Fabrication of ZnWO4/Carbon Black Nanocomposites Modified Glassy Carbon Electrode for Enhanced Electrochemical Determination of Ciprofloxacin in Environmental Water Samples
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of ZnWO4/CB
2.3. Characterizations
2.4. Modification of the GCE/ZnWO4/CB Electrode
3. Result and Discussion
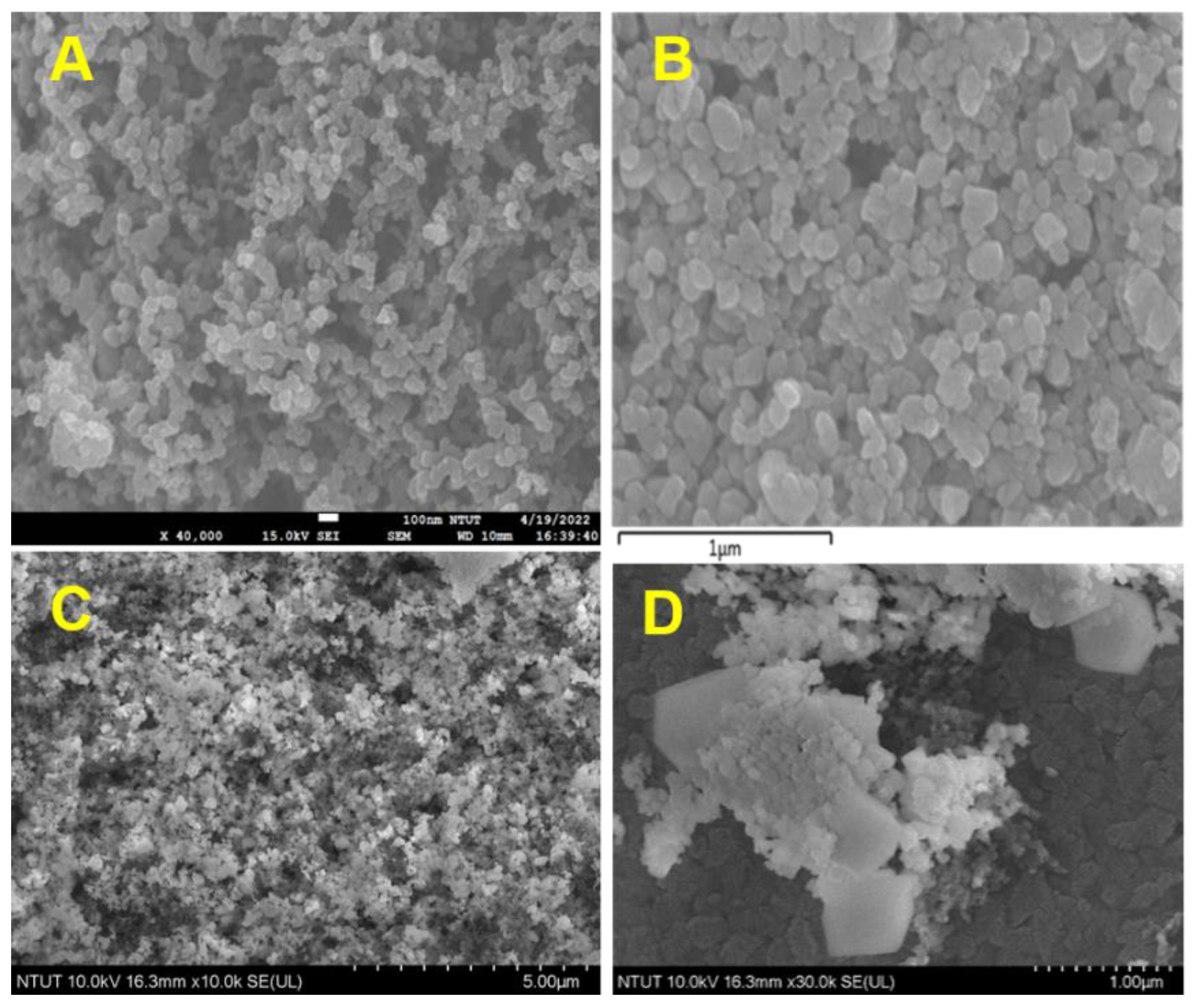
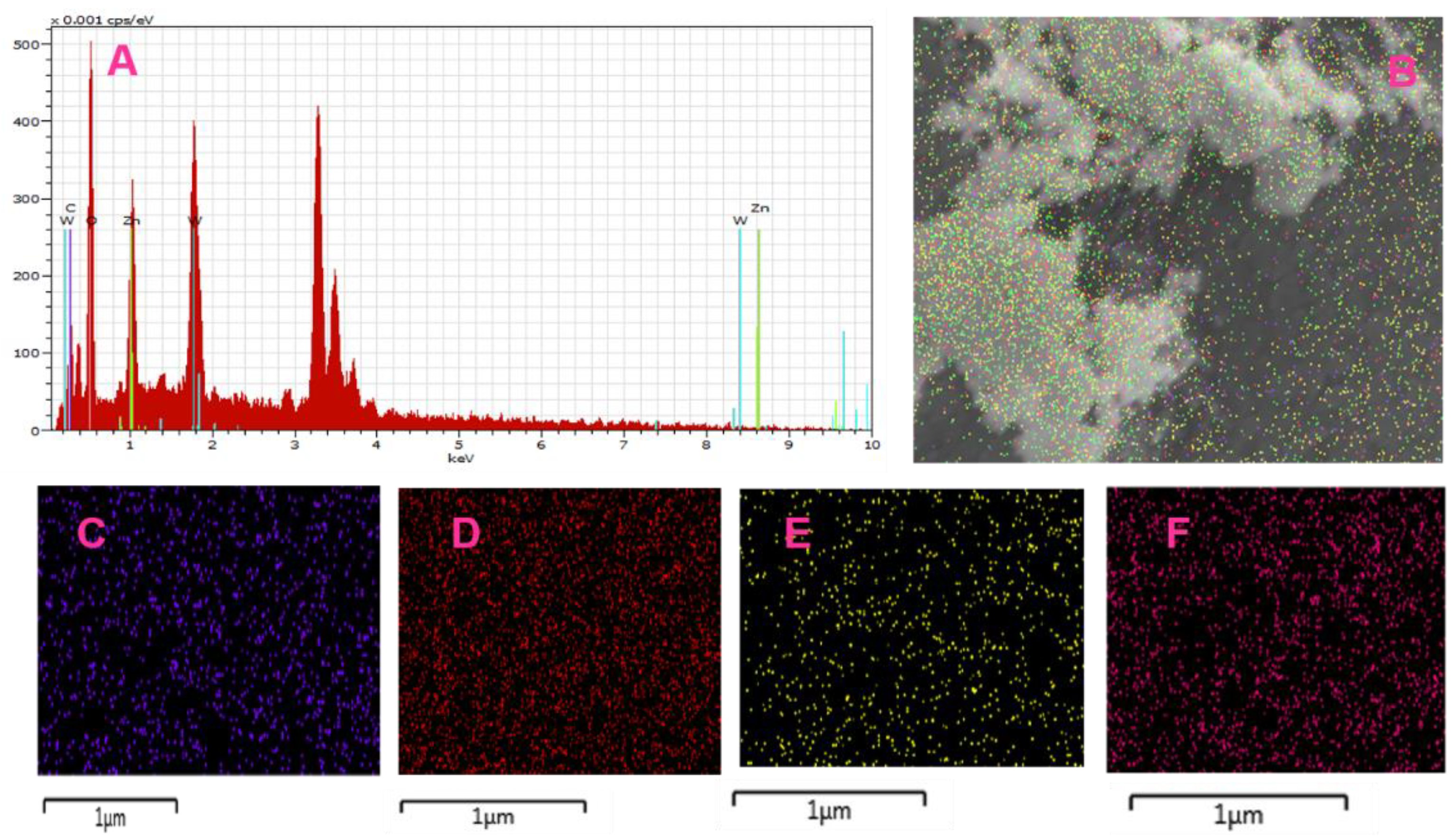
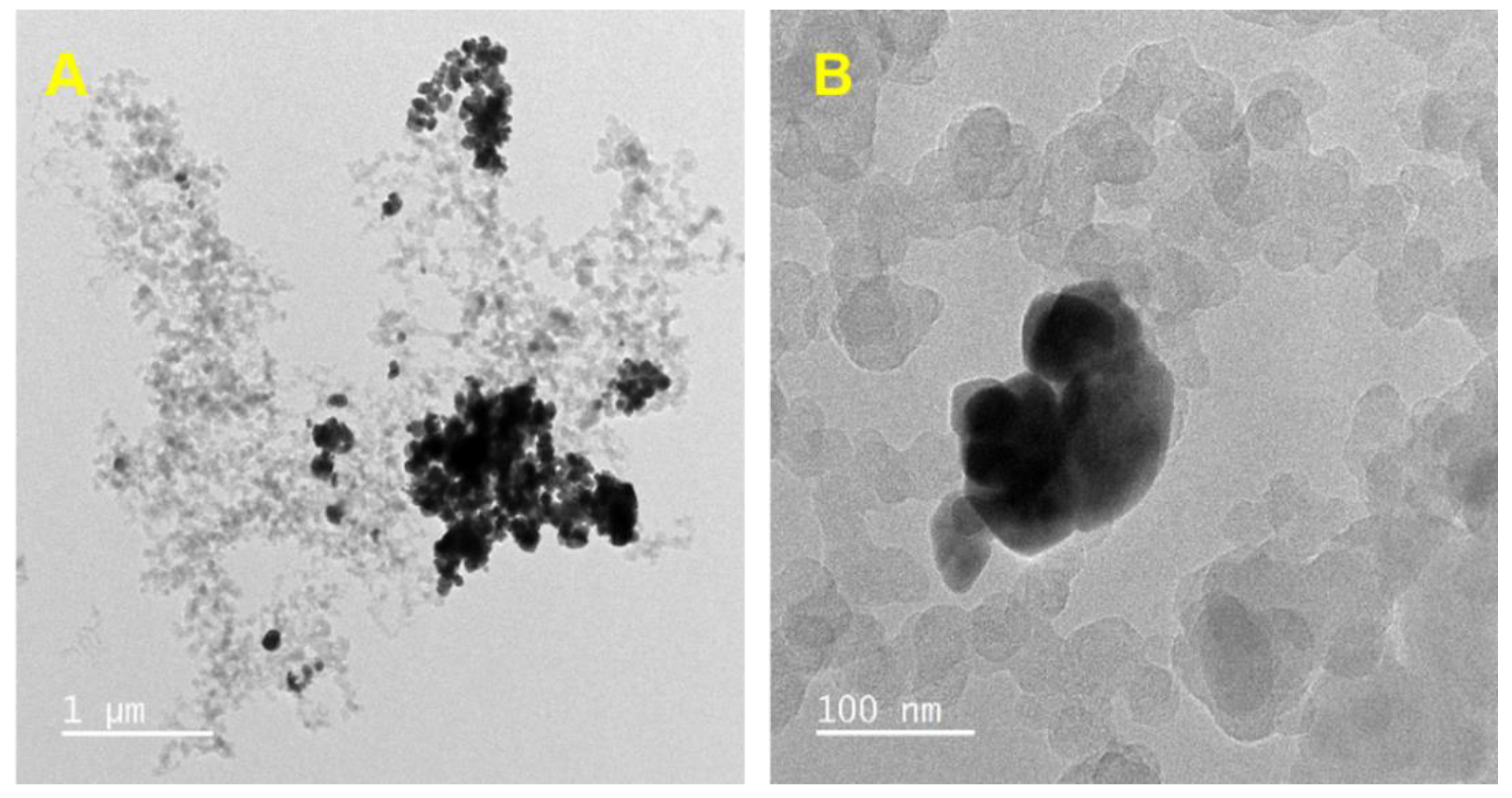
3.1. Characterization
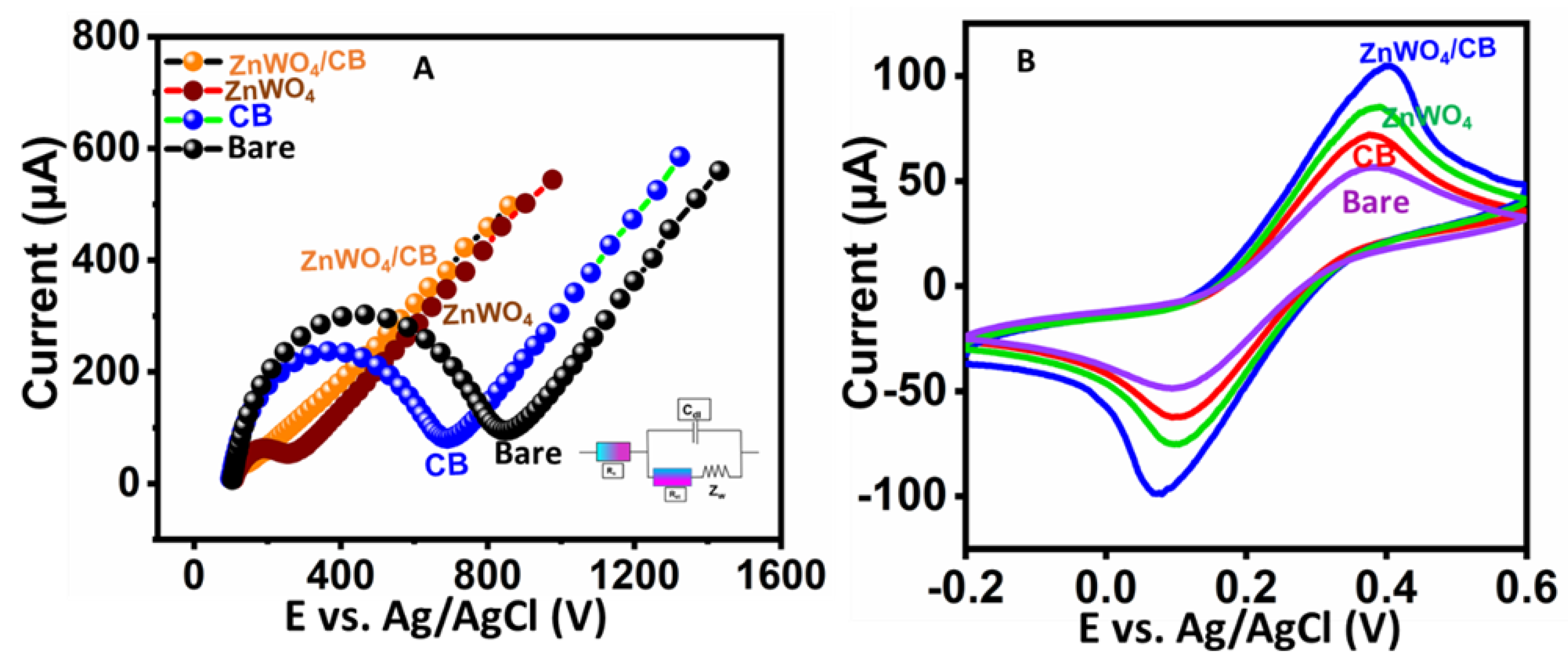
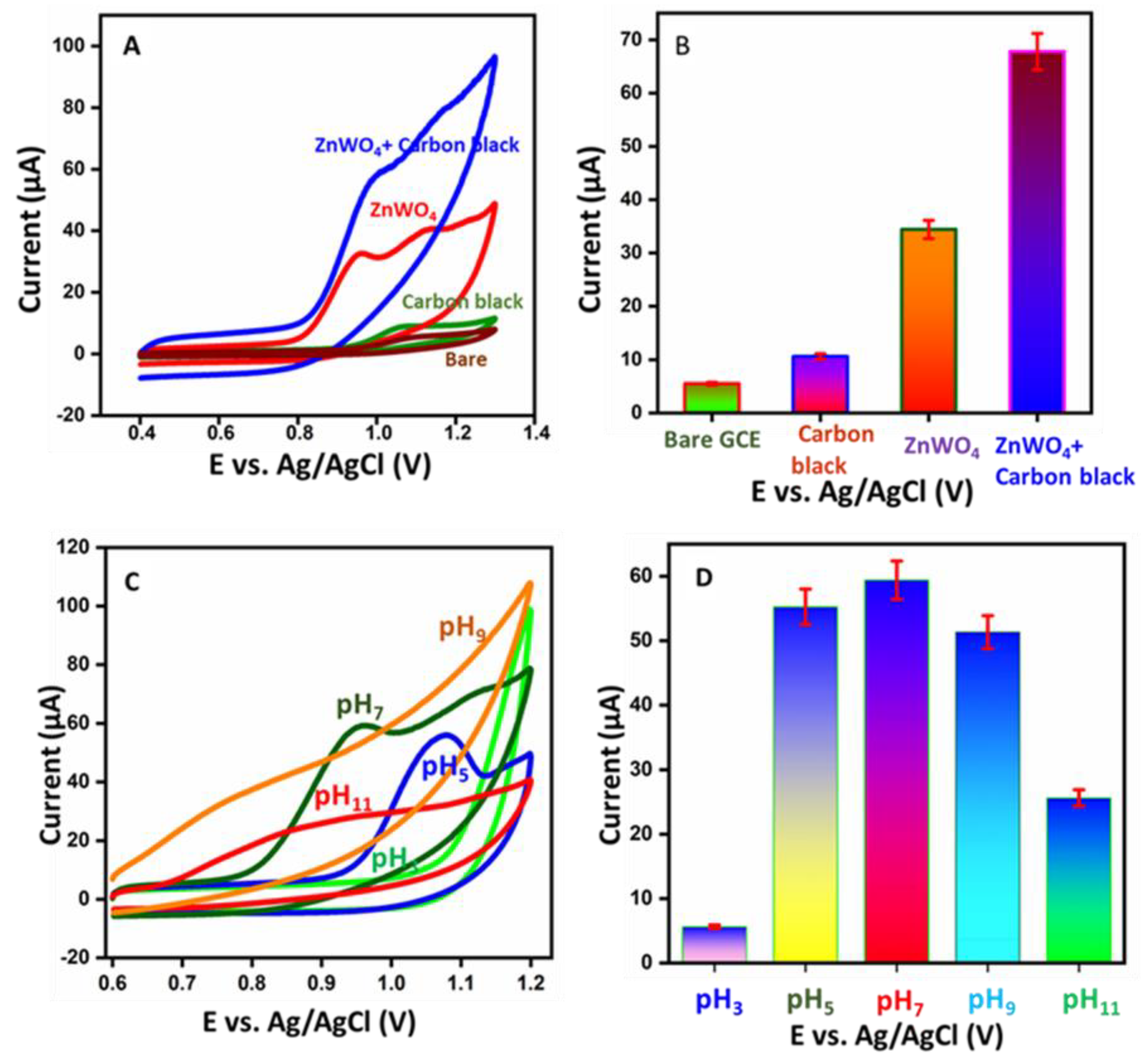
3.2. Electrochemical Performance of Different Modified Electrodes
3.3. Electrochemical Oxidation of Various Modified Electrodes and Different pH
3.4. Effect of Different Concentrations and the Scan Rates
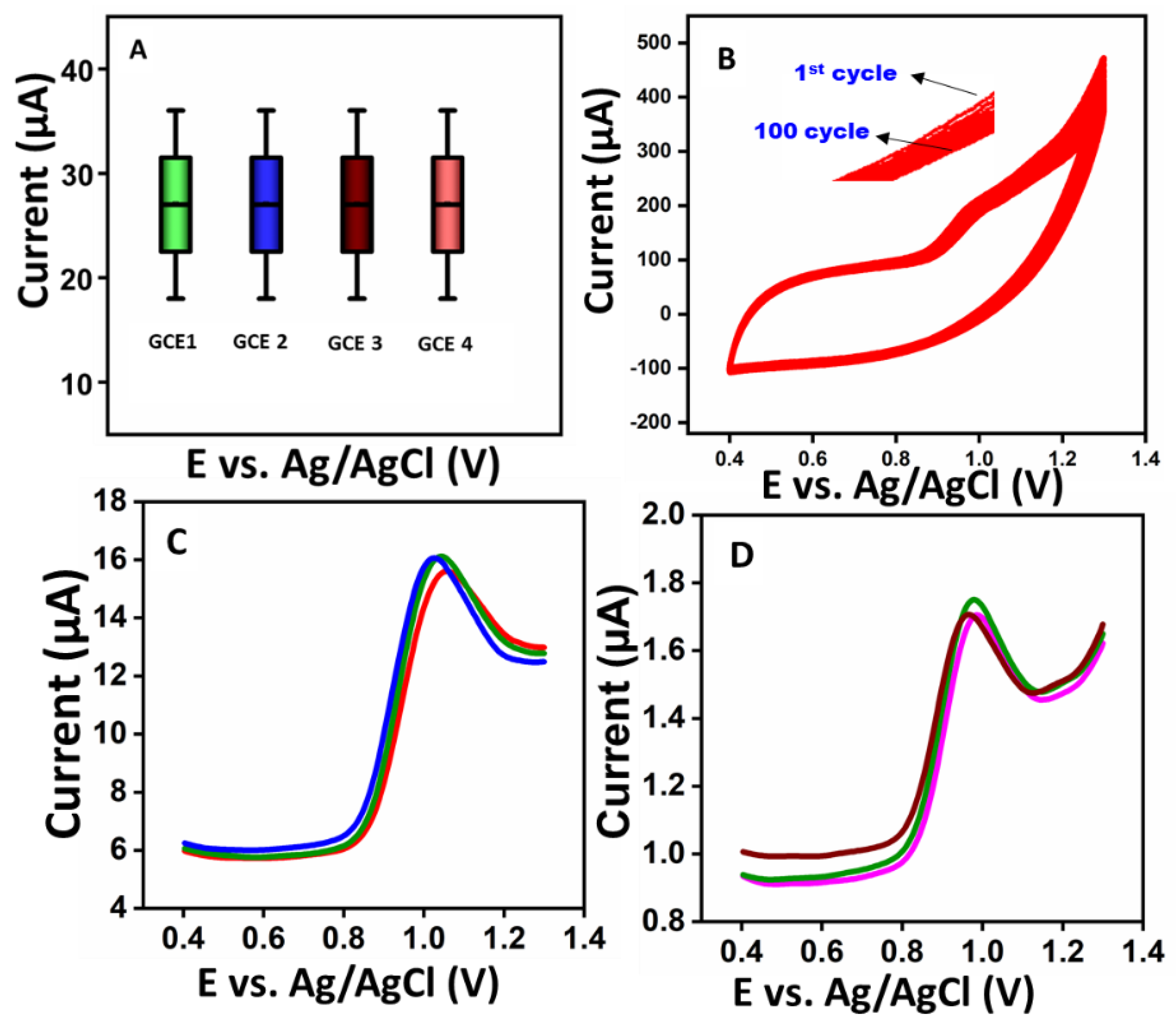
3.5. Differential Pulse Voltammetry (DPV), Interference Studies, and Repeatability of CIP
3.6. Reproducibility, Stability, and Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, J.M.; Priyanka, R.N.; Mathew, B. Bimetallic Ag–Au nanoparticles as pH dependent dual sensing probe for Mn (II) ion and ciprofloxacin. Microchem. J. 2020, 155, 104686. [Google Scholar] [CrossRef]
- Jalalvand, A.R. A study originated from combination of electrochemistry and chemometrics for investigation of the inhibitory effects of ciprofloxacin as a potent inhibitor on cytochrome P450. Microchem. J. 2020, 157, 105104. [Google Scholar] [CrossRef]
- Matulewicz, K.; Kazmierski, L.; Wisniewski, M.; Roszkowski, S.; Roszkowski, K.; Kowalczyk, O.; Roy, A.; Tylkowski, B.; Bajek, A. Ciprofloxacin and graphene oxide combination new face of a known drug. Materials 2020, 13, 4224. [Google Scholar] [CrossRef]
