Peculiarity of the Structure and Luminescence of Glasses in La2S3-Ga2S3-GeS2:Pr3+ System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
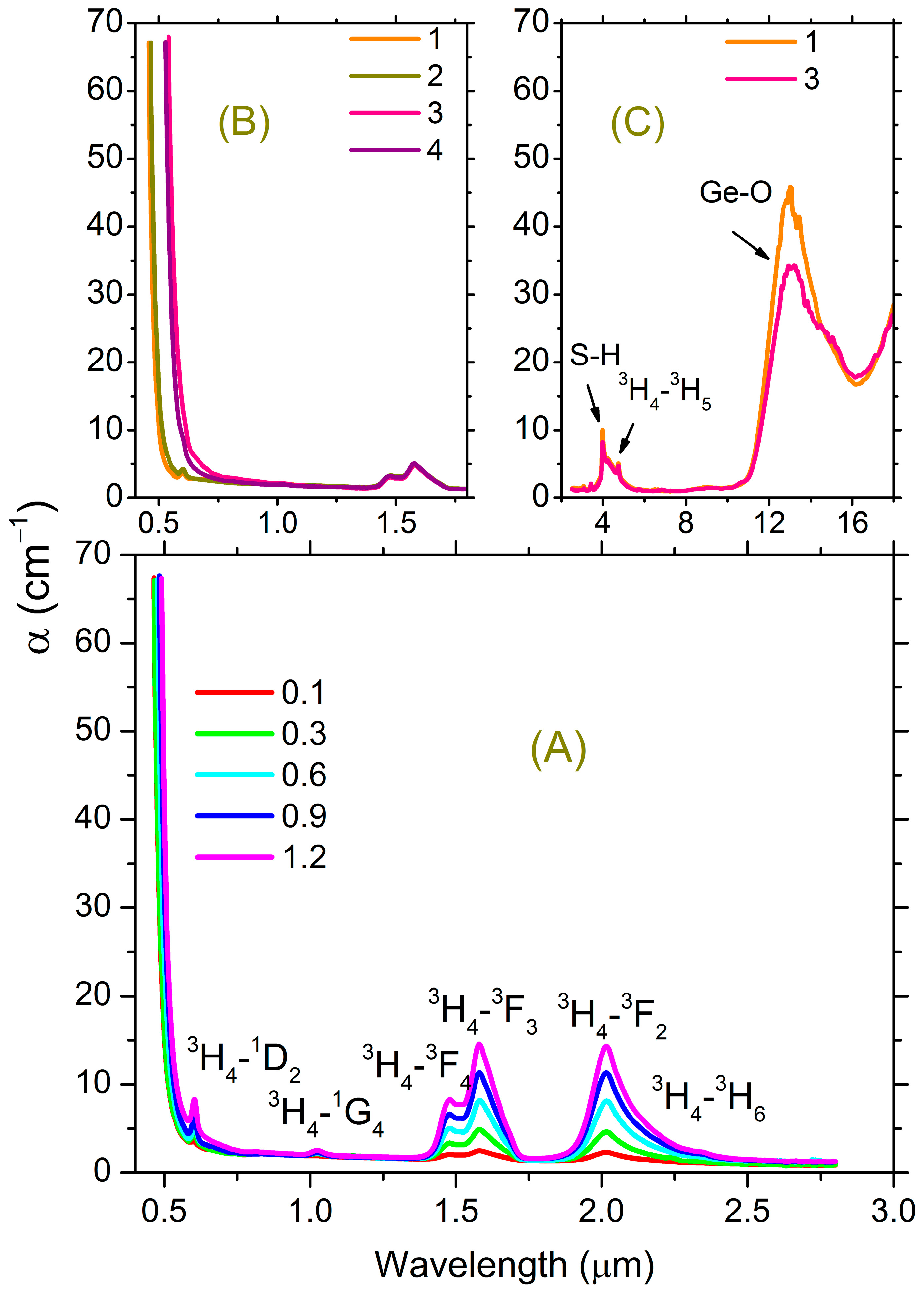
3.1. Optical Absorption Spectroscopy
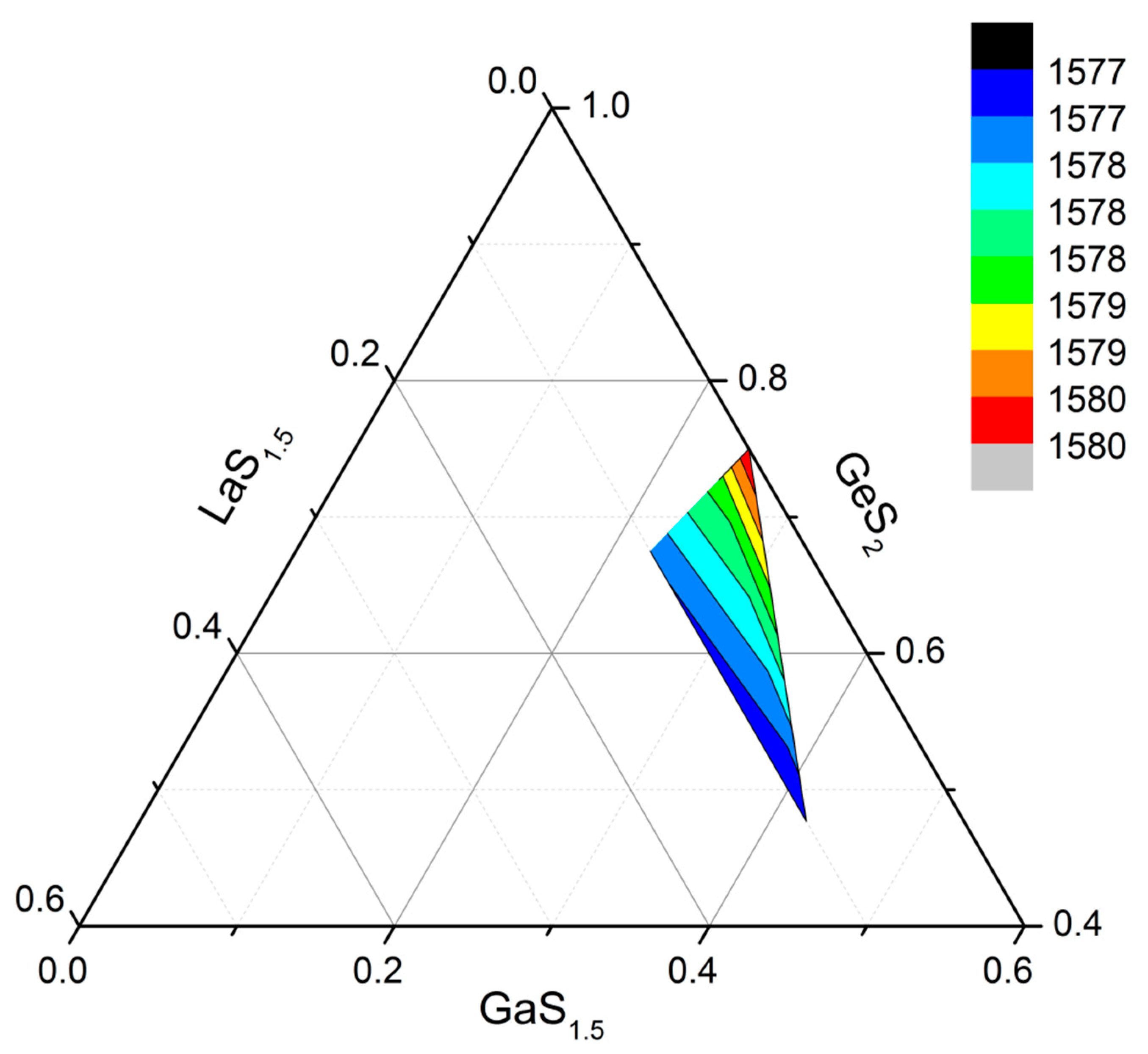
3.2. The Structure of the Studied Glasses according to Raman Spectroscopy Data
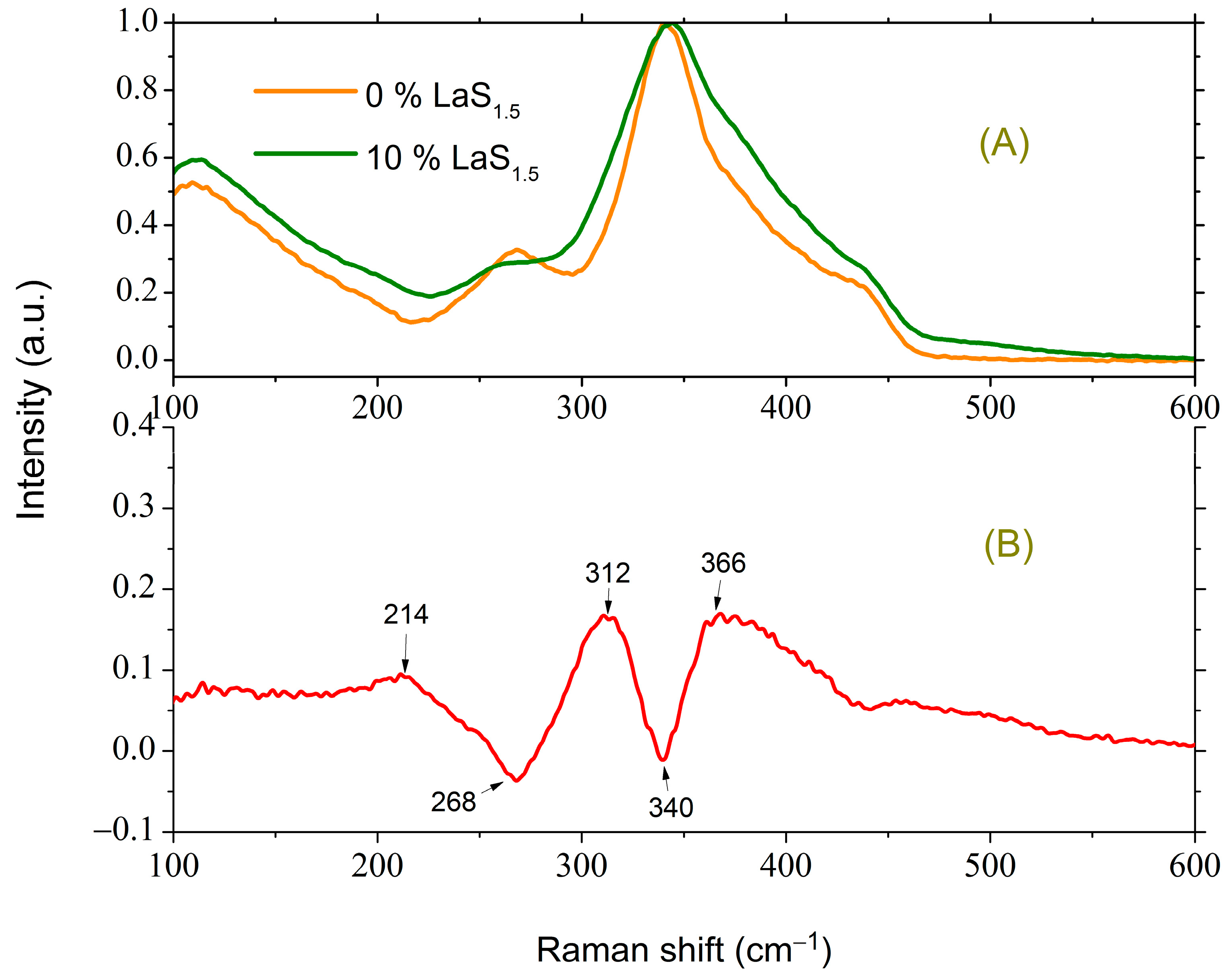
3.2.1. Initial Glasses of the Ga2S3-GeS2 System and the Influence of the La2S3 Addition
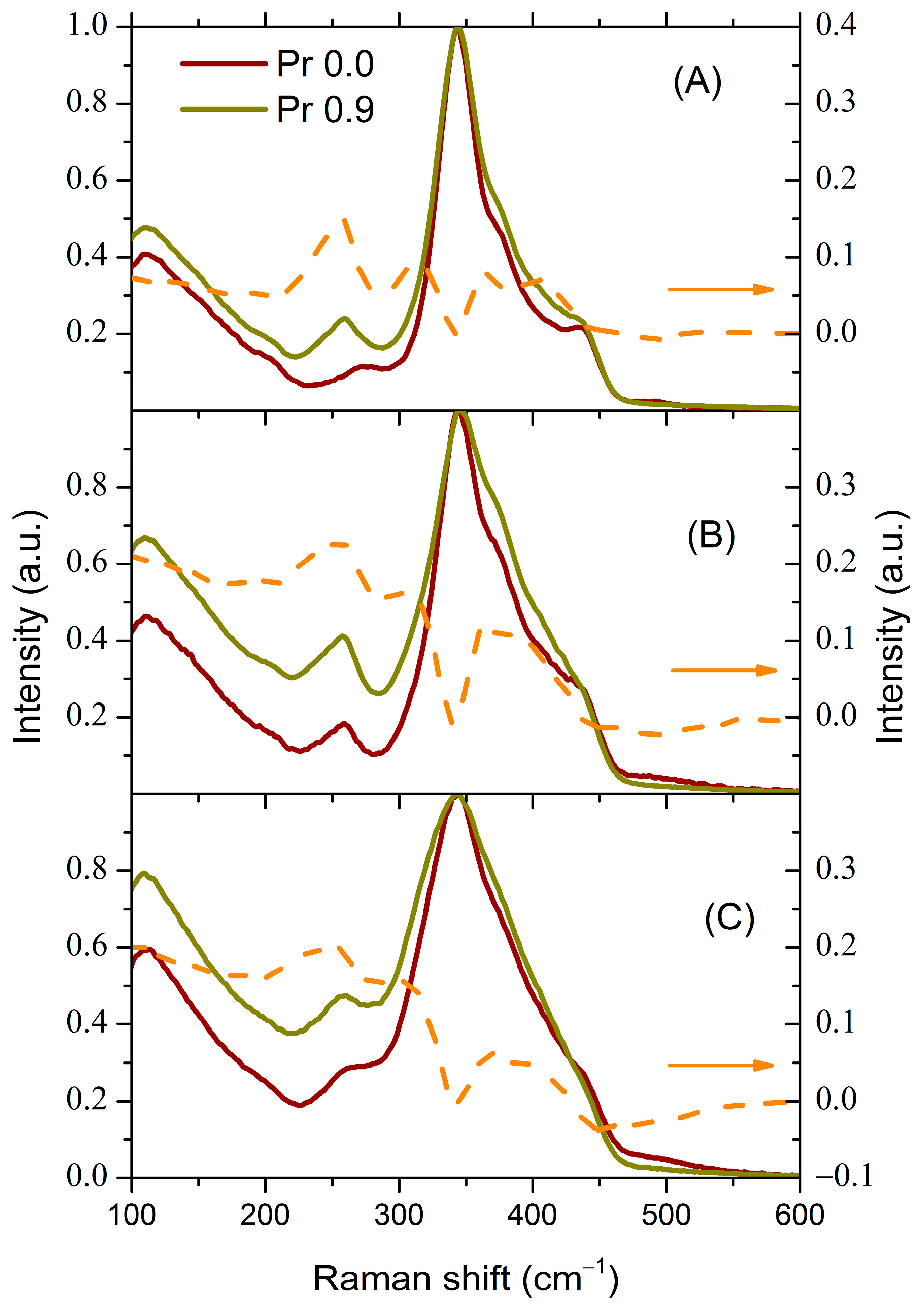
3.2.2. Influence of the Introduction of Pr on the Structure of Glasses according to Raman Spectroscopy Data
3.2.3. Peculiarities of the Raman Spectra in the High-Frequency Region

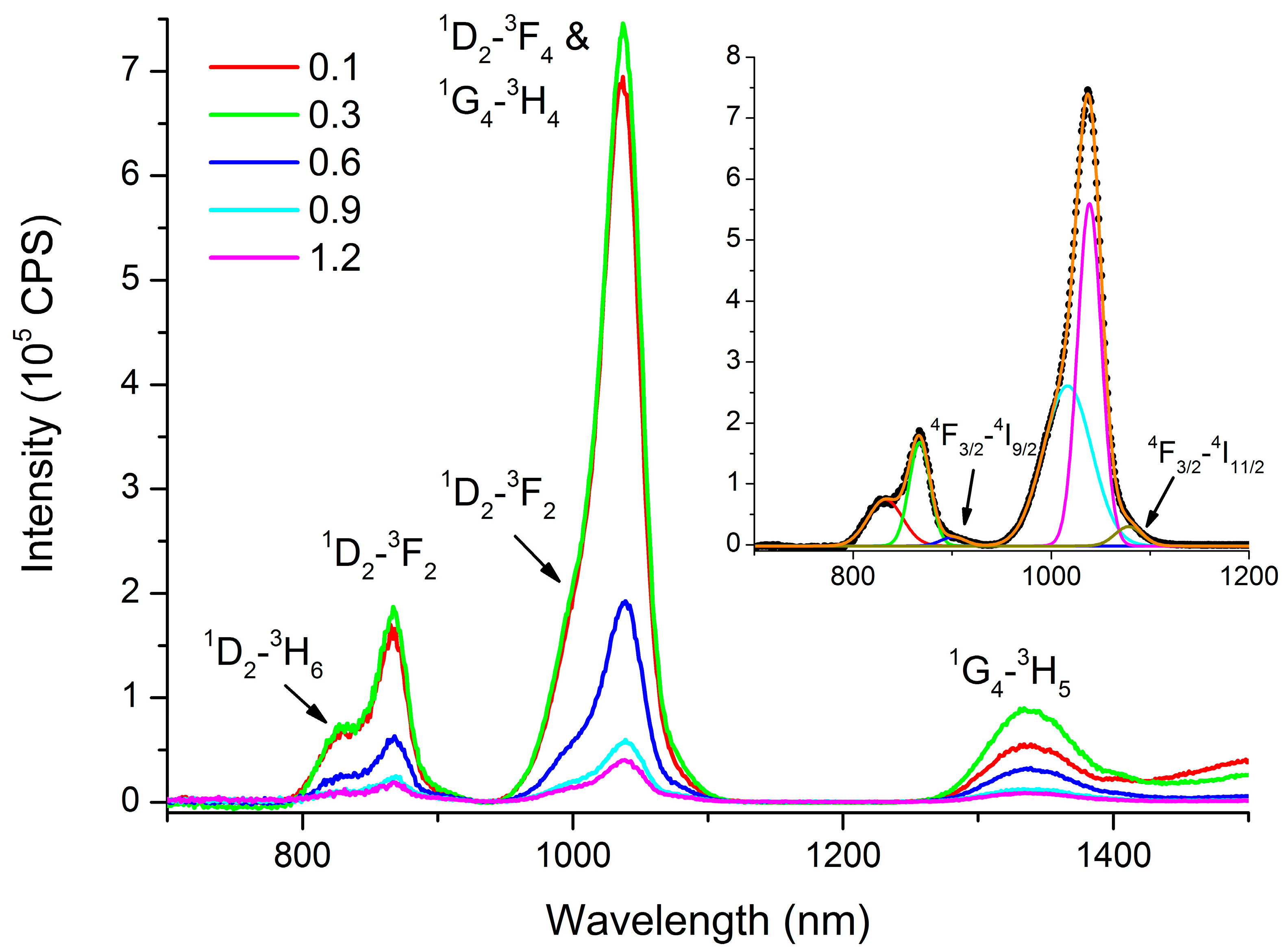
3.3. Luminescent Properties
3.4. Parameters of the Judd–Ofelt Theory for Synthesized Glasses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Cui, J.; Xu, C.; Xu, Y.; Farrell, G. Mid-Infrared Spectral Properties of Rare Earth Ion Doped Chalcogenide Glasses and Fibers. In Mid-Infrared Fluoride and Chalcogenide Glasses and Fibers. Progress in Optical Science and Photonics; Springer: Singapore, 2022; Volume 18. [Google Scholar] [CrossRef]
- Jahromi, K.E.; Pan, Q.; Høgstedt, L.; Friis, S.M.M.; Khodabakhsh, A.; Moselund, P.M.; Harren, F.J.M. Mid-infrared supercontinuum-based upconversion detection for trace gas sensing. Opt. Express 2019, 27, 24469–24480. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, S.; Kachenoura, A.; Costet, N.; Bensalah, K.; Tariel, H.; Senhadji, L. Noninvasive detection of bladder cancer using mid-infrared spectra classification. Expert Syst. Appl. 2017, 89, 333–342. [Google Scholar] [CrossRef]
- Lucas, P.; Coleman, G.J.; Jiang, S.B.; Luo, T.; Yang, Z.Y. Chalcogenide glass fibers: Optical window tailoring and suitability for bio-chemical sensing. Opt. Mater. 2015, 47, 530–536. [Google Scholar] [CrossRef]
