Influence of Long-Term Storage and UV Light Exposure on Characteristics of Polyurethane Foams for Cryogenic Insulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PUR Foam Samples
2.2.2. Thermal Stability Tests
2.2.3. Artificial Ageing by Exposing Samples to UV Light
3. Results and Discussion
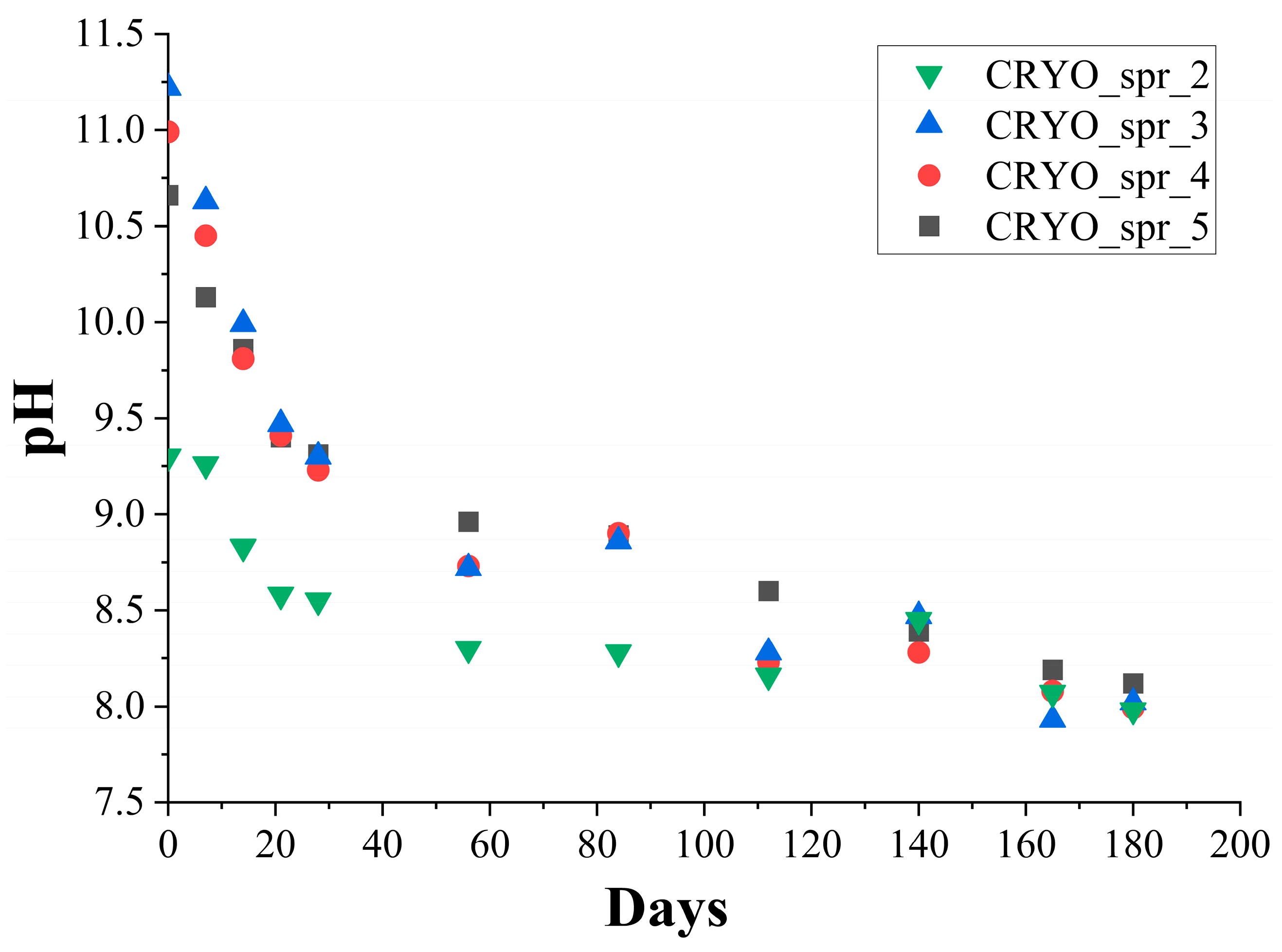
3.1. The Effect of Long-Term Storage on PUR Foams’ Kinetic Parameters
3.2. PUR Foam’s Thermal Stability Tests
3.3. Artificial Ageing by Exposing Samples to UV Light
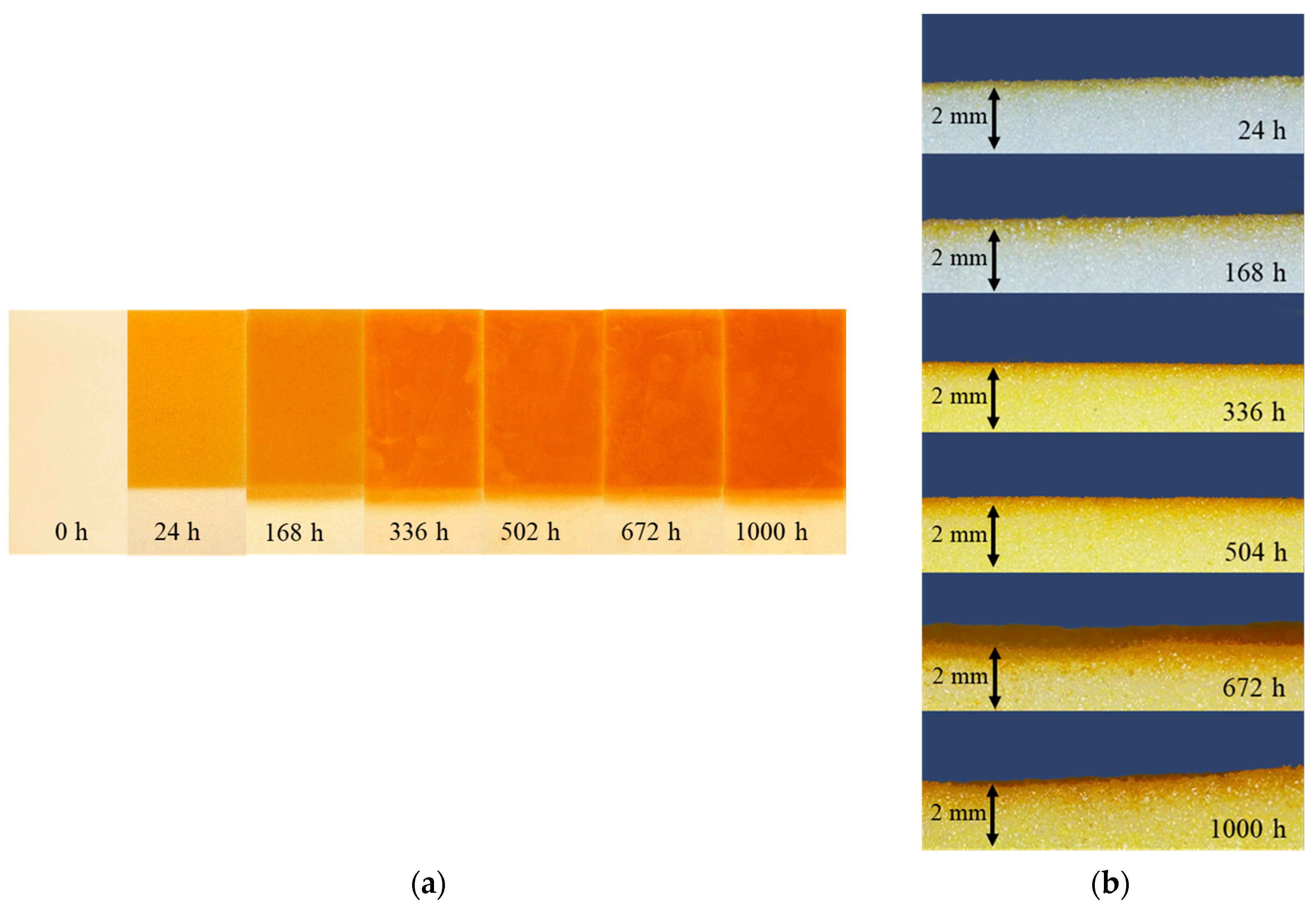
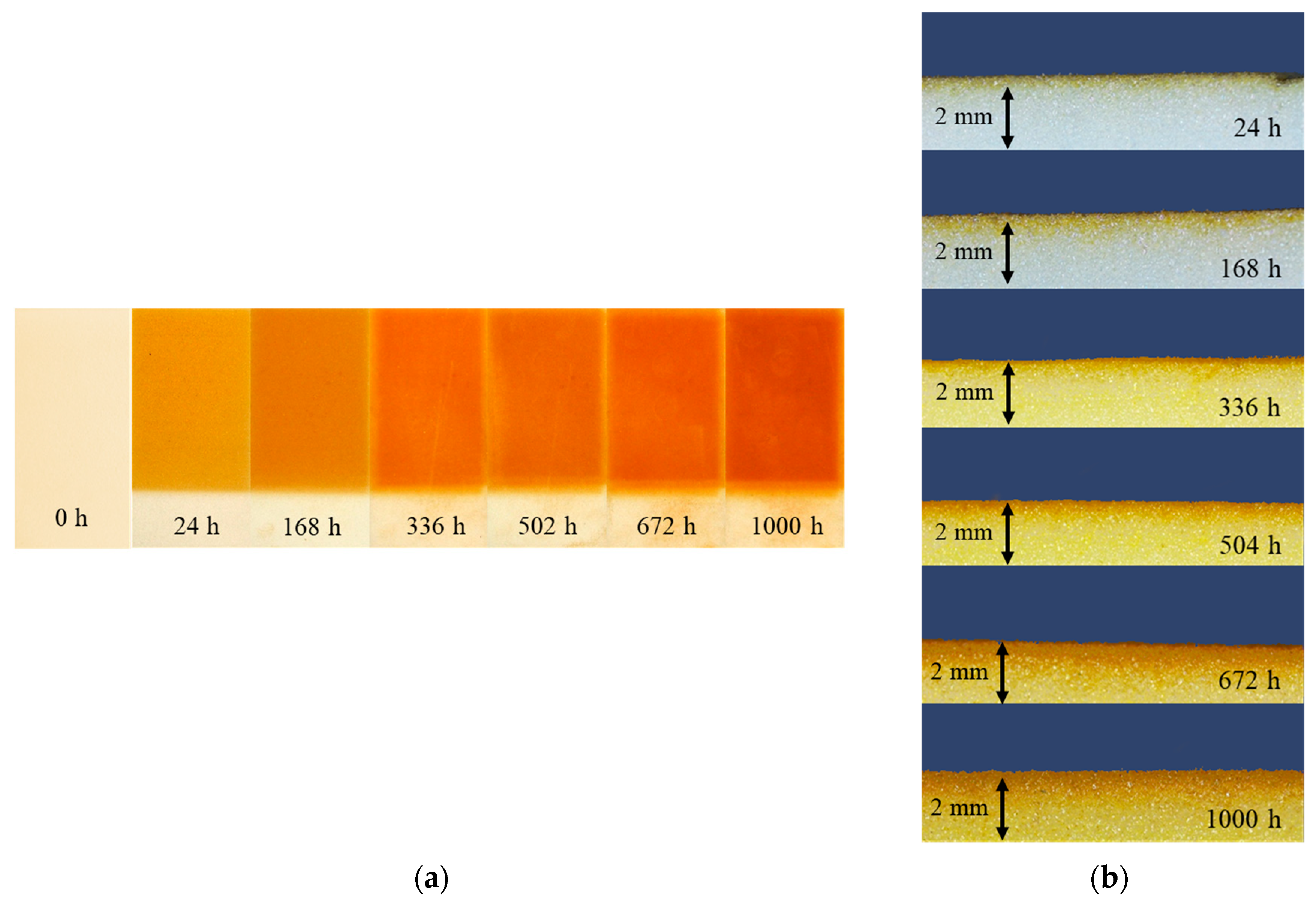
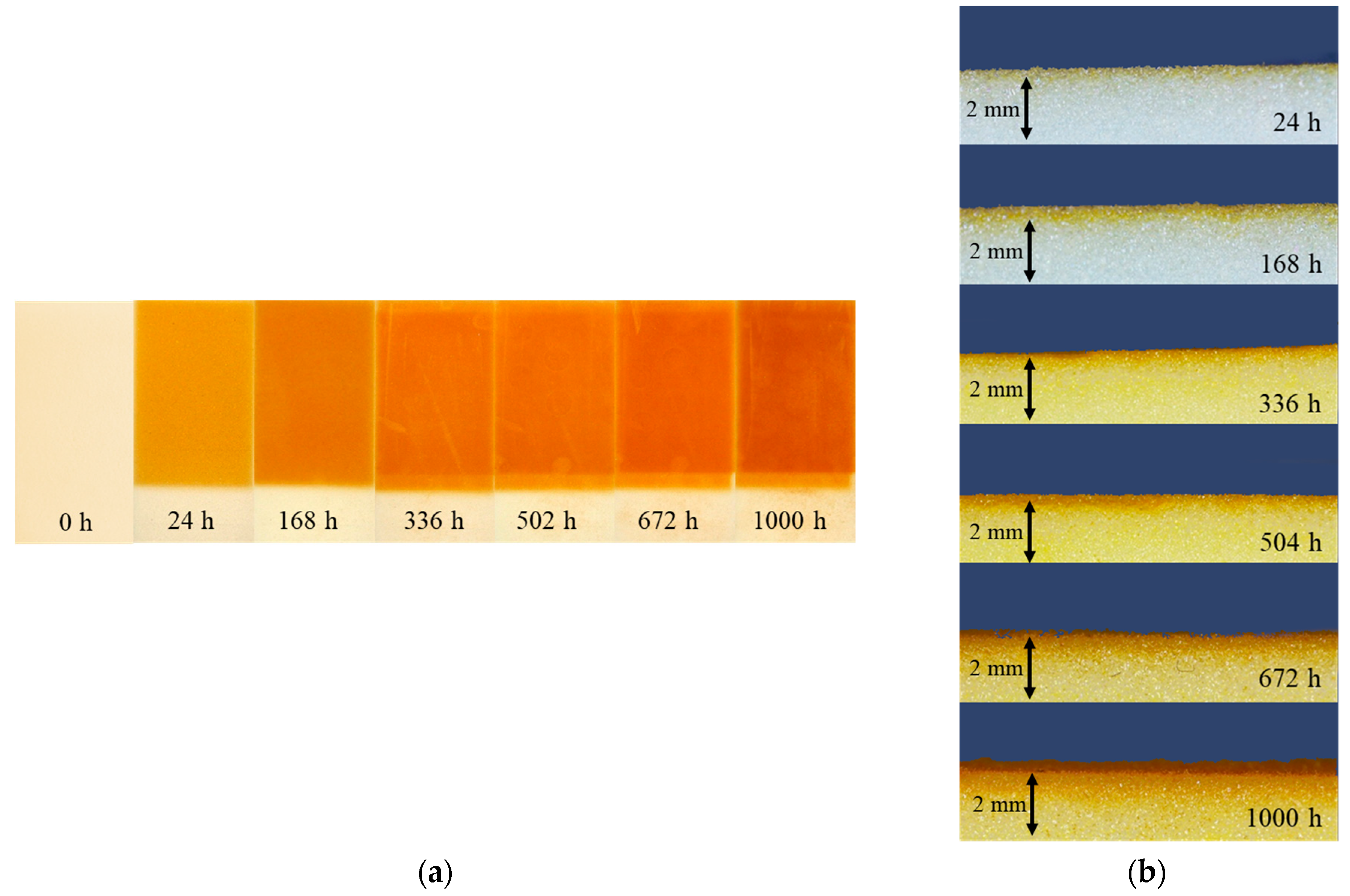
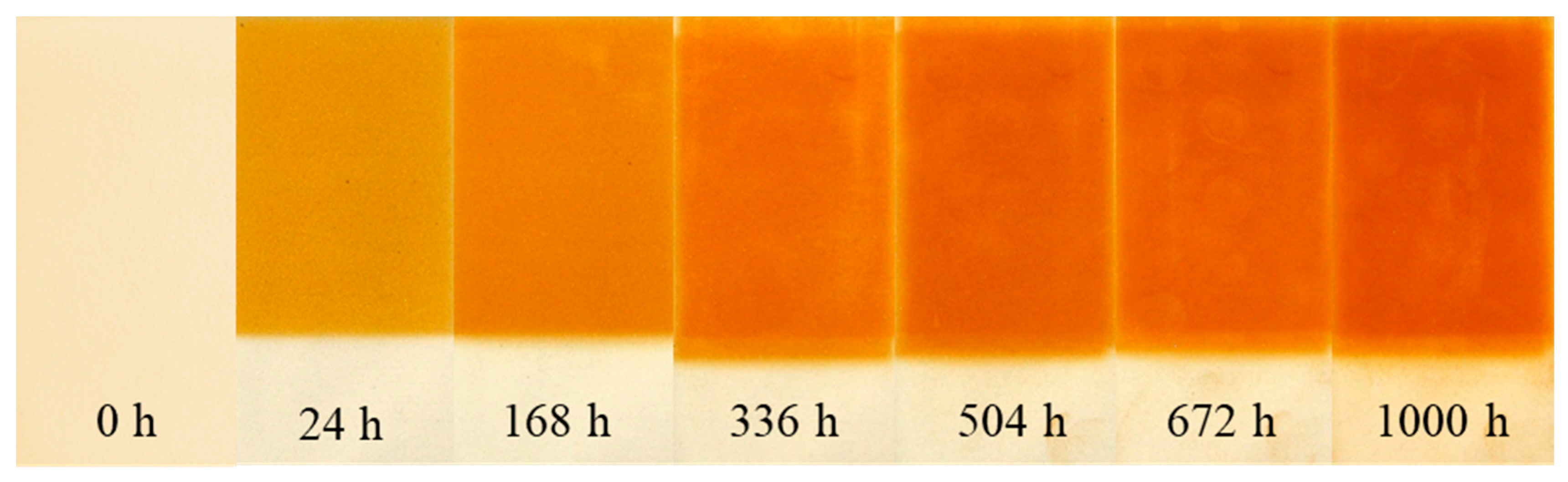
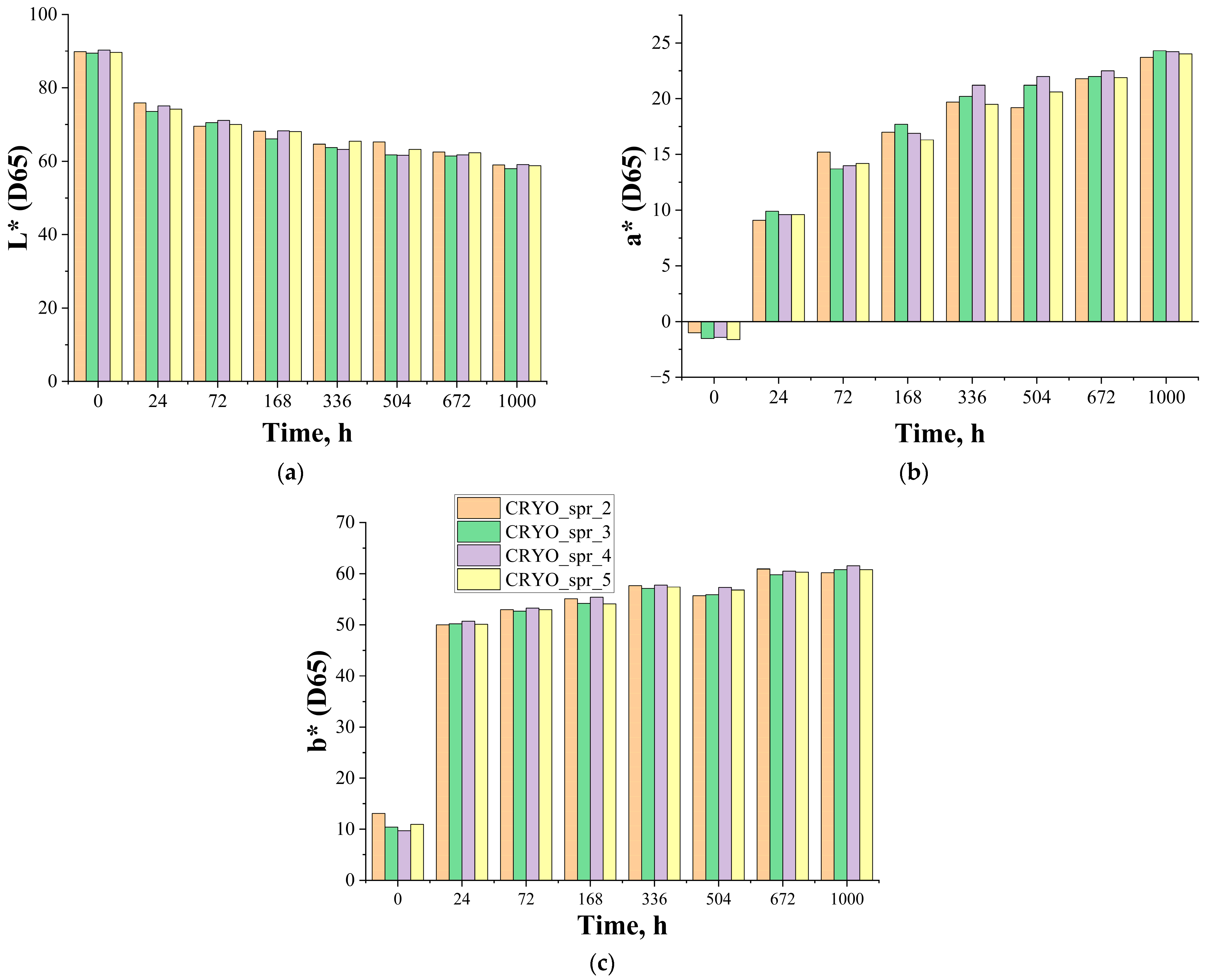
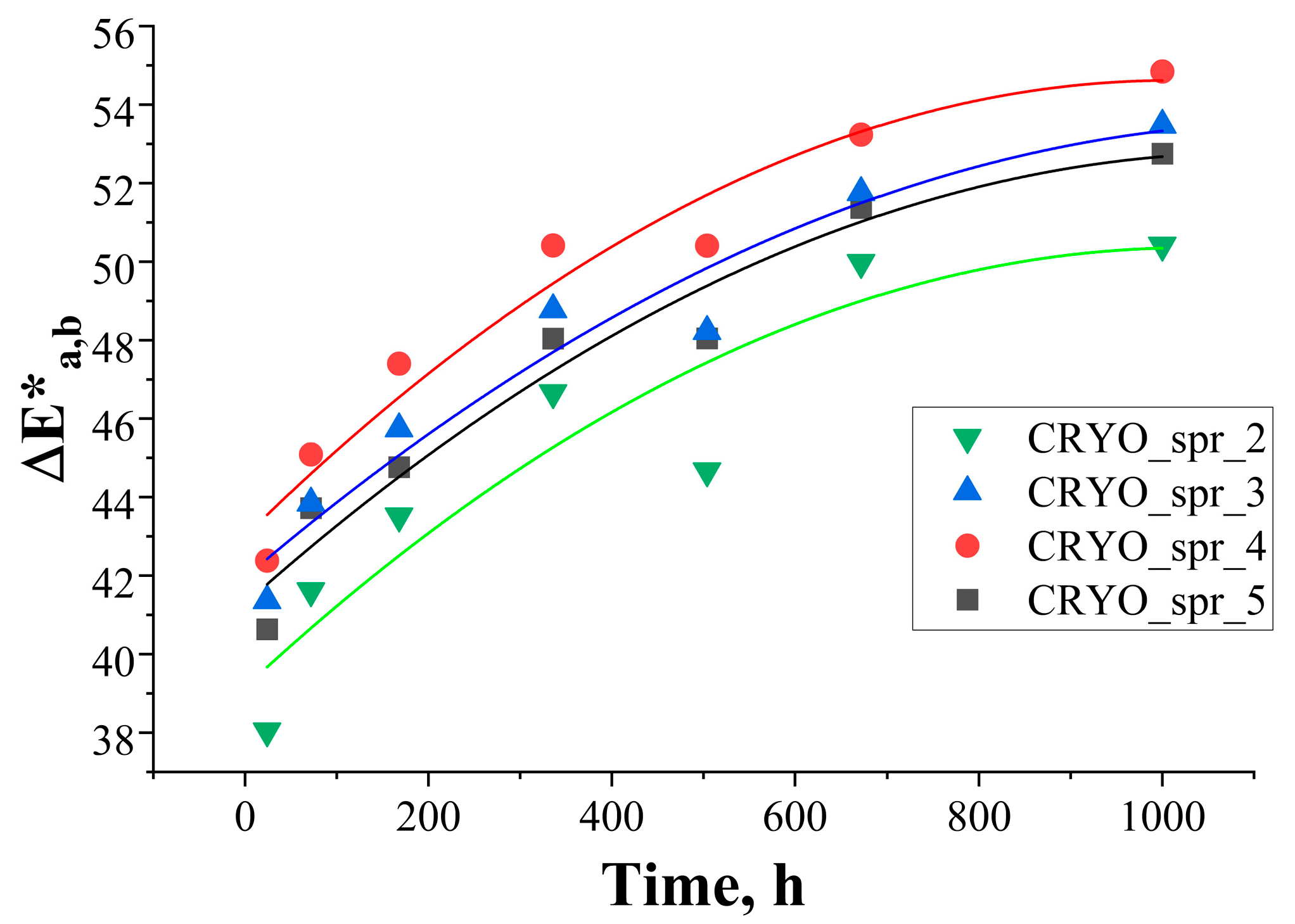
3.3.1. Change of Color
3.3.2. Thickness of Degraded Layer
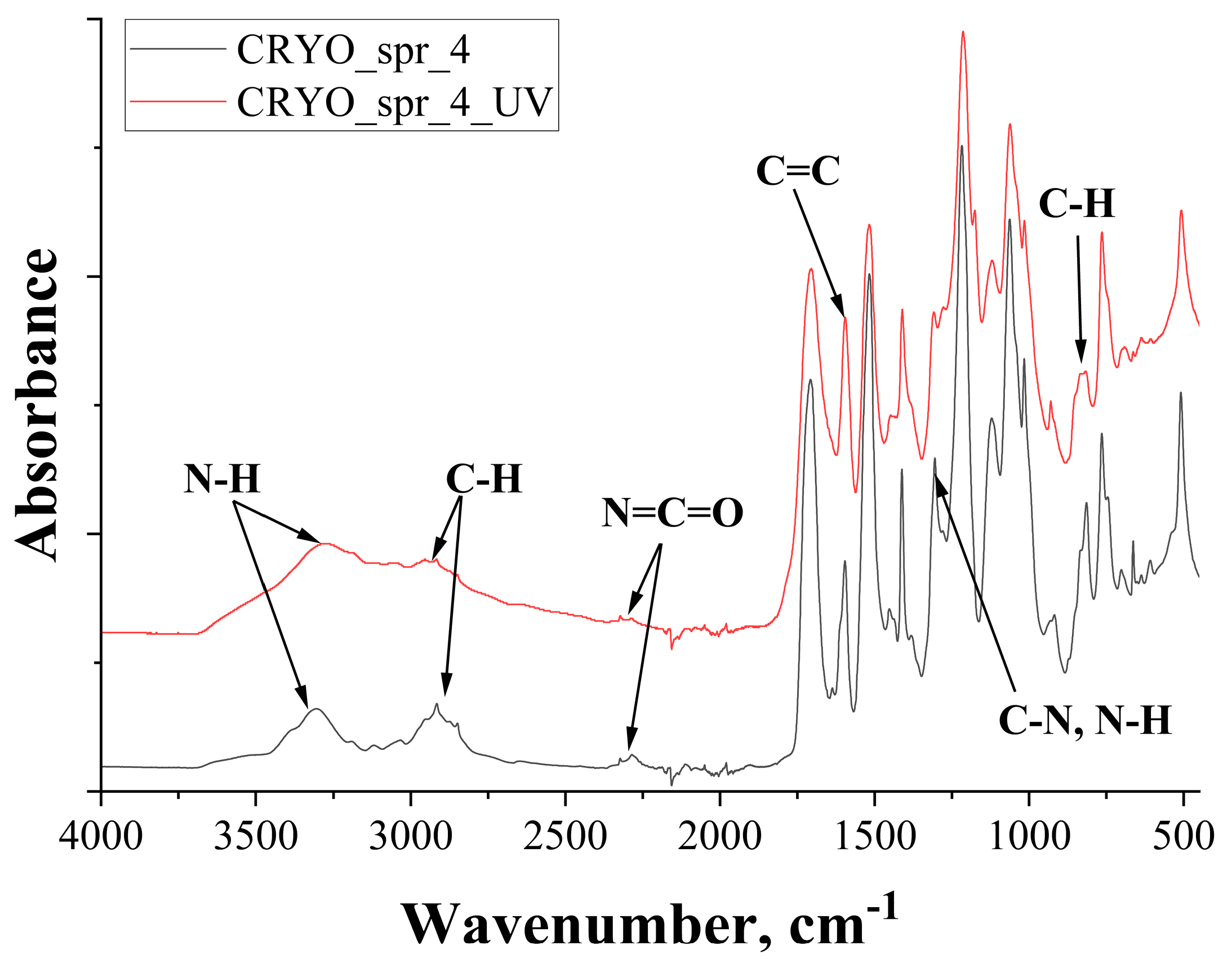
3.3.3. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Song, C.Y.; Cho, D.Y. Cryogenic compressive strength and thermal deformation of reinforced polyurethane foam material for membrane type LNG carrier. Key Eng. Mater. 2018, 773, 30–39. [Google Scholar] [CrossRef]
- Yu, Y.H.; Kim, B.G.; Lee, D.G. Cryogenic reliability of composite insulation panels for liquefied natural gas (LNG) ships. Compos. Struct. 2012, 94, 462–468. [Google Scholar] [CrossRef]