- Nguyen, D.; Hui, A.; Weeks, A.; Heynen, M.; Joyce, E.; Sheardown, H.; Jones, L. Release of ciprofloxacin-HCl and dexamethasone phosphate by hyaluronic acid containing silicone polymers. Materials 2012, 5, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Basyooni, M.A.; Zaki, S.E.; Ertugrul, S.; Yilmaz, M.; Eker, Y.R. Fast response of CO2 room temperature gas sensor based on mixed valence phases in molybdenum and tungsten oxide nanostructured thin films. Ceram. Int. 2019, 19, 33761–33767. [Google Scholar] [CrossRef]
- Shanmugana, S.; Gorjianb, S.; Elsheikhc, A.H.; Essa, F.A.; Omarad, Z.M.; Raghue, A.V. Investigation into the effects of SiO2/TiO2 nanolayer on the thermal performance of solar box type cooker. Energy Sources A Recovery Util. Environ. Eff. 2021, 43, 2724–2737. [Google Scholar] [CrossRef]
- Pollap, A.; Baran, K.; Kuszewska, N.; Kochana, J. Electrochemical sensing of ciprofloxacin and paracetamol in environmental water using titanium sol-based sensor. J. Electroanal. Chem. 2020, 878, 114574. [Google Scholar] [CrossRef]
- Reddy, K.R.; Brahman, P.K.; Suresh, L. Fabrication of high-performance disposable screen printed electrochemical sensor for ciprofloxacin sensing in biological samples. Measurement 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Azriouil, M.M.M.; Ettadili, F.E.; Laghrib, F.; Farahi, A.; Saqrane, S.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Recent trends on electrochemical determination of antibiotic Ciprofloxacin in biological fluids, pharmaceutical formulations, environmental resources and foodstuffs: Direct and indirect approaches. Food Chem. Toxicol. 2022, 168, 113378. [Google Scholar] [CrossRef]
- Hu, X.; Goud, K.Y.; Kumar, V.S.; Catanante, G.; Li, Z.; Zhu, Z.; Marty, J.L. Disposable electrochemical aptasensor based on carbon nanotubes-V2O5-chitosan nanocomposite for detection of ciprofloxacin. Sens. Actuators B Chem. 2018, 268, 278–286. [Google Scholar] [CrossRef]
- Islam, R.; Singh, V.; Ammeter, D.; Schweizer, F.; Kuss, S. Electrochemical characterization of the antibiotic hybrid ciprofloxacin-tobramycin. Electrochem. Commun. 2020, 119, 106825. [Google Scholar] [CrossRef]