- Kumta, P.N.; Risbud, S.H. Rare-earth chalcogenides—An emerging class of optical materials. J. Mater. Sci. 1994, 29, 1135–1158. [Google Scholar] [CrossRef]
- Shaw, L.B.; Cole, B.; Thielen, P.A.; Sanghera, J.S.; Aggarwal, I.D. Mid-wave IR and long-wave IR laser potential of rare-earth doped chalcogenide glass fiber. IEEE J. Quant. Electron. 2001, 48, 1127–1137. [Google Scholar] [CrossRef]
- Tver’yanovich, Y.S.; Tverjanovich, A. Rare-earth doped chalcogenide glasses. In Semiconducting Chalcogenide Glass; Fairman, R., Ushkov, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2004; pp. 169–207. [Google Scholar]
- Tverjanovich, A.; Tveryanovich, Y.S.; Loheider, S. Raman spectra of gallium sulfide based glasses. J. Non-Cryst. Solids 1996, 208, 49–55. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, J.M.; Ryou, S.Y. Raman Spectroscopic Analysis on the Solubility Mechanism of La3+ in GeS2-Ga2S3 Glasses. J. Non-Cryst. Solids 1998, 238, 115–123. [Google Scholar] [CrossRef]
- Li, L.; Jiao, Q.; Lin, C.; Dai, S.; Nie, Q. Structural characterization and compositional dependence of the optical properties of Ge-Ga-La-S chalcohalide glass system. Opt. Mater. 2018, 78, 295–301. [Google Scholar] [CrossRef]
- Higuchi, H.; Kanno, R.; Kawamoto, Y.; Takahashi, M.; Kadono, K. Physicochemical properties and structure of Ga2S3–GeS2–La2S3–Ln2S3 glasses (Ln=yttrium or lanthanoids). Phys. Chem. Glasses 1999, 40, 49–53. [Google Scholar]
- Wang, J.; Brocklesby, W.S.; Lincoln, J.R.; Townsend, J.E.; Payne, D.N. Local structures of rare-earth ions in glasses: The ‘crystal-chemistry’ approach. J. Non-Cryst. Solids 1993, 163, 261–267. [Google Scholar] [CrossRef]
- Yang, A.; Sun, M.; Ren, H.; Lin, H.; Feng, X.; Yang, Z. Dy3+-doped Ga2S3-Sb2S3-La2S3 chalcogenide glass for mid-infrared fiber laser medium. J. Lumin. 2021, 237, 118169. [Google Scholar] [CrossRef]
