Development of Polyhydroxybutyrate-Based Packaging Films and Methods to Their Ultrasonic Welding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plasticized PHB Films
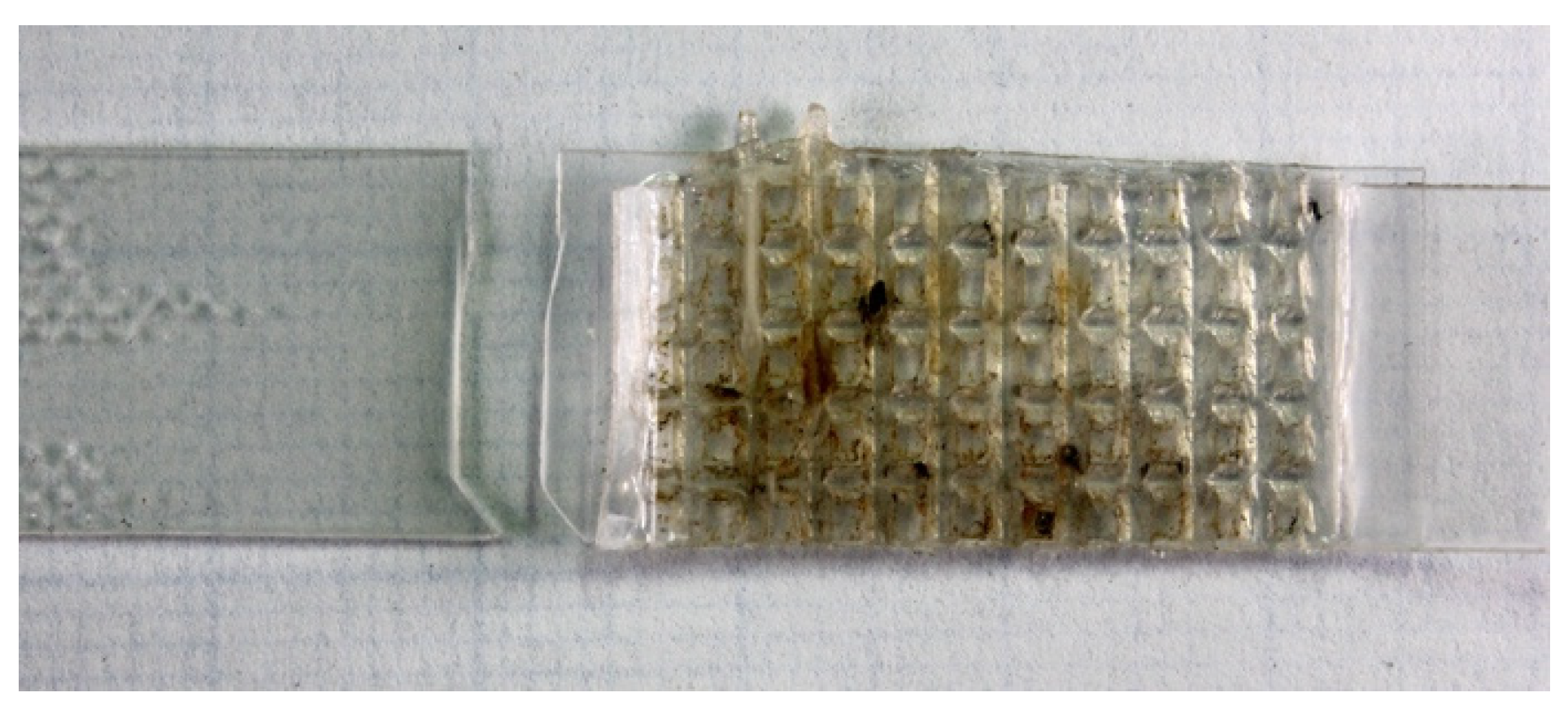
2.3. Welding
2.4. Characterization Methods
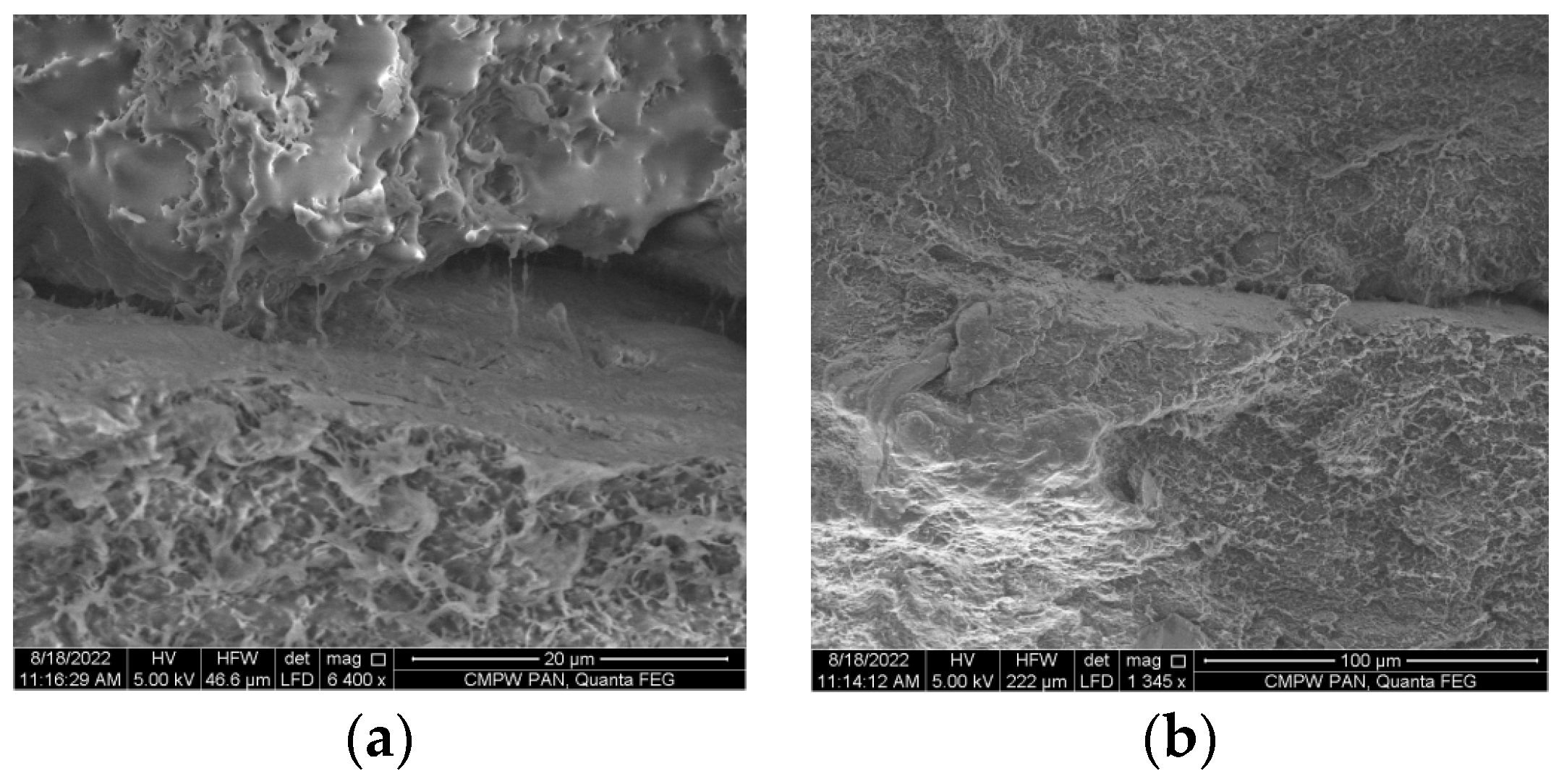
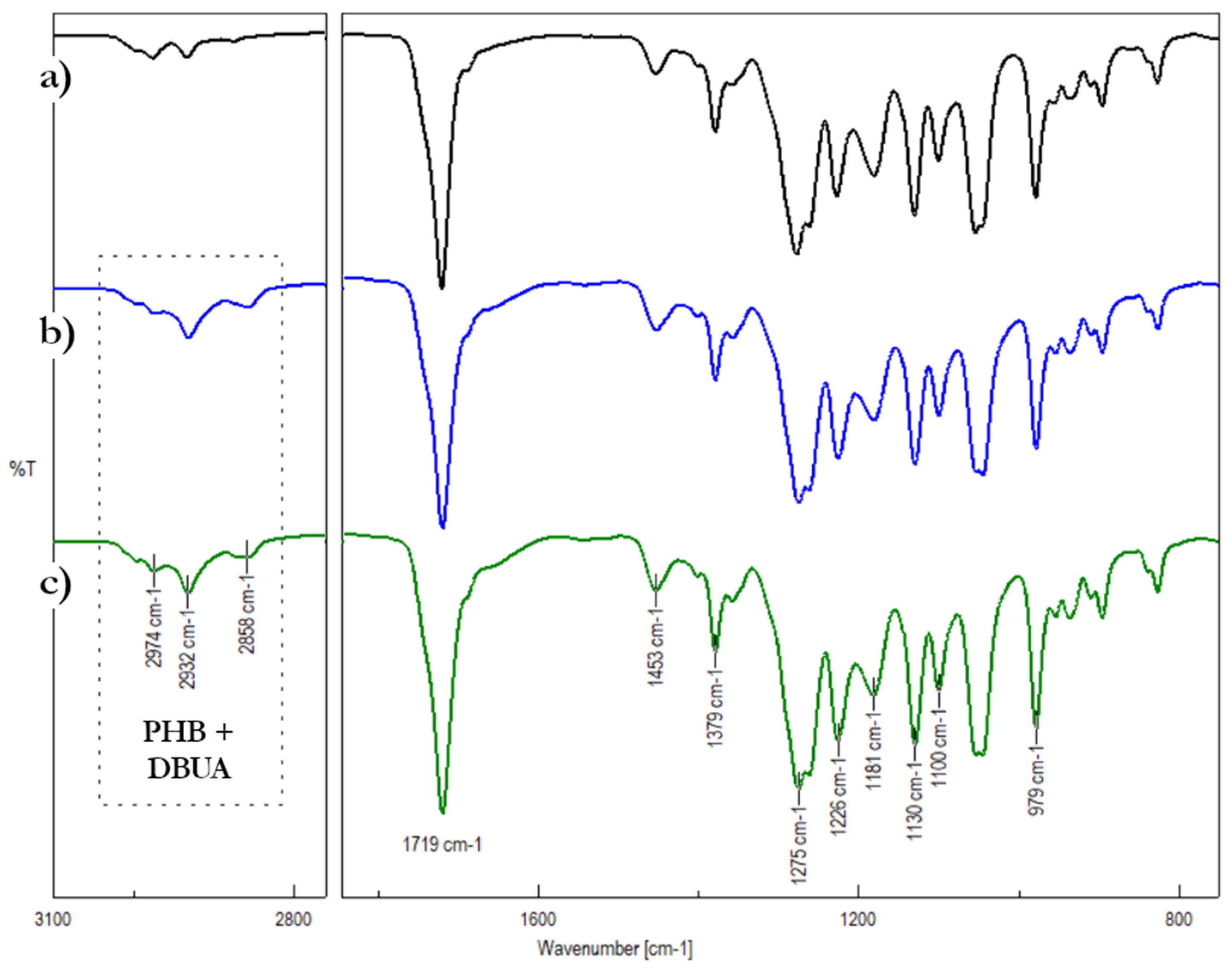
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panaitescu, D.M.; Ionita, E.R.; Nicolae, C.A.; Gabor, A.R.; Ionita, M.D.; Trusca, R.; Lixandru, B.E.; Codita, I.; Dinescu, G. Poly(3-Hydroxybutyrate) Modified by Nanocellulose and Plasma Treatment for Packaging Applications. Polymers 2018, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Nassira, H.; Sánchez-Ferrer, A.; Adamcik, J.; Handschin, S.; Mahdavi, H.; Taheri Qazvini, N.; Mezzenga, R. Gelatin–Graphene Nanocomposites with Ultralow Electrical Percolation Threshold. Adv. Mater. 2016, 28, 6914–6920. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.J. Biological Degradation of Synthetic Polyesters-Enzymes as Potential Catalysts for Polyester Recycling. Process Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Frone, A.N.; Nicolae, C.A.; Eremia, M.C.; Tofan, V.; Ghiurea, M.; Chiulan, I.; Radu, E.; Damian, C.M.; Panaitescu, D.M. Low Molecular Weight and Polymeric Modifiers as Toughening Agents in Poly(3-hydroxybutyrate) Films. Polymers 2020, 12, 2446. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ko, Y.G.; Deri, F. Biodegradable Polymer Blends and Composites: An Overview. Polym. Sci.-Ser. A 2014, 56, 812–829. [Google Scholar] [CrossRef]
- de França, J.O.C.; da Silva Valadares, D.; Paiva, M.F.; Dias, S.C.L.; Dias, J.A. Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review. Polymers 2022, 14, 2317. [Google Scholar] [CrossRef]