- Jalal, N.R.; Madrakian, T.; Afkhami, A.; Ghamsari, M. Polyethylenimine@ Fe3O4@ carbon nanotubes nanocomposite as a modifier in glassy carbon electrode for sensitive determination of ciprofloxacin in biological samples. J. Electroanal. Chem. 2019, 833, 281–289. [Google Scholar] [CrossRef]
- Babu, B.; Koutavarapu, R.; Shim, J.; Kim, J.; Yoo, K. Enhanced solar-light-driven photocatalytic and photoelectrochemical properties of zinc tungsten oxide nanorods anchored on bismuth tungsten oxide nanoflakes. Chemosphere 2021, 268, 129346. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Moradi, F.; Shahmoradi, B.; Rezaee, R.; Lee, S.M. The photocatalytic removal of diazinon from aqueous solutions using tungsten oxide doped zinc oxide nanoparticles immobilized on glass substrate. J. Mol. Liq. 2020, 297, 111918. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Reddy, C.V.; Syed, K.; Reddy, K.R.; Saleh, T.A.; Lee, D.Y.; Aminabhavi, T.M. Novel Z-scheme binary zinc tungsten oxide/nickel ferrite nanohybrids for photocatalytic reduction of chromium (Cr (VI)), photoelectrochemical water splitting and degradation of toxic organic pollutants. J. Hazard. Mater. 2022, 423, 127044. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Yue, Y.; Wang, X.; Srikhaow, A.; Sriprachuabwong, C.; Qin, J. Strongly coupled tungsten oxide/carbide heterogeneous hybrid for ultrastable aqueous rocking-chair zinc-ion batteries. J. Chem. Eng. 2021, 426, 131893. [Google Scholar] [CrossRef]
- Fu, R.; Yang, J.; Chang, W.C.; Chang, W.C.; Chang, C.M.; Lin, D.; Shieh, H.P.D. The influence of annealing temperature on amorphous indium-zinc-tungsten oxide thin-film transistors. Phys. Status Solidi 2018, 215, 1700785. [Google Scholar] [CrossRef]
