Simulating and Predicting the Mechanical Behavior of Electrospun Scaffolds for Cardiac Patches Fabrication
Abstract
:1. Introduction
2. Materials and Methods
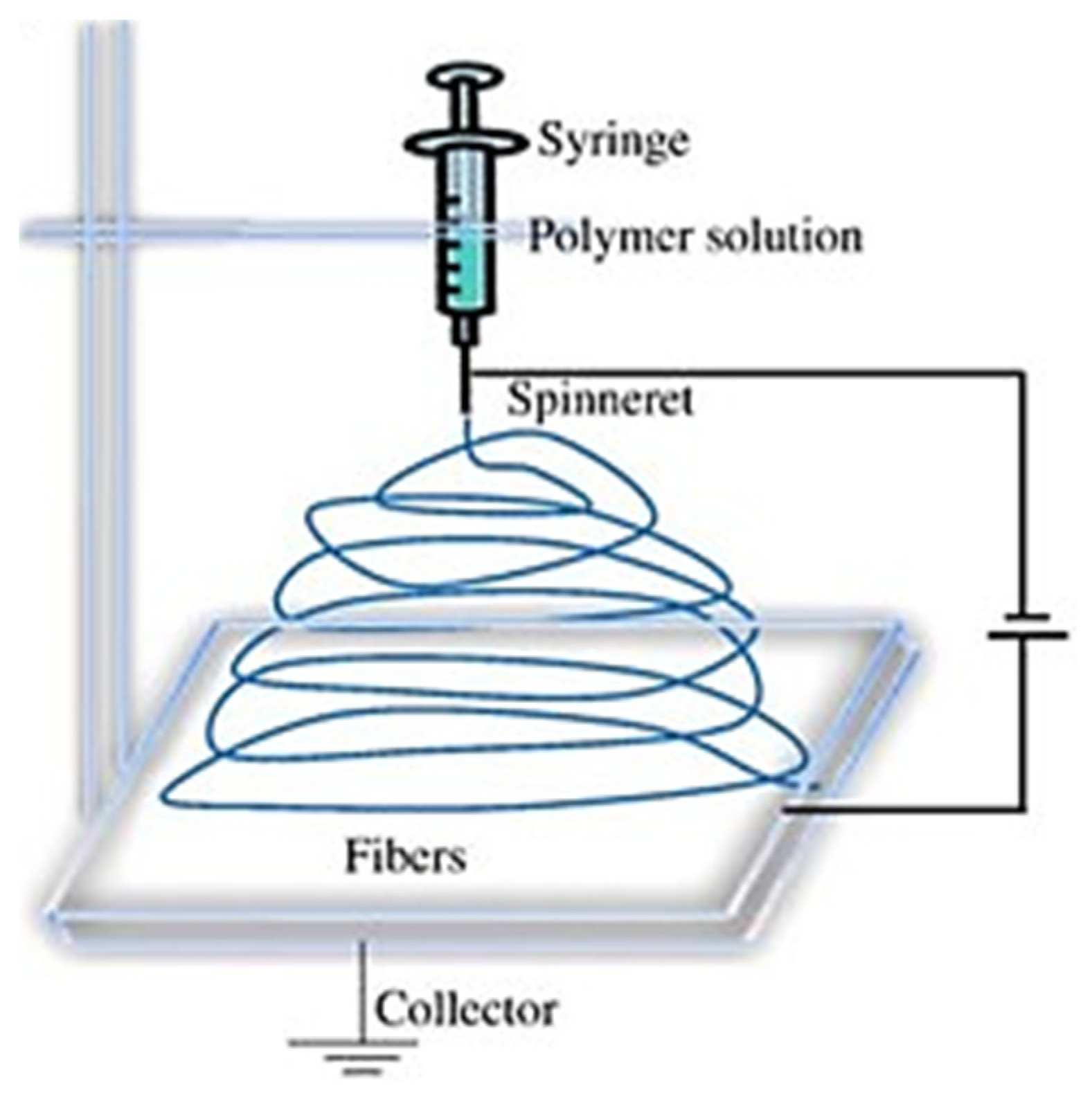
2.1. Material and Sample Preparation with Electrospinning
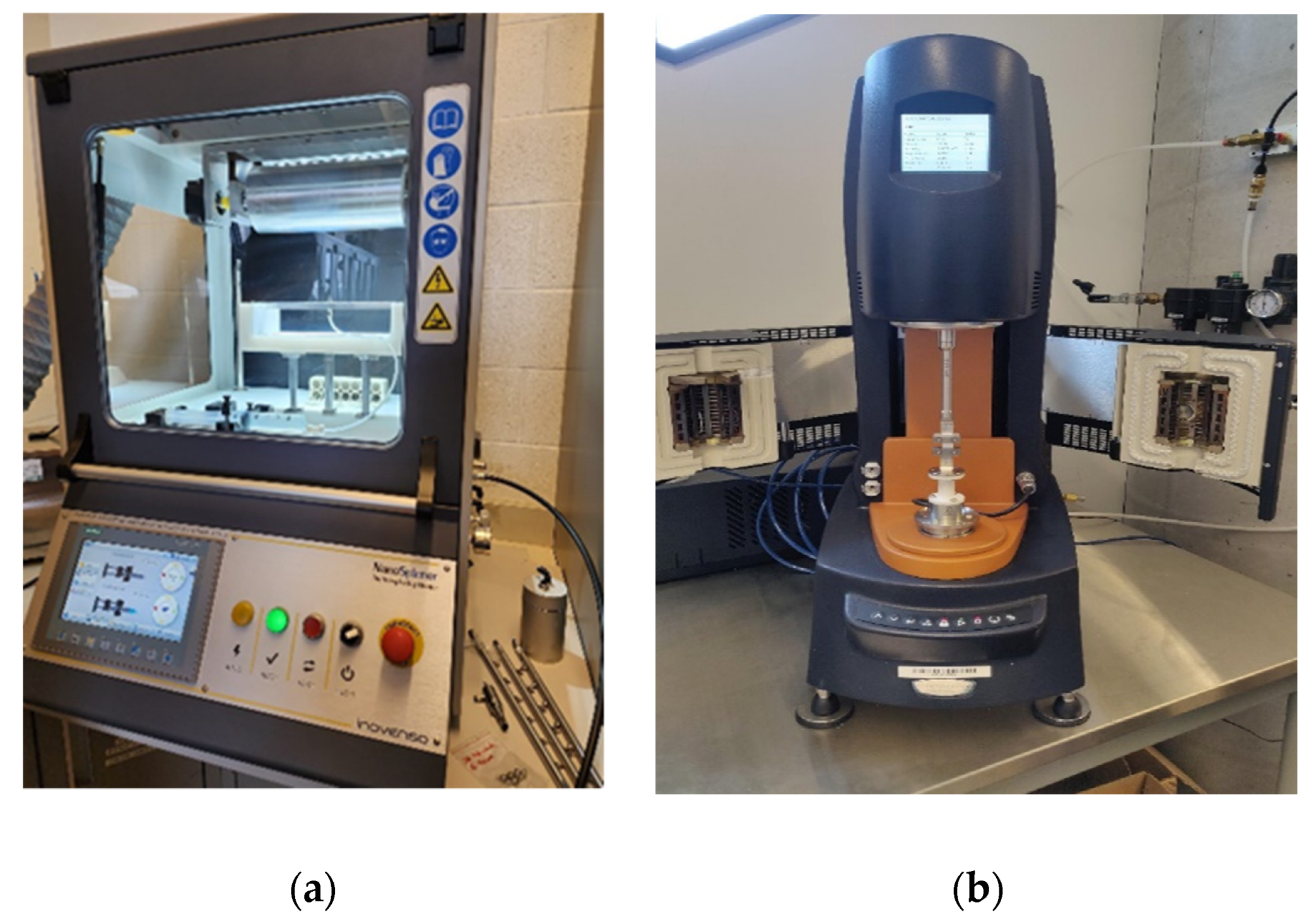
2.2. Experimental Setup for Mechanical Testing
2.3. Modeling the Viscoelastic Behavior of Electrospun Scaffolds
2.3.1. Mimicking the Mechanical Behavior of the Native Cardiac Tissue
2.3.2. Transient and Stress-Softening Effects
2.4. Simulations and Predictions of the Viscoelastic Behavior of Electrospun Scaffolds
3. Results and Discussion
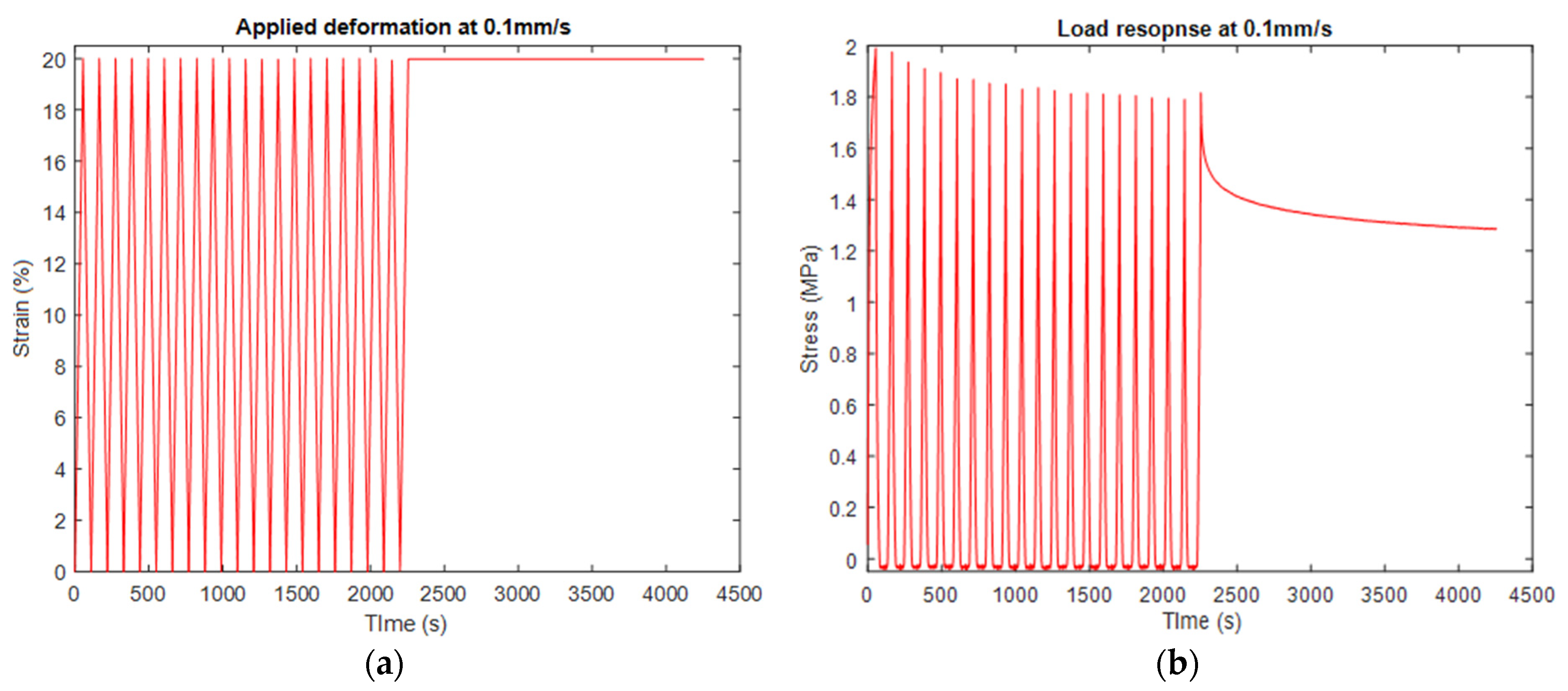
3.1. Experimental Tests
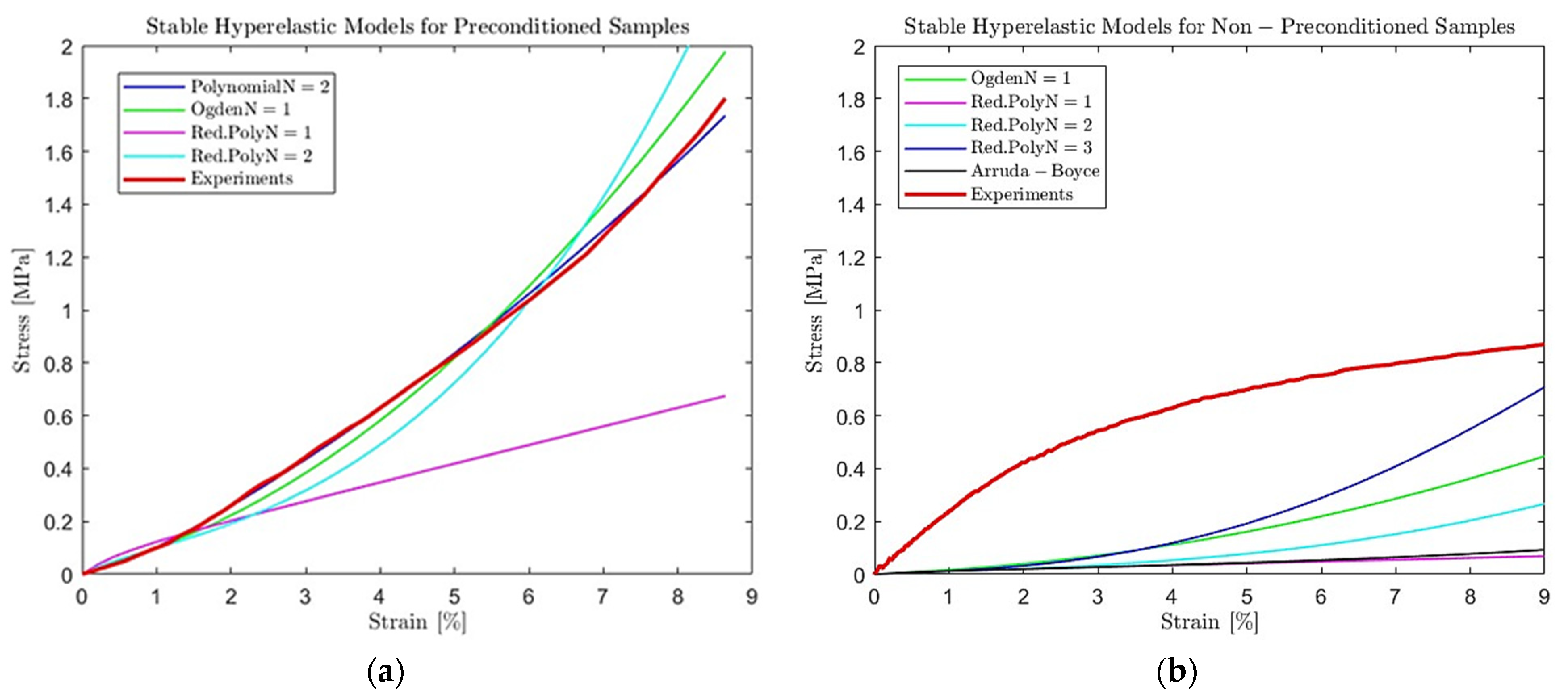
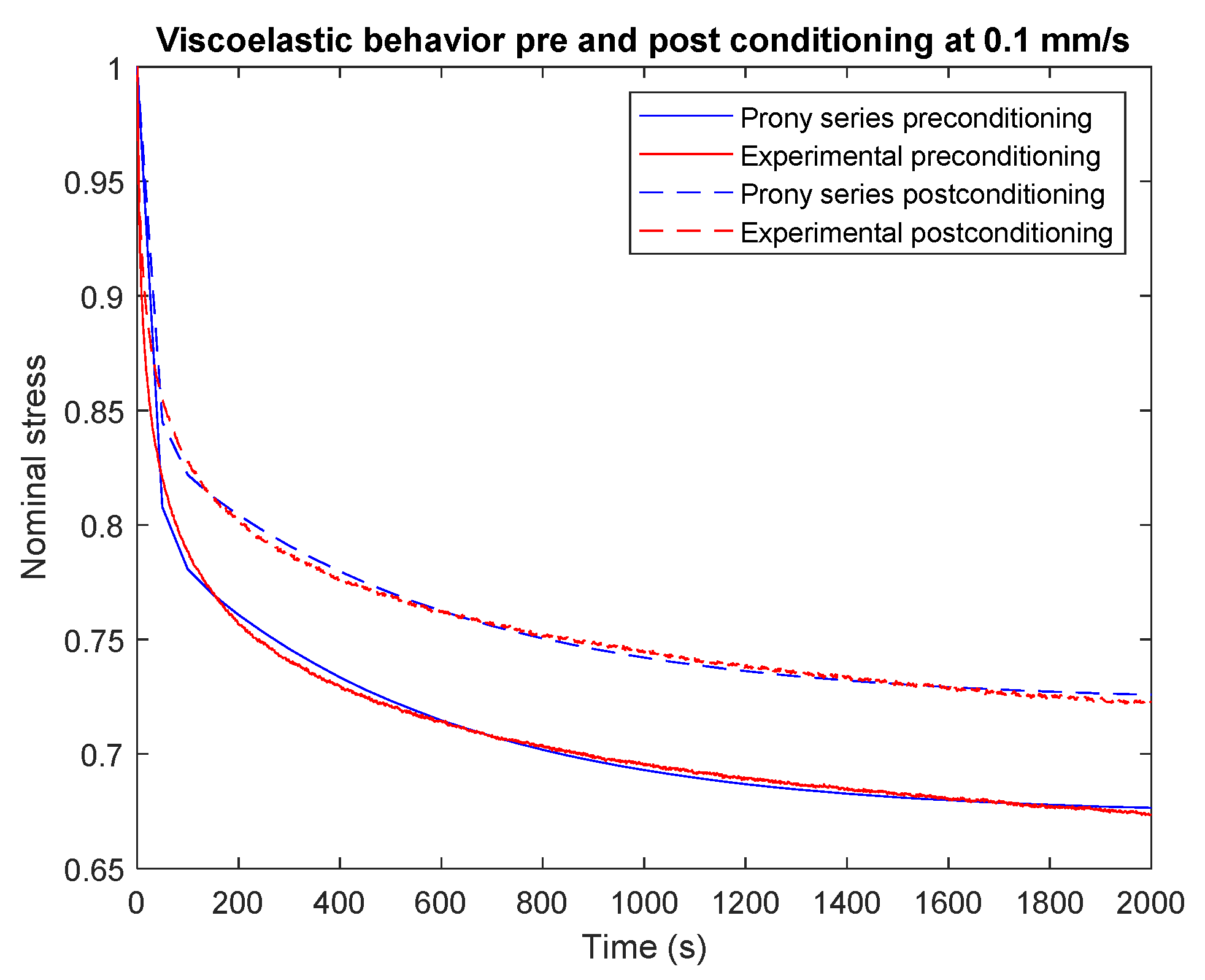
3.2. Modeling Scaffolds’ Viscoelastic Behavior Results
3.3. Simulating Scaffolds’ Viscoelastic Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engel, F.B. Cardiomyocyte Proliferation: A Platform for Mammalian Cardiac Repair. Cell Cycle 2005, 4, 1360–1363. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.