- Evangelista, A.C.J.; de Morais, J.F.; Tam, V.; Soomro, M.; Di Gregorio, L.T.; Haddad, A.N. Evaluation of Carbon Nanotube Incorporation in Cementitious Composite Materials. Materials 2019, 12, 1504. [Google Scholar] [CrossRef]
- Vasu, D.; Keyan, A.K.; Moorthi, P.; Sakthinathan, S.; You, Y.; Chiu, T.W. 2D-Layered Bi-Functional Direct Solid-Z-Scheme Heterogenous Vanadium and Oxygen Doped Graphitic Carbon Nitride Single Layered Nanosheet Catalysis for Detection and Photocatalytic Removal of Toxic Heavy Metal. Mater. Chem. Phys. 2023, 295, 127065. [Google Scholar] [CrossRef]
- Torres, M.G.; Galego Fernández, N.; Ortiz Del Toro, P.; Paneque, M.R. Ciencias Nucleares Raman Spectroscopy of Poly(3-hydroxybutryrate) Modified with Poly (vinyl acetate) by Radiation-Induced Copolymerization. Nucleus 2007, 42, 42–45. [Google Scholar]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent Advances in the Development of Biodegradable PHB-Based Toughening Materials: Approaches, Advantages and Applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Samek, O.; Obruča, S.; Šiler, M.; Sedláček, P.; Benešová, P.; Kučera, D.; Márova, I.; Ježek, J.; Bernatová, S.; Zemánek, P. Quantitative Raman Spectroscopy Analysis of Polyhydroxyalkanoates Produced by Cupriavidus Necator H16. Sensors 2016, 16, 1808. [Google Scholar] [CrossRef]
- Nosal, H.; Moser, K.; Warzała, M.; Holzer, A.; Stańczyk, D.; Sabura, E. Selected Fatty Acids Esters as Potential PHB-V Bioplasticizers: Effect on Mechanical Properties of the Polymer. J. Polym. Environ. 2021, 29, 38–53. [Google Scholar] [CrossRef]
- Oprică, M.G.; Uşurelu, C.D.; Frone, A.N.; Gabor, A.R.; Nicolae, C.A.; Vasile, V.; Panaitescu, D.M. Opposite Roles of Bacterial Cellulose Nanofibers and Foaming Agent in Polyhydroxyalkanoate-Based Materials. Polymers 2022, 14, 5358. [Google Scholar] [CrossRef] [PubMed]