- Reddy, C.; Koutavarapu, R.; Reddy, I.N.; Shim, J. Effect of a novel one-dimensional zinc tungsten oxide nanorods anchored two-dimensional graphitic carbon nitride nanosheets for improved solar-light-driven photocatalytic removal of toxic pollutants and photoelectrochemical water splitting. J. Mater. Sci. Mater. Electron 2021, 32, 33–46. [Google Scholar] [CrossRef]
- Shad, N.A.; Bajwa, S.Z.; Amin, N.; Taj, A.; Hameed, S.; Khan, Y.; Khan, W.S. Solution growth of 1D zinc tungstate (ZnWO4) nanowires; design, morphology, and electrochemical sensor fabrication for selective detection of chloramphenicol. J. Hazard. Mater. 2019, 367, 205–214. [Google Scholar] [CrossRef]
- Hou, C.; Tang, W.; Zhang, C.; Wang, Y.; Zhu, N. A novel and sensitive electrochemical sensor for bisphenol A determination based on carbon black supporting ferroferric oxide nanoparticles. Electrochim. Acta 2014, 144, 324–331. [Google Scholar] [CrossRef]
- Arduini, F.; Zanardi, C.; Cinti, S.; Terzi, F.; Moscone, D.; Palleschi, G.; Seeber, R. Effective electrochemical sensor based on screen-printed electrodes modified with a carbon black-Au nanoparticles composite. Sens. Actuators B Chem. 2015, 212, 536–543. [Google Scholar] [CrossRef]
- Deroco, P.B.; Melo, I.G.; Silva, L.S.; Eguiluz, K.I.; Salazar, B.G.R.; Fatibello, F.O. Carbon black supported Au–Pd core-shell nanoparticles within a di-hexadecyl phosphate film for the development of hydrazine electrochemical sensor. Sens. Actuators B Chem. 2018, 256, 535–542. [Google Scholar] [CrossRef]
- Cinti, S.; Colozza, N.; Cacciotti, I.; Moscone, D.; Polomoshnov, M.; Sowade, E.; Arduini, F. Electroanalysis moves towards paper-based printed electronics: Carbon black nanomodified inkjet-printed sensor for ascorbic acid detection as a case study. Sens. Actuators B Chem. 2018, 265, 155–160. [Google Scholar] [CrossRef]