-H.; Melnychenko, I.; Wasmeier, G.; Didié, M.; Naito, H.; Nixdorff, U.; Hess, A.; Budinsky, L.; Brune, K.; Michaelis, B.; et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006, 12, 452–458. [Google Scholar] [CrossRef]
- Dvir, T.; Kedem, A.; Ruvinov, E.; Levy, O.; Freeman, I.; Landa, N.; Holbova, R.; Feinberg, M.S.; Dror, S.; Etzion, Y.; et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc. Natl. Acad. Sci. USA 2009, 106, 14990–14995. [Google Scholar] [CrossRef]
- Duling, R.R.; Dupaix, R.B.; Katsube, N.; Lannutti, J. Mechanical Characterization of Electrospun Polycaprolactone (PCL): A Potential Scaffold for Tissue Engineering. J. Biomech. Eng. 2008, 130, 011006. [Google Scholar] [CrossRef]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-caprolactone)-based electrospun nano-featured substrate for tissue engineering applications: A review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef]
- Feiner, R.; Shapira, A.; Dvir, T. Scaffolds for Tissue Engineering of Functional Cardiac Muscle; Woodhead Publishing: Sawston, UK, 2019; pp. 685–703. [Google Scholar]
- Silva, P.E.S.; De Abreu, F.V.; Godinho, M.H. Shaping helical electrospun filaments: A review. Soft Matter 2017, 13, 6678–6688. [Google Scholar] [CrossRef]
