In-Situ Al-Mg Alloy Base Composite Reinforced by Oxides and Intermetallic Compounds Resulted from Decomposition of ZrW2O8 during Multipass Friction Stir Processing
Abstract
1. Introduction
2. Materials and Methods
3. Results
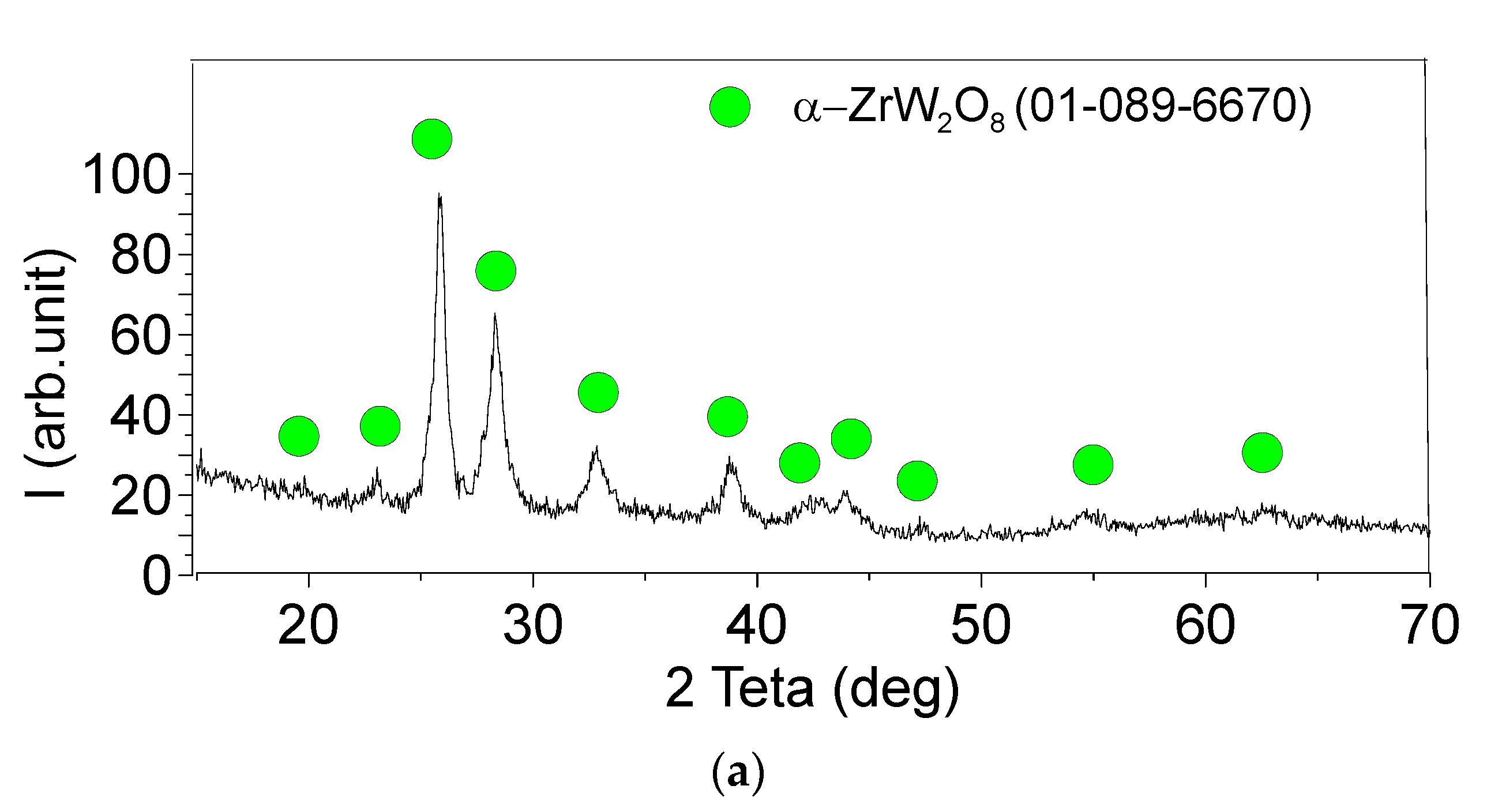
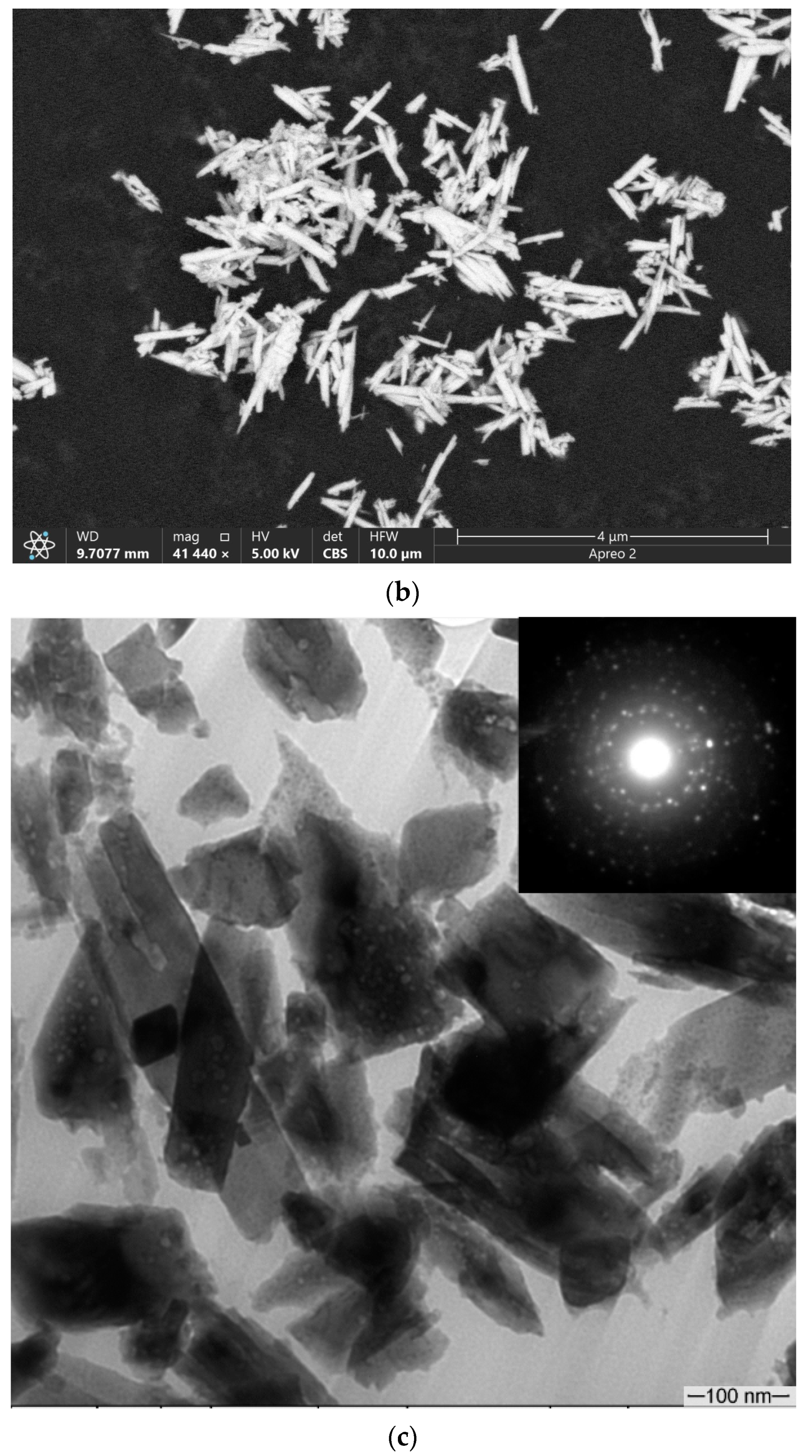
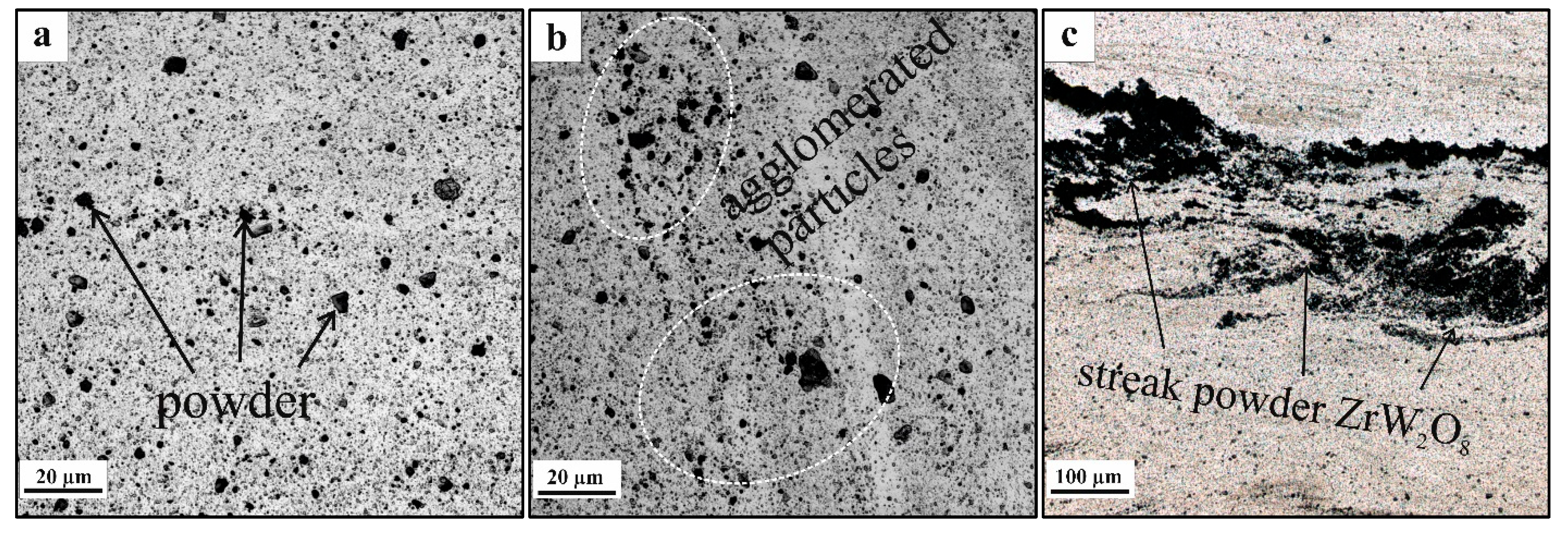
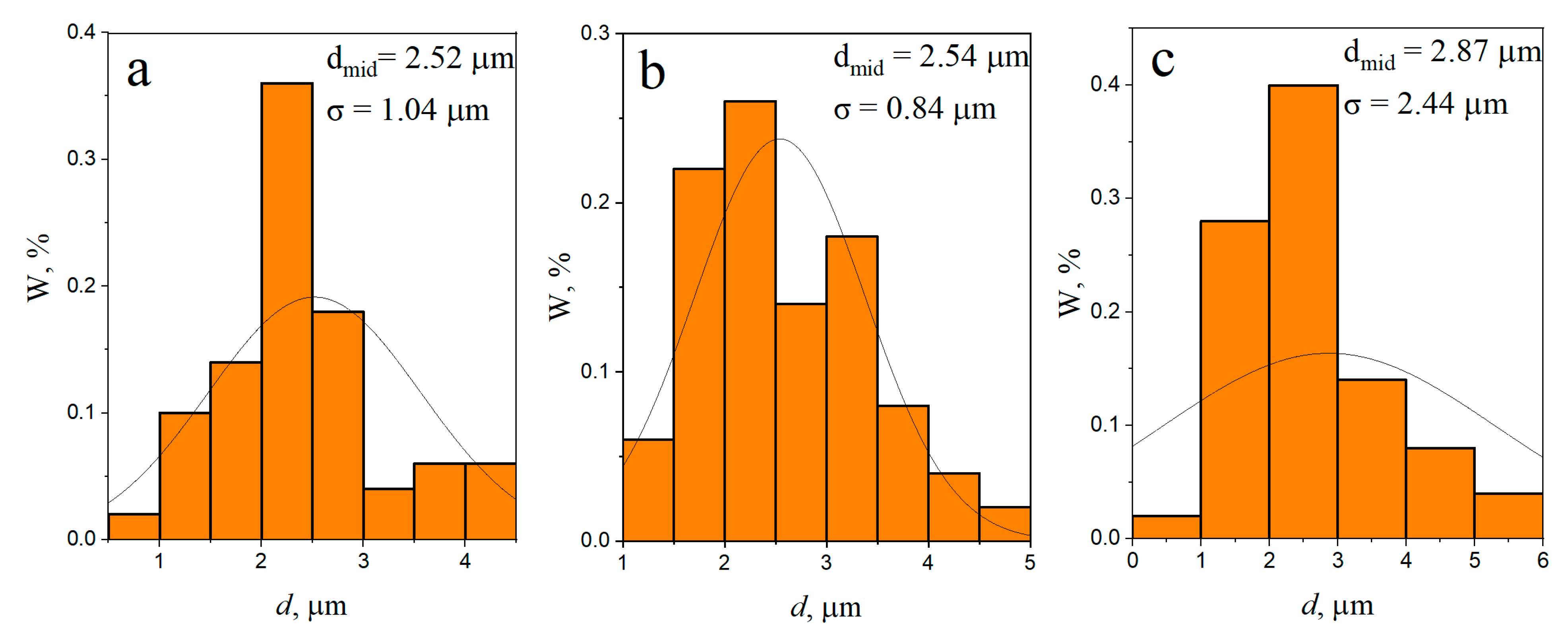
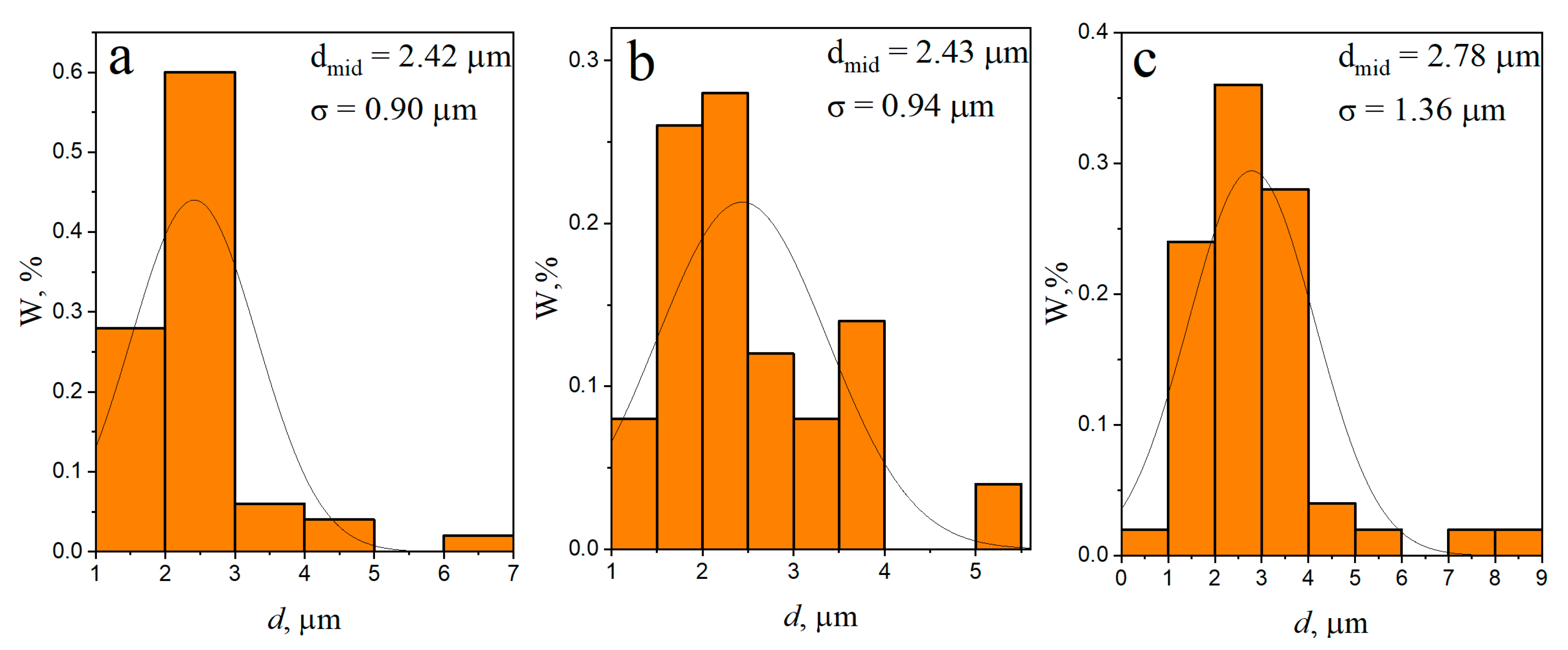
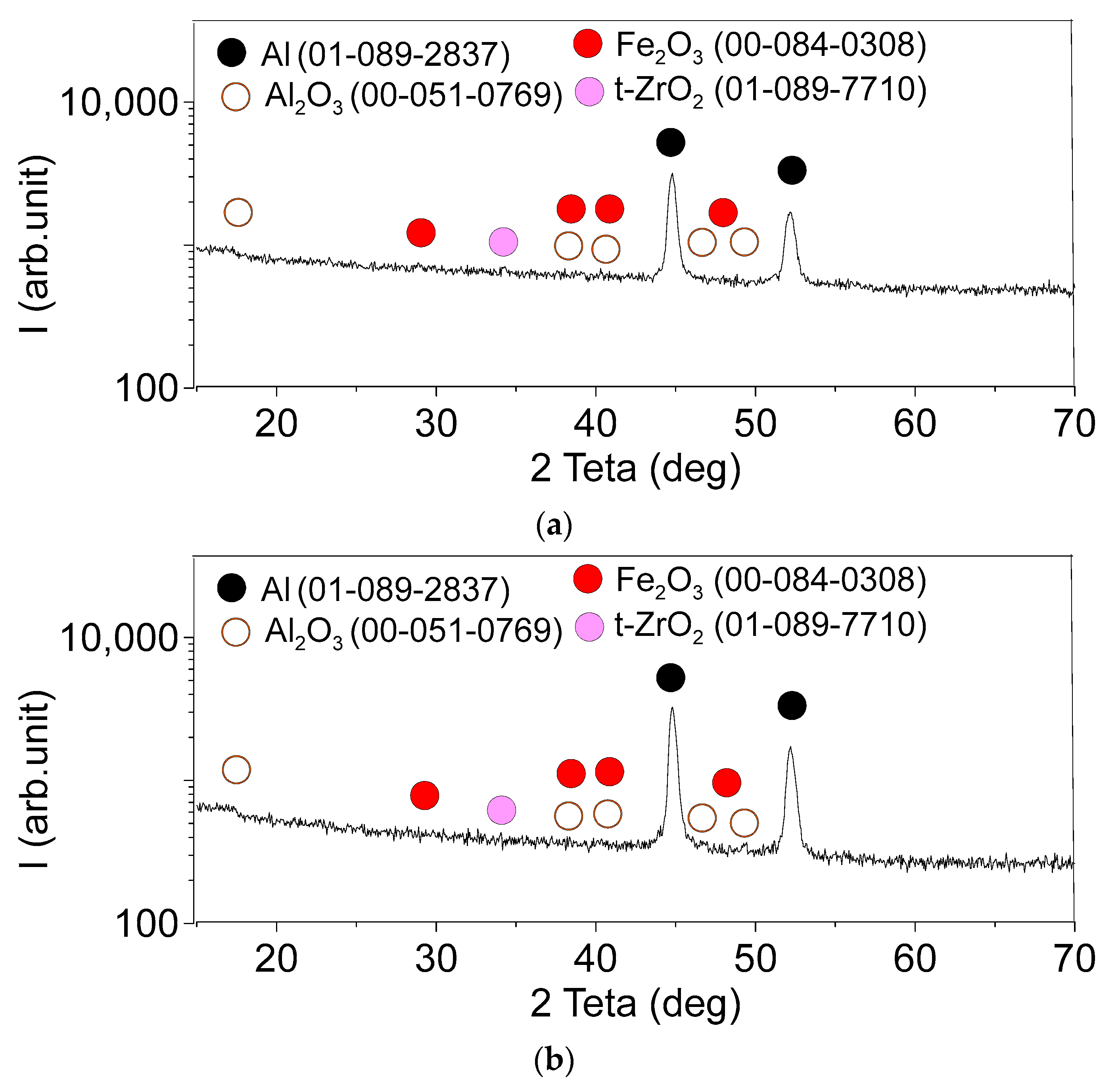
3.1. Microstructure and Phase Compound of ZrW2O8 Reinforcement Particles
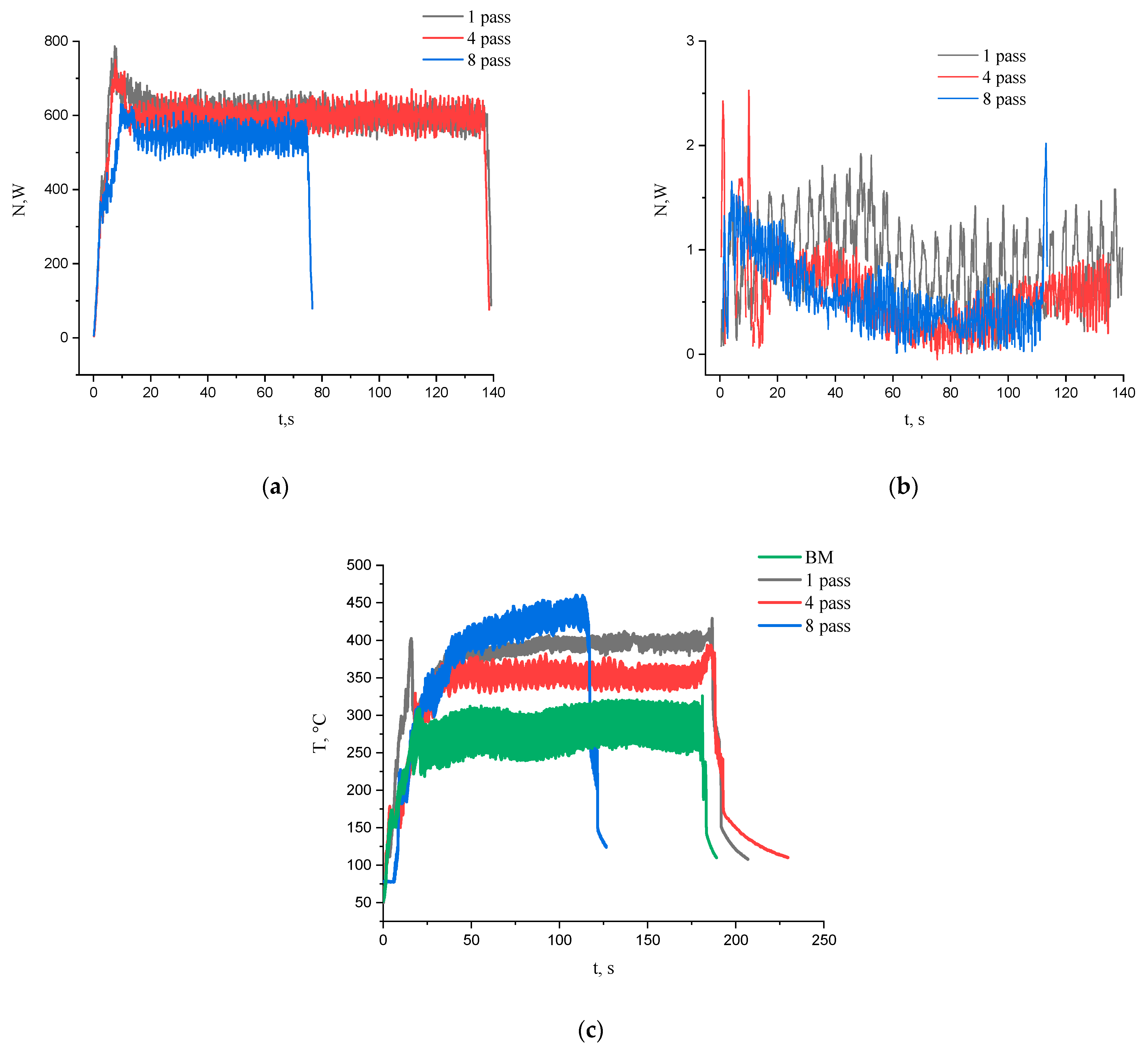
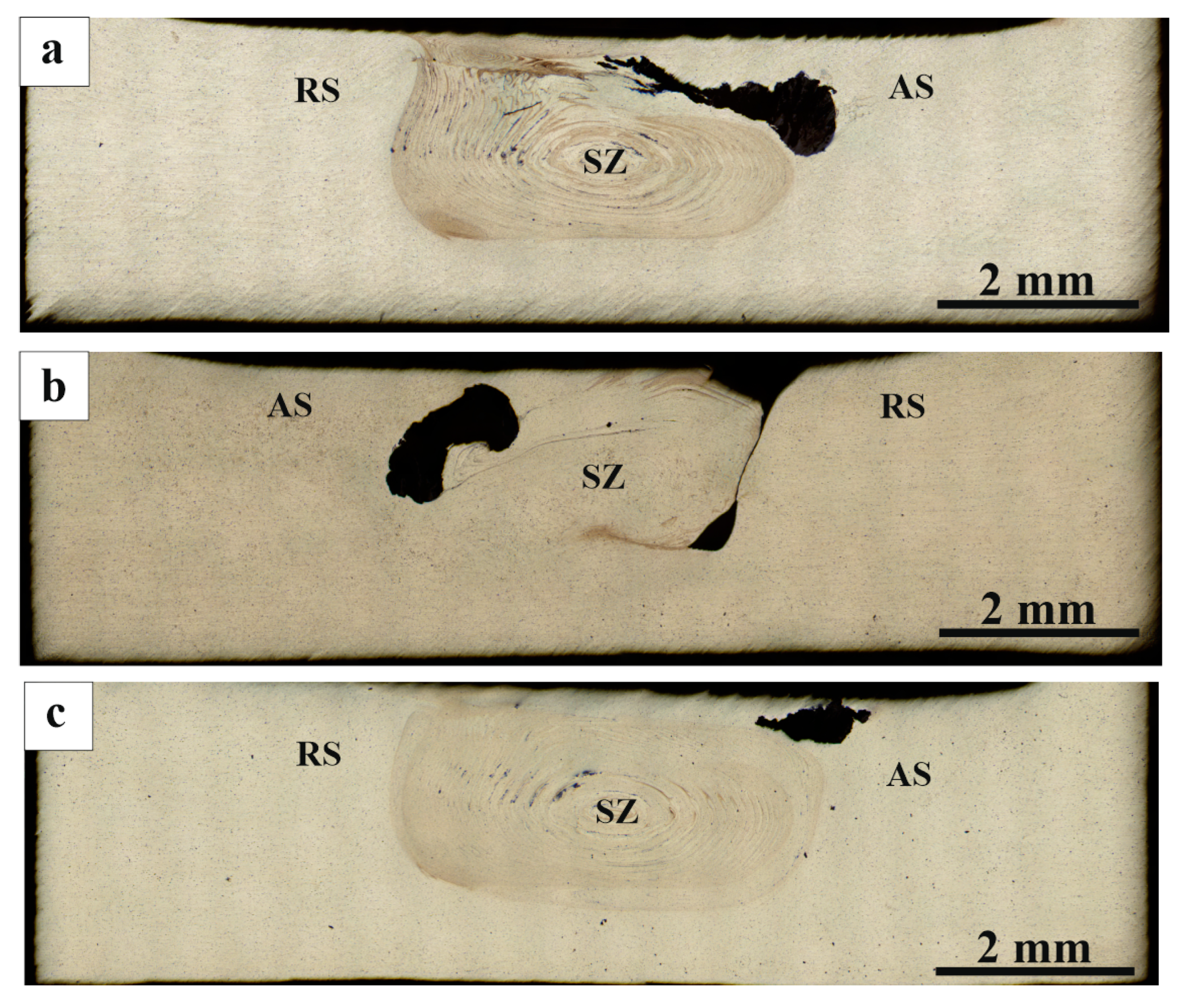
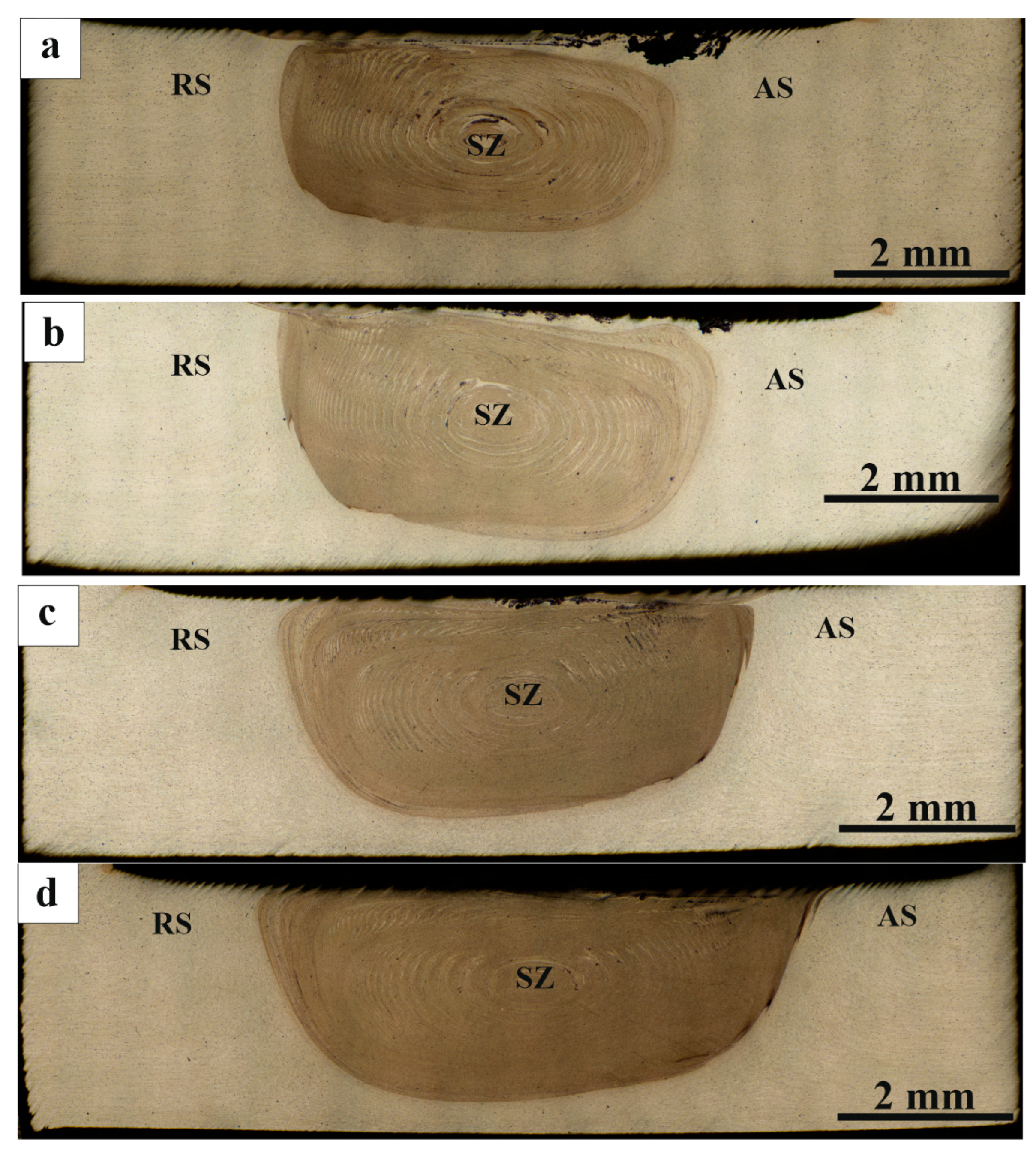
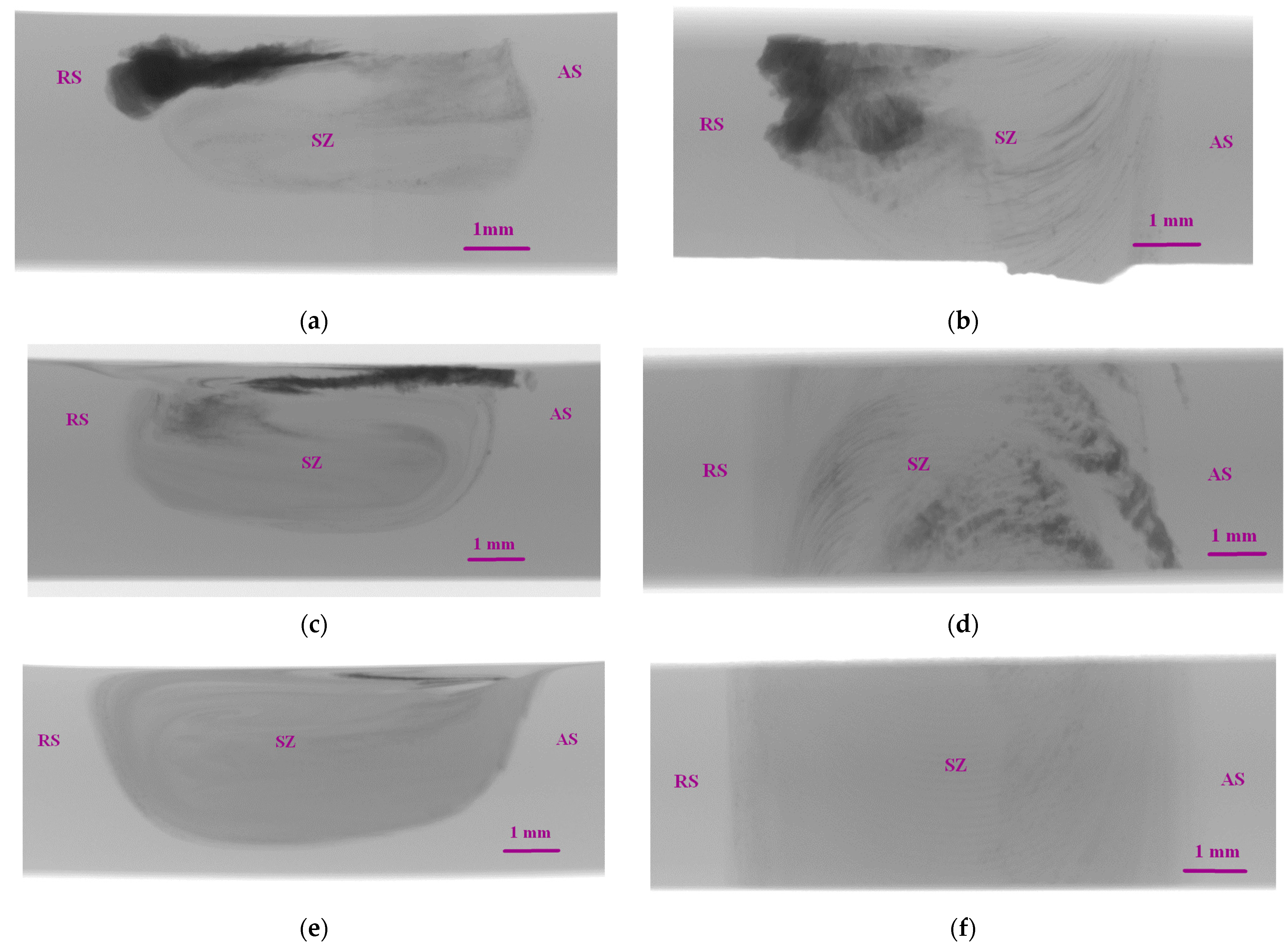
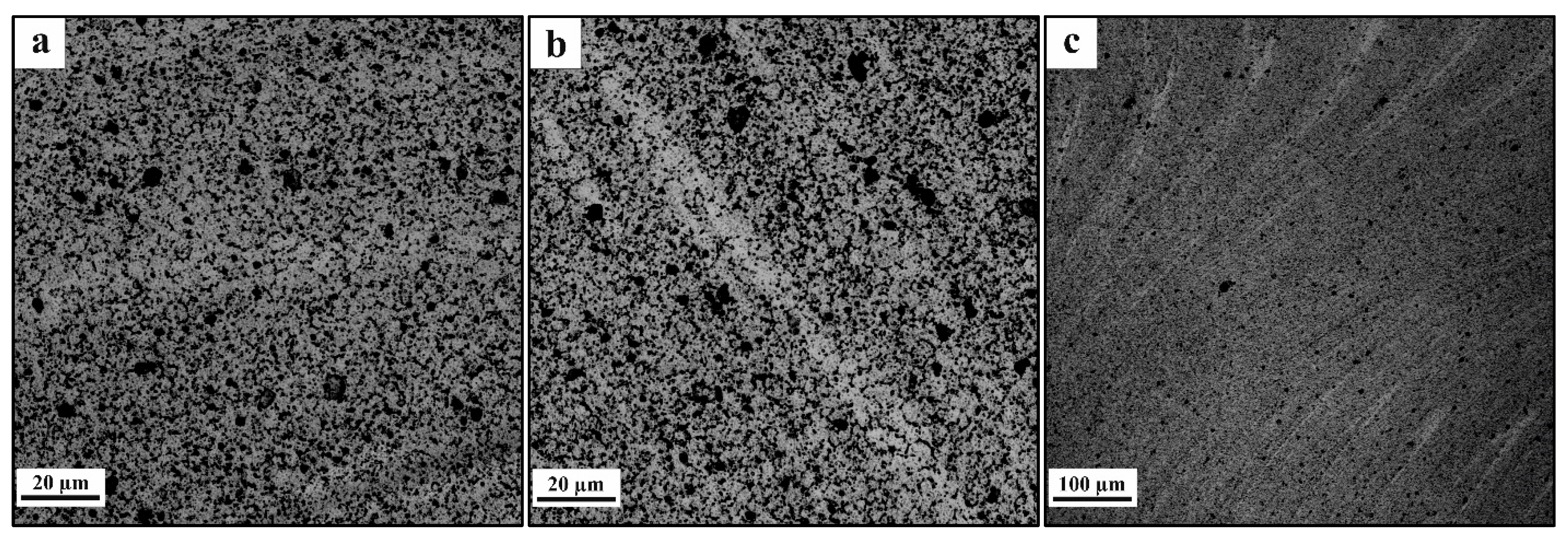
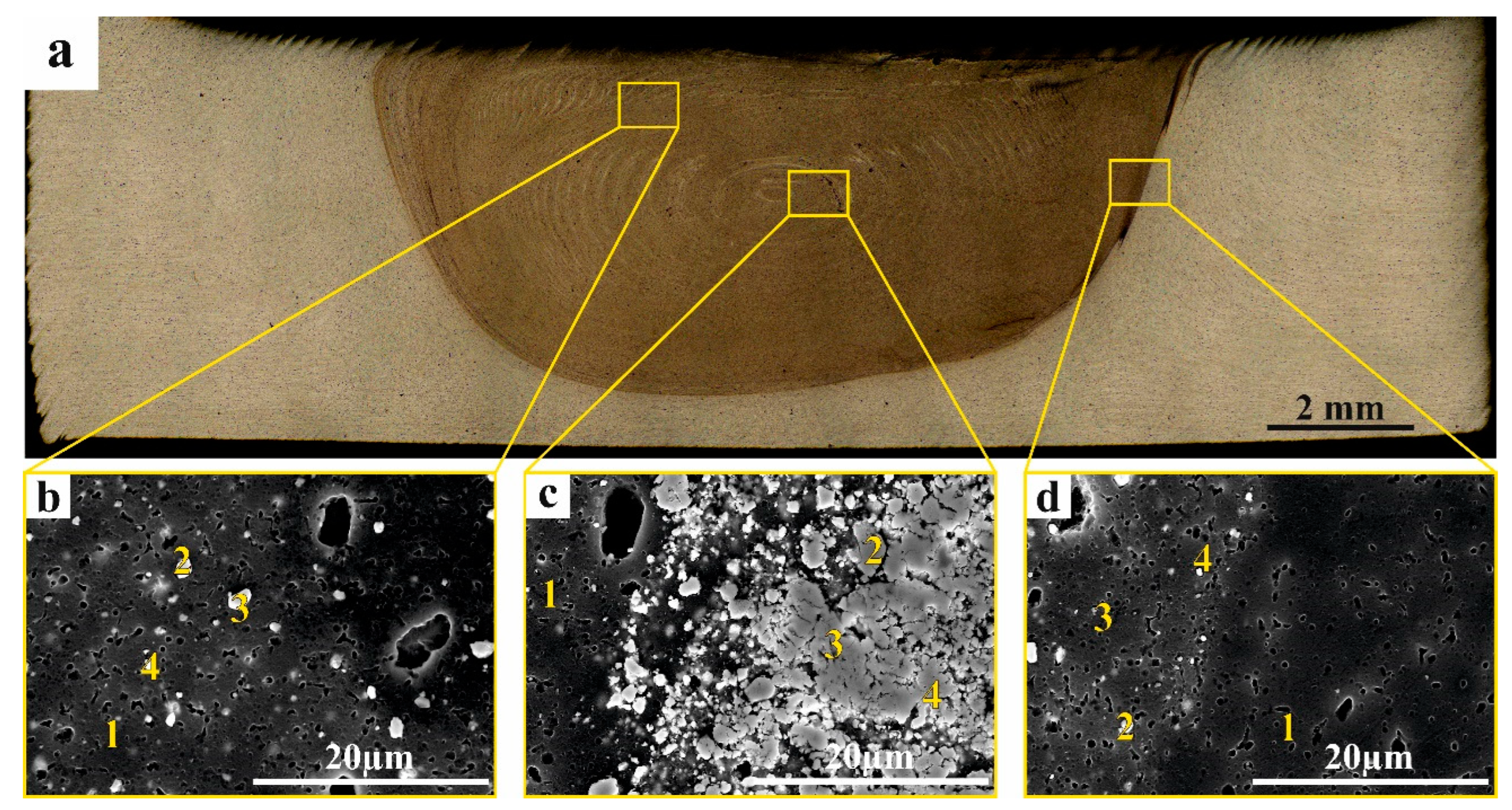
3.2. Macrostructure and Microstructure Evaluations of FSP-ed Composites
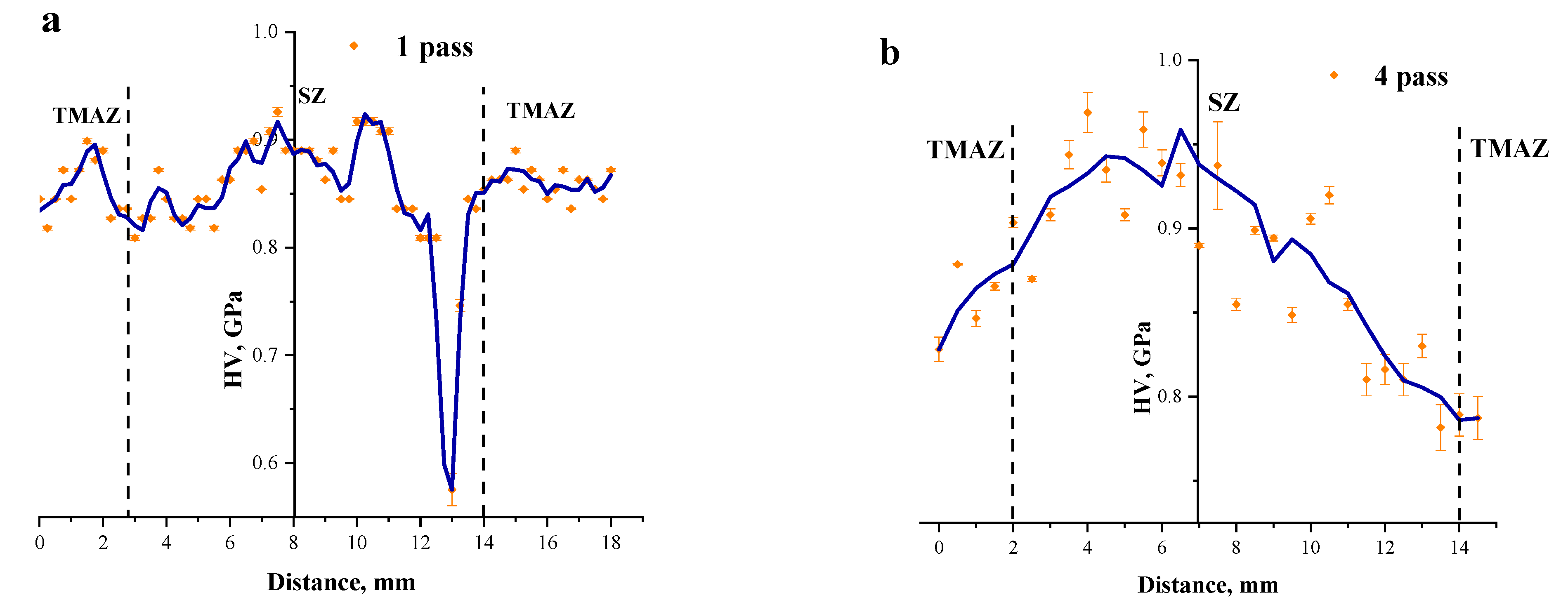
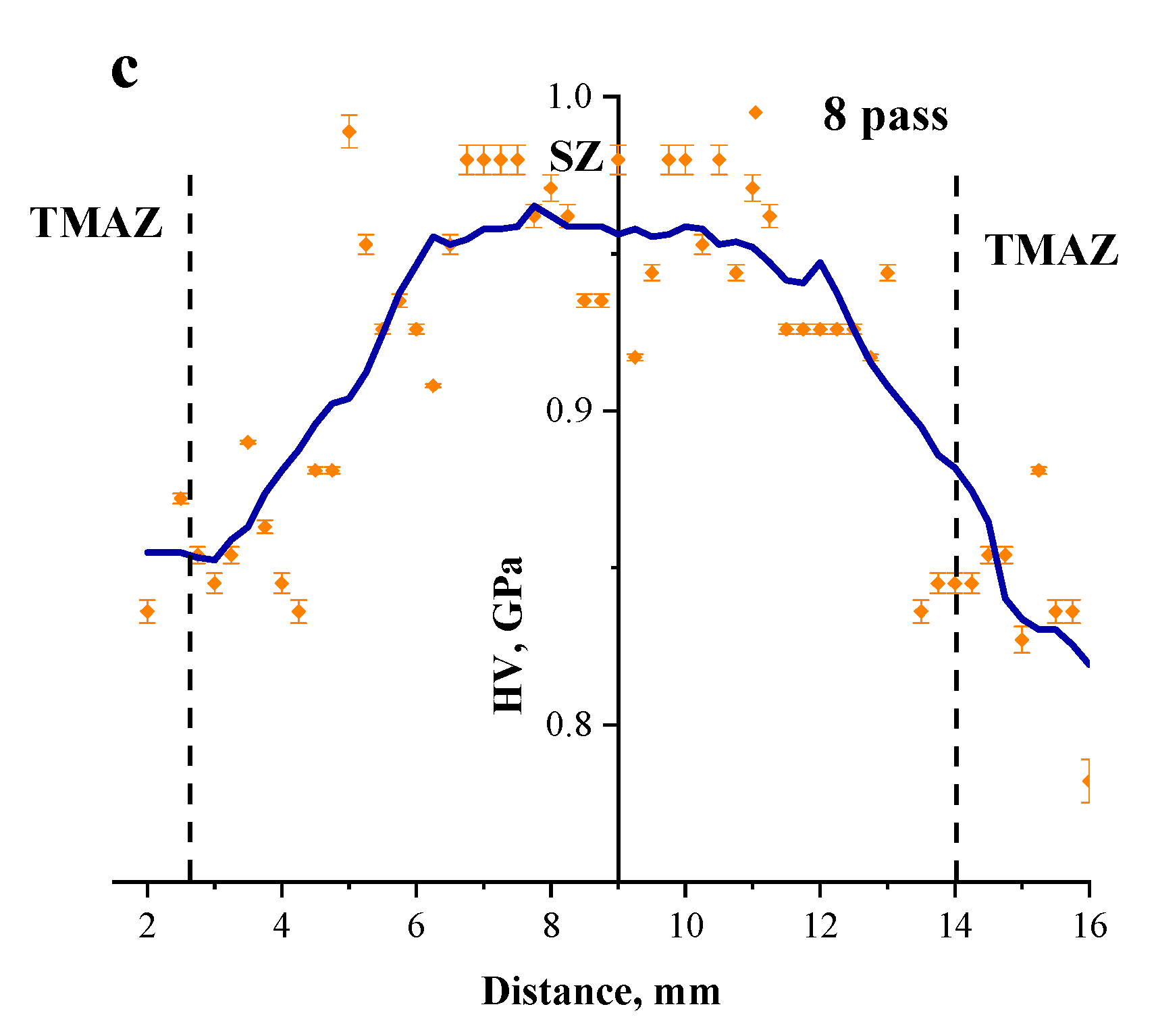
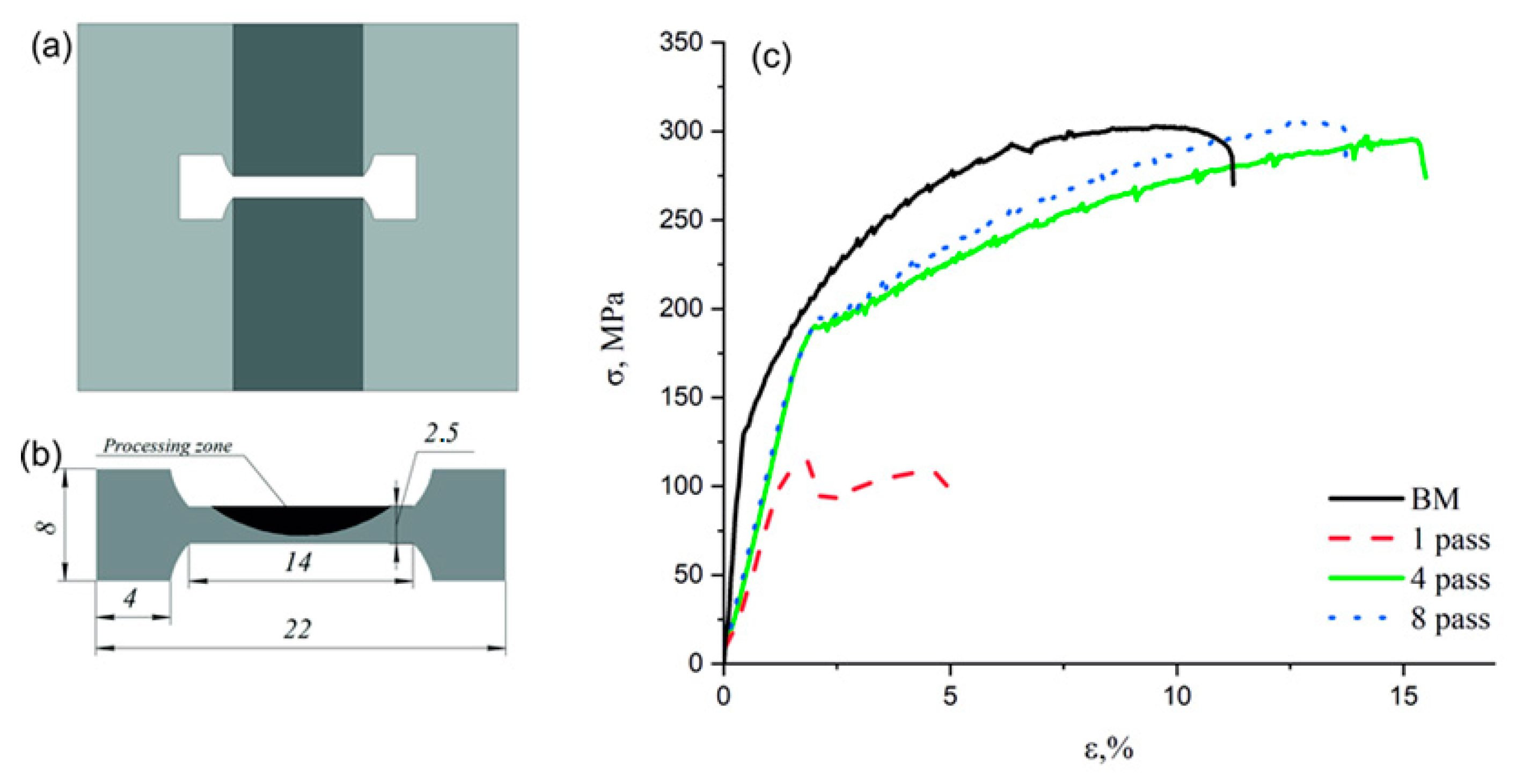
3.3. Microhardness Profiles of the FSP-ed RPPs/AA5056 Composites
3.4. Ultimate Tensile Strength and the Engineering Strain of the FSP-ed RPPs/AA5056 Composites
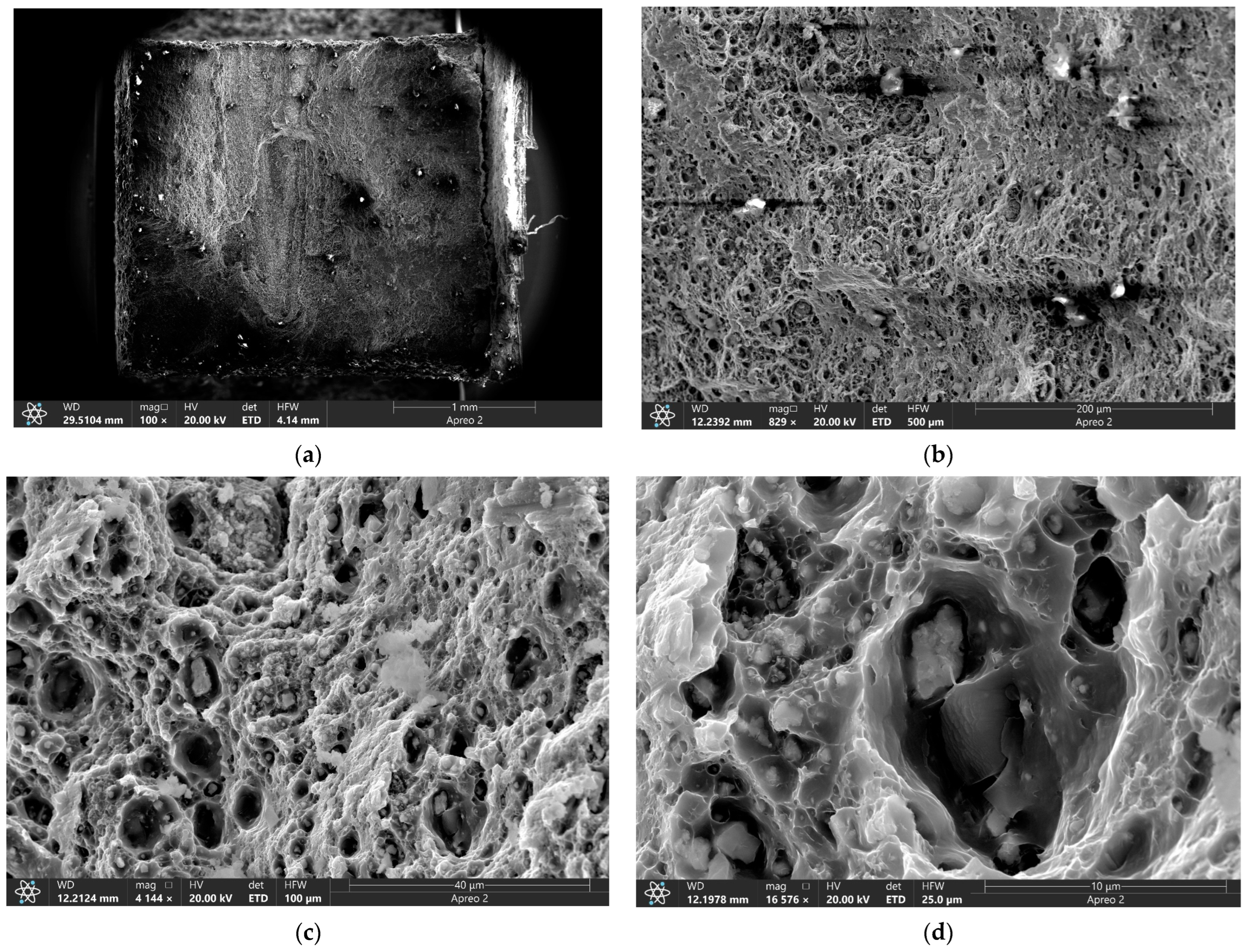
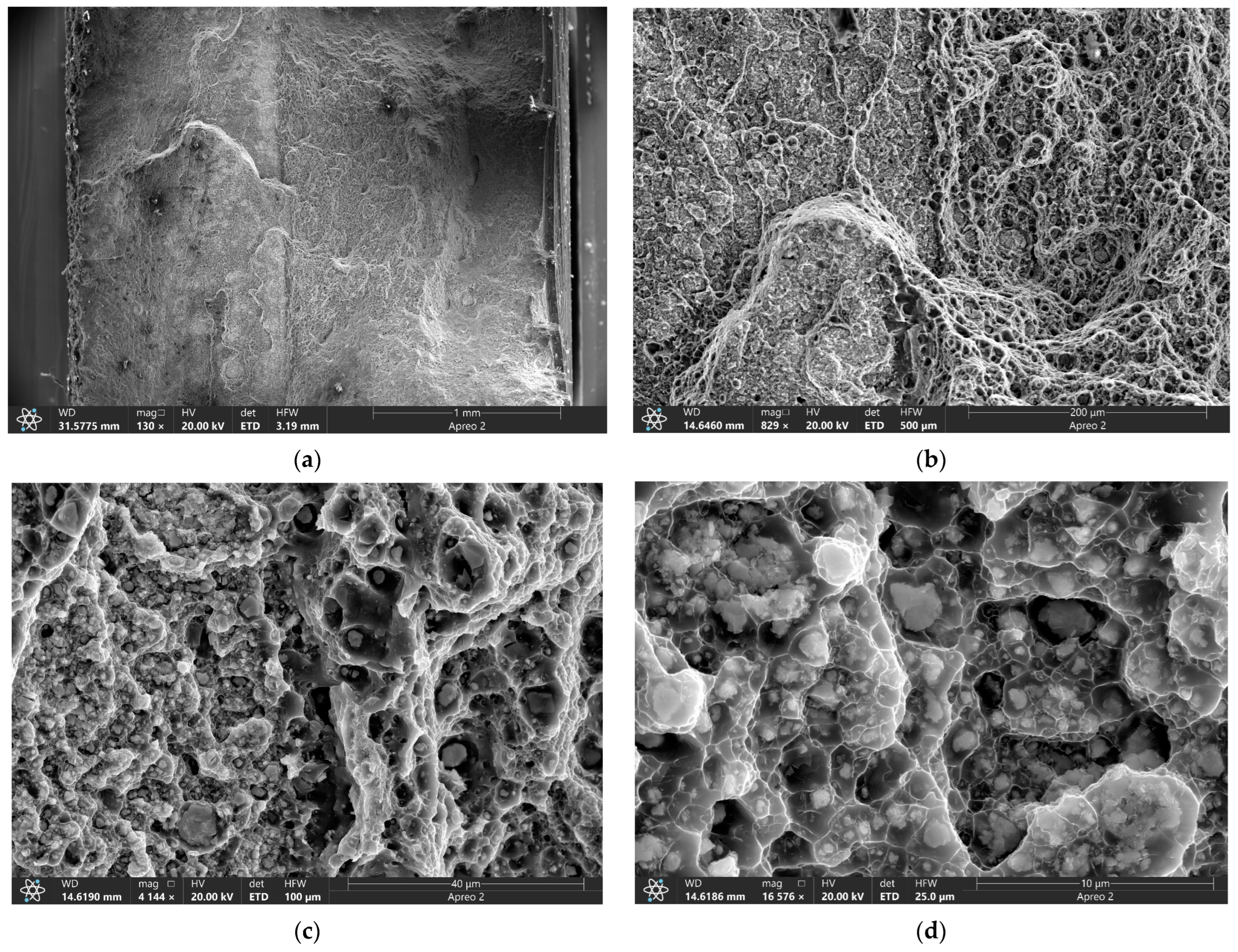
3.5. Tribological Behavior of the FSP-ed RPPs/AA5056 Composites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ujah, C.O.; Kallon, D.V.V. Trends in Aluminium Matrix Composite Development. Crystals 2022, 12, 1357. [Google Scholar] [CrossRef]
- Gill, R.S.; Samra, P.S.; Kumar, A. Effect of different types of reinforcement on tribological properties of aluminium metal matrix composites (MMCs)—A review of recent studies. Mater. Today Proc. 2022, 56, 3094–3101. [Google Scholar] [CrossRef]