- Messaoud, N.B.; Lahcen, A.A.; Dridi, C.; Amine, A. Ultrasound assisted magnetic imprinted polymer combined sensor based on carbon black and gold nanoparticles for selective and sensitive electrochemical detection of Bisphenol A. Sens. Actuators B Chem. 2018, 276, 304–312. [Google Scholar] [CrossRef]
- Baccarin, M.; Santos, F.A.; Vicentini, F.C.; Zucolotto, V.; Janegitz, B.C.; Fatibello, F.O. Electrochemical sensor based on reduced graphene oxide/carbon black/chitosan composite for the simultaneous determination of dopamine and paracetamol concentrations in urine samples. J. Electroanal. Chem. 2017, 799, 436–443. [Google Scholar] [CrossRef]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello, F.O. Electrochemical biosensors based on nanostructured carbon black: A review. J. Nanomater. 2017, 2017, 4571614. [Google Scholar] [CrossRef]
- Santhiago, M.; Corrêa, C.C.; Bernardes, J.S.; Pereira, M.P.; Oliveira, L.J.; Strauss, M.; Bufon, C.C. Flexible and foldable fully-printed carbon black conductive nanostructures on paper for high-performance electronic, electrochemical, and wearable devices. ACS Appl. Mater. Interfaces 2017, 9, 24365–24372. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mazzaracchio, V.; Cacciotti, I.; Moscone, D.; Arduini, F. Carbon black-modified electrodes screen-printed onto paper towel, waxed paper, and parafilm M. Sensors 2017, 17, 2267. [Google Scholar] [CrossRef]
- Goulart, L.A.; Guaraldo, T.T.; Lanza, M.R. A novel electrochemical sensor based on Printex L6 carbon black carrying CuO/Cu2O nanoparticles for propylparaben determination. Electroanalysis 2018, 30, 2967–2976. [Google Scholar] [CrossRef]
- Delgado, K.P.; Raymundo, P.P.A.; Campos, A.M.; Oliveira, J.O.N.; Janegitz, B.C. Ultralow cost electrochemical sensor made of potato starch and carbon black nanoballs to detect tetracycline in waters and milk. Electroanalysis 2018, 30, 2153–2159. [Google Scholar] [CrossRef]