- Lee, C.S.; Lee, J.M. Failure analysis of reinforced polyurethane foam-based LNG insulation structure using damage-coupled finite element analysis. Compos. Struct. 2014, 107, 231–245. [Google Scholar] [CrossRef]
- Sharifzadeh, S.; Verstraete, D.; Hendrick, P. Cryogenic hydrogen fuel tanks for large hypersonic cruise vehicles. Int. J. Hydrogen Energy 2015, 40, 12798–12810. [Google Scholar] [CrossRef]
- Li, K.; Wu, Z.; Liu, M.; Xu, X.; Xu, W. An application of reinforced polyurethane foam in design of the common bulkhead for cryogenic tanks. Mater. Sci. Forum 2020, 975, 182–187. [Google Scholar] [CrossRef]
- Darkow, N.; Fischer, A.; Scheufler, H.; Hellmann, H.; Gerstmann, J. Concept Development of a Cryogenic Tank Insulation for Reusable Launch Vehicle. In Proceedings of the 8th European Conference for Aeronautics and Space Sciences (EUCASS), Madrid, Spain, 1–4 July 2019. [Google Scholar] [CrossRef]
- Desai, S.; Thakore, I.M.; Sarawade, B.D.; Devi, S. Effect of polyols and diisocyanates on thermo-mechanical and morphological properties of polyurethanes. Eur. Polym. J. 2000, 36, 711–725. [Google Scholar] [CrossRef]
