Additives in Nanocrystalline Tin Dioxide: Recent Progress in the Characterization of Materials for Gas Sensor Applications
Abstract
:1. Introduction
- p-elements—Sb and F;
- 3d-, 4d-, and 5d-elements—Ti-Zn, Nb, Mo, and W;
- Rare earth elements—La and Ce;
- Precious metals and platinum group metals—Pt, Pd, Ru, Rh, Au, and Ag.
2. Gross Quantitative Elemental Composition
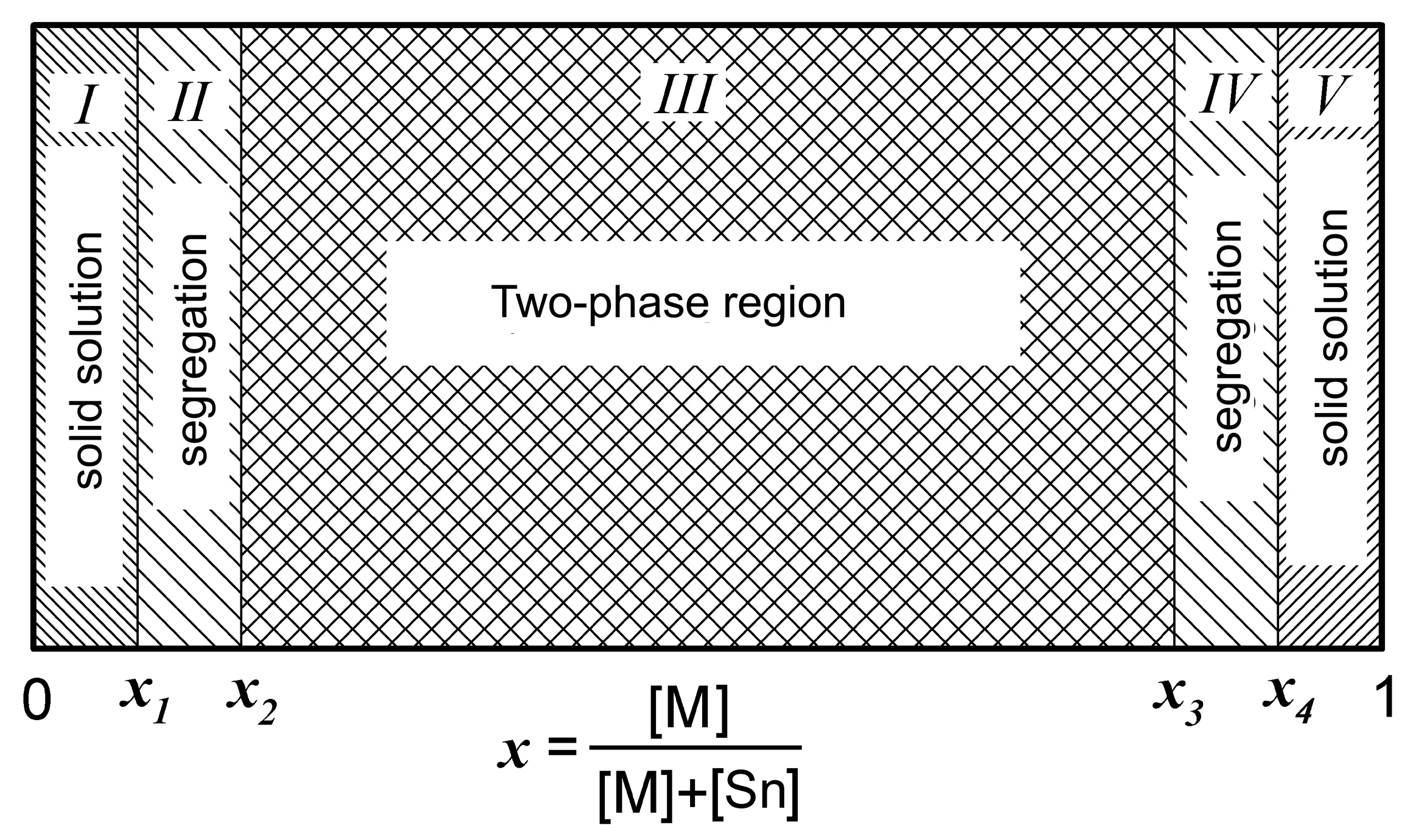
3. Phase Composition
4. Surface Composition and Electronic State of Additives
5. Distribution of Additives between the Volume and the Surface of SnO2 Crystallites
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ginley, D.S.; Hosono, H.; Paine, D.C. (Eds.) Handbook on Transparent Conductors; Springer: New York, NY, USA, 2011. [Google Scholar]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Sharma, H.; Guchhait, A.; Chakrabarty, N.; Bamola, P.; Liu, Q.; Saianand, G.; Krishna, A.M.S.; Mukhopadhyay, S.; Dey, A.; et al. Tin oxide for optoelectronic, photovoltaic and energy storage devices: A review. J. Mater. Chem. A 2021, 9, 16621–16684. [Google Scholar] [CrossRef]
- Hosono, H.; Mishima, Y.; Takezoe, H.; Mackenzie, K.J.D. (Eds.) Nanomaterials: From Research to Applications; Elsevier Ltd.: London, UK, 2006. [Google Scholar]
- Walsh, A.; Silva, L.F.D.; Wei, S.H. Origins of band gap renormalization in degenerately doped semiconductors. Phys. Rev. B 2008, 78, 075211. [Google Scholar] [CrossRef]
- Minami, T. Transparent conducting oxide semiconductors for transparent electrodes. Semicond. Sci. Technol. 2005, 20, S35–S44. [Google Scholar] [CrossRef]
- Ozel, K.; Yildiz, A. High-detectivity ultraviolet-B photodetector based on SnO2 thin film/Si heterojunction. Semicond. Sci. Technol. 2021, 36, 095001. [Google Scholar] [CrossRef]
- Hassun, H.K.; Hussein, B.H.; Salman, E.M.T.; Shaban, A.H. Photoelectric properties of SnO2:Ag/P–Si heterojunction photodetector. Energy Rep. 2020, 6, 46–54. [Google Scholar] [CrossRef]
- Yan, J.; Chen, Y.; Wang, X.; Fu, Y.; Wang, J.; Sun, J.; Dai, G.; Tao, S.; Gao, Y. High-performance solar-blind SnO2 nanowire photodetectors assembled using optical tweezers. Nanoscale 2019, 11, 2162–2169. [Google Scholar] [CrossRef]
- Adepu, V.; Kunchur, A.; Kolli, C.S.R.; Siddhartha, S.; Mattela, V.; Sahatiya, P. High-Performance Visible Light Photodetector Based on 1D SnO2 Nanofibers with a Ti3C2Tx (MXene) Electron Transport Layer. ACS Appl. Nano Mater. 2022, 5, 6852–6863. [Google Scholar] [CrossRef]
- Praveen, S.; Veeralingam, S.; Badhulika, S. A Flexible Self-Powered UV Photodetector and Optical UV Filter Based on β-Bi2O3/SnO2 Quantum Dots Schottky Heterojunction. Adv. Mater. Interfaces 2021, 8, 2100373. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, X.; Yao, R.; Luo, D.; Liu, X.; Zou, W.; Guo, C.; Xu, Z.; Ning, H.; Peng, J. Research and Progress of Transparent, Flexible Tin Oxide Ultraviolet Photodetector. Crystals 2021, 11, 1479. [Google Scholar] [CrossRef]
- Wu, P.; Wang, S.; Li, X.; Zhang, F. Advances in SnO2-based perovskite solar cells: From preparation to photovoltaic applications. J. Mater. Chem. A 2021, 9, 19554–19588. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; Shin, T.J.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, Q.; Zhang, L.; Han, C.; Gao, H.; Zhang, Y.; Yan, H. SnO2-based electron transporting layer materials for perovskite solar cells: A review of recent progress. J. Energy Chem. 2019, 35, 144–167. [Google Scholar] [CrossRef]
- Park, S.Y.; Zhu, K. Advances in SnO2 for Efficient and Stable n–i–p Perovskite Solar Cells. Adv. Mater. 2022, 34, 2110438. [Google Scholar] [CrossRef]
- Bondarchuk, A.N.; Corrales-Mendoza, I.; Aguilar-Martínez, J.A.; García-Pérez, U.M.; Marken, F. Porous and conductive SnO2 ceramics as a promising nanostructured substrate to host photocatalytic hematite coatings: Towards low cost solar-driven water splitting. Catal. Commun. 2023, 174, 106593. [Google Scholar] [CrossRef]
- Bondarchuk, A.N.; Corrales-Mendoza, I.; Aguilar-Martínez, J.A.; Tomás, S.A.; Gómez-Caiceros, D.A.; Hernández-Méndez, A.; Marken, F. A BiVO4 photoanode grown on porous and conductive SnO2 ceramics for water splitting driven by solar energy. Ceram. Int. 2020, 46, 9040–9049. [Google Scholar] [CrossRef]
- Mihaiu, M.S.; Scarlat, O.; Zuca, S.; Zaharescu, M. Advanced SnO2-Based Ceramics: Synthesis, Structure, Properties. In Advances in Ceramics—Synthesis and Characterization, Processing and Specific Applications; Sikalidis, C., Ed.; InTechOpen: London, UK, 2011; pp. 101–126. Available online: http://www.intechopen.com/books/advances-in-ceramics-synthesis-and-characterization-processing-andspecific-applications/advanced-sno2-based-ceramics-synthesis-structure-properties (accessed on 22 August 2023).