- Fleischer, S.; Feiner, R.; Dvir, T. Cardiac tissue engineering: From matrix design to the engineering of bionic hearts. Regen. Med. 2017, 12, 275–284. [Google Scholar] [CrossRef] [PubMed]
- D’hooge, J.; Konofagou, E.; Jamal, F.; Heimdal, A.; Barrios, L.; Bijnens, B.; Thoen, J.; Van de Werf, F.; Sutherland, G.; Suetens, P. Two-dimensional ultrasonic strain rate measurement of the human heart in vivo. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Sommer, G.; Schriefl, A.J.; Andrä, M.; Sacherer, M.; Viertler, C.; Wolinski, H.; Holzapfel, G.A. Biomechanical properties and microstructure of human ventricular myocardium. Acta Biomater. 2015, 24, 172–192. [Google Scholar] [CrossRef] [PubMed]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Bischoff, J.E.; Arruda, E.M.; Grosh, K. Finite element modeling of human skin using an isotropic, nonlinear elastic constitutive model. J. Biomech. 2000, 33, 645–652. [Google Scholar] [CrossRef]
- AlAttar, A.; Gkouti, E.; Czekanski, A. Multistep deformation of helical fiber electrospun scaffold toward cardiac patches development. J. Mech. Behav. Biomed. Mater. 2023, 147, 106157. [Google Scholar] [CrossRef]