- NASA External Tank Thermal Protection System. NASA Facts 2005, Pub 8-4039, 1–4. Available online: https://www.nasa.gov/wp-content/up-427loads/2016/08/114022main_tps_fs.pdf (accessed on 25 October 2023).
- Zhang, X.B.; Yao, L.; Qiu, L.M.; Gan, Z.H.; Yang, R.P.; Ma, X.J.; Liu, Z.H. Experimental study on cryogenic moisture uptake in polyurethane foam insulation material. Cryogenics 2012, 52, 810–815. [Google Scholar] [CrossRef]
- Wilken, J.; Callsen, S.; Daub, D.; Fischer, A.; Liebisch, M.; Rauh, C.; Reimer, T.; Scheufler, H.; Sippel, M. Testing combined cryogenic insulation and thermal protection systems for reusable stages. In Proceedings of the 72nd International Astronautical Congress (IAC), Dubai, United Arab Emirates, 25–29 October 2021; Volume D2, pp. 1–14. [Google Scholar]
- Szelinski, B.; Lange, H.; Rottger, C.; Sacher, H.; Weiland, S.; Zell, D. Development of an innovative sandwich common bulkhead for cryogenic upper stage propellant tank. Acta Astronaut. 2012, 81, 200–213. [Google Scholar] [CrossRef]
- Sture, B.; Vevere, L.; Kirpluks, M.; Godina, D.; Fridrihsone, A.; Cabulis, U. Polyurethane foam composites reinforced with renewable fillers for cryogenic insulation. Polymers 2021, 13, 4089. [Google Scholar] [CrossRef]