- Batista, D.P.; Campanha, V.F.; Fatibello, F.O. An electrochemical sensor for the simultaneous determination of paracetamol and codeine using a glassy carbon electrode modified with nickel oxide nanoparticles and carbon black. Electroanalysis 2015, 27, 2214–2220. [Google Scholar] [CrossRef]
- Reddy, K.R.; Jeong, H.M.; Lee, Y.; Raghu, A.V. Synthesis of MWCNTs-core/Thiophene polymer-sheath composite nanocables by a cationic surfactant-assisted chemical oxidative polymerization and their structural properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1477–1484. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Q.; Chen, T.; Li, J.; Liu, Y.; Shan, X.; Jia, J. Recent advances in electrochemical sensors for antibiotics and their applications. Chin. Chem. Lett. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. Glassy Carbon Electrodes Film-Modified with Acidic Functionalities. A Review. Electroanalysis 2012, 24, 1481–1500. [Google Scholar] [CrossRef]
- Ibrahim, I.R.; Matori, K.A.; Ismail, I.; Awang, Z.; Rusly, S.N.A.; Nazlan, R.; Ertugrul, M. A study on microwave absorption properties of carbon black and Ni0. 6Zn0. 4Fe2O4 nanocomposites by tuning the matching-absorbing layer structures. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Liang, Q.; Li, Y.; Yu, H. CoS/ZnWO4 composite with band gap matching: Simple impregnation synthesis, efficient dye sensitization system for hydrogen production. J. Nanoparticle Res. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Fan, X.; Chen, X. Facile synthesis of NFL-ZnWO4 for pseudocapacitor applications. In Proceedings of the MATEC Web of Conferences, Lisbon, Portugal, 25–27 September 2019; Volume 272, p. 01005. [Google Scholar]
- Xie, A.J.; Chen, Y.; Luo, S.P.; Tao, Y.W.; Jin, Y.S.; Li, W.W. Electrochemical detection of ciprofloxacin based on graphene modified glassy carbon electrode. Mater. Technol. 2015, 30, 362–367. [Google Scholar] [CrossRef]
