Microstructure and Corrosion Behavior of Zinc/Hydroxyapatite Multi-Layer Coating Prepared by Combining Cold Spraying and High-Velocity Suspension Flame Spraying
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Preparation of Cold-Sprayed Zn Coating
2.2. Preparation of Zn/HA-DL Coating and Zn/HA-ML Coating
2.3. Characterization of Phase and Microstructure
2.4. Bonding Strength Test
2.5. Corrosion and Immersion Testing
3. Results and Discussion
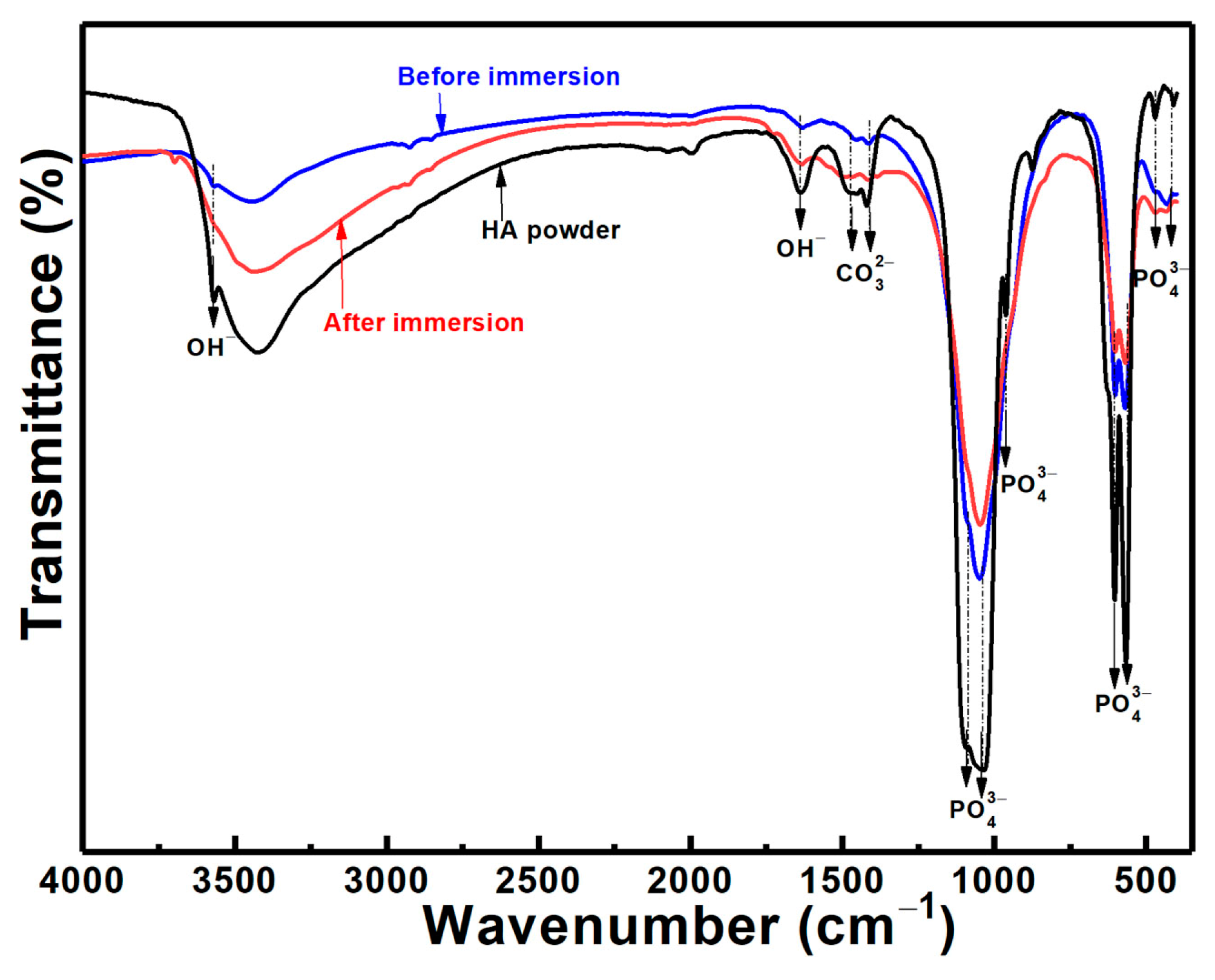
3.1. Phase Structure
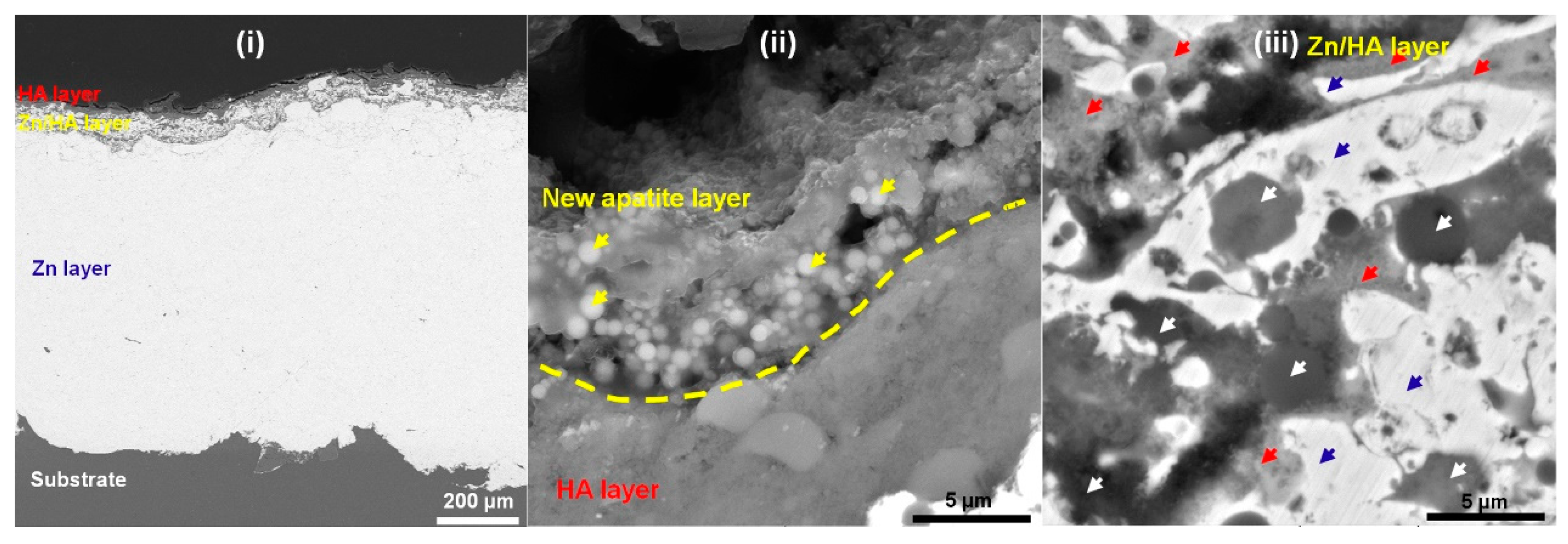
3.2. Cross-Sectional Microstructures
3.3. Bonding Strength
3.4. Potentiodynamic Polarization Property
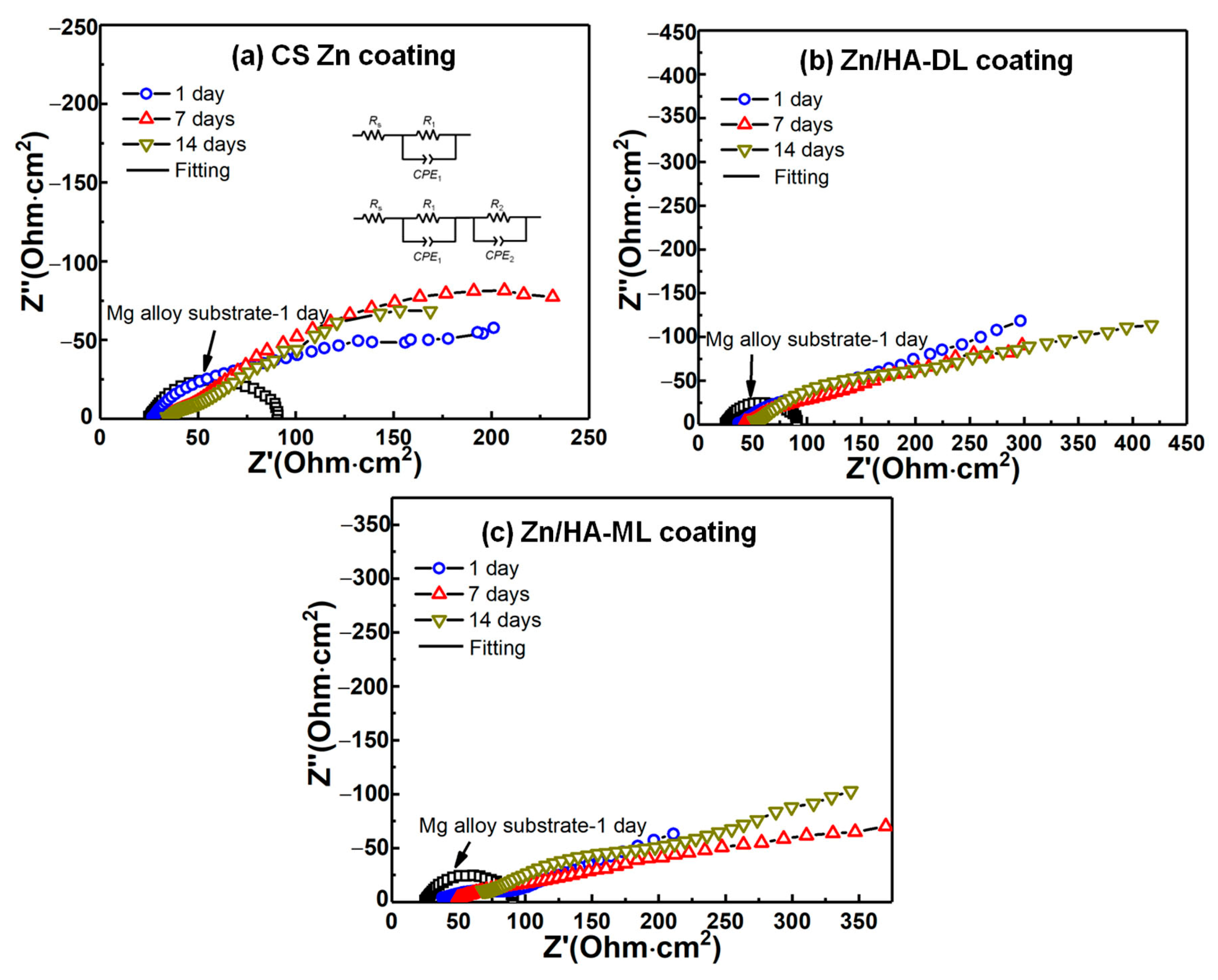
3.5. Electrochemical Impedance Spectroscopy (EIS)
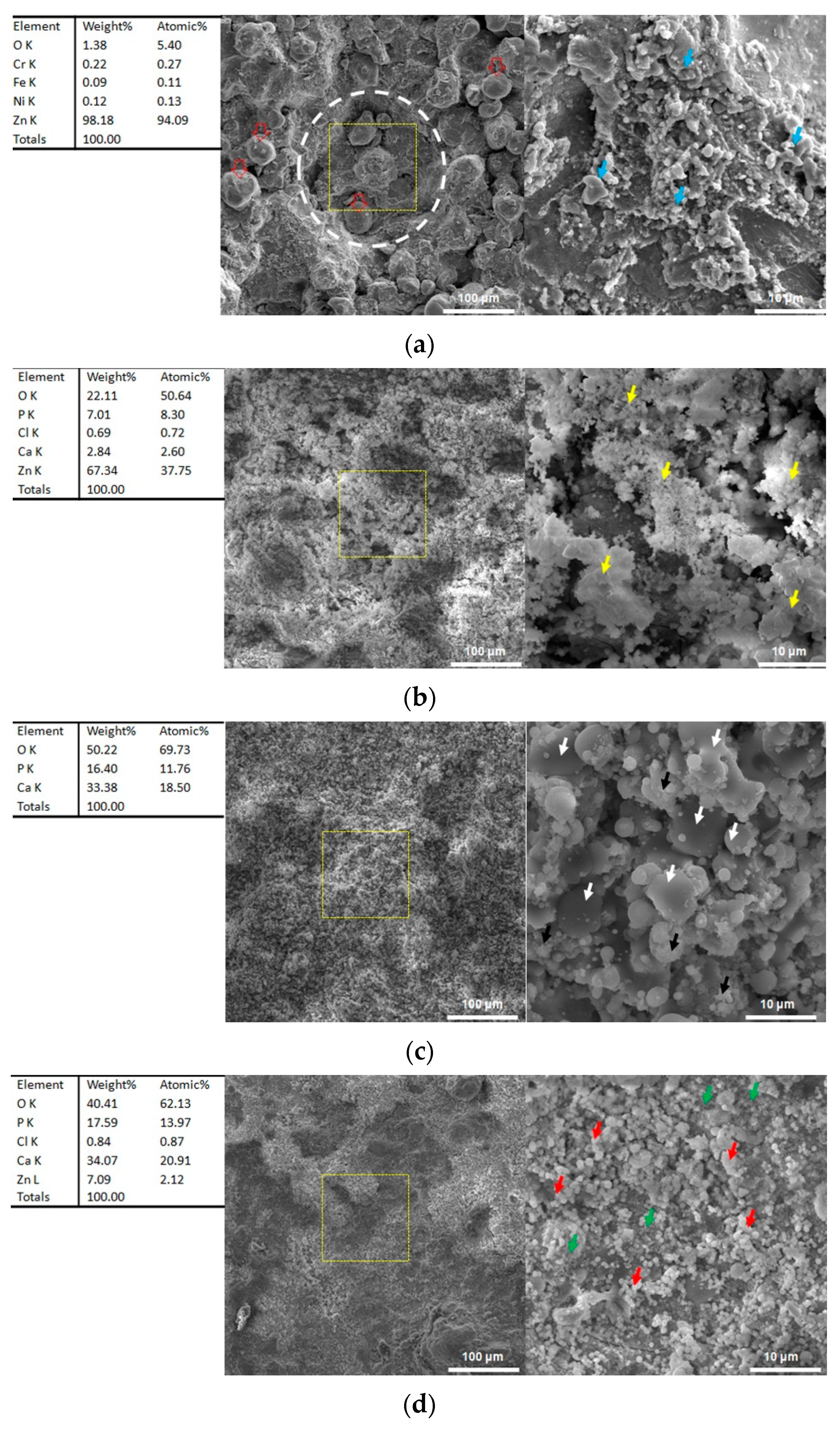
3.6. Microstructures after Immersion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for Orthopedic Implants—A Review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Wan, P.; Tan, L.; Yang, K. Surface Modification on Biodegradable Magnesium Alloys as Orthopedic Implant Materials to Improve the Bio-adaptability: A Review. J. Mater. Sci. Technol. 2016, 32, 827–834. [Google Scholar] [CrossRef]
- Mahto, V.K.; Singh, A.K.; Malik, A. Surface Modification Techniques of Magnesium-based Alloys for Implant Applications. J. Coat. Technol. Res. 2023, 20, 433–455. [Google Scholar] [CrossRef]
- Rajan, S.T.; Arockiarajan, A. A Comprehensive Review of Properties of the Biocompatible Thin Films on Biodegradable Mg Alloys. Biomed. Mater. 2023, 18, 012002. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.; Bellucci, D.; Cannillo, V. Functionally Graded Materials for Orthopedic Applications—An Update on Design and Manufacturing. Biotechnol. Adv. 2016, 34, 504–531. [Google Scholar] [CrossRef]
- Shahzamanian, M.M.; Banerjee, R.; Dahotre, N.B.; Srinivasa, A.R.; Reddy, J.N. Analysis of Stress Shielding Reduction in Bone Fracture Fixation Implant Using Functionally Graded Materials. Compos. Struct. 2023, 321, 117262. [Google Scholar] [CrossRef]