- Rogalsky, S.; Tarasyuk, O.; Vashchuk, A.; Davydenko, V.; Motrunich, S.; Cherniavska, T.; Papeikin, O.; Rogalsky, S.; Tarasyuk, O.; Vashchuk, A.; et al. Synthesis and Evaluation of N,N-Dibutylundecenamide as New Eco-Friendly Plasticizer for Polyvinyl Chloride. J. Mater. Sci. 2022, 57, 6102–6114. [Google Scholar] [CrossRef]
- Barbosa, J.L.; Perin, G.B.; Felisberti, M.I. Plasticization of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with an Oligomeric Polyester: Miscibility and Effect of the Microstructure and Plasticizer Distribution on Thermal and Mechanical Properties. ACS Omega 2021, 6, 3278–3290. [Google Scholar] [CrossRef]
- Sikorska, W.; Rydz, J.; Wolna-Stypka, K.; Musioł, M.; Adamus, G.; Kwiecień, I.; Janeczek, H.; Duale, K.; Kowalczuk, M. Forensic Engineering of Advanced Polymeric Materials-Part V: Prediction Studies of Aliphatic-Aromatic Copolyester and Polylactide Commercial Blends in View of Potential Applications as Compostable Cosmetic Packages. Polymers 2017, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Junior, R.R.; dos Santos, C.A.S.; Ito, N.M.; Suqueira, A.N.; Lackner, M.; dos Santos, D.J. PHB Processability and Property Improvement with Linear-Chain Polyester Oligomers Used as Plasticizers. Polymers 2022, 14, 4197. [Google Scholar] [CrossRef]
- Klinstein, L.; Frantz, J.; Grewell, D.; Lebron, K. ANTEC® Anaheim 2017. In Proceedings of the Plastics Technology Conference, Anaheim, CA, USA, 8–10 May 2017; pp. 474–477. [Google Scholar]
- Paul, U.C.; Fragouli, D.; Bayer, I.S.; Zych, A.; Athanassiou, A. Effect of Green Plasticizer on the Performance of Microcrystalline Cellulose/Polylactic Acid Biocomposites. ACS Appl. Polym. Mater. 2021, 3, 3071–3081. [Google Scholar] [CrossRef]