- Singh, H.; Singh, K.; Vardhan, S.; Mohan, S.; Singh, G. A comprehension review of dry sliding wear on aluminum matrix composites. Mater. Today Proc. 2022, 58, 886–894. [Google Scholar] [CrossRef]
- Charan, T.P.; Suresh, R.; Reddy, M.C.S. A review on Fabrication and testing methods of aluminium metal matrix nano composites for various applications. Mater. Today Proc. 2022, 56., 1137–1142. [Google Scholar] [CrossRef]
- Srivyas, P.D.; Charoo, M.S. Aluminum metal matrix composites a review of reinforcement; mechanical and tribological behavior. Int. J. Eng. Technol. 2018, 7, 117–122. [Google Scholar] [CrossRef]
- Matsumoto, A.; Kobayashi, K.; Nishio, T.; Ozaki, K. Fabrication and thermal expansion of Al-ZrW2O8 composites by pulse current sintering process. Mater. Sci. Forum. 2003, 426, 2279–2283. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Zhang, M.; Wu, G. In-situ Raman spectroscopy study of thermal mismatch stress and negative thermal expansion behaviours of ZrW2O8 in ZrW2O8/Al composite. J. Alloys Compd. 2017, 718, 356–360. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Liu, S.; Zhou, T.; Jokisaari, J.R.; Wu, G. Microstructure and thermal expansion analysis of porous ZrW2O8/Al composite. J. Alloys. Compd. 2016, 670, 182–187. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, C.; Zhang, Q.; Pei, R. Decomposition of ZrW2O8 in Al matrix and the influence of heat treatment on ZrW2O8/Al–Si thermal expansion. Scr. Mater. 2015, 96, 29–32. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, Y.; Zhang, Q.; Meng, Q.; Zhang, L.; Kobayashi, E.; Wu, G. Near-zero thermal expansion of ZrW2O8/Al–Si composites with three dimensional interpenetrating network structure. Compos. Part B Eng. 2021, 211, 108678. [Google Scholar] [CrossRef]