- Koventhan, C.; Vinothkumar, V.; Chen, S.M.; Sangili, A. Highly sensitive electrode materials for the voltammetric determination of nitrofurantoin based on zinc cobaltate nanosheets. New J. Chem. 2020, 44, 12036–12047. [Google Scholar] [CrossRef]
- De, S.C.C.; Alves, G.F.; Lisboa, T.P.; Matos, M.A.C.; Matos, R.C. Low cost paper-based electrochemical sensor for the detection of ciprofloxacin in honey and milk samples. J. Food Compos. Anal. 2022, 112, 104700. [Google Scholar]
- Zhao, J.; Huang, P.; Jin, W. Electrochemical sensor based on TiO2/polyvinyl alcohol nanocomposite for detection of ciprofloxacin in rainwater. Int. J. Electrochem. Sci. 2021, 16, 211018. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhao, S. A new electrochemical sensor for simultaneous detection of sulfamethoxazole and trimethoprim antibiotics based on graphene and ZnO nanorods modified glassy carbon electrode. Microchem. J. 2020, 159, 105440. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.C.; Balram, D. Electrochemical detection of an antibiotic drug chloramphenicol based on a graphene oxide/hierarchical zinc oxide nanocomposite. Inorg. Chem. Front. 2019, 6, 82–93. [Google Scholar] [CrossRef]
- Kumar, D.R.; Manoj, D.; Santhanalakshmi, J. Optimization of oleylamine Fe3O4/MWCNTs nanocomposite modified GC electrode for electrochemical determination of ofloxacin. J. Nanosci. Nanotechnol. 2014, 14, 5059. [Google Scholar] [CrossRef]
- Sawkar, R.R.; Shanbhag, M.M.; Tuwar, S.M.; Mondal, K.; Shetti, N.P. Sodium dodecyl sulfate mediated graphene sensor for electrochemical detection of the antibiotic drug: Ciprofloxacin. Materials 2022, 15, 7872. [Google Scholar] [CrossRef] [PubMed]






| Different Electrodes | Linear Range (µM) | LOD (µM) | References |
|---|---|---|---|
| a TiO2/b AuNPs/Nafion/c CMK-3 | 0.108 | [7] | |
| d AuNP/e CHI/f SPCE | - | 0.001 | [8] |
| g CNT-h V2O5CS/i SPCE | - | 3.0 | [10] |