- Voronova, M.I.; Gurina, D.L.; Surov, O.V. Polycaprolactone Polymer Mixtures Reinforced by Cellulose Nanocrystals: Experimental and Simulation Studies. Polym. J. 2022, 14, 340–376. [Google Scholar]
- Danch, A.; Karolus, M.; Burian, A. Structural Studies of Poly(4-Methyl-1-Pentene) Dendrites. Polymers 2000, 4240, 33–37. [Google Scholar]
- Karolus, M.; Łagiewka, E. Crystallite Size and Lattice Strain in Nanocrystalline Ni-Mo Alloys Studied by Rietveld Refinement. J. Alloys Compd. 2004, 367, 235–238. [Google Scholar] [CrossRef]
- Marcoaldi, C.; Pardo-Figuerez, M.; Prieto, C.; Arnal, C.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Electrospun Multilayered Films Based on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), Copolyamide 1010/1014, and Electrosprayed Nanostructured Silica. Nanomaterials 2023, 13, 972. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line Profiles of Neutron Powder-Diffraction Peaks for Structure Refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Min, J.; Park, J.-H.; Yoon, H.H.; Choy, Y.B. Ultrasonic Welding Method to Fabricate Polymer Microstructure Encapsulating Protein with Minimum Damage. Macromol. Res. 2008, 16, 570–573. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.W.; Wei, Y.H.; Wu, H.S.; Wang, S.S. Isolation and Purification of Bacterial Poly(3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- Lorenzo, F.R.; Lorenzo, M.P.; Castilla, L.H.; Álvarez Rodríguez, J.A.; Iglesias, S.; Gómez, S.; Fernández Montenegro, J.M.; Rueda, E.; Diez-Montero, R.; Garcia, J.; et al. Monitoring PHB Production in Synechocystis sp. with Hyperspectral Images. Water Sci. Technol. 2022, 86, 211–226. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Riemschneider, M. Effect of Melt Processing on Crystallization Behavior and Rheology of Poly(3-hydroxybutyrate) (PHB) and Its Blends. Macromol. Mater. Eng. 2002, 287, 363–372. [Google Scholar] [CrossRef]