- Yan, W.; Zhang, H.; Wang, X.; You, C.; Li, Z. Characterization of electrical conductivity and temperature sensitivity of Cr/Sb-modified SnO2 ceramics. J. Mater. Sci. Mater. Electron. 2020, 31, 4040–4049. [Google Scholar] [CrossRef]
- Jiang, G.; Li, Z.; You, C.; Hao, W.; Ma, Z.; Zhang, H. Temperature sensitivity and electrical stability of Sb/Mn co-doped SnO2 ceramics. J. Mater. Sci. Mater. Electron. 2021, 32, 16945–16955. [Google Scholar] [CrossRef]
- Medvedovski, E. Tin oxide-based ceramics of high density obtained by pressureless sintering. Ceram. Int. 2017, 43, 8396–8405. [Google Scholar] [CrossRef]
- Masteghin, M.G.; Orlandi, M.O.; Bueno, P.R. Varistor technology based on SnO2. In Tin Oxide Materials: Synthesis, Properties, Applications; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 321–343. [Google Scholar]
- Lee, J.H.; You, Y.J.; Saeed, M.A.; Kim, S.H.; Choi, S.H.; Kim, S.; Lee, S.Y.; Park, J.S.; Sim, J.W. Undoped tin dioxide transparent electrodes for efficient and cost-effective indoor organic photovoltaics. NPG Asia Mater. 2021, 13, 43. [Google Scholar] [CrossRef]
- Zhang, S.T.; Foldyna, M.; Roussel, H.; Consonni, V.; Pernot, E.; Schmidt-Mende, L.; Rapenne, L.; Jiménez, C.; Deschanvres, J.L.; Muñoz-Rojas, D.; et al. Tuning the properties of F:SnO2 (FTO) nanocomposites with S:TiO2 nanoparticles—Promising hazy transparent electrodes for photovoltaics applications. J. Mater. Chem. C 2017, 5, 91–102. [Google Scholar] [CrossRef]
- Park, S.H.; Oh, Y.K.; Lim, Y.J.; Shaozheng, C.; Lee, S.J.; Kim, H.K. Thermally stable and transparent F-doped SnO2 (FTO)/Ag/FTO films for transparent thin film heaters used in automobiles. Ceram. Int. 2023, 49, 2419–2426. [Google Scholar] [CrossRef]
- Seok, H.; Lee, J.; Park, J.; Lim, S.; Kim, H. Transparent Conducting Electrodes for Quantum Dots Light Emitting Diodes. Isr. J. Chem. 2019, 59, 729–746. [Google Scholar] [CrossRef]
- Koo, B.R.; Bae, J.W.; Ahn, H.J. Optoelectronic multifunctionality of combustion-activated fluorine-doped tin oxide films with high optical transparency. Ceram. Int. 2019, 45, 10260–10268. [Google Scholar] [CrossRef]
- Zum Felde, U.; Haase, M.; Weller, H. Electrochromism of Highly Doped Nanocrystalline SnO2:Sb. J. Phys. Chem. B 2000, 104, 9388–9395. [Google Scholar] [CrossRef]
- Goei, R.; Ong, A.J.; Tan, J.H.; Loke, J.Y.; Lua, S.K.; Mandler, D.; Magdassi, S.; Tok, A.L.Y. Nd–Nb Co-doped SnO2/α-WO3 Electrochromic Materials: Enhanced Stability and Switching Properties. ACS Omega 2021, 6, 26251–26261. [Google Scholar] [CrossRef]
- Tu, J.; Zhao, G.; Wang, W.; Wang, X.; Xia, X.; Gu, C. Multicolor electrochromic film based on SnO2/V2O5 core/shell structure for adaptive camouflage. J. Mater. Chem. C 2019, 7, 5702–5709. [Google Scholar]
- Kim, K.H.; Koo, B.R.; Ahn, H.J. Effects of Sb-doped SnO2–WO3 nanocomposite on electrochromic performance. Ceram. Int. 2019, 45, 15990–15995. [Google Scholar] [CrossRef]
- Jo, M.H.; Koo, B.R.; Ahn, H.J. Accelerating F-doping in transparent conducting F-doped SnO2 films for electrochromic energy storage devices. Ceram. Int. 2020, 46, 25066–25072. [Google Scholar] [CrossRef]
- Kong, L.B.; Ruan, S.; Xiao, Z.; Li, X.; Zhou, K.; Su, H.; Wang, C.; Zhang, T. Tin oxide-based electrochromics. In Tin Oxide Materials: Synthesis, Properties, Applications; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 489–517. [Google Scholar]
- Chang, H.; Huang, C.H.; Nomura, K. Low-Temperature Solution-Processed n-Channel SnO2 Thin-Film Transistors and High-Gain Zero-VGS-Load Inverter. ACS Appl. Electron. Mater. 2021, 3, 4943–4949. [Google Scholar] [CrossRef]
- Cao, H.; Liang, L. Tin oxide-based thin-film transistors and their circuits. In Tin Oxide Materials: Synthesis, Properties, Applications; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 441–476. [Google Scholar]
- Meng, X.; Valitova, I.; Santato, C.; Cicoira, F. Tin dioxide ion-gated transistors. In Tin Oxide Materials: Synthesis, Properties, Applications; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 477–488. [Google Scholar]
- Dahl, P.I.; Barnett, A.O.; Monterrubio, F.A.; Colmenares, L.C. The use of tin oxide in fuel cells. In Tin Oxide Materials: Synthesis, Properties, Applications; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 379–410. [Google Scholar]
- Notohara, H.; Urita, K.; Moriguchi, I. Tin oxide electrodes in Li and Na-ion batteries. In Tin Oxide Materials: Synthesis, Properties, Applications; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 411–439. [Google Scholar]
- Manjunathan, P.; Shanbhag, G.V. Application of tin oxide-based materials in catalysis. In Tin Oxide Materials: Synthesis, Properties, Applications; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 519–553. [Google Scholar]
- Staerz, A.; Suzuki, T.; Weimar, U.; Barsan, N. SnO2: The most important base material for semiconducting metal oxide-based materials. In Tin Oxide Materials: Synthesis, Properties, Applications; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 345–377. [Google Scholar]
- Gaskov, A.; Rumyantseva, M.; Marikutsa, A. Tin oxide nanomaterials: Active centers and gas sensor properties. In Tin Oxide Materials: Synthesis, Properties, Applications; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–218. [Google Scholar]
- Masuda, Y. Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuators B 2022, 364, 131876. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Current state of knowledge on metal oxide based gas sensing mechanism. Sens. Actuators B 2022, 358, 131531. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Gaskov, A.M. Chemical modification of nanocrystalline metal oxides: Effect of the real structure and surface chemistry on the sensor properties. Russ. Chem. Bull. Int. Ed. 2008, 57, 1106–1125. [Google Scholar] [CrossRef]
- Krivetskiy, V.V.; Rumyantseva, M.N.; Gaskov, A.M. Chemical modification of nanocrystalline tin dioxide for selective gas sensors. Russ. Chem. Rev. 2013, 82, 917–941. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunction for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinghe Arachchige, H.M.M.; Sisman, O.; Comini, E. Metal oxide-based heterostructures for gas sensors—A review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nanoheterostructures: A review. Sens. Actuators B 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef]
- Degler, D.; Weimar, U.; Barsan, N. Current Understanding of the Fundamental Mechanisms of Doped and Loaded Semiconducting Metal-Oxide-Based Gas Sensing Materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef]
- Bordes-Richard, E. Multicomponent Oxides in Selective Oxidation of Alkanes Theoretical Acidity versus Selectivity. Top. Catal. 2008, 50, 82–89. [Google Scholar] [CrossRef]
- Duffy, J.A. Chemical bonding in the oxides of elements: A new appraisal. J. Solid State Chem. 1986, 62, 145–157. [Google Scholar] [CrossRef]
- Duffy, J.A. Ionic-covalent character of metal and nonmetal oxides. J. Phys. Chem. 2006, 110, 13245–13248. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, R.T. An Interpretation of Bond Lengths and a Classification of Bonds. Science 1951, 114, 670–672. [Google Scholar] [CrossRef]
- Sanderson, R.T. The interrelationship of bond dissociation energies and contributing bond energies. J. Am. Chem. Soc. 1975, 97, 1367–1372. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Elliot, J.A.W. Gibbsian Surface Thermodynamics. J. Phys. Chem. B 2020, 124, 10859–10878. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Shen, Y.G. Effects of amorphous matrix on the grain growth kinetics in two-phase nanostructured films: A Monte Carlo study. Acta Mater. 2004, 52, 729–736. [Google Scholar] [CrossRef]
- Miodownik, M.; Holm, E.A.; Hassold, G.N. Highly parallel computer simulation of particle pinning: Zener vindicated. Scripta Mater. 2000, 42, 1173–1177. [Google Scholar] [CrossRef]
- Arbiol, J.; Morante, J.R.; Bouvier, P.; Pagnier, T.; Makeeva, E.; Rumyantseva, M.; Gaskov, A. SnO2/MoO3-nanostructure and alcohol detection. Sens. Actuators B 2006, 118, 156–162. [Google Scholar] [CrossRef]
- Vladimirova, S.A.; Rumyantseva, M.N.; Filatova, D.G.; Chizhov, A.S.; Khmelevsky, N.O.; Konstantinova, E.A.; Kozlovsky, V.F.; Marchevsky, A.V.; Karakulina, O.M.; Hadermann, J.; et al. Cobalt location in p-CoOx/n-SnO2 nanocomposites: Correlation with gas sensor performances. J. Alloys Compd. 2017, 721, 249–260. [Google Scholar] [CrossRef]
- Eshmakov, R.S.; Filatova, D.G.; Konstantinova, E.A.; Rumyantseva, M.N. Effect of manganese distribution on sensor properties of SnO2/MnOx nanocomposites. Nanomaterials 2023, 13, 1437. [Google Scholar] [CrossRef] [PubMed]
- Krivetskiy, V.; Garshev, A.; Marikutsa, A.; Ivanov, V.; Krotova, A.; Filatova, D.; Konstantinova, E.; Naberezhnyi, D.; Khmelevsky, N.; Kots, P.; et al. Enhancement of Lewis acidity of Cr-doped nanocrystalline SnO2 and its effect on surface NH3 oxidation and the sensory detection pattern. ChemPhysChem 2019, 20, 1985–1996. [Google Scholar]
- Rockenberger, J.; zum Felde, U.; Tischer, M.; Tröger, L.; Haase, M.; Weller, H. Near edge X-ray absorption fine structure measurements (XANES) and extended X-ray absorption fine structure measurements (EXAFS) of the valence state and coordination of antimony in doped nanocrystalline SnO2. J. Chem. Phys. 2000, 112, 4296–4304. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M.; Konstantinova, E.A.; Grishina, D.A.; Deygen, D.M. CO and NH3 Sensor Properties and Paramagnetic Centers of Nanocrystalline SnO2 Modified by Pd and Ru. Thin Solid Films 2011, 520, 904–908. [Google Scholar] [CrossRef]
- Wolkenstein, T. Electronic Processes on Semiconductor Surfaces during Chemisorption; Springer: New York, NY, USA, 1991. [Google Scholar]
- Mowat, I.A.; Putyera, K.; Wang, X.; Newman, J.; Vitarelli, J. Materials Characterization and Problem Solving. Available online: http://www.ceramicindustry.com/articles/91508-materials-characterization-and-problem-solving/ (accessed on 22 August 2023).