- Kityk, I.V.; Halyan, V.V.; Yukhymchuk, V.O.; Strelchuk, V.V.; Ivashchenko, I.A.; Zhydachevskyy, Y.; Suchocki, A.; Olekseyuk, I.D.; Kevshyn, A.H.; Piasecki, M. NIR and visible luminescence features of erbium doped Ga2S3-La2S3 glasses. J. Non-Cryst. Solids 2018, 498, 380–385. [Google Scholar] [CrossRef]
- Lima, S.M.; Sampaio, J.A.; Catunda, T.; de Camargo, A.S.S.; Nunes, L.A.O.; Baesso, M.L.; Hewak, D.W. Spectroscopy, thermal and optical properties of Nd3+-doped chalcogenide glasses. J. Non-Cryst. Solids 2001, 284, 274–281. [Google Scholar] [CrossRef]
- Churbanov, M.F.; Denker, B.I.; Galagan, B.I.; Koltashev, V.V.; Plotnichenko, V.G.; Sverchkov, S.E.; Sukhanov, M.V.; Velmuzhov, A.P. Peculiarities of 1.6-7.5 µm Pr3+ luminescence in Ge36Ga5Se59 glass. Opt. Mater. Express 2019, 9, 4154–4164. [Google Scholar] [CrossRef]
- Shi, L.; Sordillo, L.A.; Rodriguez-Contreras, A.; Alfano, R. Transmission in near-infrared optical windows for deep brain imaging. J. Biophotonics 2016, 9, 38–43. [Google Scholar] [CrossRef]
- Sordillo, D.C.; Sordillo, L.A.; Sordillo, P.P.; Alfano, R.R. Fourth Near-Infrared Optical Window for Assessment of Bone and other Tissues. SPIE 2016, 9689, 499–506. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Jia, X.; Yang, L.; Long, N.; Liu, Z.; Dai, S. Mid-infrared emission properties of Pr3+-doped Ge–Sb–Se–Ga–I chalcogenide glasses. Opt. Mater. Express 2018, 8, 992–1000. [Google Scholar] [CrossRef]
- Nazabal, V.; Adam, J.-L. Infrared luminescence of chalcogenide glasses doped with rare earth ions and their potential applications. Opt. Mater. X 2022, 15, 100168. [Google Scholar] [CrossRef]
- Walsh, B.M. Judd-Ofelt Theory: Principles and Practices. In Advances in Spectroscopy for Lasers and Sensing; Di Bartolo, B., Forte, O., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 403–433. [Google Scholar] [CrossRef]