- Raza, M.; Alrobei, H.; Malik, R.A.; Hussain, A.; Alzaid, M.; Saleem, M.; Imran, M. Structural, Fatigue Behavior, and Mechanical Properties of Zirconium Tungstate-Reinforced Casted A356 Aluminum Alloy. Metals 2020, 10, 1492. [Google Scholar] [CrossRef]
- Harlow, D.G.; Nardiello, J.; Payne, J. The effect of constituent particles in aluminum alloys on fatigue damage evolution: Statistical observations. Int. J. Fatigue 2010, 32, 505–511. [Google Scholar] [CrossRef]
- Tajiri, A.; Nozaki, T.; Uematsu, Y.; Kakiuchi, T. Fatigue limit prediction of large scale cast aluminum alloy A356. Procedia Mater. Sci. 2014, 3, 924–929. [Google Scholar] [CrossRef]
- Kandpal, B.C.; Kumar, J.; Singh, H. Fabrication and characterisation of Al2O3/aluminium alloy 6061 composites fabricated by Stir casting. Mater. Today Proc. 2017, 4, 2783–2792. [Google Scholar] [CrossRef]
- Hashim, J.; Looney, L.; Hashmi, M.S.J. Metal matrix composites: Production by the stir casting method. J. Mater. Process. Technol. 1999, 92, 1–7. [Google Scholar] [CrossRef]
- Shadrin, V.S.; Kulkov, S.N. Structure and Properties of Al-ZrW2O8 Pseudo Alloys. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 1045, 012039. [Google Scholar] [CrossRef]
- Staia, M.H.; Cruz, M.; Dahotre, N.B. Microstructural and tribological characterization of an A-356 aluminum alloy superficially modified by laser alloying. Thin Solid Films 2000, 377, 665–674. [Google Scholar] [CrossRef]
- Otani, Y.; Sasaki, S. Effects of the addition of silicon to 7075 aluminum alloy on microstructure, mechanical properties, and selective laser melting processability. Mater. Sci. Eng. A 2020, 777, 139079. [Google Scholar] [CrossRef]