- Roy, S. Functionally Graded Coatings on Biomaterials: A Critical Review. Mater. Today Chem. 2020, 18, 100375. [Google Scholar] [CrossRef]
- Sathish, M.; Radhika, N.; Saleh, B. A Critical Review on Functionally Graded Coatings: Methods, Properties, and Challenges. Compos. Part B Eng. 2021, 225, 109278. [Google Scholar] [CrossRef]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.-K. Recent Advances in the Synthesis, Functionalization and Biomedical Applications of Hydroxyapatite: A Review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef]
- Tang, J.R.; Zhao, Z.P.; Liu, H.S.; Cui, X.Y.; Wang, J.Q.; Xiong, T.Y. A Novel Bioactive Ta/hydroxyapatite Composite Coating Fabricated by Cold Spraying. Mater. Lett. 2019, 250, 197–201. [Google Scholar] [CrossRef]
- Yao, H.-L.; Zou, Y.-L.; Bai, X.-B.; Wang, H.-T.; Ji, G.-C.; Chen, Q.-Y. Microstructures, Mechanical Properties and Electrochemical Behaviors of Nano-structured HA/Ti Composite Coatings Deposited by High-Velocity Suspension Flame Spray (HVSFS). Ceram. Int. 2018, 44, 13024–13030. [Google Scholar] [CrossRef]
- Yao, H.-L.; Hu, X.-Z.; Wang, H.-T.; Chen, Q.-Y.; Bai, X.-B.; Zhang, M.-X.; Ji, G.-C. Microstructure and Corrosion Behavior of Thermal-Sprayed Hydroxyapatite/Magnesium Composite Coating on the Surface of AZ91D Magnesium Alloy. J. Therm. Spray Technol. 2019, 28, 495–503. [Google Scholar] [CrossRef]
- Hu, G.W.; Zeng, L.C.; Du, H.; Fu, X.D.; Jin, X.X.; Deng, M.; Zhao, Y.J.; Liu, X.W. The Formation Mechanism and Bio-corrosion Properties of Ag/HA Composite Conversion Coating on the Extruded Mg-2Zn-1Mn-0.5Ca Alloy for Bone Implant Application. Surf. Coat. Technol. 2017, 325, 127–135. [Google Scholar] [CrossRef]
- Zhuo, X.R.; Wu, Y.N.; Ju, J.; Liu, H.; Jiang, J.H.; Hu, Z.C.; Bai, J.; Xue, F. Recent Progress of Novel Biodegradable Zinc Alloys: From the Perspective of Strengthening and Toughening. J. Mater. Res. Technol. 2022, 17, 244–269. [Google Scholar] [CrossRef]
- Łatka, L.; Pawłowski, L.; Winnicki, M.; Sokołowski, P.; Małachowska, A.; Kozerski, S. Review of Functionally Graded Thermal Sprayed Coatings. Appl. Sci. 2020, 10, 5153. [Google Scholar] [CrossRef]