- Brenes-Granados, D.; Cubero-Sesin, J.M.; Orozco Gutiérrez, F.; Vega-Baudrit, J.; Gonzalez-Paz, R. Variation of physical properties of rigid polyurethane foams synthesized from renewable sources with different commercial catalysts. J. Renew. Mater. 2017, 5, 280–289. [Google Scholar] [CrossRef]
- Fesmire, J.E.; Coffman, B.E.; Sass, J.P.; Williams, M.K.; Smith, T.M.; Meneghelli, B.J. Cryogenic moisture uptake in foam insulation for space launch vehicles. J. Spacecr. Rocket. 2012, 49, 220–230. [Google Scholar] [CrossRef]
- Recupido, F.; Lama, G.C.; Ammendola, M.; Bossa, F.D.L.; Minigher, A.; Campaner, P.; Morena, A.G.; Tzanov, T.; Ornelas, M.; Barros, A.; et al. Rigid composite bio-based polyurethane foams: From synthesis to LCA analysis. Polymer 2023, 267, 125674. [Google Scholar] [CrossRef]
- Yu, Y.H.; Choi, I.; Nam, S.; Lee, D.G. Cryogenic characteristics of chopped glass fiber reinforced polyurethane foam. Compos. Struct. 2014, 107, 476–481. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.M.; Park, S.; Park, K.H.; Lee, J.M. Synthesis and cryogenic mechanical properties of CO2-blown carbon-reinforced polyurethane foam. J. Cell. Plast. 2018, 54, 743–763. [Google Scholar] [CrossRef]