| j PBE | - | 4.96 | [40] |
| TiO2/k PVA-GCE | 10–120 | 0.04 | [41] |
| l GR-m ZnO/GCE | 1–180 | 0.4 | [42] |
| n GO/ZnO/GCE | 0.2–7.2 | 0.01 | [43] |
| o OLA-p Fe3O4/q MWCNTs/GCE | 0.01–8.9 | 0.06 | [44] |
| r SDS·s Gr/t CPE | 0.3–100 | 0.029 | [45] |
| u GCE/v ZnWO4/w CB | 0.02–120 | 0.02 | This work |
| Real Samples | Added | Found | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| River water | 10 | 9 | 98.5 | |
| 20 | 19 | 98.1 | 4.20% | |
| 30 | 29 | 97.6 | ||
| Tap water | 10 | 9.5 | 99.2 | |
| 20 | 19 | 98.5 | 3.60% | |
| 30 | 29.5 | 98.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariappan, K.; Alagarsamy, S.; Chen, S.-M.; Sakthinathan, S. Fabrication of ZnWO4/Carbon Black Nanocomposites Modified Glassy Carbon Electrode for Enhanced Electrochemical Determination of Ciprofloxacin in Environmental Water Samples. Materials 2023, 16, 741. https://doi.org/10.3390/ma16020741
Mariappan K, Alagarsamy S, Chen S-M, Sakthinathan S. Fabrication of ZnWO4/Carbon Black Nanocomposites Modified Glassy Carbon Electrode for Enhanced Electrochemical Determination of Ciprofloxacin in Environmental Water Samples. Materials. 2023; 16(2):741. https://doi.org/10.3390/ma16020741
Chicago/Turabian StyleMariappan, Kiruthika, Saranvignesh Alagarsamy, Shen-Ming Chen, and Subramanian Sakthinathan. 2023. "Fabrication of ZnWO4/Carbon Black Nanocomposites Modified Glassy Carbon Electrode for Enhanced Electrochemical Determination of Ciprofloxacin in Environmental Water Samples" Materials 16, no. 2: 741. https://doi.org/10.3390/ma16020741
APA StyleMariappan, K., Alagarsamy, S., Chen, S.-M., & Sakthinathan, S. (2023). Fabrication of ZnWO4/Carbon Black Nanocomposites Modified Glassy Carbon Electrode for Enhanced Electrochemical Determination of Ciprofloxacin in Environmental Water Samples. Materials, 16(2), 741. https://doi.org/10.3390/ma16020741