- Ghanbari, A.; Bordbar-Khiabani, A.; Warchomicka, F.; Sommitsch, C.; Yarmand, B.; Zamanian, A. PEO/Polymer Hybrid Coatings on Magnesium Alloy to Improve Biodegradation and Biocompatibility Properties. Surf. Interfaces 2023, 36, 102495. [Google Scholar] [CrossRef]
- Liu, W.; Yan, Z.; Ma, X.; Geng, T.; Wu, H.; Li, Z. Mg-MOF-74/MgF2 Composite Coating for Improving the Properties of Magnesium Alloy Implants: Hydrophilicity and Corrosion Resistance. Materials 2018, 11, 396. [Google Scholar] [CrossRef]
- Bulina, N.V.; Makarova, S.V.; Baev, S.G.; Matvienko, A.A.; Gerasimov, K.B.; Logutenko, O.A.; Bystrov, V.S. A Study of Thermal Stability of Hydroxyapatite. Minerals 2021, 11, 1310. [Google Scholar] [CrossRef]
- Bagheri, P.; Saber-Samandari, S.; Sadeghi, A.; Akhtarian, S.; Doostmohammadi, A. Hydroxyapatite Nanoparticles Coating on Ti-6Al-4V Substrate Using Plasma Spray Method. Trans. IMF 2023, 101, 158–168. [Google Scholar] [CrossRef]
- Liao, T.Y.; Biesiekierski, A.; Berndt, C.C.; King, P.C.; Ivanova, E.P.; Thissen, H.; Kingshott, P. Multifunctional Cold Spray Coatings for Biological and Biomedical Applications: A Review. Prog. Surf. Sci. 2022, 97, 100654. [Google Scholar] [CrossRef]
- Chavan, N.M.; Kiran, B.; Jyothirmayi, A.; Phani, P.S.; Sundararajan, G. The Corrosion Behavior of Cold Sprayed Zinc Coatings on Mild Steel Substrate. J. Therm. Spray Technol. 2013, 22, 463–470. [Google Scholar] [CrossRef]
- Yao, H.-L.; Yi, Z.-H.; Yao, C.; Zhang, M.-X.; Wang, H.-T.; Li, S.-B.; Bai, X.-B.; Chen, Q.-Y.; Ji, G.-C. Improved Corrosion Resistance of AZ91D Magnesium Alloy Coated by Novel Cold-sprayed Zn-HA/Zn Double-layer Coatings. Ceram. Int. 2020, 46, 7687–7693. [Google Scholar] [CrossRef]
- Zhou, X.; Mohanty, P. Electrochemical Behavior of Cold Sprayed Hydroxyapatite/titanium Composite in Hanks’ Solution. Electrochim. Acta 2012, 65, 134–140. [Google Scholar] [CrossRef]