- Edigarov, V.R.; Akhtulov, A.L.; Dadayan, S.E.; Maly, V.V. Friction-Electric Modification of the Surfaces of Machine Parts with Tungsten Carbides. Key Eng. Mater. 2022, 910, 538–543. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, P.; Akhtar, F. Aluminium matrix tungsten aluminide and tungsten reinforced composites by solid-state difusion mechanism. Sci. Rep. 2017, 7, 12391. [Google Scholar] [CrossRef]
- Gandra, J.; Krohn, H.; Miranda, R.M.; Vilaca, P.; Quintino, L.; dos Santos, J.F. Friction surfacing—A review. J. Mater. Process. Technol. 2014, 214, 1062–1093. [Google Scholar] [CrossRef]
- Seidi, E.; Miller, S.F. Lateral friction surfacing: Experimental and metallurgical analysis of different aluminum alloy depositions. J. Mater. Res. Technol. 2021, 15, 5948–5967. [Google Scholar] [CrossRef]
- Mane, K.M.; Hosmani, S.S. Friction stir surface processing of Al 6061 alloy: Role of surface alloying with copper and heat-treatment. Trans. Indian Inst. Met. 2018, 71, 1411–1425. [Google Scholar] [CrossRef]
- Zykova, A.P.; Tarasov, S.Y.; Chumaevskiy, A.V.; Kolubaev, E.A. A Review of Friction Stir Processing of Structural Metallic Materials: Process, Properties, and Methods. Metals 2020, 10, 772. [Google Scholar] [CrossRef]
- Srivatsan, T.S.; Al-Hajri, M. The fatigue and final fracture behavior of SiC particle reinforced 7034 aluminum matrix composites. Compos. Part B Eng. 2002, 33, 391–404. [Google Scholar] [CrossRef]
- Zykova, A.; Vorontsov, A.; Chumaevskii, A.; Gurianov, D.; Gusarova, A.; Kolubaev, E.; Tarasov, S. Structural evolution of contact parts of the friction stir processing heat-resistant nickel alloy tool used for multi-pass processing of Ti6Al4V/ (Cu+Al) system. Wear 2022, 488, 204138. [Google Scholar] [CrossRef]
- Seidi, E.; Miller, S.F. A Novel Approach to Friction Surfacing: Experimental Analysis of Deposition from Radial Surface of a Consumable Tool. Coatings 2020, 10, 1016. [Google Scholar] [CrossRef]