- Mishra, R.K.; Zachariah, A.K.; Thomas, S. Energy-Dispersive X-Ray spectroscopy Techniques for Nanomaterials. In Microscopy Methods in Nanomaterials Characterization: Micro and Nano Technologies; Thomas, S., Tomas, R., Zachariah, A.K., Mishra, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 383–405. [Google Scholar]
- Chen, Z.; Weyland, M.; Sang, X.; Xu, W.; Dycus, J.H.; LeBeau, J.M.; D’Alfonso, A.J.; Allen, L.J.; Findlay, S.D. Quantitative atomic resolution elemental mapping via absolute-scale energy dispersive X-ray spectroscopy. Ultramicroscopy 2016, 168, 7–16. [Google Scholar] [CrossRef]
- Ross, R.J. (Ed.) Microelectronics Failure Analysis Desk Reference, 6th ed.; ASM International: Detroit, MI, USA, 2011. [Google Scholar]
- Xu, W.; Dycus, J.H.; LeBeau, J.M. Numerical modeling of specimen geometry for quantitative energy dispersive X-ray spectroscopy. Ultramicroscopy 2017, 184A, 100–108. [Google Scholar] [CrossRef]
- Bilovol, V.; Herme, C.; Jacobo, S.; Cabrera, A.F. Study of magnetic behavior of Fe-doped SnO2 powders prepared by chemical method. Mater. Chem. Phys. 2012, 135, 334–339. [Google Scholar] [CrossRef]
- García-Tecedor, M.; Maestre, D.; Cremades, A.; Piqueras, J. Growth and characterization of Cr doped SnO2 microtubes with resonant cavity modes. J. Mater. Chem. C 2016, 4, 5709–5716. [Google Scholar] [CrossRef]
- Padmaja, B.; Dhanapandian, S.; Ashokkumar, K.; Krishnakumar, N. Cobalt ions doped SnO2 nanoparticles for enhanced photocatalytic and supercapacitor applications. Inorg. Chem. Commun. 2023, 155, 110948. [Google Scholar] [CrossRef]
- Shaikh, F.I.; Chikhale, L.P.; Mulla, I.S.; Suryavanshi, S.S. Synthesis, characterization and enhanced acetone sensing performance of Pd loaded Sm doped SnO2 nanoparticles. Ceram. Int. 2017, 43, 10307–10315. [Google Scholar] [CrossRef]
- Berenguer, R.; Quijada, C.; Morallón, E. Electrochemical characterization of SnO2 electrodes doped with Ru and Pt. Electrochim. Acta 2009, 54, 5230–5238. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Gulina, L.B.; Cho, B.K.; Han, S.H.; Tolstoy, V.P. SnO2–Au nanocomposite synthesized by successive ionic layer deposition method: Characterization and application in gas sensors. Mater. Chem. Phys. 2011, 128, 433–441. [Google Scholar] [CrossRef]
- Liu, D.; Pan, J.; Tang, J.; Liu, W.; Bai, S.; Luo, R. Ag decorated SnO2 nanoparticles to enhance formaldehyde sensing properties. J. Phys. Chem. Solids 2019, 124, 36–43. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Kovalenko, V.V.; Gaskov, A.M.; Pagnier, T.; Machon, D.; Arbiol, J.; Morante, J.R. Nanocomposites SnO2/Fe2O3: Wet chemical synthesis and nanostructure characterization. Sens. Actuators B 2005, 109, 64–74. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Panday, M.; Upadhyay, G.K.; Purohit, L.P. Sb incorporated SnO2 nanostructured thin films for CO2 gas sensing and humidity sensing applications. J. Alloys Compd. 2022, 904, 164053. [Google Scholar] [CrossRef]
- Chen, X.; Liu, T.; Wu, R.; Yu, J.; Yin, X. Gas sensors based on Pd-decorated and Sb-doped SnO2 for hydrogen detection. J. Ind. Eng. Chem. 2022, 115, 491–499. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, J.-Y.; Lee, J.-H.; Kim, H.W.; Hishita, S.; Kim, S.S. Enhancement of gas sensing by implantation of Sb-ions in SnO2 nanowires. Sens. Actuators B 2020, 304, 127307. [Google Scholar] [CrossRef]
- Feng, Z.; Gaiardo, A.; Valt, M.; Fabbri, B.; Casotti, D.; Krik, S.; Vanzetti, L.; Ciana, M.D.; Fioravanti, S.; Caramori, S.; et al. Investigation on Sensing Performance of Highly Doped Sb/SnO2. Sensors 2022, 22, 1233. [Google Scholar] [CrossRef]
- Raheem, Z.H.A.; Jebur, E.K. Effect of mixing ratio on gas sensitivity of SnO2:TiO2 thin film against oxidizing and reducing gasses. AIP Conf. Proc. 2020, 2290, 050025. [Google Scholar]
- Kusior, A.; Zych, L.; Zakrzewska, K.; Radecka, M. Photocatalytic activity of TiO2/SnO2 nanostructures with controlled dimensionality/complexity. Appl. Surf. Sci. 2019, 471, 973–985. [Google Scholar] [CrossRef]
- Łyson-Sypien, B.; Kusior, A.; Rekas, M.; Żukrowski, J.; Gajewska, M.; Michalow-Mauke, K.; Graule, T.; Radecka, M.; Zakrzewska, K. Nanocrystalline TiO2/SnO2 heterostructures for gas sensing. Beilstein J. Nanotechnol. 2017, 8, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Krotova, A.A.; Krivetskiy, V.V.; Rumyantseva, M.N.; Filatova, D.G. Study of the Chromium Distribution in New Materials Based on Tin Dioxide by Inductively Coupled Plasma–Mass Spectrometry. Mosc. Univ. Chem. Bull. 2019, 74, 10–13. [Google Scholar] [CrossRef]
- Huang, J.Q.; Liu, Y.K.; Wu, Y.M.; Li, X.M. Influence of Mn Doping on the Sensing Properties of SnO2 Nanobelt to Ethanol. Am. J. Anal. Chem. 2017, 8, 60–71. [Google Scholar] [CrossRef]
- Gandhi, T.I.; Babu, R.R.; Ramamurthi, K.; Arivanandhan, M. Effect of Mn doping on the electrical and optical properties of SnO2 thin films deposited by chemical spray pyrolysis technique. Thin Solid Films 2016, 598, 195–203. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, X.; Peng, H.; Fang, X.; Liu, W.; Ying, J.; Yu, F.; Wang, X. SnO2-based solid solutions for CH4 deep oxidation: Quantifying the lattice capacity of SnO2 using an X-ray diffraction extrapolation method. Chin. J. Catal. 2016, 37, 1293–1302. [Google Scholar] [CrossRef]
- Kovalenko, V.V.; Rumyantseva, M.N.; Fabritchnyi, P.B.; Gaskov, A.M. The unusual distribution of the constituants in the (Fe2O3)0.8(SnO2)0.2 nanocomposite evidenced by 57Fe and 119Sn Mössbauer spectroscopy. Mendeleev Commun. 2004, 14, 140–141. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Y.; Lu, G.; Qi, Y.; Wu, H.; Yang, Z.; Han, H.; Zhang, X.; Wu, L.; Wang, Z.; et al. Transition metal elements-doped SnO2 for ultrasensitive and rapid ppb-level formaldehyde sensing. Heliyon 2023, 9, e13486. [Google Scholar] [CrossRef]
- Soussi, L.; Garmim, T.; Karzazi, O.; Rmili, A.; El Bachiri, A.; Louardi, A.; Erguig, H. Effect of (Co, Fe, Ni) doping on structural, optical and electrical properties of sprayed SnO2 thin film. Surf. Interfaces 2020, 19, 100467. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Man, J.; Chen, C. Preparation of high-performance Fe-doped SnO2 humidity sensor and its application in respiration detection. Sens. Actuators A 2023, 362, 114644. [Google Scholar] [CrossRef]
- Filatova, D.G.; Eskina, V.V.; Baranovskaya, V.B.; Vladimirova, S.A.; Gaskov, A.M.; Rumyantseva, M.N.; Karpov, Y.A. Determination of gold and cobalt dopants in advanced materials based on tin oxide by slurry sampling high-resolution continuum source graphite furnace atomic absorption spectrometry. Spectrochim. Acta B 2018, 140, 1–4. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, H.; Hu, J.; Lv, T.; Rong, Q.; Zhang, Y.; Zi, B.; Chen, M.; Zhang, D.; Wei, J.; et al. Formaldehyde gas sensor with extremely high response employing cobalt-doped SnO2 ultrafine nanoparticles. Nanoscale Adv. 2022, 4, 824–836. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Boris, I.; Brinzari, V.; Han, S.H.; Cho, B.K. The role of doping effect on the response of SnO2-based thin film gas sensors: Analysis based on the results obtained for Co-doped SnO2 films deposited by spray pyrolysis. Sens. Actuators B 2013, 182, 112–124. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, Y.; Jin, R.; Wang, T.; Liu, F.; Wang, C.; Yan, X.; Sun, P.; Lu, G. Gas sensor based on cobalt-doped 3D inverse opal SnO2 for air quality monitoring. Sens. Actuators B 2022, 350, 130807. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Yang, M.; Zhang, X.; Cheng, X.; Zhou, X.; Gao, S.; Xu, Y.; Huo, L. Three-in-one Ni doped porous SnO2 nanorods sensor: Controllable oxygen vacancies content, surface site activation and low power consumption for highly selective NO2 monitoring. Sens. Actuators B 2023, 382, 133550. [Google Scholar] [CrossRef]