- Yang, X.W.; Li, W.Y.; Yu, S.Q.; Xu, Y.X.; Hu, K.W.; Zhao, Y.B. Electrochemical Characterization and Microstructure of Cold Sprayed AA5083/Al2O3 Composite Coatings. J. Mater. Sci. Technol. 2020, 59, 117–128. [Google Scholar] [CrossRef]
- Chen, X.; Ji, G.C.; Bai, X.B.; Yao, H.L.; Chen, Q.Y.; Zou, Y.L. Microstructures and Properties of Cold Spray Nanostructured HA Coatings. J. Therm. Spray Technol. 2018, 27, 1344–1355. [Google Scholar] [CrossRef]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of Doping in Hydroxyapatite as Coating Material on Biomedical Implants by Plasma Spraying Method: A Review. Ceram. Int. 2021, 47, 4426–4445. [Google Scholar] [CrossRef]
- Hirai, S.; Nishinaka, K.; Shimakage, K.; Uo, M.; Watari, F. Hydroxyapatite Coating on Titanium Substrate by the Sol-Gel Process. J. Am. Ceram. Soc. 2004, 87, 29–34. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M. Electrophoretic Deposition of Fiber Hydroxyapatite/titania Nanocomposite Coatings. Ceram. Int. 2018, 44, 622–630. [Google Scholar] [CrossRef]
- Gaona, M.; Lima, R.S.; Marple, B.R. Nanostructured Titania/hydroxyapatite Composite Coatings Deposited by High Velocity Oxy-fuel (HVOF) Spraying. Mater. Sci. Eng. A Struct. 2007, 458, 141–149. [Google Scholar] [CrossRef]
- Hahn, B.-D.; Park, D.-S.; Choi, J.-J.; Ryu, J.; Yoon, W.-H.; Kim, K.-H.; Park, C.; Kim, H.-E. Dense Nanostructured Hydroxyapatite Coating on Titanium by Aerosol Deposition. J. Am. Ceram. Soc. 2009, 92, 683–687. [Google Scholar] [CrossRef]
- Chatelain, D.; Denoirjean, A.; Guipont, V.; Rossignol, F.; Tessier-Doyen, N. Influence of the Thermal Treatment of A Hydroxyapatite Powder on the Characteristics of Coatings Deposited by Cold Gas Spraying. Surf. Coat. Technol. 2022, 446, 128697. [Google Scholar] [CrossRef]
- Stiegler, N.; Bellucci, D.; Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Sola, A. High-Velocity Suspension Flame Sprayed (HVSFS) Hydroxyapatite Coatings for Biomedical Applications. J. Therm. Spray Technol. 2012, 21, 275–287. [Google Scholar] [CrossRef]
- ASTM C633; Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings. ASTM International: West Conshohocken, PA, USA, 2008.