- Lu, T.; Wang, J.; Yang, R.; Wang, T.J. A Constitutive Model for Soft Materials Incorporating Viscoelasticity and Mullins Effect. J. Appl. Mech. 2016, 84, 021010. [Google Scholar] [CrossRef]
- Gkouti, E.; Yenigun, B.; Czekanski, A. Transient Effects of Applying and Removing Strain on the Mechanical Behavior of Rubber. Materials 2020, 13, 4333. [Google Scholar] [CrossRef]
- Gkouti, E.; Yenigun, B.; Jankowski, K.; Czekanski, A. Experimental study of Mullins effect in natural rubber for different stretch conditions. In Proceedings of the International Design Engineering Technical Conferences and Computers and Information in Engineering Conference (ASME2020), Virtual, 17–19 August 2020. [Google Scholar]
- Ogden, R.W.; Roxburgh, D.G. A pseudo–elastic model for the Mullins effect in filled rubber. Proc. R. Soc. A Math. Phys. Eng. Sci. 1999, 455, 2861–2877. [Google Scholar] [CrossRef]
- Horgan, C.O. A note on a class of generalized neo-Hookean models for isotropic incompressible hyperelastic materials. Int. J. Non-Linear Mech. 2021, 129, 103665. [Google Scholar] [CrossRef]