- Kurańska, M.; Cabulis, U.; Prociak, A.; Polaczek, K.; Uram, K.; Kirpluks, M. Scale-Up and Testing of Polyurethane Bio-Foams as Potential Cryogenic Insulation Materials. Materials 2022, 15, 3469. [Google Scholar] [CrossRef] [PubMed]
- Grzęda, D.; Węgrzyk, G.; Nowak, A.; Komorowska, G.; Szczepkowski, L.; Ryszkowska, J. Effect of Different Amine Catalysts on the Thermomechanical and Cytotoxic Properties of ‘Visco’-Type Polyurethane Foam for Biomedical Applications. Materials 2023, 16, 1527. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.; Bordado, J.C. Recent developments in polyurethane catalysis: Catalytic mechanisms review. Catal. Rev.-Sci. Eng. 2004, 46, 31–51. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Transitioning to Low GWP Alternatives in Building/Construction Foams. EPA-430-F-11-005, February 2011. Available online: http://www.epa.gov/ozone/downloads/EPA_HFC_ConstFoam.pdf (accessed on 25 October 2023).
- Yakushin, V.; Cabulis, U.; Fridrihsone, V.; Kravchenko, S.; Pauliks, R. Properties of polyurethane foam with fourth-generation blowing agent. e-Polymers 2021, 21, 763–769. [Google Scholar] [CrossRef]
- Deindorf, J. 4th generation blowing agents: Chances and Challenges with HFOs. In Proceedings of the Rigid Technical Seminar Feipur 2018 by Evonik.