- Kokubo, T.; Takadama, H. How Useful is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Li, W.-Y.; Li, C.-J.; Yang, G.-J. Effect of Impact-Induced Melting on Interface Microstructure and Bonding of Cold-sprayed Zinc Coating. Appl. Surf. Sci. 2010, 257, 1516–1523. [Google Scholar] [CrossRef]
- Yuan, S.D.; Ma, Y.; Li, X.Y.; Ma, Z.; Yang, H.; Mu, L.T. Fabrication and Microstructure of ZnO/HA Composite with In Situ Formation of Second-Phase ZnO. Materials 2020, 13, 3948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial Properties of ZnO Nanomaterials: A Review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Chen, K.; Boyle, K.P. Elastic Properties, Thermal Expansion Coefficients, and Electronic Structures of Mg and Mg-Based Alloys. Metall. Mater. Trans. A 2009, 40, 2751–2760. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhao, H.; Hu, Y.H.; Ye, C.H.; Zhang, L.D. Thermal Expansion Behavior of Hexagonal Zn Nanowires. J. Cryst. Growth 2007, 305, 8–11. [Google Scholar] [CrossRef]
- Miyazaki, H.; Ushiroda, I.; Itomura, D.; Hirashita, T.; Adachi, N.; Ota, T. Thermal Expansion of Hydroxyapatite Between −100 °C and 50 °C. Mater. Sci. Eng. C Mater. 2009, 29, 1463–1466. [Google Scholar] [CrossRef]
- Tomaszek, R.; Pawlowski, L.; Gengembre, L.; Laureyns, J.; Le Maguer, A. Microstructure of Suspension Plasma Sprayed Multilayer Coatings of Hydroxyapatite and Titanium Oxide. Surf. Coat. Technol. 2007, 201, 7432–7440. [Google Scholar] [CrossRef]
- Cannillo, V.; Lusvarghi, L.; Sola, A. Production and Characterization of Plasma-sprayed TiO2-Hydroxyapatite Functionally Graded Coatings. J. Eur. Ceram. Soc. 2008, 28, 2161–2169. [Google Scholar] [CrossRef]
- Marzbanrad, B.; Toyserkani, E.; Jahed, H. Characterization of Single- and Multilayer Cold-spray Coating of Zn on AZ31B. Surf. Coat. Technol. 2021, 416, 127155. [Google Scholar] [CrossRef]
- Cheng, D.; Xu, Q.; Lavernia, E.J.; Trapaga, G. The Effect of Particle Size and Morphology on the In-flight Behavior of Particles During High-velocity Oxyfuel Thermal Spraying. Metall. Mater. Trans. B 2001, 32, 525–535. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Yang, C.-Y. Mechanical and Histological Evaluation of A Plasma Sprayed Hydroxyapatite Coating on A Titanium Bond Coat. Ceram. Int. 2013, 39, 6509–6516. [Google Scholar] [CrossRef]
