | Max. EQE [%] |
|---|---|---|---|---|
| Without PVPy | 516 | 82.03 | 0.0076 | 0.003 |
| With PVPy interlayer | 518 | 367.9 | 0.084 | 0.026 |
| EL λmax [nm] | Max. Luminance [cd/m2] | Max. Current Efficiency [cd/A] | Max. EQE [%] | |
|---|---|---|---|---|
| 10 mg/mL 2000 rpm (45 nm) | 518 | 1082 | 0.204 | 0.064 |
| 5 mg/mL 500 rpm (40 nm) | 518 | 2439 | 0.29 | 0.091 |
| 5 mg/mL 2000 rpm (25 nm) | 516 | 927.2 | 0.1123 | 0.035 |
| Thickness [nm] | EQE [%] | RMS [nm] |
|---|---|---|
| 54 | 0.03 | 3.093 |
| 40 | 0.09 | 2.854 |
| 25 | 0.04 | 3.434 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, H.J.; Kiguye, C.; Gong, M.; Park, J.H.; Kim, G.-H.; Kim, J.Y. Enhanced Performance of Inverted Perovskite Quantum Dot Light-Emitting Diode Using Electron Suppression Layer and Surface Morphology Control. Materials 2023, 16, 7171. https://doi.org/10.3390/ma16227171
Kwak HJ, Kiguye C, Gong M, Park JH, Kim G-H, Kim JY. Enhanced Performance of Inverted Perovskite Quantum Dot Light-Emitting Diode Using Electron Suppression Layer and Surface Morphology Control. Materials. 2023; 16(22):7171. https://doi.org/10.3390/ma16227171
Chicago/Turabian StyleKwak, Hee Jung, Collins Kiguye, Minsik Gong, Jun Hong Park, Gi-Hwan Kim, and Jun Young Kim. 2023. "Enhanced Performance of Inverted Perovskite Quantum Dot Light-Emitting Diode Using Electron Suppression Layer and Surface Morphology Control" Materials 16, no. 22: 7171. https://doi.org/10.3390/ma16227171
APA StyleKwak, H. J., Kiguye, C., Gong, M., Park, J. H., Kim, G.-H., & Kim, J. Y. (2023). Enhanced Performance of Inverted Perovskite Quantum Dot Light-Emitting Diode Using Electron Suppression Layer and Surface Morphology Control. Materials, 16(22), 7171. https://doi.org/10.3390/ma16227171