- Yamada, S.; Wang, Y.; Asakawa, N.; Yoshie, N.; Inoue, Y. Crystalline Structural Change of Bacterial Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Narrow Compositional Distribution. Macromolecules 2001, 34, 4659–4661. [Google Scholar] [CrossRef]
- Izumi, C.M.S.; Temperini, M.L.A. FT-Raman Investigation of Biodegradable Polymers: Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Vib. Spectrosc. 2010, 54, 127–132. [Google Scholar] [CrossRef]
- Doppler, P.; Gasser, C.; Kriechbaum, R.; Ferizi, A.; Spadiut, O. In Situ Quantification of Polyhydroxybutyrate in Photobioreactor Cultivations of Synechocystis sp. Using an Ultrasound-Enhanced Atr-Ftir Spectroscopy Probe. Bioengineering 2021, 8, 129. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.N.; Leckie, J.O. Effect of Membrane Chemistry and Coating Layer on Physiochemical Properties of Thin Film Composite Polyamide RO and NF Membranes. I. FTIR and XPS Characterization of Polyamide and Coating Layer Chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of Membrane Chemistry and Coating Layer on Physiochemical Properties of Thin Film Composite Polyamide RO and NF Membranes II. Membrane Physiochemical Properties and Their Dependence on Polyamide and Coating Layers. Desalination 2009, 242, 168–182. [Google Scholar] [CrossRef]
- Bloembergen, S.; Holden, D.A.; Bluhm, T.L.; Hamer, G.K.; Marchessault, R.H. Isodimorphism in Synthetic Poly(/3-hydroxybutyrate-co-/3-hydroxyvalerate): Stereoregular Copolyesters from Racemic/9-Lactones. Macromolecules 1989, 22, 1663–1669. [Google Scholar] [CrossRef]