- Kweh, S.W.K.; Khor, K.A.; Cheang, P. Plasma-sprayed Hydroxyapatite (HA) Coatings with Flame-spheroidized Feedstock: Microstructure and Mechanical Properties. Biomaterials 2000, 21, 1223–1234. [Google Scholar] [CrossRef]
- ISO 13779-2; Lmplants for Surgery Hydroxyapatite—Part 2: Thermally Sprayed Coatings of Hydroxyapatite. ISO: Richmond, VA, USA, 2018.
- List, A.; Huang, C.; Wiehler, L.; Gieseler, C.-P.; Schulze, M.; Gärtner, F.; Klassen, T. Influence of Ductility on Fracture in Tensile Testing of Cold Gas Sprayed Deposits. J. Therm. Spray Technol. 2023, 32, 1780–1795. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of Corrosion Rates Measured by the Tafel Extrapolation Method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- ASTM G102-2015; Standard Practice for Calculation of Corrosion Rates and Related Informationfrom Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015.
- Cui, Z.Y.; Ge, F.; Lin, Y.; Wang, L.W.; Lei, L.; Tian, H.Y.; Yu, M.D.; Wang, X. Corrosion Behavior of AZ31 Magnesium Alloy in the Chloride Solution Containing Ammonium Nitrate. Electrochim. Acta 2018, 278, 421–437. [Google Scholar] [CrossRef]
- Vlasa, A.; Varvara, S.; Pop, A.; Bulea, C.; Muresan, L.M. Electrodeposited Zn-TiO2 Nanocomposite Coatings and Their Corrosion Behavior. J. Appl. Electrochem. 2010, 40, 1519–1527. [Google Scholar] [CrossRef]
- Chakradhar, R.P.S.; Chandra Mouli, G.; Barshilia, H.; Srivastava, M. Improved Corrosion Protection of Magnesium Alloys AZ31B and AZ91 by Cold-Sprayed Aluminum Coatings. J. Therm. Spray Technol. 2021, 30, 371–384. [Google Scholar] [CrossRef]
- Wei, Y.K.; Luo, X.T.; Li, C.X.; Li, C.J. Optimization of In-Situ Shot-Peening-Assisted Cold Spraying Parameters for Full Corrosion Protection of Mg Alloy by Fully Dense Al-Based Alloy Coating. J. Therm. Spray Technol. 2017, 26, 173–183. [Google Scholar] [CrossRef]