- Weber, M.J. Spontaneous emission probabilities and quantum efficiencies for excited states of Pr3+ in LaF3. J. Chem. Phys. 1968, 48, 4774–4780. [Google Scholar]
- Bagde, G.D.; Londhe, C.T.; Bagde, A.G. Morphological Studies on Spray Deposited Lanthanum Sulphide (La2S3) Thin Films. Key Eng. Mater. 2016, 705, 283–288. [Google Scholar] [CrossRef]
- Ho, C.-H.; Chen, H.-H. Optically decomposed near-band edge structure and excitonic transitions in Ga2S3. Sci. Rep. 2014, 4, 6143. [Google Scholar] [CrossRef] [PubMed]
- Kokh, K.A.; Huang, Z.-M.; Huang, J.-G.; Gao, Y.-Q.; Uralbekov, B.; Panomarev, J.; Lapin, I.N.; Svetlichnyi, V.A.; Lanskii, G.V.; Andreev, Y.M. Study of Ga2S3 crystals grown from melt and PbCl2 flux. Mater. Res. Bull. 2016, 84, 462–467. [Google Scholar] [CrossRef]
- Caraman, I.; Evtodiev, S.; Untila, D.; Palachi, L.; Susu, O.; Evtodiev, I.; Kantser, V. Optical and photoelectric properties of planar structures obtained by thermal annealing of Ga2S3 plates in Zn vapors. Phys. Status Solidi A 2017, 214, 1700808. [Google Scholar] [CrossRef]
- Bletskan, D.I.; Kabatsiy, V.N.; Frolova, V.V. Peculiarities of the Absorption Edge and Photoconductivity Spectra of (GeS2)x(Bi2S3)1-x Glasses. Chalcogenide Lett. 2007, 4, 119–126. [Google Scholar]
- Onari, S.; Taniyama, M.; Kiyomoto, S.; Makino, T.; Matsuishi, K. Optical Properties of the Amorphous Semiconductor (GeS2)1-x(Bi2S3)x System under High Pressure. Phys. Status Solidi B 1999, 211, 263–267. [Google Scholar] [CrossRef]
- Lucovsky, G.; Galeener, F.L.; Keezer, R.C.; Geils, R.H.; Six, H.A. Structural interpretation of the infrared and Raman spectra of glasses in the alloy system Ge1−xSx. Phys. Rev. B 1974, 10, 5134–5146. [Google Scholar] [CrossRef]