- Zaccone, M.; Patel, M.K.; De Brauwer, L.; Nair, R.; Montalbano, M.L.; Monti, M.; Oksman, K. Influence of Chitin Nanocrystals on the Crystallinity and Mechanical Properties of Poly(hydroxybutyrate) Biopolymer. Polymers 2022, 14, 562. [Google Scholar] [CrossRef] [PubMed]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, Characterization and Antimicrobial Properties of Electrospun Polylactide Films Containing Allium ursinum L. Extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Schawea, J.E.K.; Bergmannb, E. Thermochimica Acts ELSEVIER Investigation of Polymer Melting by Temperature Modulated Differential Scanning Calorimetry and It’s Description Using Kinetic Models. Thermochim. Acta 1997, 304, 179–186. [Google Scholar] [CrossRef]
- Kovačević, Z.; Flinčec Grgac, S.; Bischof, S. Progress in Biodegradable Flame Retardant Nano-Biocomposites. Polymers 2021, 13, 741. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and Potential Application of Short-Chain-Length Polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef]
- Hong, J.K.; Kim, S.B.; Lyou, E.S.; Lee, T.K. Microbial Phenomics Linking the Phenotype to Fonction: The Potential of Raman Spectroscopy. J. Microbiol. 2021, 59, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.J.; Robbins, K.E.; Kelly, C.A. Secondary Crystallisation and Degradation in P(3HB-Co-3HV): An Assessment of Long-Term Stability. Polym. J. 2018, 50, 365–373. [Google Scholar] [CrossRef]
- Quispe, M.M.; Lopez, O.V.; Boina, D.A.; Stumbé, J.F.; Villar, M.A. Glycerol-Based Additives of Poly(3-hydroxybutyrate) Films. Polym. Test. 2021, 93, 107005. [Google Scholar] [CrossRef]
- Schawe, J.E.K.; Hütter, T.; Heitz, C.; Alig, I.; Lellinger, D. Stochastic Temperature Modulation: A New Technique in Temperature-Modulated DSC. Thermochim. Acta 2006, 446, 147–155. [Google Scholar] [CrossRef]