- Reddy, G.; Rao, K.; Mohandas, T. Friction surfacing: Novel technique for metal matrix composite coating on aluminium silicon alloy. Surf. Eng. 2009, 25, 25–30. [Google Scholar] [CrossRef]
- Janakiraman, S.; Udaya Bhat, K. Formation of Composite Surface during Friction Surfacing of Steel with Aluminium. Adv. Tribol. 2012, 4, 614278. [Google Scholar] [CrossRef]
- Gubanov, A.I.; Dedova, E.S.; Pavel, E.P.; Filatov, E.Y.; Kardash, T.Y.; Korenev, S.V.; Kulkov, S.N. Some peculiarities of zirconium tungstate synthesis by thermal decomposition of hydrothermal precursors. Thermochim. Acta 2014, 597, 19–26. [Google Scholar] [CrossRef]
- Tarasov, S.Y.; Rubtsov, V.E.; Kolubaev, E.A. A proposed diffusion-controlled wear mechanism of alloy steel friction stir welding (FSW) tools used on an aluminum alloy. Wear 2014, 318, 130–134. [Google Scholar] [CrossRef]
- Ekstrom, T.; Tilley, R.J.D. The stability of the perovskite-type zirconium tungsten bronze ZrxWO3. J. Solid State Chem. 1976, 19, 227–233. [Google Scholar] [CrossRef]
- Kalashnikov, K.N.; Tarasov, S.Y.; Chumaevskii, A.V.; Fortuna, S.V.; Eliseev, A.A.; Ivanov, A.N. Towards aging in a multipass friction stir–processed AA2024. Int. J. Adv. Manuf. Technol. 2019, 1, 2121–2132. [Google Scholar] [CrossRef]
- Grigoriev, A.S.; Shilko, E.V.; Dmitriev, A.I.; Tarasov, S.Y. Suppression of wear in dry sliding friction induced by negative thermal expansion. Phys. Rev. E 2020, 102, 042801. [Google Scholar] [CrossRef]
- Radu, I.; Li, D.Y. Effects of ZrW2O8 and tungsten additions on the temperature range in which a pseudoelastic TiNi alloy retains its maximum wear resistance. Wear 2007, 263, 858–865. [Google Scholar] [CrossRef]
- Radu, I.; Li, D.Y. Enlarging the temperature range for maximum wear resistance of TiNi alloy using a NTE phase. Mater. Sci. Forum. 2007, 539, 3261–3266. [Google Scholar] [CrossRef]