- Holomb, R.; Johansson, P.; Mitsa, V.; Rosola, I. Local structure of technologically modified g-GeS2: Resonant Raman and absorption edge spectroscopy combined with ab initio calculations. Philos. Mag. 2005, 85, 2947–2960. [Google Scholar] [CrossRef]
- Masselin, P.; Le Coq, D.; Cuisset, A.; Bychkov, E. Spatially resolved Raman analysis of laser induced refractive index variation in chalcogenide glass. Opt. Mater. Express 2012, 2, 1768–1775. [Google Scholar] [CrossRef]
- Jackson, K.; Briley, A.; Grossman, S.; Porezag, D.V.; Pederson, M.R. Raman-active modes of a-GeSe2 and a-GeS2: A first-principles study. Phys. Rev. B 1999, 60, R14985–R14989. [Google Scholar] [CrossRef]
- Lucovsky, G.; Nemanich, R.J.; Galeener, F.L. Amorphous and Liquid Semiconductors: Proceedings of the 7th International Conference on Amorphous and Liquid Semiconductors, Edinburgh, Scotland, 1977; Spear, W.E., Stevenson, G.G., Eds.; University of Edinburgh: Edinburgh, Scotland, 1977; pp. 130–134. [Google Scholar]
- Tverjanovich, A.; Tveryanovich, Y.S.; Shahbazova, C. Structure and Luminescent Properties of Glasses in the GeS2–Ga2S3–Sb2S3:Pr3+ System. Materials 2023, 16, 4672. [Google Scholar] [CrossRef]
- Ishibashi, T.; Takebe, H.; Morinaga, K. Glass forming region and structure of vitreous RS-Ga2S3 (R = Ca, Sr, Ba). J. Ceram. Soc. Jpn. 2003, 111, 308–311. [Google Scholar] [CrossRef]
- Julien, C.; Barnier, S.; Massot, M.; Chbani, N.; Cai, X.; Loireau-Lozac’h, A.M.; Guittard, M. Raman and infrared spectroscopic studies of Ge-Ga-Ag- sulphide glasses. Mater. Sci. Eng. B 1994, 22, 191–200. [Google Scholar] [CrossRef]
- Koudelka, L.; Pisárčik, M.; Baidakova, O.L. The effect of MnS- and MnCl2-doping on the structure of GeS2-Ga2S3 glasses. J. Mater. Sci. Lett. 1989, 8, 1161–1162. [Google Scholar] [CrossRef]