- Vincent, J.L.; Miller, T.I.; Tadao, Y.; Ozbas, B.; Burdeniuc, J.J.; Keller, R.J. Catalysts for Improved Spray Foam System Stability and Reactivity with Low GWP Blowing Agents. Chemistry 2013, corpus ID: 115146060.
- Azevedo, E.C.; Nascimento, E.M.; Chierice, G.O.; Neto, S.C.; Lepienski, C.M. UV and gamma irradiation effects on surface properties of polyurethane derivate from castor oil. Polimeros 2013, 23, 305–311. [Google Scholar] [CrossRef]
- Paberza, A.; Stiebra, L.; Cabulis, U. Photodegradation of polyurethane foam obtained from renewable resource-pulp production byproducts. J. Renew. Mater. 2015, 3, 19–27. [Google Scholar] [CrossRef]
- Costantini, R.; Nodari, L.; La Nasa, J.; Modugno, F.; Bonasera, L.; Rago, S.; Zoleo, A.; Legnaioli, S.; Tomasin, P. Preserving the Ephemeral: A Micro-Invasive Study on a Set of Polyurethane Scenic Objects from the 1960s and 1970s. Polymers 2023, 15, 2111. [Google Scholar] [CrossRef] [PubMed]
- Rus, A.Z.M.; Kemp, T.J.; Clark, A.J. Degradation studies of polyurethanes based on vegetable oils. Part 1. Photodegradation. Prog. React. Kinet. Mech. 2008, 33, 363–391. [Google Scholar] [CrossRef]
- Yakushin, V.; Rundans, M.; Holynska, M.; Sture, B.; Cabulis, U. Influence of Reactive Amine-Based Catalysts on Cryogenic Properties of Rigid Polyurethane Foams for Space and On-Ground Applications. Materials 2023, 16, 2798. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yu, K.; Qian, K. Thermal degradation study of rigid polyurethane foams containing tris(1-chloro-2-propyl)phosphate and modified aramid fiber. Polym. Test. 2018, 67, 159–168. [Google Scholar] [CrossRef]
- Boquillon, N.; Fringant, C. Polymer networks derived from curing of epoxidised linseed oil: Influence of different catalysts and anhydride hardeners. Polymer 2000, 41, 8603–8613. [Google Scholar] [CrossRef]