- Gu, C.; Guan, W.; Liu, X.; Gao, L.; Wang, L.; Shim, J.-J.; Huang, J. Controlled synthesis of porous Ni-doped SnO2 microstructures and their enhanced gas sensing properties. J. Alloys Compd. 2017, 692, 855–864. [Google Scholar] [CrossRef]
- Inderan, V.; Arafat, M.M.; Kumar, S.; Haseeb, A.S.M.A.; Jiang, Z.-T.; Altarawneh, M.; Lee, H.L. Study of structural properties and defects of Ni-doped SnO2 nanorods as ethanol gas sensors. Nanotechnology 2017, 28, 265702. [Google Scholar] [CrossRef]
- Manikandan, V.; Petrila, I.; Vigneselvan, S.; Mane, R.S.; Vasile, B.; Dharmavarapu, R.; Lundgaard, S.; Juodkazis, S.; Chandrasekarang, J. A reliable chemiresistive sensor of nickel-doped tin oxide (Ni-SnO2) for sensing carbon dioxide gas and humidity. RSC Adv. 2020, 10, 3796–3804. [Google Scholar] [CrossRef]
- Zhang, L.; He, J.; Jiao, W. Synthesis and gas sensing performance of NiO decorated SnO2 vertical standing nanotubes composite thin films. Sens. Actuators B 2019, 281, 326–334. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Pandey, A.; Satpati, B.; Tomar, M.; Gupta, V.; Mohapatra, S. Enhanced CO gas sensing properties of Cu doped SnO2 nanostructures prepared by a facile wet chemical method. Phys. Chem. Chem. Phys. 2016, 18, 18846. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Sun, J.; Xue, N.; Wang, W.; Luo, Z.; Liang, Q.; Zhou, T.; Quan, H.; Cai, H.; Tang, K.; et al. Cu-doped SnO2/rGO nanocomposites for ultrasensitive H2S detection at low temperature. Microsyst. Nanoeng. 2023, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tong, Y.; Zhang, J.; Fang, X.; Xu, J.; Liu, F.; Liu, J.; Zhong, W.; Lebedeva, O.E.; Wang, X. Investigation of lattice capacity effect on Cu2+-doped SnO2 solid solution catalysts to promote reaction performance toward NOx-SCR with NH3. Chin. J. Catal. 2020, 41, 877–888. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Liu, S. SnO2 Nanostructure with Well-Engineered Crystal Facets by Zn Doping for Chemical Sensing Applications. Cryst. Growth Des. 2020, 20, 2742–2752. [Google Scholar] [CrossRef]
- Minau, S.; Atkinson, I.; Mocioiu, O.; Toader, A.; Tenea, E.; Zaharescu, M. Phase formation mechanism in the ZnO-SnO2 binary system. Rev. Roum. Chim. 2011, 56, 465–472. [Google Scholar]
- Boomashri, M.; Perumal, P.; Khan, A.; El-Toni, A.M.; Ansari, A.A.; Gupta, R.K.; Murahari, P.; Kumar, K.D.A. Zinc influence on nanostructured tin oxide (SnO2) films as ammonia sensor at room temperature. Surf. Interfaces 2021, 25, 101195. [Google Scholar] [CrossRef]
- Khamfoo, K.; Wisitsoraat, A.; Punginsang, M.; Tuantranont, A.; Liewhiran, C. Selectivity towards acetylene gas of flame-spray-made Nb-substituted SnO2 particulate thick films. Sens. Actuators B 2021, 349, 130808. [Google Scholar] [CrossRef]
- Gao, S.; Guan, H.; Wang, H.; Yang, X.; Yang, W.; Li, Q. Creation of SnxNb1−xO2 solid solution through heavy Nb-doping in SnO2 to boost its photocatalytic CO2 reduction to C2+ products under simulated solar illumination. J. Adv. Ceram. 2022, 11, 1404–1416. [Google Scholar] [CrossRef]
- Zhu, Y.; Shan, W.; Lian, Z.; Liu, J.; Zhang, Y.; He, H. Effects of impregnation sequence on the NH3-SCR activity and hydrothermal stability of a Ce-Nb/SnO2 catalyst. J. Environ. Sci. 2023, 138, 450–457. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Konstantinova, E.A.; Shatalova, T.B.; Gaskov, A.M. Active sites on nanocrystalline tin dioxide surface: Effect of palladium and ruthenium oxides clusters. J. Phys. Chem. C 2014, 118, 21541–21549. [Google Scholar] [CrossRef]
- Kou, X.; Meng, F.; Chen, K.; Wang, T.; Sun, P.; Liu, F.; Yan, X.; Sun, Y.; Liu, F.; Shimanoe, K.; et al. High performance acetone gas sensor based on Ru-doped SnO2 nanofibers. Sens. Actuators B 2020, 320, 128292. [Google Scholar] [CrossRef]
- Li, J.; Xian, J.; Wang, W.; Cheng, K.; Zeng, M.; Zhang, A.; Wu, S.; Gao, X.; Lu, X.; Liu, J.-M. Ultrafast response and high-sensitivity acetone gas sensor based on porous hollow Ru-doped SnO2 nanotubes. Sens. Actuators B 2022, 352, 131061. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Raja, S.; Babu, R.R.; Vasuki, G. Influence of Ru doping on the structural, morphological, optical, electrical and optoelectronic properties of SnO2 thin films. J. Phys. Chem. Solids 2023, 174, 111117. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Huang, J.; Luo, W.; Yu, J.; Fang, X.; Lebedeva, O.E.; Wang, X. The influence of RuO2 distribution and dispersion on the reactivity of RuO2-SnO2 composite oxide catalysts probed by CO oxidation. ChemCatChem 2019, 11, 2473–2483. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Koziej, D.; Hubner, M.; Barsan, N.; Weimar, U.; Sikorazc, M.; Grunwaldt, J.-D. Operando X-ray absorption spectroscopy studies on Pd-SnO2 based sensors. Phys. Chem. Chem. Phys. 2009, 11, 8620–8625. [Google Scholar] [CrossRef] [PubMed]
- Marikutsa, A.V.; Rumyantseva, M.N.; Yashina, L.V.; Gaskov, A.M. Role of surface hydroxyl groups in promoting room temperature CO sensing by Pd-modified nanocrystalline SnO2. J. Solid State Chem. 2010, 183, 2389–2399. [Google Scholar] [CrossRef]
- Gschwend, P.M.; Schenk, F.M.; Gogos, A.; Pratsinis, S.E. Acetone Sensing and Catalytic Conversion by Pd-Loaded SnO2. Materials 2021, 14, 5921. [Google Scholar] [CrossRef] [PubMed]
- Schierbaum, K.D.; Kirner, U.K.; Geiger, J.F.; Göpel, W. Schottky-barrier and conductivity gas sensors based upon Pd/SnO2 and Pt/TiO2. Sens. Actuators B 1991, 4, 87–94. [Google Scholar] [CrossRef]
- Rettenmaier, C.; Arán-Ais, R.M.; Timoshenko, J.; Rizo, R.; Jeon, H.S.; Kühl, S.; Chee, S.W.; Bergmann, A.; Cuenya, B.R. Enhanced Formic Acid Oxidation over SnO2-decorated Pd Nanocubes. ACS Catal. 2020, 10, 14540–14551. [Google Scholar] [CrossRef]
- Gaidi, M.; Hazemann, J.L.; Matko, I.; Chenevier, B.; Rumyantseva, M.; Gaskov, A.; Labeau, M. Role of Pt aggregates in Pt/SnO2 thin films used as gas sensors: Investigation of the catalytic effect. J. Electrochem. Soc. 2000, 147, 3131–3139. [Google Scholar] [CrossRef]
- Kutukov, P.; Rumyantseva, M.; Krivetskiy, V.; Filatova, D.; Batuk, M.; Hadermann, J.; Khmelevsky, N.; Aksenenko, A.; Gaskov, A. Influence of mono- and bimetallic PtOx, PdOx, PtPdOx clusters on CO sensing by SnO2 based gas sensors. Nanomaterials 2018, 8, 917. [Google Scholar] [CrossRef]