- Treloar, L.R.G. Stress-Strain Data for Vulcanized Rubber under Various Types of Deformation. Rubber Chem. Technol. 1944, 17, 813–825. [Google Scholar] [CrossRef]
- AlAttar, A.; Czekanski, A. Sensitivity study for optimizing electrospun helix fibers production for cardiac scaffold. In Proceedings of the Canadian Society for Mechanical Engineering International Congress 2021 (CSME2021), Charlottetown, PE, Canada, 28–30 June 2011. [Google Scholar]
- Gkouti, E.; Czekanski, A. Modeling the viscoelastic behavior of electrospun scaffolds used for cardiac patches development. In Proceedings of the Canadian Society for Mechanical Engineering International Congress (CSME2023), Sherbrooke, QC, Canada, 28–31 May 2023. [Google Scholar]
- Yoon, J.; Yang, H.S.; Lee, B.S.; Yu, W.R. Recent progress in coaxial electrospinning: New parameters, various structures, and wide applications. Adv. Mater. 2018, 30, 1704765. [Google Scholar] [CrossRef] [PubMed]
- Behtaj, S.; Karamali, F.; Masaeli, E.; Anissimov, Y.G.; Rybachuk, M. Electrospun PGS/PCL, PLLA/PCL, PLGA/PCL and pure PCL scaffolds for retinal progenitor cell cultivation. Biochem. Eng. J. 2021, 166, 107846. [Google Scholar] [CrossRef]