- Xia, D.D.; Yang, F.; Zheng, Y.F.; Liu, Y.S.; Zhou, Y.S. Research Status of Biodegradable Metals Designed for Oral and Maxillofacial Applications: A Review. Bioact. Mater. 2021, 6, 4186–4208. [Google Scholar] [CrossRef] [PubMed]
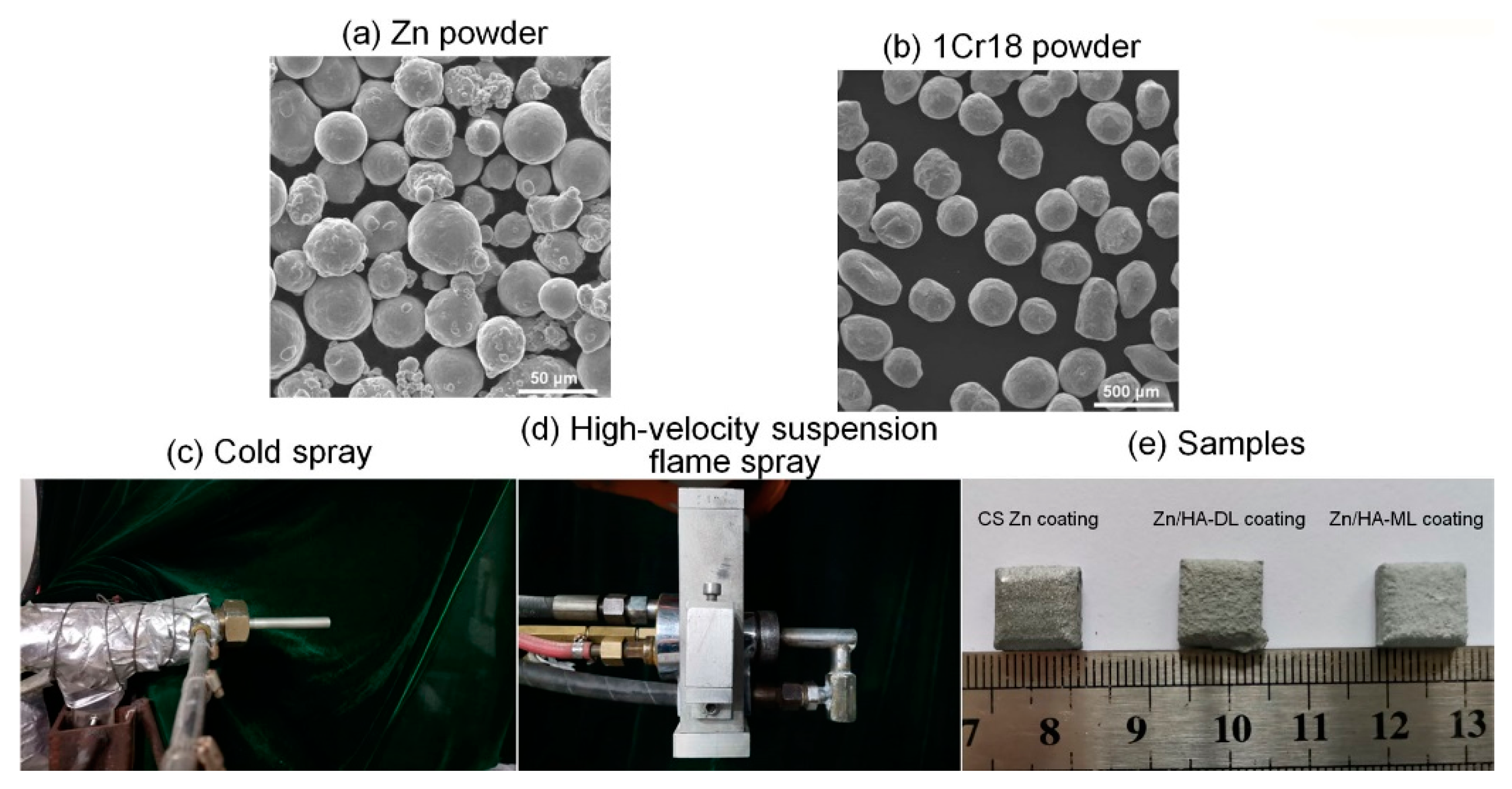
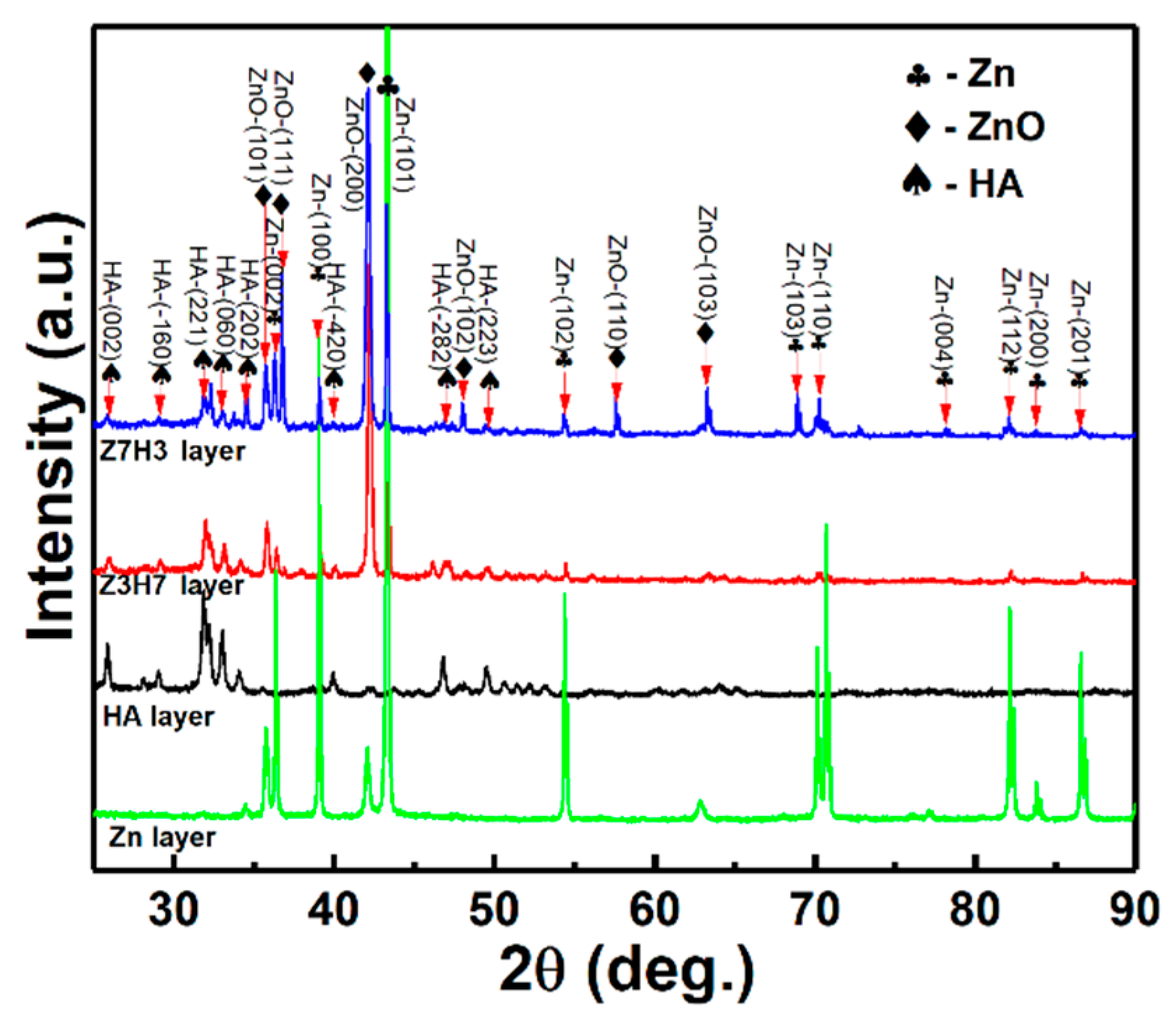

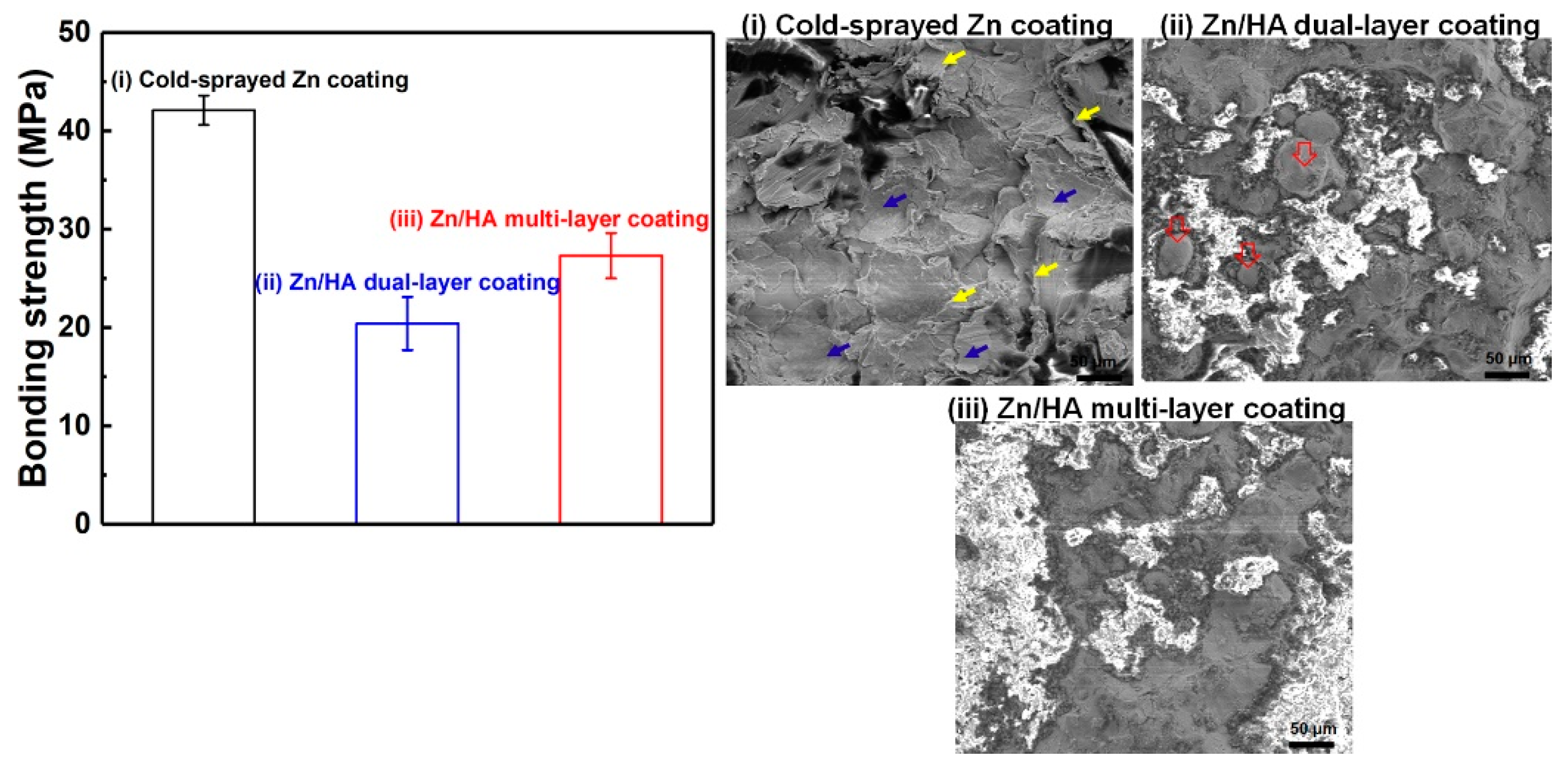
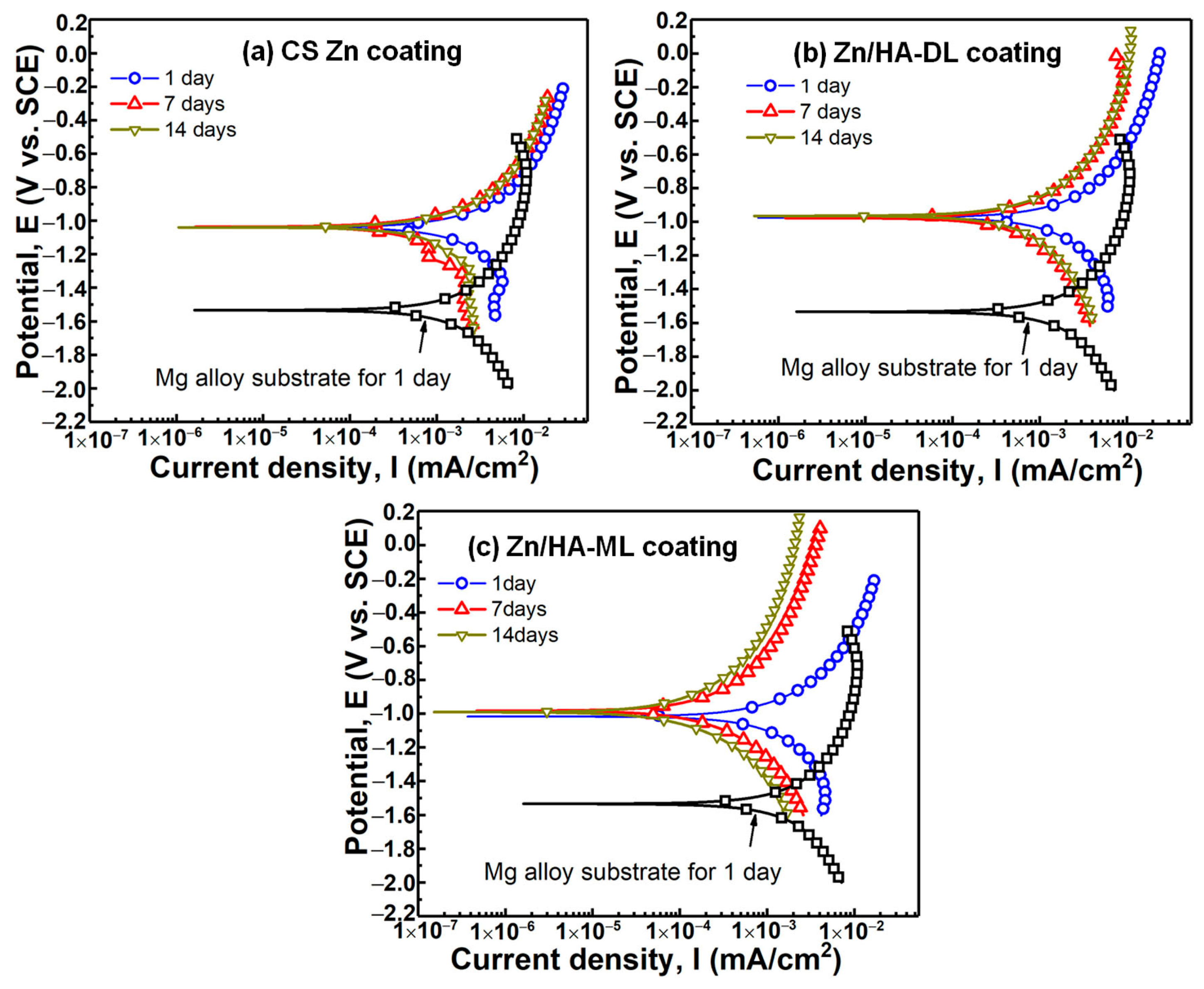
| Fuel (propane) flow rate, slpm | 27 |
| Oxygen flow rate, slpm | 181 |
| Suspension flow rate, mL/min | 40 |
| Spray distance, mm | 100 |
| Torch traverse speed, mm/s | 500 |
| Samples | Immersion Time (Day) | βα (mV) | βc (mV) | Icorr (mA/cm2) | Ecorr (V) | Corrosion Rate (mm/Year) |
|---|---|---|---|---|---|---|
| AZ31B sub. | 1 | 690.6 ± 59.9 | 417.6 ± 144.3 | 3.12 ± 0.75 | −1.54 ± 0.01 | 29.9 ± 0.9 |
| CS Zn coating | 1 | 455.8 ± 29.7 | 265.5 ± 82.3 | 1.41 ± 0.82 | −1.03 ± 0.02 | 16.5 ± 3.6 |
| 7 | 678.4 ± 43.3 | 412.9 ± 281.8 | 0.95 ± 0.52 | −1.04 ± 0.06 | 11.2 ± 4.2 | |
| 14 | 517.5 ± 45.1 | 314.8 ± 109.6 | 0.73 ± 0.12 | −1.11 ± 0.09 | 8.5 ± 1.2 | |
| Zn/HA-DL coating | 1 | 413.2 ± 60.1 | 291.1 ± 36.8 | 1.06 ± 0.31 | −0.94 ± 0.01 | 12.5 ± 3.7 |
| 7 | 451.1 ± 32.7 | 312.1 ± 20.8 | 0.56 ± 0.04 | −0.97 ± 0.01 | 6.7 ± 0.6 | |
| 14 | 368.3 ± 47.3 | 283.7 ± 17.9 | 0.37 ± 0.12 | −0.97 ± 0.02 | 4.4 ± 1.5 | |
| Zn/HA-ML coating | 1 | 417.2 ± 17.3 | 336.1 ± 25.1 | 0.88 ± 0.27 | −0.99 ± 0.02 | 10.3 ± 3.2 |
| 7 | 296.7 ± 19.5 | 311.5 ± 45.8 | 0.24 ± 0.11 | −0.98 ± 0.01 | 2.8 ± 1.4 | |
| 14 | 281.81 ± 67.3 | 318.5 ± 16.1 | 0.14 ± 0.07 | −0.99 ± 0.03 | 1.7 ± 0.9 |
| Samples | Immersion Time (Day) | Rs (ohm·cm2) | R1 (ohm·cm2) | CPE1 (mF·cm−2·sn) | n1 | R2 (ohm·cm2) | CPE2 (mF·cm−2·sn) | n2 | Rp (R1 + R2) (ohm·cm2) |
|---|---|---|---|---|---|---|---|---|---|
| AZ31B sub. | 1 | 27.2 ± 5.6 | - | - | - | 21.5 ± 5.8 | 0.0511 ± 0.0112 | 0.767 ± 0.019 | 21.5 ± 5.8 |
| CS Zn coating | 1 | 26.8 ± 1.1 | 36.5 ± 19.2 | 0.0017 ± 0.0008 | 0.832 ± 0.071 | 100.2 ± 42.6 | 0.0121 ± 0.0014 | 0.607 ± 0.131 | 136.7 ± 51.2 |
| 7 | 30.9 ± 4.28 | 25.9 ± 12.4 | 0.0021 ± 0.0006 | 0.722 ± 0.149 | 163.6 ± 50.4 | 0.0151 ± 0.0062 | 0.486 ± 0.117 | 189.5 ± 78.1 | |