- Nanocomposites, P.T.; Antunes, A.; Popelka, A.; Aljarod, O.; Hassan, M.K.; Kasak, P.; Luyt, A.S. Accelerated Weathering Effects on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and PHBV/TiO2 Nanocomposites. Polymers 2020, 12, 1743. [Google Scholar]
- Panaitescu, D.M.; Nicolae, C.A.; Frone, A.N.; Chiulan, I.; Stanescu, P.O.; Draghici, C.; Iorga, M.; Mihailescu, M. Plasticized Poly(3-Hydroxybutyrate) with Improved Melt Processing and Balanced Properties. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Version, D. Crystallization phenomena in bacterial poly [(R)-3-hydroxybutyrate]: 2. Embrittlement and rejuvenation. Polymer 1993, 34, 4089–4094. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and Morphology of a Bacterial Thermoplastic: Poly-3-Hyd Roxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]



| Component | Structure | Characteristics |
|---|---|---|
| Polyhydroxybutyrate (PHB) | 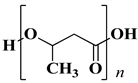 | d = 1.25 g/cm3 Tmelt = 170–176 °C |
| N,N-dibutylundecenoylamide (DBUA) | 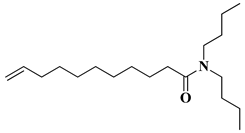 | TΔm = 5% = 219 °C |
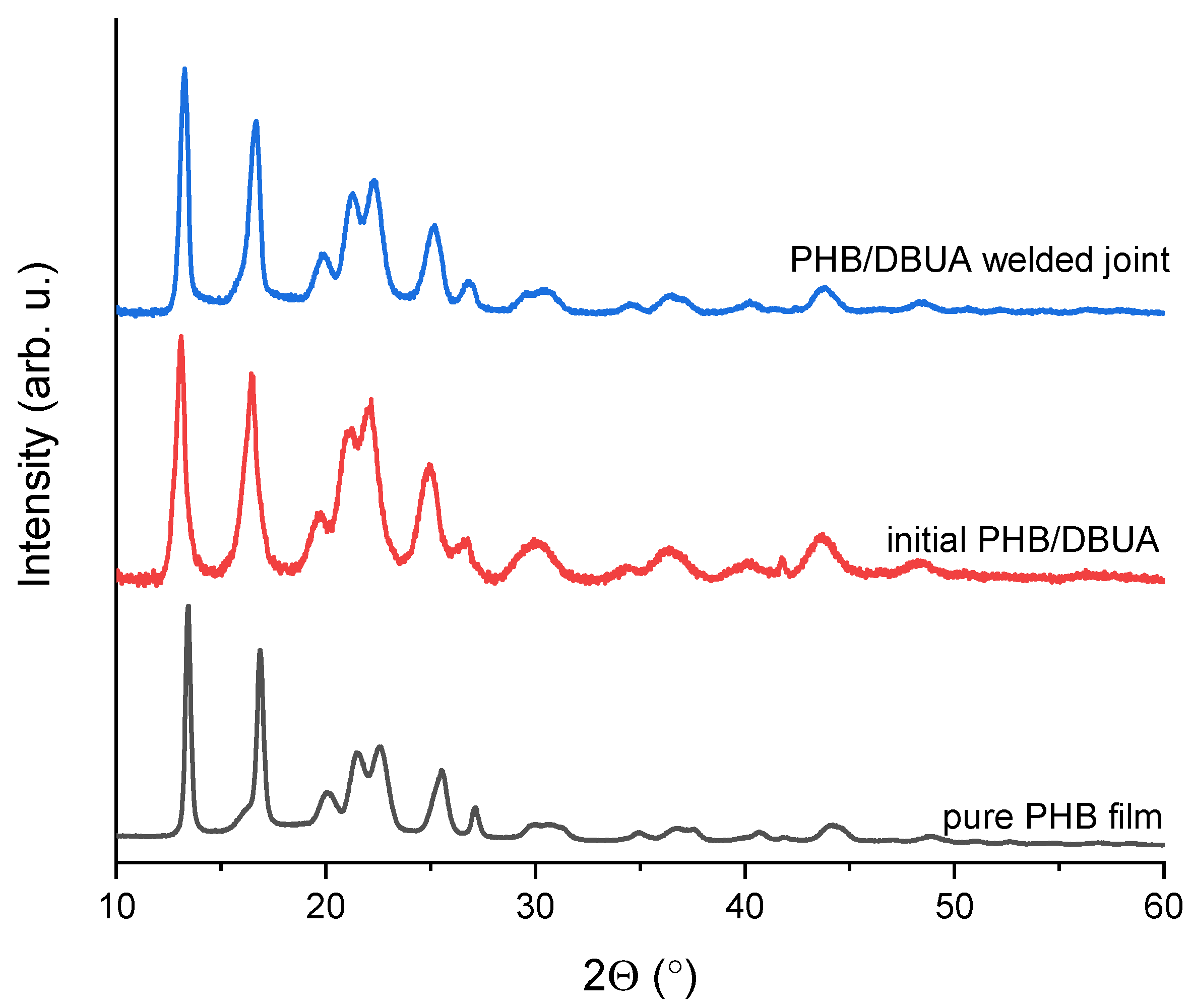
| Model Parameters, ICDD, Card No. 00-068-1411 | Pure PHB Film | Initial PHB/DBUA Film | PHB/DBUA Welded Joint | |
|---|---|---|---|---|
| Lattice parameters, Å | a = 5.73, | a = 5.682, | a = 5.679, | a = 5.678, |
| b = 13.098, | b = 13.046, | b = 13.058, | b = 13.063, | |
| c = 5.958 | c = 5.946 | c = 5.95 | c = 5.968 | |
| Crystallite size, nm | - | 89 ± 10 | 9.7 ± 0.3 | 100 ± 26 |
| Lattice strain, % | - | 2.98 ± 0.04 | 0.84 ± 0.22 | 3.55 ± 0.08 |