- Tammanoon, N.; Wisitsoraat, A.; Phokharatkul, D.; Tuantranont, A.; Phanichphant, S.; Yordsri, V.; Liewhiran, C. Highly sensitive acetone sensors based on flame-spray-made La2O3-doped SnO2 nanoparticulate thick films. Sens. Actuators B 2018, 262, 245–262. [Google Scholar] [CrossRef]
- Choi, J.Y.; Oh, T.S. CO sensitivity of La2O3-doped SnO2 thick film gas sensor. Thin Solid Films 2013, 547, 230–234. [Google Scholar] [CrossRef]
- Leangtanom, P.; Wisitsoraat, A.; Jaruwongrungsee, K.; Chanlek, N.; Phanichphant, S.; Kruefu, V. Highly sensitive and selective ethylene gas sensors based on CeOx-SnO2 nanocomposites prepared by a Co-precipitation method. Mater. Chem. Phys. 2020, 254, 123540. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Huang, D.; Wang, X.; Cai, L.; Chen, Y.; Wang, W.; Song, Y.; Han, G.; Zhen, B. A high-performance ethanol gas sensor based on Ce-doped SnO2 nanomaterials prepared by the Pechini method. Mater. Sci. Semicond. Proc. 2022, 137, 106188. [Google Scholar] [CrossRef]
- Kotchasak, N.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Yordsri, V.; Liewhiran, C. Highly sensitive and selective detection of ethanol vapor using flame-spray-made CeOx-doped SnO2 nanoparticulate thick films. Sens. Actuators B 2018, 255, 8–21. [Google Scholar] [CrossRef]
- Pagnier, T.; Boulova, M.; Galerie, A.; Gaskov, A.; Lucazeau, G. Reactivity of SnO2 –CuO nanocrystalline materials with H2S: A coupled electrical and Raman spectroscopic study. Sens. Actuators B 2000, 71, 134–139. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Platonov, V.; Garshev, A.; Rumyantseva, M. Low temperature HCHO detection by SnO2/TiO2@Au and SnO2/TiO2@Pt: Understanding by in-situ DRIFT spectroscopy. Nanomaterials 2021, 11, 2049. [Google Scholar] [CrossRef]
- Chen, G.; Ji, S.; Li, H.; Kang, X.; Chang, S.; Wang, Y.; Yu, G.; Lu, J.; Claverie, J.; Sang, Y.; et al. High-Energy Faceted SnO2-Coated TiO2 Nanobelt Heterostructure for Near-Ambient Temperature-Responsive Ethanol Sensor. ACS Appl. Mater. Interfaces 2015, 7, 24950–24956. [Google Scholar] [CrossRef]
- Ng, S.; Prášek, J.; Zazpe, R.; Pytlíček, Z.; Spotz, Z.; Rodriguez Pereira, J.; Michalička, J.; Přikryl, J.; Krbal, M.; Sopha, H.; et al. Atomic Layer Deposition of SnO2-Coated Anodic One-Dimensional TiO2 Nanotube Layers for Low Concentration NO2 Sensing. ACS Appl. Mater. Interfaces 2020, 12, 33386–33396. [Google Scholar] [CrossRef] [PubMed]
- Marzec, A.; Radecka, M.; Maziarz, W.; Kusior, A.; Pędzich, Z. Structural, optical and electrical properties of nanocrystalline TiO2, SnO2 and their composites obtained by the sol–gel method. J. Eur. Ceram. Soc. 2016, 36, 2981–2989. [Google Scholar] [CrossRef]
- Xu, H.; Ju, J.; Li, W.; Zhang, J.; Wang, J.; Cao, B. Superior triethylamine-sensing properties based on TiO2/SnO2 n–n heterojunction nanosheets directly grown on ceramic tubes. Sens. Actuators B 2016, 228, 634–642. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G. Study on TiO2-SnO2 core-shell heterostructure nanofibers with different work function and its application in gas sensor. Sens. Actuators B 2017, 248, 812–819. [Google Scholar] [CrossRef]
- Nowak, P.; Maziarz, W.; Rydosz, A.; Kowalski, K.; Ziąbka, M.; Zakrzewska, K. SnO2/TiO2 Thin Film n-n Heterostructures of Improved Sensitivity to NO2. Sensors 2020, 20, 6830. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Kim, K.M.; Kwon, Y.J.; Choi, H.; Park, J.K.; Jeong, Y.K. ALD-assisted synthesis of V2O5 nanoislands on SnO2 nanowires for improving NO2 sensing performance. Appl. Surf. Sci. 2020, 509, 144821. [Google Scholar] [CrossRef]
- Staerz, A.; Gao, X.; Cetmi, F.; Ming, Z.; Weimar, U.; Zhang, T.; Barsan, N. Dominant Role of Heterojunctions in Gas Sensing with Composite Materials. ACS Appl. Mater. Interfaces 2020, 12, 21127–21132. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Moon, Y.K.; Kim, T.-H.; Park, S.-W.; Kim, K.B.; Kang, Y.C.; Lee, J.-H. A New Strategy for Detecting Plant Hormone Ethylene Using Oxide Semiconductor Chemiresistors: Exceptional Gas Selectivity and Response Tailored by Nanoscale Cr2O3 Catalytic Overlayer. Adv. Sci. 2020, 7, 1903093. [Google Scholar] [CrossRef]
- An, Y.; Wang, T.; Li, T.; Yang, H.; Yu, H.; Xia, L.; Huang, X. Ultra-high gas sensing properties of porous Cr2O3@SnO2 composite toward NOx based on the p-p heterostructure. Ceram. Int. 2022, 48, 21982–21987. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Lu, Z.; Xu, L.; Zhang, Q.; Zeng, W. Synthesis of Cr2O3 Nanoparticle-Coated SnO2 Nanofibers and C2H2 Sensing Properties. Front. Mater. 2019, 6, 63. [Google Scholar] [CrossRef]
- Singh, A.; Verma, A.; Yadav, B.C. MnO2-SnO2 Based Liquefied Petroleum Gas Sensing Device for Lowest Explosion Limit Gas Concentration. ECS Sens. Plus 2022, 1, 025201. [Google Scholar] [CrossRef]
- Bigiani, L.; Zappa, D.; Maccato, C.; Gasparotto, A.; Sada, C.; Comini, E.; Barreca, D. Hydrogen Gas Sensing Performances of p-Type Mn3O4 Nanosystems: The Role of Built-in Mn3O4/Ag and Mn3O4/SnO2 Junctions. Nanomaterials 2020, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.-T.; Wu, S.-S.; Dastan, D.; Nie, N.; Liu, Y.; Li, Z.-G.; Zhou, Y.-W.; Li, J.; Faik, A.; Shan, K.; et al. Sensing selectivity of SnO2-Mn3O4 nanocomposite sensors for the detection of H2 and CO gases. Surf. Interfaces 2021, 25, 101190. [Google Scholar] [CrossRef]
- Jayababu, N.; Poloju, M.; Ramana Reddy, M.V. Facile synthesis of SnO2-Fe2O3 core-shell nanostructures and their 2-methoxyethanol gas sensing characteristics. J. Alloys Compd. 2019, 780, 523–533. [Google Scholar] [CrossRef]
- Sun, P.; Zhou, X.; Wang, C.; Shimanoe, K.; Lu, G.; Yamazoe, N. Hollow SnO2/α-Fe2O3 spheres with a double-shell structure for gas sensors. J. Mater. Chem. A 2014, 2, 1302–1308. [Google Scholar] [CrossRef]
- Salah, B.; Ayesh, A.I. Fabrication of H2S sensitive gas sensors formed of SnO2–Fe2O3 composite nanoparticles. Mater. Chem. Phys. 2021, 266, 124597. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, W.; Meng, X.; Ruan, A.; Su, P.; Yang, H. Enhanced ethanol sensing properties based on spherical-coral-like SnO2 nanorods decorated with α-Fe2O3 nanocrystallites. Sens. Actuators B 2018, 261, 505–514. [Google Scholar] [CrossRef]
- Yan, S.; Xue, J.; Wu, Q. Synchronous synthesis and sensing performance of α-Fe2O3/SnO2 nanofiber heterostructures for conductometric C2H5OH detection. Sens. Actuators B 2018, 275, 322–331. [Google Scholar] [CrossRef]
- Li, X.-B.; Wang, D.; Gao, C.; Zhao, X.; Sun, S.; Ren, Y.; Zhang, Q.; Zhang, J.; Dang, W.; Zhao, Y.-X.; et al. Isobutanol gas sensor based on Fe2O3–SnO2 heterostructure nanostructure. Vacuum 2023, 218, 112624. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Na, H.G.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, T.W.; Mirzaei, A.; Kim, H.W. Attachment of Co3O4 layer to SnO2 nanowires for enhanced gas sensing properties. Sens. Actuators B 2017, 239, 180–182. [Google Scholar] [CrossRef]
- Kim, H.; Cai, Z.; Chang, S.-P.; Park, S. Improved sub-ppm acetone sensing properties of SnO2 nanowire-based sensor by attachment of Co3O4 nanoparticles. J. Mater. Res. Technol. 2020, 9, 1129–1136. [Google Scholar] [CrossRef]