- Pislaru, C.; Abraham, T.P.; Belohlavek, M. Strain and strain rate echocardiography. Curr. Opin. Cardiol. 2002, 17, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Heimdal, A.; Støylen, A.; Torp, H.; Skjærpe, T. Real-Time Strain Rate Imaging of the Left Ventricle by Ultrasound. J. Am. Soc. Echocardiogr. 1998, 11, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Arruda, E.M.; Boyce, M.C. A three-dimensional consitutive model for the large stretch behavior of rubber elastic materials. J. Mech. Phys. Solids 1993, 41, 389–412. [Google Scholar] [CrossRef]


| Condition | Length (mm) |
|---|---|
| Initial length | 28 mm |
| Deformed (after testing completion) | 31.2 mm (11.6% from the initial length) |
| Recovered (24 h resting) | 29.7 mm (6% from the initial length) |
| Hyperelastic Models | Preconditioned Samples | Non-Preconditioned Samples |
|---|---|---|
| Polynomial N = 2 | ✓ | - |
| Ogden N = 1 | ✓ | ✓ |
| Red. Polynomial N = 1 | ✓ | ✓ |
| Red. Polynomial N = 2 | ✓ | ✓ |
| Red. Polynomial N = 3 | - | ✓ |
| Arruda–Boyce | - | ✓ |
| Polynomial Model Parameters | Prony Series Parameters | ||