| Biopolymer System | Crystallization from Melt | Cold Crystallization | Melting | Xc [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TMc [°C] | ΔHMc J/g] | TCc [°C] | ΔHCc [J/g] | Tm1 [°C] | L [Å] | Tm2 [°C] | L [Å] | ΔHm [J/g] | ||
| Pure PHB | 92.2 | 82.9 | 99.8 | 8.8 | - | - | 173.7 | 96.4 | 101.2 | 69.4 |
| PHB/DBUA initial | 53.6 | 43.9 | 85.8 | 11.3 | 136.8 | 10.1 | 158.4 | 25.1 | 64.2 | 62.8 |
| PHB/DBUA welded | 61.1 | 49.2 | 86.8 | 13.4 | 143.4 | 18.1 | 161.3 | 27.1 | 69.6 | 68.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talaniuk, V.; Godzierz, M.; Vashchuk, A.; Iurhenko, M.; Chaber, P.; Sikorska, W.; Kobyliukh, A.; Demchenko, V.; Rogalsky, S.; Szeluga, U.; et al. Development of Polyhydroxybutyrate-Based Packaging Films and Methods to Their Ultrasonic Welding. Materials 2023, 16, 6617. https://doi.org/10.3390/ma16206617
Talaniuk V, Godzierz M, Vashchuk A, Iurhenko M, Chaber P, Sikorska W, Kobyliukh A, Demchenko V, Rogalsky S, Szeluga U, et al. Development of Polyhydroxybutyrate-Based Packaging Films and Methods to Their Ultrasonic Welding. Materials. 2023; 16(20):6617. https://doi.org/10.3390/ma16206617
Chicago/Turabian StyleTalaniuk, Viktoriia, Marcin Godzierz, Alina Vashchuk, Maksym Iurhenko, Paweł Chaber, Wanda Sikorska, Anastasiia Kobyliukh, Valeriy Demchenko, Sergiy Rogalsky, Urszula Szeluga, and et al. 2023. "Development of Polyhydroxybutyrate-Based Packaging Films and Methods to Their Ultrasonic Welding" Materials 16, no. 20: 6617. https://doi.org/10.3390/ma16206617
APA StyleTalaniuk, V., Godzierz, M., Vashchuk, A., Iurhenko, M., Chaber, P., Sikorska, W., Kobyliukh, A., Demchenko, V., Rogalsky, S., Szeluga, U., & Adamus, G. (2023). Development of Polyhydroxybutyrate-Based Packaging Films and Methods to Their Ultrasonic Welding. Materials, 16(20), 6617. https://doi.org/10.3390/ma16206617