- Hassan, N.N.M.; Shamsudin, Z.S.; Rus, A.Z.M.; Sulong, N. Investigation of renewable polymer composite from waste oil endurance to UV irradiation exposure by using FTIR and UV-Vis. J. Mech. Eng. 2018, 5, 53–61. [Google Scholar]
- Rashvand, M.; Ranjbar, Z.; Rastegar, S. Nano zinc oxide as a UV-stabilizer for aromatic polyurethane coatings. Prog. Org. Coat. 2011, 71, 362–368. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Liu, D.; Sun, Z.; Wang, H. Effects of Additives on Weather-Resistance Properties of Polyurethane Films Exposed to Ultravioler Radiation and Ozone Atmosphere. J. Nanomater. 2014, 2014, 487343. [Google Scholar] [CrossRef]
- Merlini, C.; Soldi, V.; Barra, G.M.O. Influence of fiber surface treatment and length on physico-chemical properties of short random banana fiber-reinforced castor oil polyurethane composites. Polym. Test. 2011, 30, 833–840. [Google Scholar] [CrossRef]
- Baysal, G.; Aydın, H.; Hoşgören, H.; Uzan, S.; Karaer, H. Improvement of Synthesis and Dielectric Properties of Polyurethane/Mt-QASs+ (Novel Synthesis). J. Polym. Environ. 2016, 24, 139–147. [Google Scholar] [CrossRef]
- Song, H.; Qi, H.; Li, N.; Zhang, X. Tribological behaviour of carbon nanotubes/polyurethane nanocomposite coatings. Micro Nano Lett. 2011, 6, 48–51. [Google Scholar] [CrossRef]
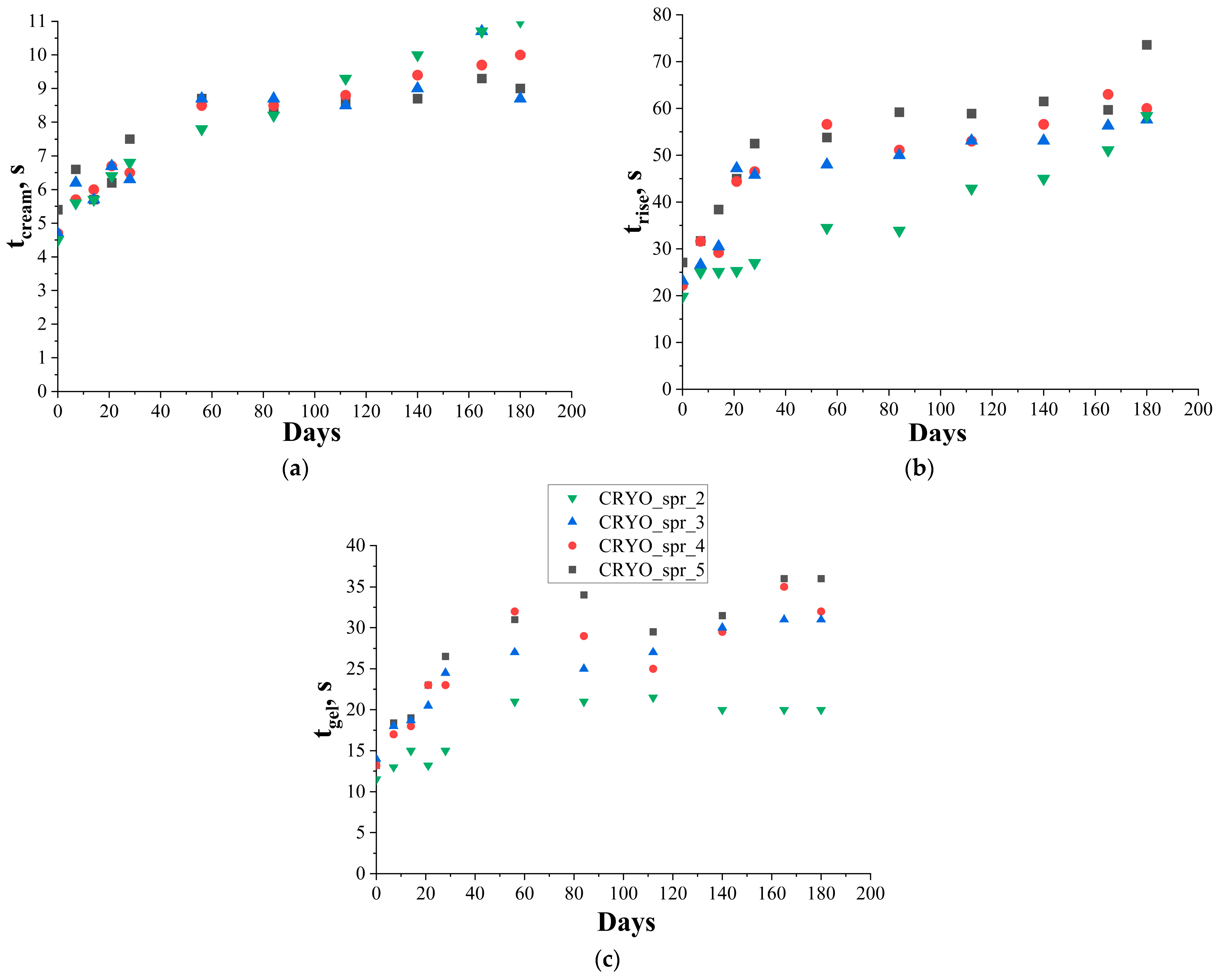
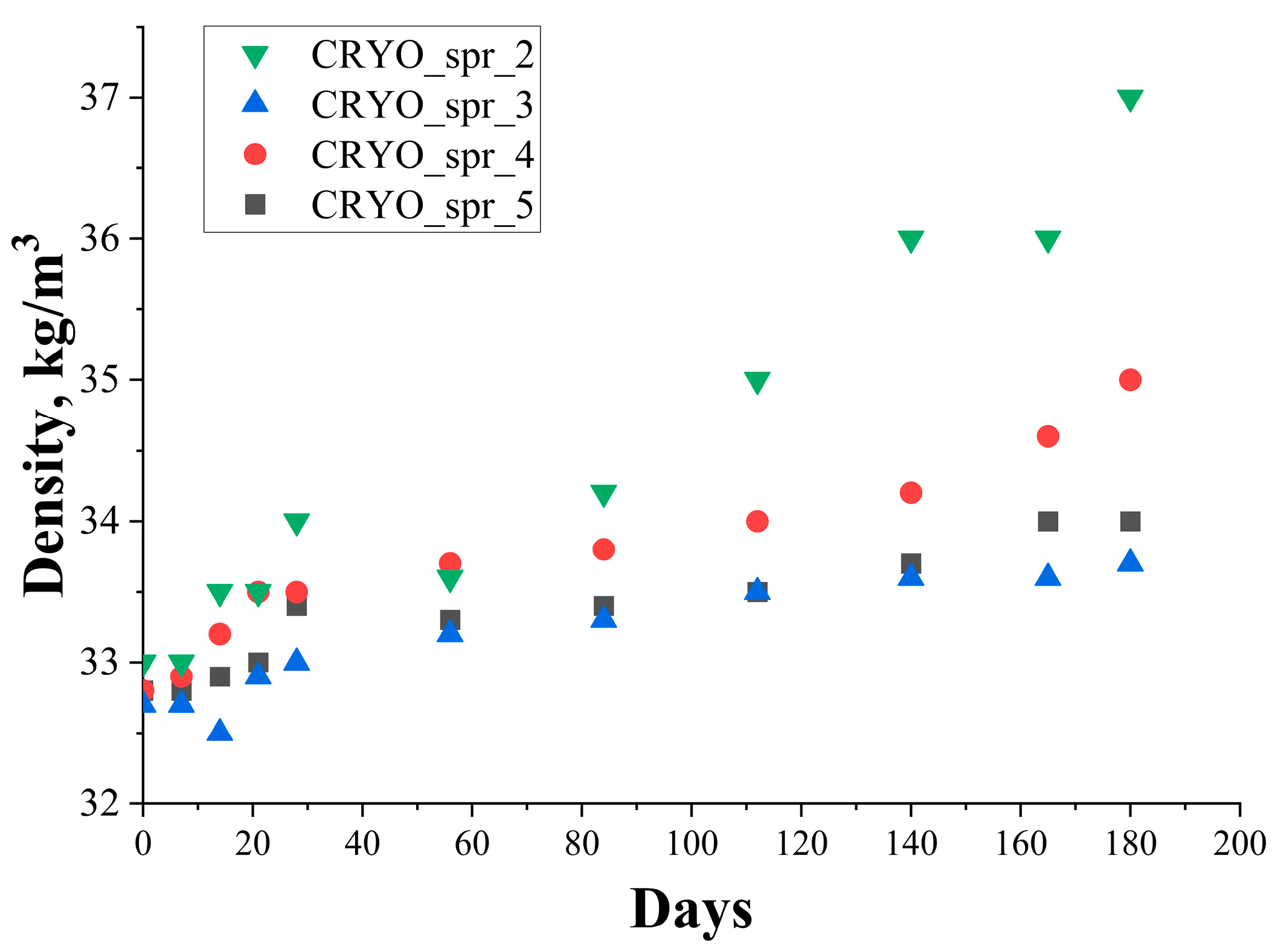
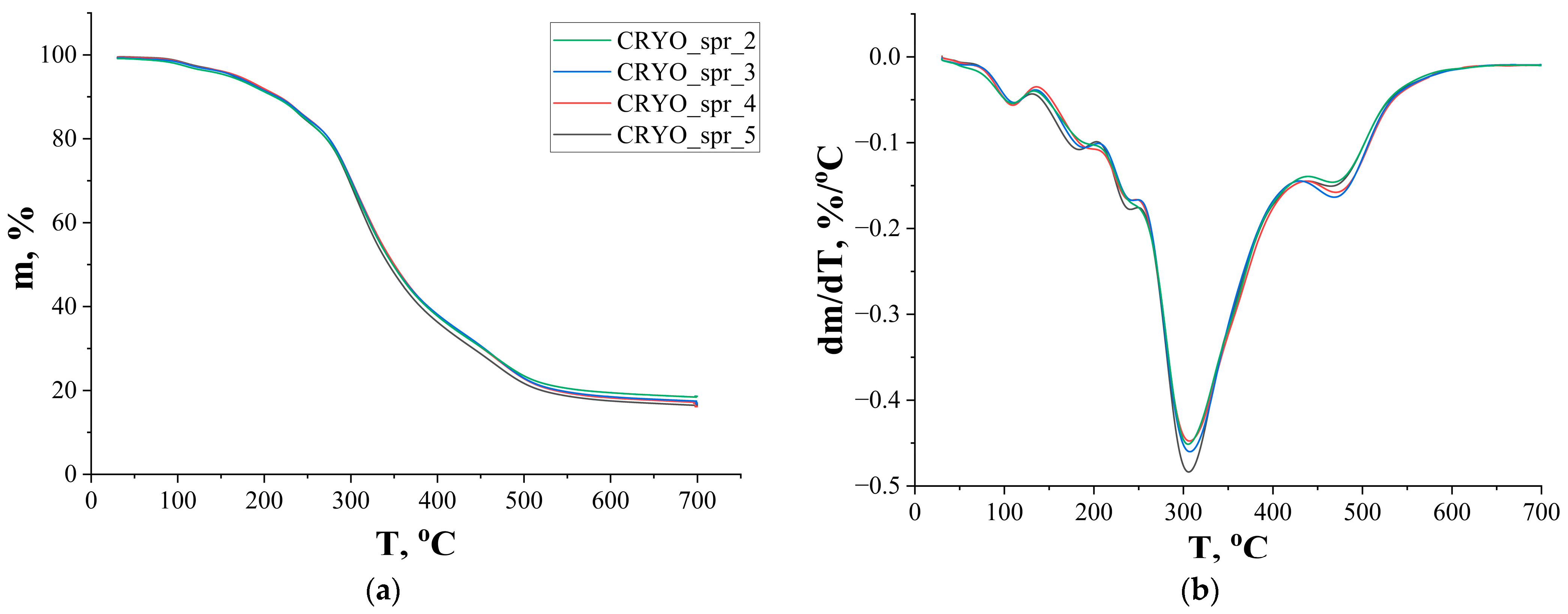
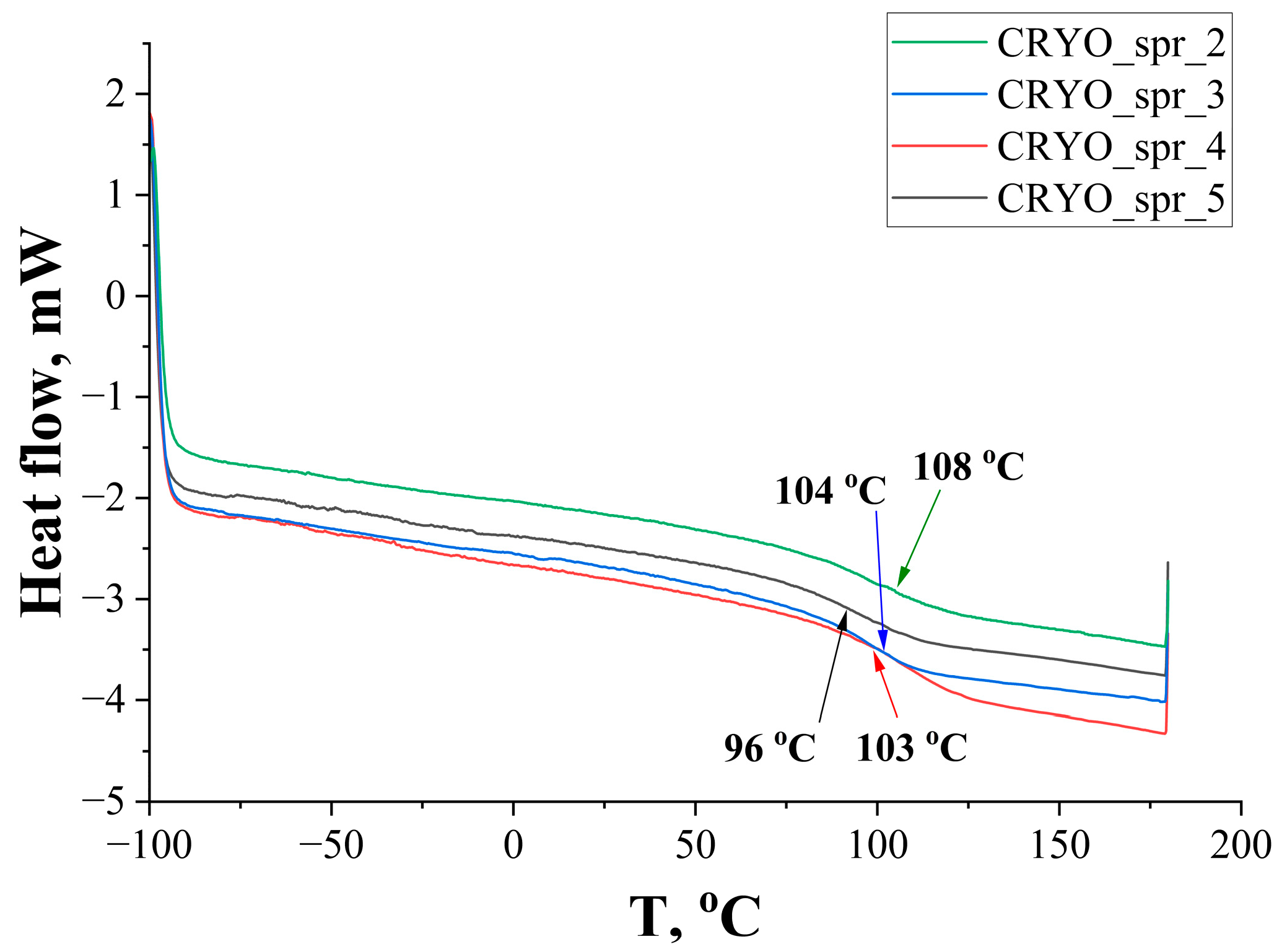

| Sample | CRYO_spr_2 | CRYO_spr_3 | CRYO_spr_4 | CRYO_spr_5 | |
|---|---|---|---|---|---|
| Polyols | Lupranol 3300 | 55 | |||
| Lupranol 3508/1 | |||||
| Lupraphen 1901/1 | |||||
| Diethylene glycol | 25 | ||||
| IXOL B 251 | 20 | ||||
| Flame retardant | TCPP | 15 | |||
| Surfactant | Silicone L-6815LV | 1.5 | |||
| Blowing agents | Solstice® LBA | 45.0 | 45.0 | 45.0 | 41.4 |
| Water | 0 | ||||