- Raza, M.H.; Kaur, N.; Comini, E.; Pinna, N. Toward Optimized Radial Modulation of the Space-Charge Region in One-Dimensional SnO2−NiO Core−Shell Nanowires for Hydrogen Sensing. ACS Appl. Mater. Interfaces 2020, 12, 4594–4606. [Google Scholar] [CrossRef]
- Niu, G.; Zhao, C.; Gong, G.; Yang, Z.; Leng, X.; Wang, F. NiO nanoparticle-decorated SnO2 nanosheets for ethanol sensing with enhanced moisture resistance. Microsyst. Nanoeng. 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Jayababu, N.; Poloju, M.; Shruthi, J.; Ramana Reddy, M.V. Semi shield driven p-n heterostructures and their role in enhancing the room temperature ethanol gas sensing performance of NiO/SnO2 nanocomposites. Ceram. Int. 2019, 45, 15134–15142. [Google Scholar] [CrossRef]
- Ju, D.; Xu, H.; Xu, Q.; Gong, H.; Qiu, Z.; Guo, J.; Zhang, J.; Cao, B. High triethylamine-sensing properties of NiO/SnO2 hollow sphere P–N heterojunction sensors. Sens. Actuators B 2015, 215, 39–44. [Google Scholar] [CrossRef]
- Park, K.-R.; Cho, H.-B.-; Lee, J.; Song, Y.; Kim, W.-B.; Cho, Y.-H. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuators B 2020, 302, 127179. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar Shringi, A.; Kumar, M. RF sputtered CuO anchored SnO2 for H2S gas sensor. Sens. Actuators B 2022, 370, 132417. [Google Scholar] [CrossRef]
- Duoc, V.T.; Hung, C.M.; Nguyen, H.; Duy, N.V.; Hieu, N.V.; Hoa, N.D. Room temperature highly toxic NO2 gas sensors based on rootstock/scion nanowires of SnO2/ZnO, ZnO/SnO2, SnO2/SnO2 and, ZnO/ZnO. Sens. Actuators B 2021, 348, 130652. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Ma, Q.; Li, H.; Che, Q.; Wang, J.; Wang, G.; Yang, P. Multilevel Effective Heterojunctions Based on SnO2/ZnO 1D Fibrous Hierarchical Structure with Unique Interface Electronic Effects. ACS Appl. Mater. Interfaces 2019, 11, 31551–31561. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, H.; Wang, H.; Yan, B.; Ma, J.; Wang, W.; Yadav, A.K.; Dong, W.; Wang, S. ZnO–SnO2 nano-heterostructures with high-energy facets for high selective and sensitive chlorine gas sensor. Ceram. Int. 2020, 46, 27499–27507. [Google Scholar] [CrossRef]
- Mao, L.-W.; Zhu, L.-Y.; Wu, T.T.; Xu, L.; Jin, X.-H.; Lu, H.-L. Excellent long-term stable H2S gas sensor based on Nb2O5/SnO2 core-shell heterostructure nanorods. Appl. Surf. Sci. 2022, 602, 154339. [Google Scholar] [CrossRef]
- Li, C.; Kim, K.; Fuchigami, T.; Asaka, T.; Kakimoto, K.; Masuda, Y. Acetone gas sensor based on Nb2O5@SnO2 hybrid structure with high selectivity and ppt-level sensitivity. Sens. Actuators B 2023, 393, 134144. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, D.; Xu, X.; Wang, X.; Zhang, N. Study on RuO2/SnO2: Novel and Active Catalysts for CO and CH4 Oxidation. ChemCatChem 2012, 4, 1122–1132. [Google Scholar] [CrossRef]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. II. Adiabatic principle of X-ray diffraction analysis of mixtures. J. Appl. Cryst. 1974, 7, 526–530. [Google Scholar] [CrossRef]
- Hu, K.; Li, Y.; Ge, C.; Bai, L.; Liu, G.; Qiao, G.; Kang, S.G.; Kim, E.J.; Wang, M. Room-temperature ppb-level NO2 sensitivity of three-dimensional ordered macroporous Au-loaded SnO2 under intermittent UV light irradiation. Sens. Actuators B 2023, 387, 133786. [Google Scholar] [CrossRef]
- Ischenko, A.A.; Lazov, M.A.; Mironova, E.V.; Putin, A.Y.; Ionov, A.M.; Storozhenko, P.A. Analysis of nanoparticles and nanomaterials using X-ray photoelectron spectroscopy. Fine Chem. Technol. 2023, 18, 135–167. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on surface characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Gunji, S.; Jukei, M.; Shimotsuma, Y.; Miura, K.; Suematsu, K.; Watanabe, K.; Shimanoe, K. Unexpected gas sensing property of SiO2/SnO2 core-shell nanofibers in dry and humid conditions. J. Mater. Chem. C 2017, 5, 6369–6376. [Google Scholar] [CrossRef]
- Gulevich, D.; Rumyantseva, M.; Marikutsa, A.; Shatalova, T.; Konstantinova, E.; Gerasimov, E.; Gaskov, A. Nanocomposites SnO2/SiO2: SiO2 Impact on the Active Centers and Conductivity Mechanism. Materials 2019, 12, 3618. [Google Scholar] [CrossRef]
- Gulevich, D.; Rumyantseva, M.; Gerasimov, E.; Marikutsa, A.; Krivetskiy, V.; Shatalova, T.; Khmelevsky, N.; Gaskov, A. Nanocomposites SnO2/SiO2 for CO gas sensors: Microstructure and reactivity in the interaction with the gas phase. Materials 2019, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.T.; Haverkamp, R.G. Electrocatalytic activity of IrO2–RuO2 supported on Sb-doped SnO2 nanoparticles. Electrochim. Acta 2010, 55, 1978–1984. [Google Scholar] [CrossRef]
- Costa, I.M.; Colmenares, Y.N.; Pizani, P.S.; Leite, E.R.; Chiquito, A.J. Sb doping of VLS synthesized SnO2 nanowires probed by Raman and XPS spectroscopy. Chem. Phys. Lett. 2018, 695, 125–130. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Huang, J.; Wang, Y.; Ren, H.; Wu, S.; Zhang, S.; Huang, W. Low-temperature CO gas sensors based on Au/SnO2 thick film. Appl. Surf. Sci. 2007, 253, 3057–3061. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Milone, C.; Galvagno, S. Role of the Au oxidation state in the CO sensing mechanism of Au/iron oxide-based gas sensors. Sens. Actuators B 2003, 93, 402. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Cheng, X.; Luo, D.; Huang, B.; Sun, S.; Li, X.; Yang, Z. Low-temperature and high-sensitivity Au-decorated thin-walled SnO2 nanotubes sensor for ethanol detection. Mater. Today Commun. 2023, 37, 107217. [Google Scholar] [CrossRef]
- Gulevich, D.; Rumyantseva, M.; Gerasimov, E.; Khmelevsky, N.; Tsvetkova, E.; Gaskov, A. Synergy effect of Au and SiO2 modification on SnO2 sensor properties in VOCs detection in humid air. Nanomaterials 2020, 10, 813. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, I.; Haruta, M. Role of Water in CO Oxidation on Gold Catalysts. Catal. Lett. 2014, 144, 1475–1486. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, L. Hollow hierarchical TiO2-SnO2-TiO2 composite nanofibers with increased active-sites and charge transfer for enhanced acetone sensing performance. Sens. Actuators B 2021, 334, 129644. [Google Scholar] [CrossRef]
- Stranick, M.A. Mn2O3 by XPS. Surf. Sci. Spectra 1999, 6, 39–46. [Google Scholar] [CrossRef]
- Stranick, M.A. MnO2 by XPS. Surf. Sci. Spectra 1999, 6, 31–38. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, J. Highly sensitive ethanol gas sensors based on Co-doped SnO2 nanobelts and pure SnO2 nanobelts. Physica E 2023, 147, 115604. [Google Scholar] [CrossRef]
- Frolov, D.D.; Kotovshchikov, Y.N.; Morozov, I.V.; Boltalin, A.I.; Fedorova, A.A.; Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M.; Sadovskaya, E.M.; Abakumov, A.M. Oxygen exchange on nanocrystalline tin dioxide modified by palladium. J. Solid State Chem. 2012, 186, 1–8. [Google Scholar] [CrossRef]
- Bahrami, B.; Khodadadi, A.; Kazemeini, M.; Mortazavi, Y. Enhanced CO sensitivity and selectivity of gold nanoparticles-doped SnO2 sensor in presence of propane and methane. Sens. Actuators B 2008, 133, 352–356. [Google Scholar] [CrossRef]