- Lucazeau, G.; Barnier, S.; Loireau-Lozac’h, A.M. Vibrational spectra, electronic transitions and short order structure of rare earth-gallium sulphide glasses. Spec. Acta Part A 1978, 34, 21–27. [Google Scholar] [CrossRef]
- Viras, F.; King, T.A. The universality of low frequency Raman scattering from amorphous solids. J. Non-Cryst. Solids 1990, 119, 65–74. [Google Scholar] [CrossRef]
- Tikhomirov, V.K.; Jha, A.; Perakis, A.; Sarantopoulou, E.; Naftaly, M.; Krasteva, V.; Li, R.; Seddon, A.B. An interpretation of the Boson peak in rare-earth ion doped glasses. J. Non-Cryst. Solids 1999, 256, 89–94. [Google Scholar] [CrossRef]
- Judd, B.R. Ionic transitions hypersensitive to environment. J. Chem. Phys. 1979, 70, 4830–4833. [Google Scholar] [CrossRef]
- Mann, M.M.; DeShazer, L.G. Energy Levels and Spectral Broadening of Neodymium Ions in Laser Glass. J. Appl. Phys. 1970, 41, 2951. [Google Scholar] [CrossRef]
- Robinson, C.C. Evidence of Sixfold Coordination of Nd3+ in Barium Rubidium Silicate Glass. J. Chem. Phys. 1971, 54, 3572. [Google Scholar] [CrossRef]
- Ponader, C.W.; Brown, G.E., Jr. Rare earth elements in silicate glass melt systems: I. Effects of composition on the coordination environments of La, Gd, and Yb. Geochim. Cosmochim. Acta 1989, 53, 2893–2903. [Google Scholar] [CrossRef]
- Quintas, A.; Majérus, O.; Lenoir, M.; Caurant, D.; Klementiev, K.; Webb, A. Effect of alkali and alkaline-earth cations on the neodymium environment in a rare-earth rich aluminoborosilicate glass. J. Non-Cryst. Solids 2008, 354, 98–104. [Google Scholar] [CrossRef]
- Drewitt, J.W.E.; Salmon, P.S.; Zeidler, A.; Benmore, C.J.; Hannon, A.C. Structure of rare-earth chalcogenide glasses by neutron and x-ray diffraction. J. Phys. Condens. Matter. 2017, 29, 225703. [Google Scholar] [CrossRef] [PubMed]