- Gnyusov, S.F.; Fedin, E.A.; Tarasov, S.Y. The effect of counterbody on tribological adaptation of an electron beam deposited HSS M2 steel coating in a range of sliding speeds and normal loads. Tribol. Int. 2021, 161, 107109. [Google Scholar] [CrossRef]
- Savchenko, N.; Sevostyanova, I.; Grigoriev, M.; Sablina, T.; Buyakov, A.; Rudmin, M.; Vorontsov, A.; Moskvichev, E.; Rubtsov, V.; Tarasov, S. Self-Lubricating Effect of WC/Y–TZP–Al2O3 Hybrid Ceramic–Matrix Composites with Dispersed Hadfield Steel Particles during High-Speed Sliding against an HSS Disk. Lubricants 2022, 10, 140. [Google Scholar] [CrossRef]
- Savchenko, N.; Sevostyanova, I.; Tarasov, S. Self-Lubricating Effect of FeWO4 Tribologically Synthesized from WC-(Fe-Mn-C) Composite during High-Speed Sliding against a HSS Disk. Lubricants 2022, 10, 86. [Google Scholar] [CrossRef]



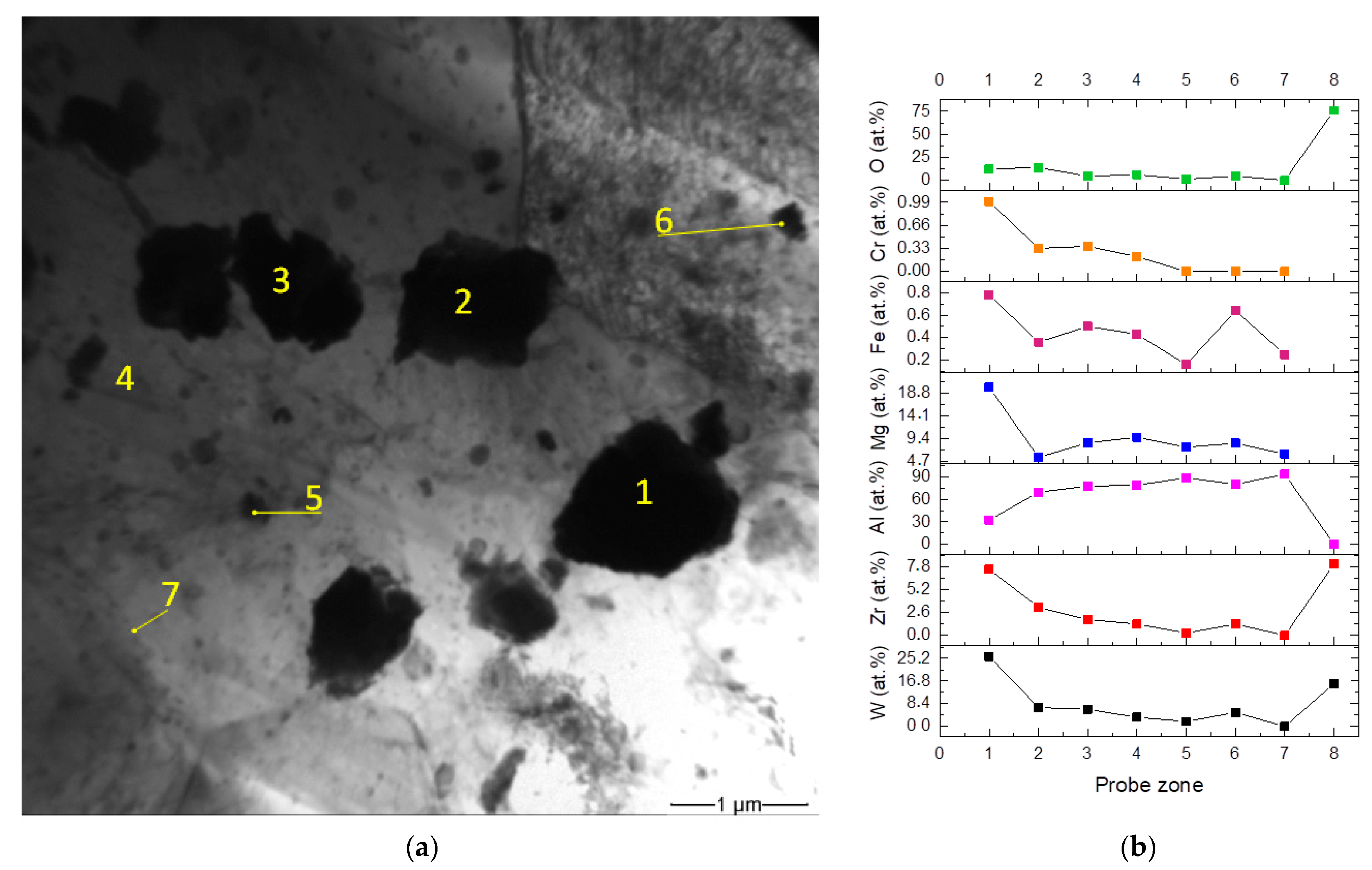
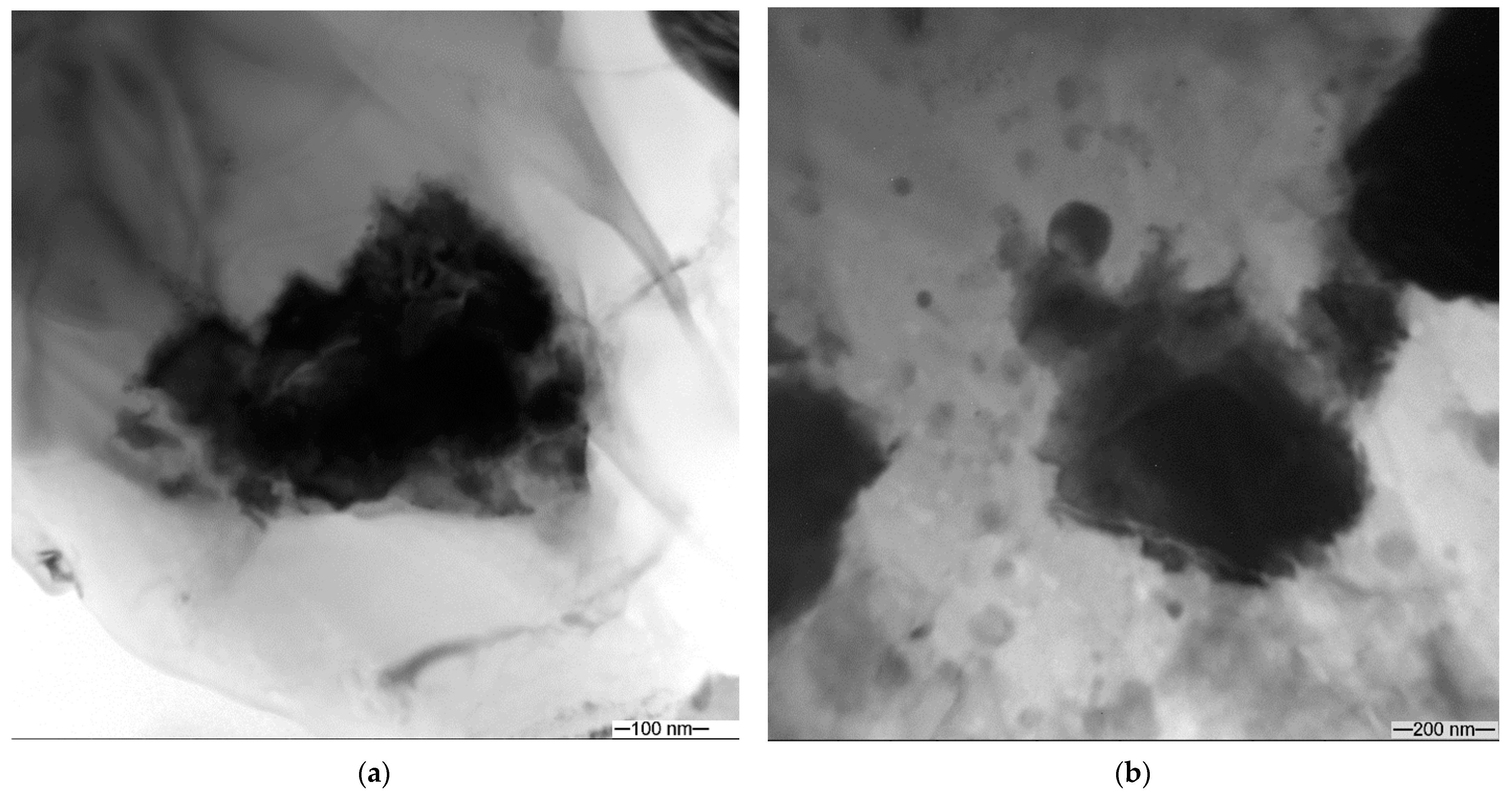

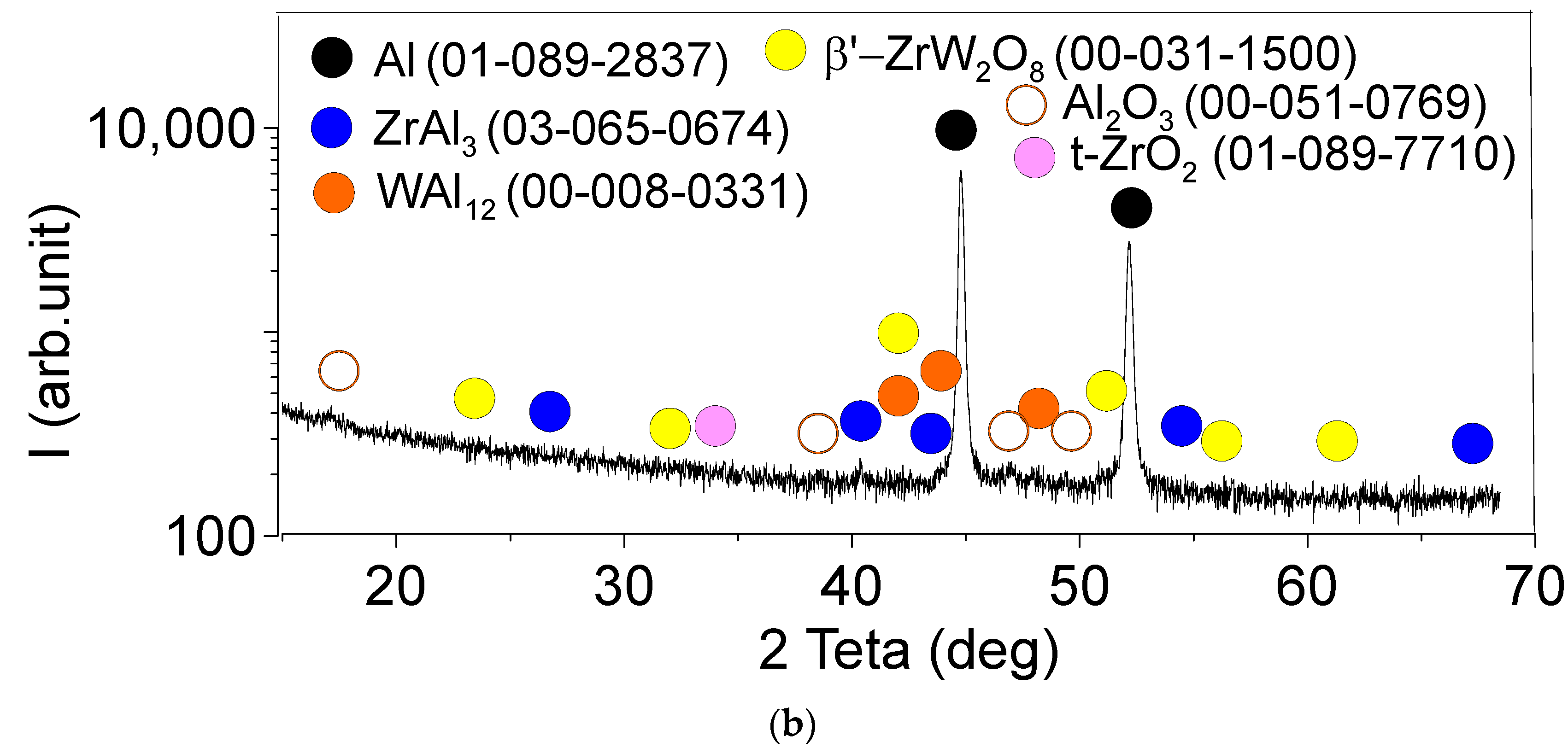

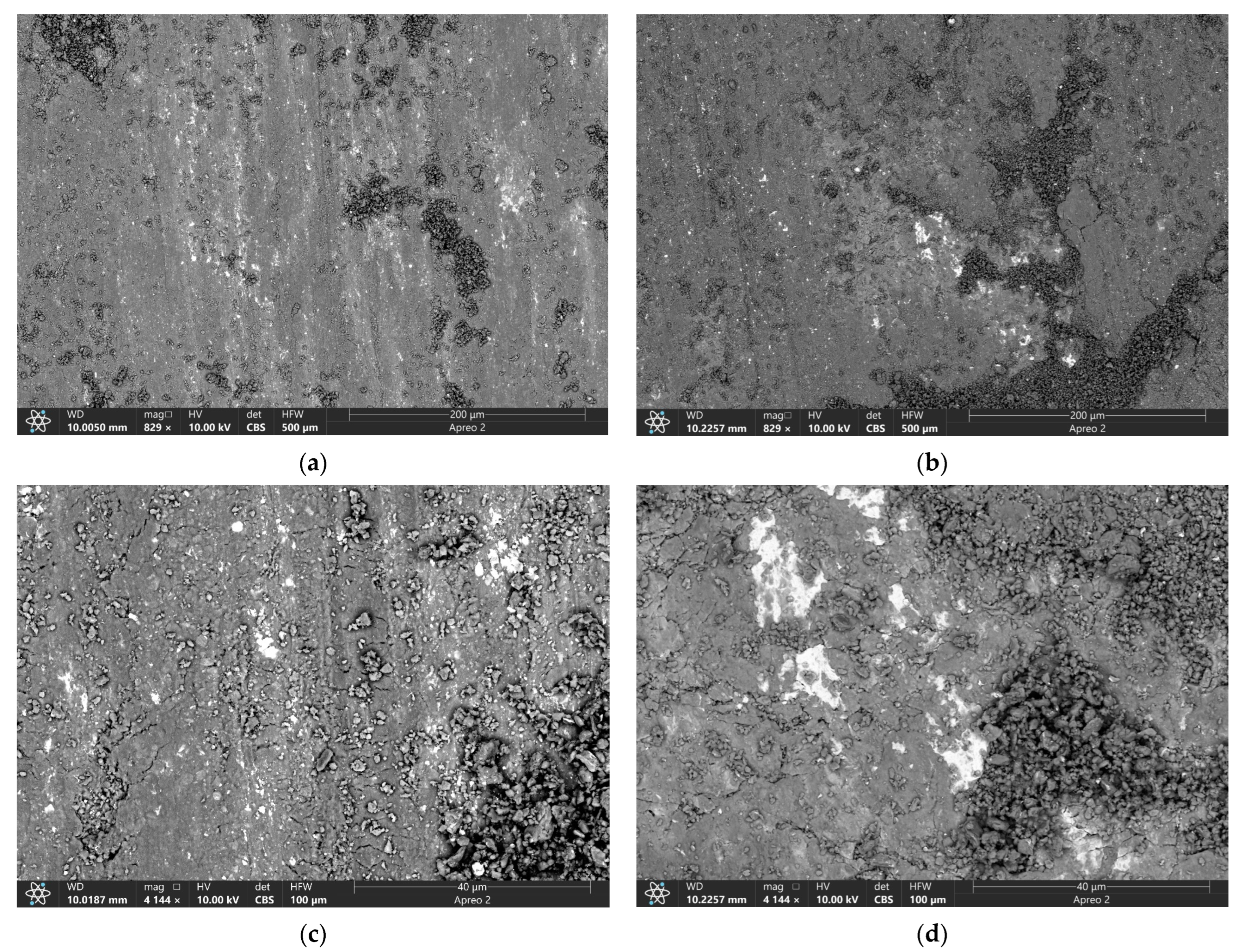
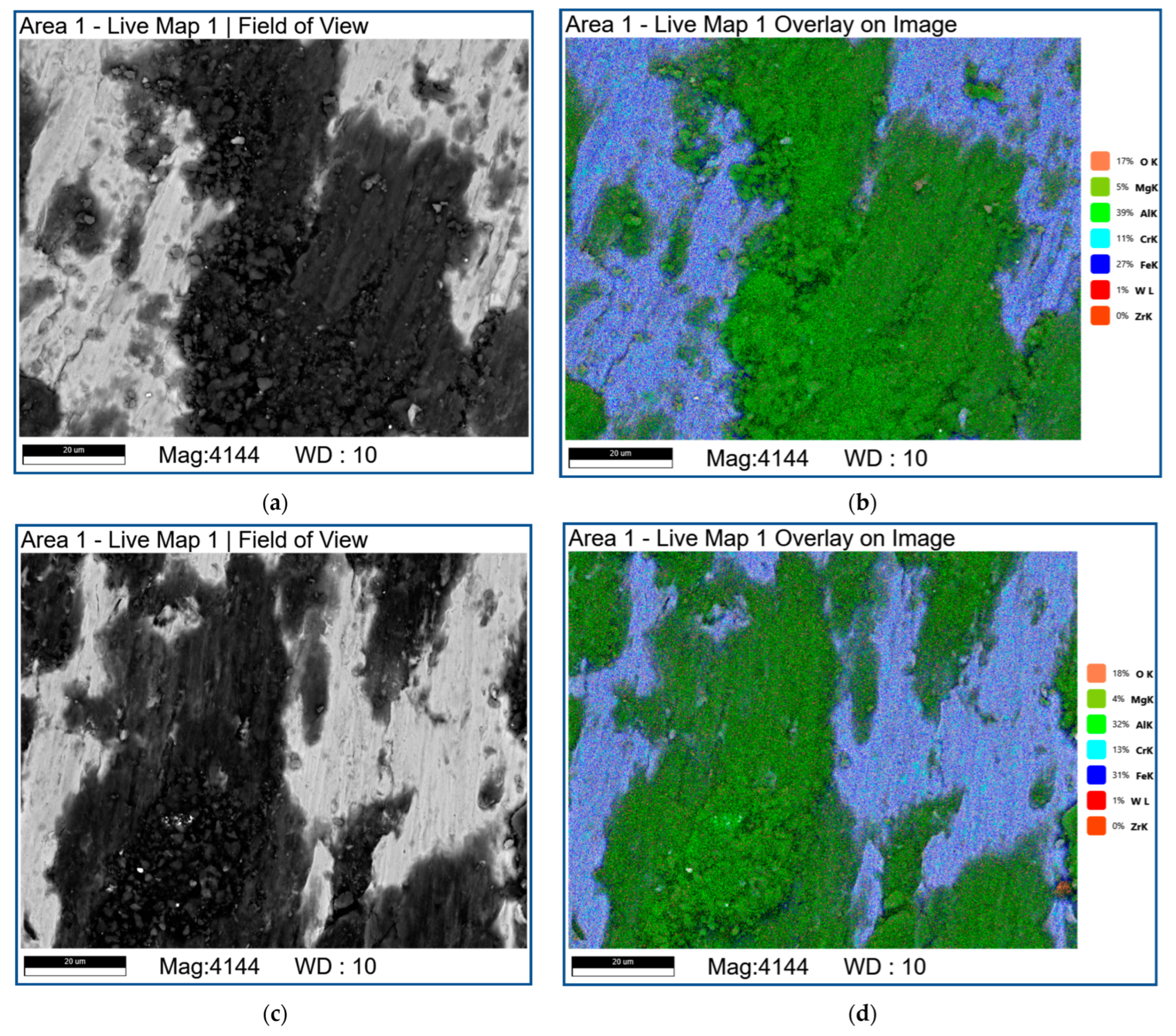
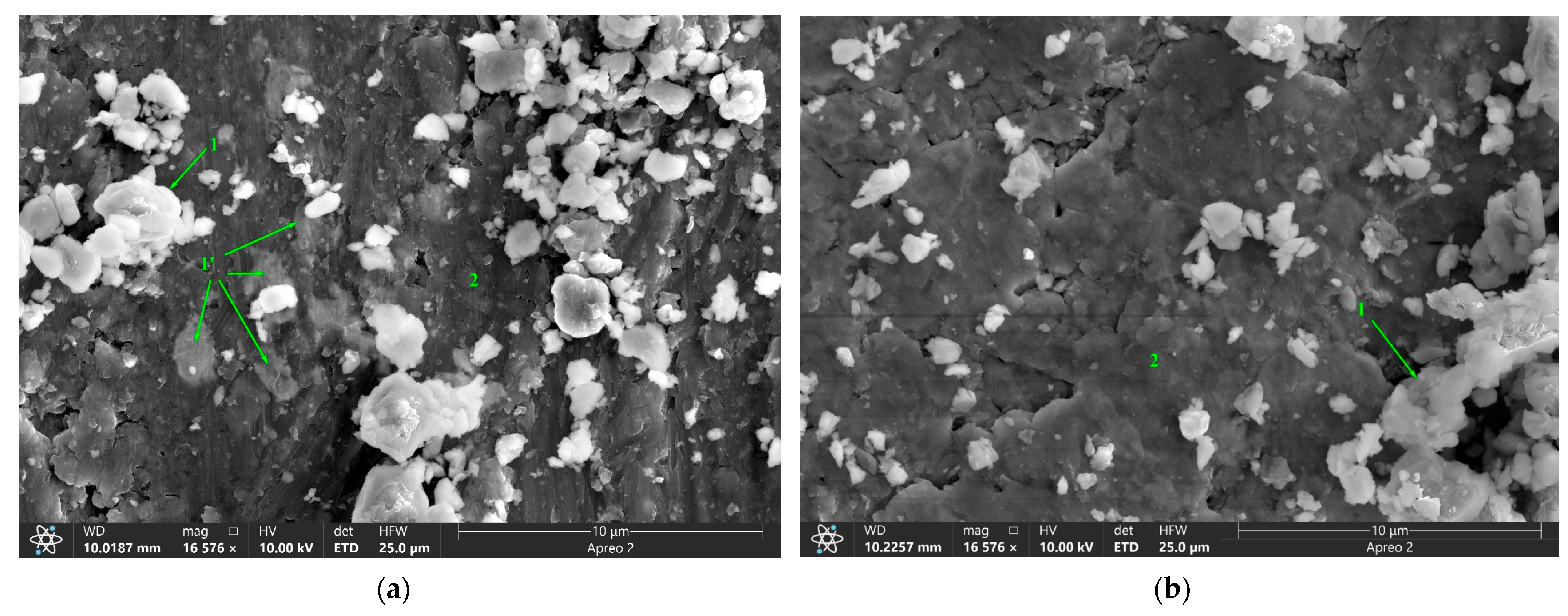
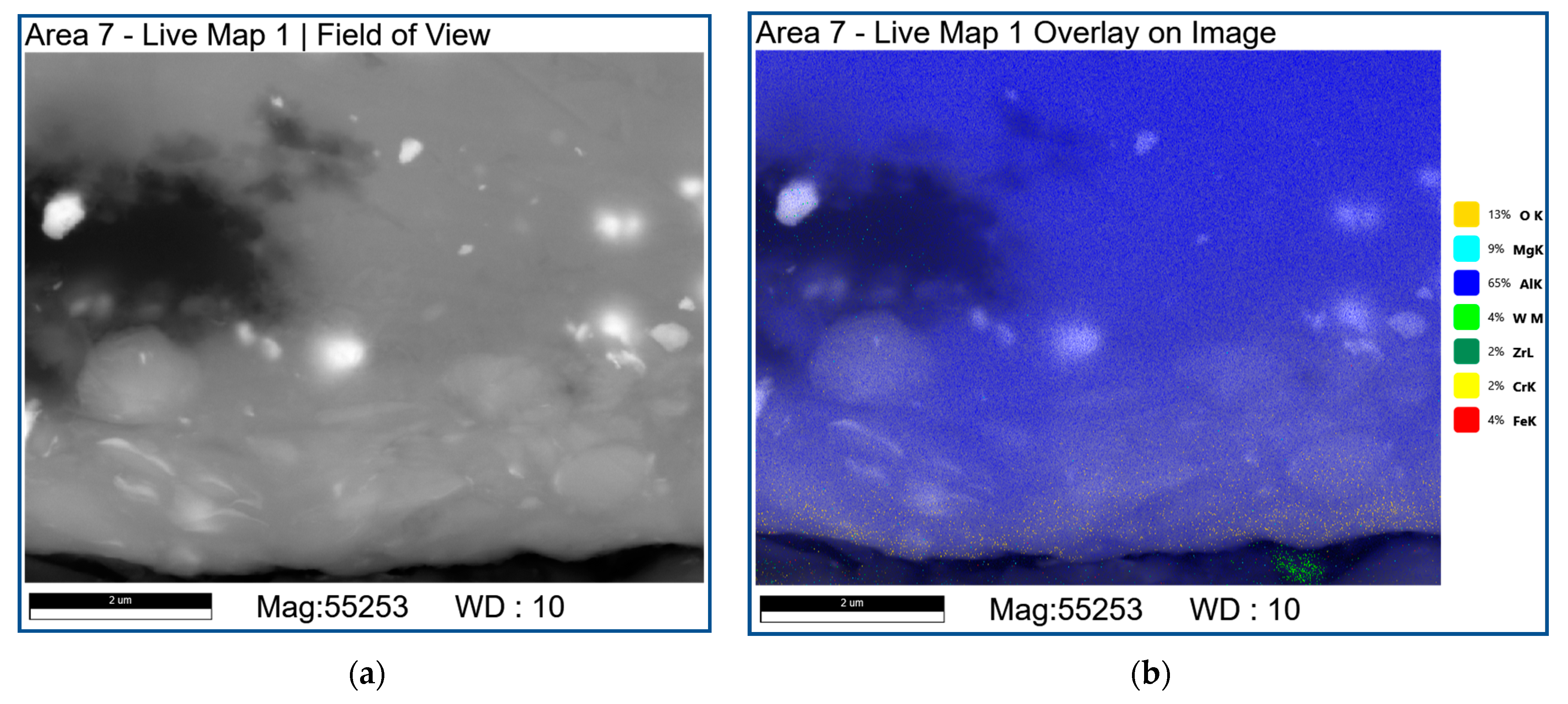
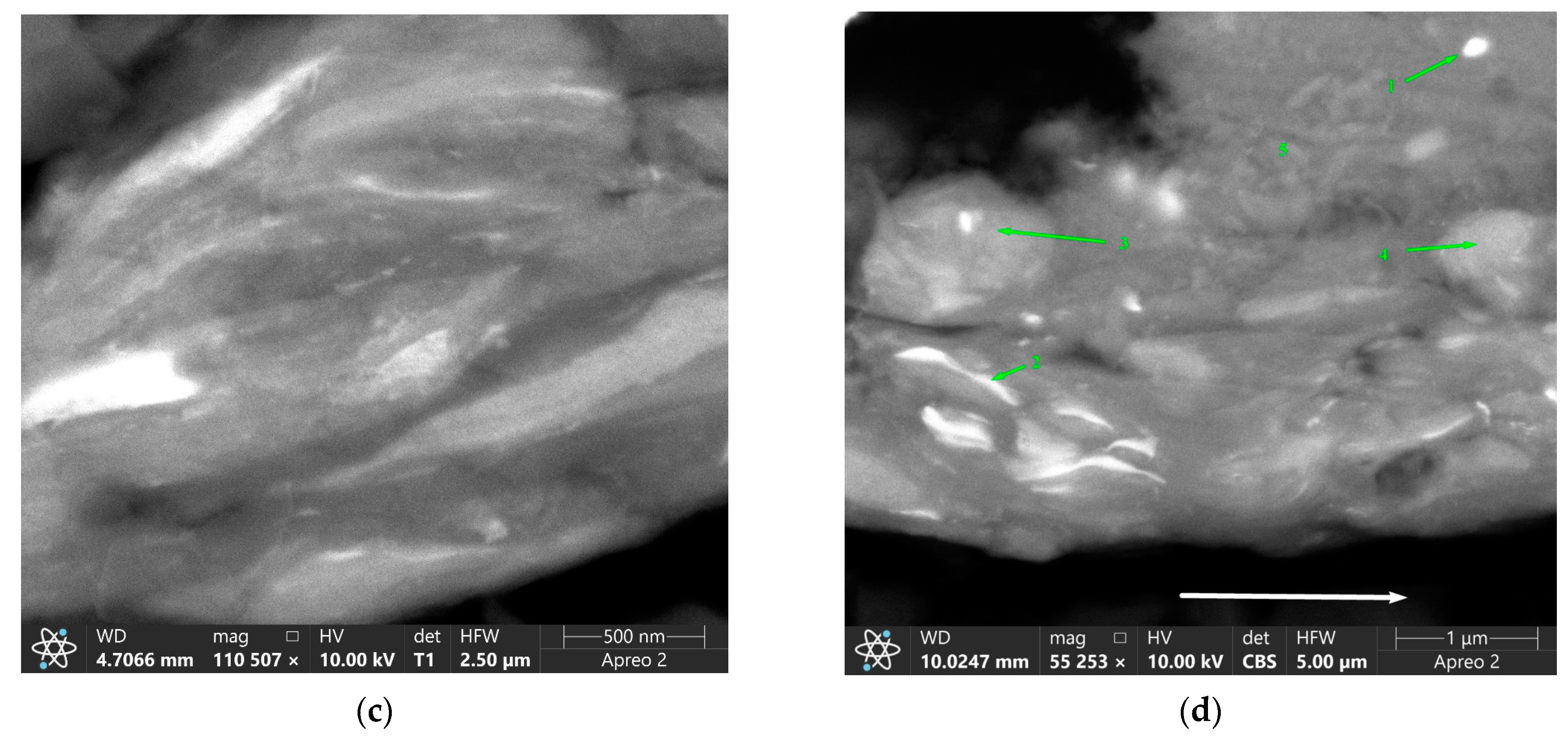
| Area | Point Number | Element, at.% | ||||
|---|---|---|---|---|---|---|
| O | Al | Mg | W | Zr | ||
| RPPs accumulation zone | 1 | 1.7 | 90.3 | 7.8 | 0.2 | 0.0 |
| 2 | 68.2 | 1.4 | 0.0 | 12.8 | 17.6 | |
| 3 | 69.2 | 1.0 | 0.0 | 13.3 | 16.5 | |
| 4 | 70.7 | 1.4 | 0.0 | 12.5 | 15.4 | |
| SZ | 1 | 1.0 | 90.7 | 8.2 | 0.1 | 0.0 |
| 2 | 24.7 | 51.4 | 8.8 | 7.0 | 8.1 | |
| 3 | 66.7 | 8.6 | 0.5 | 14.1 | 10.1 | |
| 4 | 40.5 | 33.0 | 8.6 | 6.7 | 11.2 | |
| TMAZ | 1 | 0.3 | 91.6 | 8.1 | 0.0 | 0.0 |