- Cerdà Belmonte, J.; Manzano, J.; Arbiol, J.; Cirera, A.; Puigcorbe, J.; Vilà, A.; Sabaté, N.; Gràcia, I.; Cané, C.; Morante, J.R. Micromachined twin gas sensor for CO and O2 quantification based on catalytically modified nano-SnO2. Sens. Actuators B 2006, 114, 881–892. [Google Scholar] [CrossRef]
- Marikutsa, A.; Krivetskiy, V.; Yashina, L.; Rumyantseva, M.; Konstantinova, E.; Ponzoni, A.; Comini, E.; Abakumov, A.; Gaskov, A. Catalytic impact of RuOx clusters to high ammonia sensitivity of tin dioxide. Sens. Actuators B 2012, 175, 186–193. [Google Scholar] [CrossRef]
- Safonova, O.V.; Delabouglise, G.; Chenevier, B.; Gaskov, A.M.; Labeau, M. CO and NO2 gas sensitivity of nanocrystalline tin dioxide thin films doped with Pd, Ru and Rh. Mater. Sci. Eng. C 2002, 21, 105–111. [Google Scholar] [CrossRef]
- Liu, T.J.; Jin, Z.G.; Feng, L.R.; Wang, T. Conducting antimony-doped tin oxide films derived from stannous oxalate by aqueous sol–gel method. Appl. Surf. Sci. 2008, 254, 6547–6553. [Google Scholar] [CrossRef]
- Noonuruk, R.; Vittayakorn, N.; Mekprasart, W.; Sritharathikhun, J.; Pecharapa, W. Sb-Doped SnO2 Nanoparticles Synthesized by Sonochemical-Assisted Precipitation Process. J. Nanosci. Nanotechnol. 2015, 15, 2564–2569. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Zhai, Z.; Jin, G.; Jiang, Q.; Zhao, Y.; Luo, C.; Quan, L. Evaluation of depletion layer width and gas-sensing properties of antimony-doped tin oxide thin film sensors. Sens. Actuators B 2015, 220, 1354–1360. [Google Scholar] [CrossRef]
- Yao, P. Effects of Sb doping level on the properties of Ti/SnO2-Sb electrodes prepared using ultrasonic spray pyrolysis. Desalination 2011, 267, 170–174. [Google Scholar] [CrossRef]
- Fabbri, E.; Rabis, A.; Kötz, R.; Schmidt, T.J. Pt nanoparticles supported on Sb-doped SnO2 porous structures: Developments and issues. Phys. Chem. Chem. Phys. 2014, 16, 13672–13681. [Google Scholar] [CrossRef]
- Zaytsev, V.B.; Zhukova, A.A.; Rumyantseva, M.N.; Dobrovolsky, A.A.; Calvo, L.; Gaskov, A.M. Antimony doped whiskers of SnO2 grown from vapor phase. J. Cryst. Growth 2010, 312, 386–390. [Google Scholar] [CrossRef]
- Filatova, D.G.; Zhukova, A.A.; Podolko, E.V.; Rumyantseva, M.N.; Gaskov, A.M.; Bolshov, M.A. Determination of antimony and tin in tin dioxide whiskers by inductively coupled plasma mass spectrometry. J. Anal. Chem. 2012, 67, 950–954. [Google Scholar] [CrossRef]
- Turkin, A.A.; Chizhov, A.S.; Seregina, I.F.; Filatova, D.G.; Karpov, Y.A. Determination of gold and antimony in advanced materials based on tin dioxide using inductively coupled plasma mass spectrometry. Inorg. Mater. 2015, 51, 1420–1422. [Google Scholar] [CrossRef]
- Lopez-Navarrete, E.; Caballero, A.; Orera, V.M.; Lázaro, F.J.; Ocaña, M. Oxidation state and localization of chromium ions in Cr-doped cassiterite and Cr-doped malayaite. Acta Mater. 2003, 51, 2371–2381. [Google Scholar] [CrossRef]
- Antony, M.P.; Jha, A.; Tathavadkar, V. Alkali roasting of Indian chromite ores: Thermodynamic and kinetic considerations. Miner. Process. Extr. Metall. 2006, 115, 71–79. [Google Scholar] [CrossRef]
- Filatova, D.G.; Rumyantseva, M.N.; Gaskov, A.M. A Method of Determining the Doping Additives of Gold and Cobalt in Semiconductor Materials Based on Tin. Dioxide. Patent RU 2649136, 29 March 2018. [Google Scholar]
- Vereda Alonso, E.; García de Torres, A.; Siles Cordero, M.T.; Cano Pavón, J.M. Multivariate optimization of the synthesis and of the microwave dissolution of biomorphic silicon carbide ceramics. Microchem. J. 2011, 97, 101–108. [Google Scholar] [CrossRef]
- Filatova, D.G.; Kutukov, P.S.; Rumyantseva, M.N.; Gaskov, A.M. Study of platinum and palladium distribution in advanced materials based on tin dioxide using inductively coupled plasma mass spectrometry (ICP-MS). Inorg. Mater. 2019, 55, 1399–1402. [Google Scholar] [CrossRef]
- Klockenkämper, R.; von Bohlen, A. Survey of sampling techniques for solids suitable for microanalysis by total-reflection X-ray fluorescence spectrometry. J. Anal. At. Spectrom. 1999, 14, 571–576. [Google Scholar] [CrossRef]
- Hellin, D.; De Gendt, S.; Valckx, N.; Mertens, P.W.; Vinckier, C. Trends in total reflection X-ray fluorescence spectrometry for metallic contamination control in semiconductor nanotechnology. Spectrochim. Acta B 2006, 61, 496–514. [Google Scholar] [CrossRef]
- Fernández Ruiz, R. Three empirical cases of the deposition morphology influence in the analytical quality of direct solid suspension measurements by total-reflection X-ray fluorescence. Spectrochim. Acta B 2009, 64, 672–678. [Google Scholar] [CrossRef]
- Filatova, D.G.; Alov, N.V.; Marikutsa, A.V.; Seregina, I.F. Ruthenium and palladium determination in advanced materials based on tin dioxide by mass spectrometry with inductively coupled plasma and total reflection X-ray fluorescence. Mosc. Univ. Chem. Bull. 2015, 70, 234–236. [Google Scholar] [CrossRef]
- Filatova, D.G.; Alov, N.V.; Sharanov, P.Y.; Marikutsa, A.V. Detecting gold in semiconducting advanced nanomaterials based on tin oxide via total reflection X-ray fluorescence analysis. Mosc. Univ. Chem. Bull. 2015, 70, 60–62. [Google Scholar] [CrossRef]
- Filatova, D.G.; Bogdanova, A.P.; Krivetskiy, V.V.; Penkina, T.N.; Rumyantseva, M.N. Quantification of Si dopant in β-Ga2O3-based semiconductor gas sensors by total reflection X-ray fluorescence spectroscopy (TXRF). Ind. Lab. Diagn. Mater. 2022, 88, 5–9. (In Russian) [Google Scholar]
| Method | Working Principle | LOD | Analytical Spot Size | Depth of Analysis | Information |
|---|---|---|---|---|---|
| Energy-dispersive X-ray spectroscopy (EDX) | Generation of characteristic X-rays from a specimen through the electron beam | 0.1–1 at% | ≥0.1 μm (combined with SEM) 0.05–0.2 nm (combined with STEM) | 0.1–3 μm | Gross elemental composition except for light elements; visualizing element distribution (EDX mapping) |
| X-ray diffraction (XRD) | Interference of monochromatic X-rays | ~1% by volume | ~20 Å to ~30 µm depending on material properties and X-ray incidence angle | Phase composition; determining the crystallographic properties of a sample | |
| Raman spectroscopy | Scattering of incident light at an energy shifted by the vibrational energy (hν) of the molecule | 100 ppb | ~1 µm | <10 µm | Chemical bonding/molecular information; phase composition; surface adsorbates |
| X-ray photoelectron spectroscopy (XPS) | The simultaneous measurement of kinetic energy and the number of electrons escaping when the sample is irradiated with a beam of X-ray radiation under high vacuum | 0.1–1 at% | ~10 μm | 10 nm | Surface elemental composition; element oxidation state |
| Inductively coupled plasma mass spectrometry (ICP MS) | Ionization of atoms in plasma and mass selection | 1–100 ppt | volume | volume | Determining trace elements (<1%) present in a sample |
| Total reflection X-ray fluorescence (TXRF) | Energy-dispersive X-ray fluorescence spectrometry on a thin layer of the sample | ng–pg | size of the collimator of the detector (8 mm diameter) | volume | Gross elemental composition except for light elements; determining trace elements (<1%) present in a sample |