- Quimby, R.S.; Aitken, B.G. Anomalous temperature quenching of fluorescence in Pr3+ doped sulfide glass. J. Appl. Phys. 1997, 82, 3992–3996. [Google Scholar] [CrossRef]
- Sattayaporn, S.; Loiseau, P.; Aka, G.; Klimin, S.; Boldyrev, K.; Mavrin, B. Fine spectroscopy and Judd-Ofelt analysis of Pr3+ doped Sr0.7La0.3Mg0.3Al11.7O19 (Pr:ASL). J. Lumin. 2020, 219, 116895. [Google Scholar] [CrossRef]
- Judd, B.R. Optical absorption intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Quimby, R.S.; Miniscalco, W.J. Modified Judd-Ofelt technique and application to optical transitions in Pr3+-doped glass. J. Appl. Phys. 1994, 75, 613–615. [Google Scholar] [CrossRef]
- Goldner, P.; Auzel, F. Application of standard and modified Judd-Ofelt theories to a praseodymium-doped fluorozirconate glass. J. Appl. Phys. 1996, 79, 7972–7977. [Google Scholar] [CrossRef]
- Dunina, E.; Kornienko, A.; Fomicheva, L. Modified theory of f-f transition intensities and crystal field for systems with anomalously strong configuration interaction. Cent. Eur. J. Phys. 2008, 6, 407–414. [Google Scholar] [CrossRef]
- Olivier, M.; Doualan, J.-L.; Nazabal, V.; Camy, P.; Adam, J.-L. Spectroscopic study and Judd–Ofelt analysis of Pr3+-doped Zr-Ba-La-Al glasses in visible spectral range. J. Opt. Soc. Am. B 2013, 30, 2032–2042. [Google Scholar] [CrossRef]
- Zhang, F.; Bi, Z.; Huang, A.; Xiao, Z. Luminescence and Judd–Ofelt analysis of the Pr3+ doped fluorotellurite glass. J. Lumin. 2015, 160, 85–89. [Google Scholar] [CrossRef]
- Wei, K.; Machewirth, D.P.; Wenzel, J.; Snitzer, E.; Sigel, G.H., Jr. Pr3+-doped Ge-Ga-S glasses for 1.3 optical fiber amplifiers. J. Non-Cryst. Solids 1995, 182, 257–261. [Google Scholar] [CrossRef]
- Hehlen, M.P.; Brik, M.G.; Kramer, K.W. 50th anniversary of the Judd–Ofelt theory: An experimentalist’s view of the formalism and its application. J. Lumin. 2013, 136, 221–239. [Google Scholar] [CrossRef]