| 14 | 33.9 ± 8.9 | 57.1 ± 7.1 | 0.0058 ± 0.0007 | 0.503 ± 0.142 | 323.3 ± 91.6 | 0.0121 ± 0.0045 | 0.546 ± 0.288 | 380.4 ± 88.6 | |
| Zn/HA-DL coating | 1 | 46.7 ± 10.4 | 118.2 ± 69.5 | 0.0013 ± 0.0005 | 0.616 ± 0.058 | 241.4 ± 89.1 | 0.0182 ± 0.0015 | 0.417 ± 0.359 | 359.5 ± 85.6 |
| 7 | 52.1 ± 4.1 | 102.2 ± 49.4 | 0.0011 ± 0.0002 | 0.582 ± 0.056 | 358.6 ± 96.4 | 0.0153 ± 0.0013 | 0.596 ± 0.081 | 460.8 ± 94.8 | |
| 14 | 60.4 ± 3.4 | 239.8 ± 86.6 | 0.0012 ± 0.0004 | 0.612 ± 0.111 | 365.2 ± 83.5 | 0.0145 ± 0.0082 | 0.731 ± 0.336 | 604.9 ± 81.8 | |
| Zn/HA-ML coating | 1 | 30.8 ± 6.9 | 76.7 ± 23.8 | 0.0019 ± 0.0004 | 0.374 ± 0.012 | 160.7 ± 56.8 | 0.0246 ± 0.0091 | 0.668 ± 0.013 | 237.4 ± 65.3 |
| 7 | 52.2 ± 10.1 | 107.8 ± 36.2 | 0.0011 ± 0.0007 | 0.543 ± 0.043 | 193.8 ± 63.9 | 0.0177 ± 0.0072 | 0.736 ± 0.143 | 301.6 ± 89.2 | |
| 14 | 66.1 ± 9.8 | 184.3 ± 62.1 | 0.0015 ± 0.0001 | 0.513 ± 0.032 | 292.9 ± 62.1 | 0.0147 ± 0.0011 | 0.714 ± 0.121 | 477.2 ± 78.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.; Hu, X.; Chen, Q.; Wang, H.; Bai, X. Microstructure and Corrosion Behavior of Zinc/Hydroxyapatite Multi-Layer Coating Prepared by Combining Cold Spraying and High-Velocity Suspension Flame Spraying. Materials 2023, 16, 6782. https://doi.org/10.3390/ma16206782
Yao H, Hu X, Chen Q, Wang H, Bai X. Microstructure and Corrosion Behavior of Zinc/Hydroxyapatite Multi-Layer Coating Prepared by Combining Cold Spraying and High-Velocity Suspension Flame Spraying. Materials. 2023; 16(20):6782. https://doi.org/10.3390/ma16206782
Chicago/Turabian StyleYao, Hailong, Xiaozhen Hu, Qingyu Chen, Hongtao Wang, and Xiaobo Bai. 2023. "Microstructure and Corrosion Behavior of Zinc/Hydroxyapatite Multi-Layer Coating Prepared by Combining Cold Spraying and High-Velocity Suspension Flame Spraying" Materials 16, no. 20: 6782. https://doi.org/10.3390/ma16206782
APA StyleYao, H., Hu, X., Chen, Q., Wang, H., & Bai, X. (2023). Microstructure and Corrosion Behavior of Zinc/Hydroxyapatite Multi-Layer Coating Prepared by Combining Cold Spraying and High-Velocity Suspension Flame Spraying. Materials, 16(20), 6782. https://doi.org/10.3390/ma16206782