|---|---|---|---|
| C10 | −0.02873762 | G1 | 0.0226086 |
| C20 | 0.00002634 | G2 | 0.11157 |
| C01 | 0.05022618 | τ1 | 8.8895 |
| C11 | 0.00101692 | τ2 | 97.497 |
| C02 | 0.02451267 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkouti, E.; Czekanski, A.; AlAttar, A. Simulating and Predicting the Mechanical Behavior of Electrospun Scaffolds for Cardiac Patches Fabrication. Materials 2023, 16, 7095. https://doi.org/10.3390/ma16227095
Gkouti E, Czekanski A, AlAttar A. Simulating and Predicting the Mechanical Behavior of Electrospun Scaffolds for Cardiac Patches Fabrication. Materials. 2023; 16(22):7095. https://doi.org/10.3390/ma16227095
Chicago/Turabian StyleGkouti, Elli, Aleksander Czekanski, and Ahmed AlAttar. 2023. "Simulating and Predicting the Mechanical Behavior of Electrospun Scaffolds for Cardiac Patches Fabrication" Materials 16, no. 22: 7095. https://doi.org/10.3390/ma16227095
APA StyleGkouti, E., Czekanski, A., & AlAttar, A. (2023). Simulating and Predicting the Mechanical Behavior of Electrospun Scaffolds for Cardiac Patches Fabrication. Materials, 16(22), 7095. https://doi.org/10.3390/ma16227095