| Catalysts | Dabco MB 20 | 0.15 | 0.15 | 0.2 | 0.2 |
| Polycat 218 | 3 | 3 | 2 | ||
| Polycat 203 | 1 | ||||
| Polycat 5 | 3 | ||||
| pMDI | Desmodur® 44V20L | 147–160 | |||
| PUR Foam | CRYO_spr_2 | CRYO_spr_3 | CRYO_spr_4 | CRYO_spr_5 |
|---|---|---|---|---|
| T5% (°C) | 158 | 166 | 169 | 165 |
| T10% (°C) | 212 | 216 | 218 | 213 |
| Tmax (°C) | 304 | 307 | 306 | 306 |
| mmax (%) | 68 | 67 | 68 | 66 |
| m500 (%) | 24 | 23 | 23 | 22 |
| m700 (%) | 18 | 17 | 17 | 16 |
| Test Duration (h) | L*(D65) | a*(D65) | b*(D65) | ΔE*a,b |
|---|---|---|---|---|
| Initial | 93.4 | −1.8 | 11.0 | 0 |
| 24 | 78.5 | 8.3 | 49.6 | 42.6 |
| 72 | 74.8 | 12.4 | 52.8 | 47.9 |
| 168 | 68.0 | 18.7 | 56.1 | 55.7 |
| 336 | 63.4 | 21.9 | 61.7 | 63.5 |
| 504 | 61.0 | 22.9 | 61.0 | 64.5 |
| 672 | 56.6 | 26.1 | 59.8 | 67.2 |
| 1000 | 55.9 | 26.5 | 59.7 | 67.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sture, B.; Yakushin, V.; Vevere, L.; Cabulis, U. Influence of Long-Term Storage and UV Light Exposure on Characteristics of Polyurethane Foams for Cryogenic Insulation. Materials 2023, 16, 7071. https://doi.org/10.3390/ma16227071
Sture B, Yakushin V, Vevere L, Cabulis U. Influence of Long-Term Storage and UV Light Exposure on Characteristics of Polyurethane Foams for Cryogenic Insulation. Materials. 2023; 16(22):7071. https://doi.org/10.3390/ma16227071
Chicago/Turabian StyleSture, Beatrise, Vladimir Yakushin, Laima Vevere, and Ugis Cabulis. 2023. "Influence of Long-Term Storage and UV Light Exposure on Characteristics of Polyurethane Foams for Cryogenic Insulation" Materials 16, no. 22: 7071. https://doi.org/10.3390/ma16227071
APA StyleSture, B., Yakushin, V., Vevere, L., & Cabulis, U. (2023). Influence of Long-Term Storage and UV Light Exposure on Characteristics of Polyurethane Foams for Cryogenic Insulation. Materials, 16(22), 7071. https://doi.org/10.3390/ma16227071