| High-resolution continuum source graphite furnace atomic absorption spectrometry (HR CS GFAAS) | Absorption of ground state-atoms of UV or visible light in the gaseous state | 10−6–10−1% | - | volume | Determining trace elements (<1%) present in a sample |
| Material | Synthesis Method | Ref. |
|---|---|---|
| SnO2/TiO2 nanobelts | I: Hydrothermal (TiO2); II: hydrothermal (SnO2) | [136] |
| SnO2/TiO2 nanotubes | I: Electrochemical anodization (TiO2); II: atomic layer deposition (SnO2) | [137] |
| SnO2/TiO2 nanopowders | I: Sol–gel (TiO2); II: sol–gel (SnO2) | [138] |
| TiO2/SnO2 nanosheets | I: Hydrothermal (SnO2); II: pulsed laser deposition (TiO2) | [139] |
| TiO2/SnO2 nanofibers | Coaxial electrospinning | [140] |
| TiO2/SnO2 thin films | I: Magnetron sputtering (SnO2); II: Langmuir–Blodgett (TiO2) | [141] |
| V2O5/SnO2 nanowires | I: Vapor–liquid–solid (SnO2); II: atomic layer deposition (V2O5) | [142] |
| Cr2O3/SnO2 nanofibers | Coaxial electrospinning | [143] |
| Cr2O3/SnO2 hollow spheres | I: Ultrasonic spray pyrolysis (SnO2); II: E-beam evaporation (Cr2O3) | [144] |
| Cr2O3/SnO2 nanocomposites | I: Sol–gel (SnO2); II: electrodeposition (Cr2O3) | [145] |
| Cr2O3/SnO2 nanofibers | I: Electrospinning (SnO2); II: impregnation (Cr2O3) | [146] |
| MnO2/SnO2 nanocomposites | Chemical precipitation with subsequent thermal annealing | [147] |
| SnO2/Mn3O4 nanocomposites | I: Plasmo-chemical deposition (Mn3O4); II: RF sputtering (SnO2) | [148] |
| Mn3O4/SnO2 nanocomposites | I: Citrate sol–gel (SnO2); II: impregnation (Mn3O4) | [149] |
| Fe2O3/SnO2 core–shell | I: Chemical precipitation (SnO2); II: sol–gel (Fe2O3) | [150] |
| Fe2O3/SnO2 hollow spheres | I: Hydrothermal (SnO2); II: hydrothermal (Fe2O3) | [151] |
| Fe2O3/SnO2 nanocomposites | Citrate sol–gel | [152] |
| Fe2O3/SnO2 nanorods | I: Hydrothermal (SnO2); II: ionic layer adsorption reaction (Fe2O3) | [153] |
| Fe2O3/SnO2 nanofibers | I: Electrospinning (SnO2); II: hard-template method (Fe2O3) | [154] |
| Fe2O3/SnO2 nanofibers | Electrospinning | [155] |
| Co3O4/SnO2 nanocomposites | I: Chemical precipitation (SnO2); II: impregnation (Co3O4) | [63] |
| Co3O4/SnO2 nanowires | I: Vapor–liquid–solid (SnO2); II: DC sputtering (Co3O4) | [156] |
| Co3O4/SnO2 nanowires | I: Vapor–liquid–solid (SnO2); II: sol–gel (Co3O4) | [157] |
| NiO/SnO2 nanowires | I: Vapor–liquid–solid (SnO2); II: atomic layer deposition (NiO) | [158] |
| NiO/SnO2 nanosheets | I: Hydrothermal (SnO2); II: hydrothermal (NiO) | [159] |
| NiO/SnO2 nanocomposites | I: Chemical precipitation (SnO2); II: sol–gel (NiO) | [160] |
| NiO/SnO2 hollow spheres | I: Hydrothermal (SnO2); II: pulsed laser deposition (NiO) | [161] |
| CuO/SnO2 hollow nanofibers | Electrospinning | [162] |
| CuO/SnO2 thin films | I: RF magnetron sputtering (SnO2); II: RF magnetron sputtering (CuO) | [163] |
| ZnO/SnO2 rootstock/scion | I: Vapor–liquid–solid (SnO2 or ZnO); II: vapor–liquid–solid (ZnO or SnO2) | [164] |
| ZnO/SnO2 nanofibers | Electrospinning | [165] |
| ZnO/SnO2 nanoheterostructures | I: Hydrothermal (SnO2); II: chemical bath deposition (ZnO) | [166] |
| SnO2/Nb2O5 core–shell | I: Hydrothermal (Nb2O5); II: atomic layer deposition (SnO2) | [167] |
| Nb2O5/SnO2 nanocomposites | I: Spin coating (SnO2 nanosheets); II: hydrothermal (Nb2O5 nanorods) | [168] |
| Nb2O5/SnO2 nanocomposites | I: Chemical precipitation (SnO2); II: impregnation (Nb2O5) | [115] |
| RuO2/SnO2 nanopowders | I: Chemical precipitation (SnO2); II: impregnation (RuO2) | [169] |
| RuO2/SnO2 nanopowders | I: Chemical precipitation (SnO2); II: deposition–precipitation (RuO2) | [120] |
| Material | Synthesis Method | Ref. |
|---|---|---|
| SnO2(Sb) thin films | Sol–gel spin coating | [83] |
| SnO2(Sb) nanopowders | Hydrothermal | [84] |
| SnO2(Sb) nanowires | Sb-ion implantation | [85] |
| (SnO2:TiO2) thin films | Pulsed laser deposition | [87] |
| (SnO2:TiO2) nanopowders | Co-precipitation | [88] |
| (SnO2:TiO2) nanopowders | Flame spray pyrolysis | [89] |
| (Cr2O3/SnO2) nanopowders | Flame spray pyrolysis | [65] |
| SnO2(Mn) nanobelts | Thermal evaporation | [91] |
| SnO2(Mn) thin films | Spray pyrolysis | [92] |
| SnO2(Mn) nanopowders | Co-precipitation | [93] |
| SnO2(Fe) thin films | Spray pyrolysis | [96] |
| SnO2(Fe) nanopowders | Co-precipitation, hydrothermal | [95] |
| SnO2(Fe) nanopowders | Co-precipitation, hydrothermal | [97] |
| SnO2(Co) thin films | Spray pyrolysis | [96] |
| SnO2(Co) thin films | Spray pyrolysis | [100] |
| SnO2(Co) nanopowders | Co-precipitation, hydrothermal | [99] |
| SnO2(Co) inverse opal | Ultrasonic nebulizing deposition with a self-assembly template | [101] |
| SnO2(Ni) thin films | Spray pyrolysis | [96] |
| SnO2(Ni) nanopowders | Co-precipitation, hydrothermal | [95] |
| SnO2(Ni) porous structures | Co-precipitation, hydrothermal | [103] |
| SnO2(Ni) nanorods | Co-precipitation, hydrothermal | [104] |
| SnO2(Ni) nanopowders | Co-precipitation, microwave treatment | [105] |
| SnO2(Cu) porous structures | Surfactant-assisted co-precipitation | [107] |
| SnO2(Cu)/rGO nanocomposites | Solvothermal | [108] |
| SnO2(Cu) nanopowders | Co-precipitation | [109] |
| SnO2(Zn) thin films | Spray pyrolysis | [112] |
| SnO2(Zn) nanostructures | Co-precipitation, hydrothermal | [110] |
| SnO2(Nb) nanopowders | Co-precipitation, hydrothermal | [114] |
| SnO2(Nb) nanopowders | Flame spray pyrolysis | [113] |
| SnO2(Ru) nanopowders | Co-precipitation | [120] |
| SnO2(Ru) nanofibers | Electrospinning | [117] |
| SnO2(Ru) nanotubes | Electrospinning | [118] |
| SnO2(Ru) thin films | Spray pyrolysis | [119] |
| Additive on SnO2 Surface | Reagent, Treatment Conditions | Analysis Method |
|---|---|---|
| Sb3+ | 10% citric acid, heating for 30 min in an ultrasonic bath. | ICP MS, TXRF |
| Cr3+ | 1% KSCN in 25% NH3 with the formation of NH4[Cr(NCS)4(NH3)2]. | ICP MS, TXRF |
| Co2+, Co3+ | HNO3:HCl = 1:3. | ICP MS, TXRF |
| PtOx, PdOx, Au0 | HNO3:HCl = 1:3. | ICP MS |
| RuOx | Oxalic acid or ascorbic acid (pH = 3), HNO3:HCl = 1:3. | ICP MS |
| MnOx | Oxalic acid (pH = 3). | ICP MS, TXRF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filatova, D.; Rumyantseva, M. Additives in Nanocrystalline Tin Dioxide: Recent Progress in the Characterization of Materials for Gas Sensor Applications. Materials 2023, 16, 6733. https://doi.org/10.3390/ma16206733
Filatova D, Rumyantseva M. Additives in Nanocrystalline Tin Dioxide: Recent Progress in the Characterization of Materials for Gas Sensor Applications. Materials. 2023; 16(20):6733. https://doi.org/10.3390/ma16206733
Chicago/Turabian StyleFilatova, Darya, and Marina Rumyantseva. 2023. "Additives in Nanocrystalline Tin Dioxide: Recent Progress in the Characterization of Materials for Gas Sensor Applications" Materials 16, no. 20: 6733. https://doi.org/10.3390/ma16206733
APA StyleFilatova, D., & Rumyantseva, M. (2023). Additives in Nanocrystalline Tin Dioxide: Recent Progress in the Characterization of Materials for Gas Sensor Applications. Materials, 16(20), 6733. https://doi.org/10.3390/ma16206733