- Bhatia, B.; Parihar, V.; Singh, S.; Verma, A.S. Spectroscopic Properties of Pr3+ in Lithium Bismuth Borate Glasses. Am. J. Condens. Matter Phys. 2013, 3, 80–88. [Google Scholar]



| No Composition | GaS1.5 (mol.%) | GeS2 (mol.%) | LaS1.5 (mol.%) |
|---|---|---|---|
| 1 | 25.00 | 75.00 | 0.00 |
| 2 | 24.25 | 72.75 | 3.00 |
| 3 | 22.50 | 67.50 | 10.00 |
| 4 | 47.00 | 53.00 | 0.00 |
| 5 | 42.30 | 47.70 | 10.00 |
| No Composition | Ω2 (10−20 cm2) | Ω4 (10−20 cm2) | Ω6 (10−20 cm2) | Radiative Lifetime from 1G4, ms |
|---|---|---|---|---|
| 1 | 14.28 | 5.19 | 7.23 | 0.411 |
| 3 | 10.68 | 6.28 | 6.43 | 0.366 |
| 5 | 10.53 | 6.10 | 6.00 | 0.334 |
| Transition | Transition Probabilities, s−1 | ||
|---|---|---|---|
| 1 | 3 | 5 | |
| 1G4-3F4 | 88.9 | 101.1 | 108.5 |
| 1G4-3F3 | 13.8 | 15.4 | 16.6 |
| 1G4-3F2 | 10.5 | 14.3 | 15.8 |
| 1G4-3H6 | 798.7 | 855.4 | 960.5 |
| 1G4-3H5 | 1356.0 | 1554.1 | 1685.4 |
| 1G4-3H4 | 166.0 | 193.8 | 209.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tverjanovich, A.; Smirnov, E. Peculiarity of the Structure and Luminescence of Glasses in La2S3-Ga2S3-GeS2:Pr3+ System. Materials 2023, 16, 7094. https://doi.org/10.3390/ma16227094
Tverjanovich A, Smirnov E. Peculiarity of the Structure and Luminescence of Glasses in La2S3-Ga2S3-GeS2:Pr3+ System. Materials. 2023; 16(22):7094. https://doi.org/10.3390/ma16227094
Chicago/Turabian StyleTverjanovich, Andrey, and Egor Smirnov. 2023. "Peculiarity of the Structure and Luminescence of Glasses in La2S3-Ga2S3-GeS2:Pr3+ System" Materials 16, no. 22: 7094. https://doi.org/10.3390/ma16227094
APA StyleTverjanovich, A., & Smirnov, E. (2023). Peculiarity of the Structure and Luminescence of Glasses in La2S3-Ga2S3-GeS2:Pr3+ System. Materials, 16(22), 7094. https://doi.org/10.3390/ma16227094