| 2 | 24.5 | 55.5 | 6.5 | 6.4 | 7.1 | |
| 3 | 68.7 | 4.5 | 0.3 | 12.2 | 14.3 | |
| 4 | 40.2 | 36.5 | 4.5 | 6.6 | 12.2 | |
| Area | Point Number | Element, at.% | ||||
|---|---|---|---|---|---|---|
| O | Al | Mg | W | Zr | ||
| RPPs accumulation zone | 1 | 0.3 | 87.1 | 12.5 | 0.1 | 0.0 |
| 2 | 45.0 | 32.5 | 3.5 | 7.7 | 11.3 | |
| 3 | 54.1 | 17.5 | 1.9 | 10.0 | 16.5 | |
| 4 | 20.9 | 58.0 | 7.7 | 6.2 | 7.2 | |
| SZ | 1 | 1.7 | 90.1 | 8.0 | 0.2 | 0.0 |
| 2 | 62.9 | 3.9 | 0.2 | 13.2 | 19.8 | |
| 3 | 69.2 | 3.5 | 0.2 | 14.0 | 13.1 | |
| 4 | 51.9 | 25.5 | 1.1 | 9.2 | 12.3 | |
| TMAZ | 1 | 0.3 | 91.2 | 8.5 | 0.0 | 0.0 |
| 2 | 20.9 | 58.0 | 7.7 | 6.2 | 7.2 | |
| 3 | 34.5 | 53.9 | 4.8 | 6.8 | 0.0 | |
| 4 | 66.0 | 10.6 | 2.4 | 7.7 | 13.3 | |
| Sample | Counterbody | Rwear Track, mm | P, H | t, min | ω, RPM | Hs, mm | Hf, mm | ΔH, mm | Iw, mm3/m |
|---|---|---|---|---|---|---|---|---|---|
| passes AA5056 | 40 × 13 | 10 | 12 | 120 | 250 | 9.30 | 8.35 | 0.95 | 9.9 × 10−3 |
| AA5056 + ZrW2O8 4 passes | 40 × 13 | 10 | 12 | 120 | 250 | 9.27 | 8.65 | 0.62 | 6.5 × 10−3 |
| AA5056 + ZrW2O8 8 passes | 40 × 13 | 10 | 12 | 120 | 250 | 9.37 | 8.87 | 0.50 | 5.2 × 10−3 |
| Sample | Point Number | Element, at. % | ||||||
|---|---|---|---|---|---|---|---|---|
| O | Al | Mg | Cr | Fe | W | Zr | ||
| 4 passes | 1 | 56.5 | 22.7 | 3.2 | 0.1 | 0.5 | 11.7 | 5.3 |
| 2 | 44.7 | 49.0 | 4.1 | 0.3 | 1.6 | 0.1 | 0.1 | |
| 8 passes | 1 | 31.9 | 48.2 | 3.9 | 0.1 | 0.3 | 10.5 | 5.1 |
| 2 | 6.6 | 84.5 | 6.8 | 0.3 | 1.7 | 0.1 | 0.0 | |
| Sample | Point Number | Element, at. % | ||||||
|---|---|---|---|---|---|---|---|---|
| O | Al | Mg | Cr | Fe | W | Zr | ||
| 8 passes | 1 | 19.4 | 69.7 | 6.3 | 0.5 | 1.4 | 2.1 | 0.8 |
| 2 | 20.1 | 69.3 | 6.9 | 0.3 | 1.2 | 1.6 | 0.7 | |
| 3 | 31.6 | 56.7 | 5.5 | 1.0 | 4.6 | 0.5 | 0.2 | |
| 4 | 28.9 | 60.7 | 5.3 | 0.8 | 3.9 | 0.3 | 0.2 | |
| 5 | 6.9 | 85.9 | 6.3 | 0.1 | 0.5 | 0.3 | 0.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumaevskii, A.; Zykova, A.; Sudarikov, A.; Knyazhev, E.; Savchenko, N.; Gubanov, A.; Moskvichev, E.; Gurianov, D.; Nikolaeva, A.; Vorontsov, A.; et al. In-Situ Al-Mg Alloy Base Composite Reinforced by Oxides and Intermetallic Compounds Resulted from Decomposition of ZrW2O8 during Multipass Friction Stir Processing. Materials 2023, 16, 817. https://doi.org/10.3390/ma16020817
Chumaevskii A, Zykova A, Sudarikov A, Knyazhev E, Savchenko N, Gubanov A, Moskvichev E, Gurianov D, Nikolaeva A, Vorontsov A, et al. In-Situ Al-Mg Alloy Base Composite Reinforced by Oxides and Intermetallic Compounds Resulted from Decomposition of ZrW2O8 during Multipass Friction Stir Processing. Materials. 2023; 16(2):817. https://doi.org/10.3390/ma16020817
Chicago/Turabian StyleChumaevskii, Andrey, Anna Zykova, Alexandr Sudarikov, Evgeny Knyazhev, Nickolai Savchenko, Alexander Gubanov, Evgeny Moskvichev, Denis Gurianov, Aleksandra Nikolaeva, Andrey Vorontsov, and et al. 2023. "In-Situ Al-Mg Alloy Base Composite Reinforced by Oxides and Intermetallic Compounds Resulted from Decomposition of ZrW2O8 during Multipass Friction Stir Processing" Materials 16, no. 2: 817. https://doi.org/10.3390/ma16020817
APA StyleChumaevskii, A., Zykova, A., Sudarikov, A., Knyazhev, E., Savchenko, N., Gubanov, A., Moskvichev, E., Gurianov, D., Nikolaeva, A., Vorontsov, A., Kolubaev, E., & Tarasov, S. (2023). In-Situ Al-Mg Alloy Base Composite Reinforced by Oxides and Intermetallic Compounds Resulted from Decomposition of ZrW2O8 during Multipass Friction Stir Processing. Materials, 16(2), 817. https://doi.org/10.3390/ma16020817









