Architecture of Nanoantioxidant Based on Mesoporous Organosilica Trp-Met-PMO with Dipeptide Skeleton
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
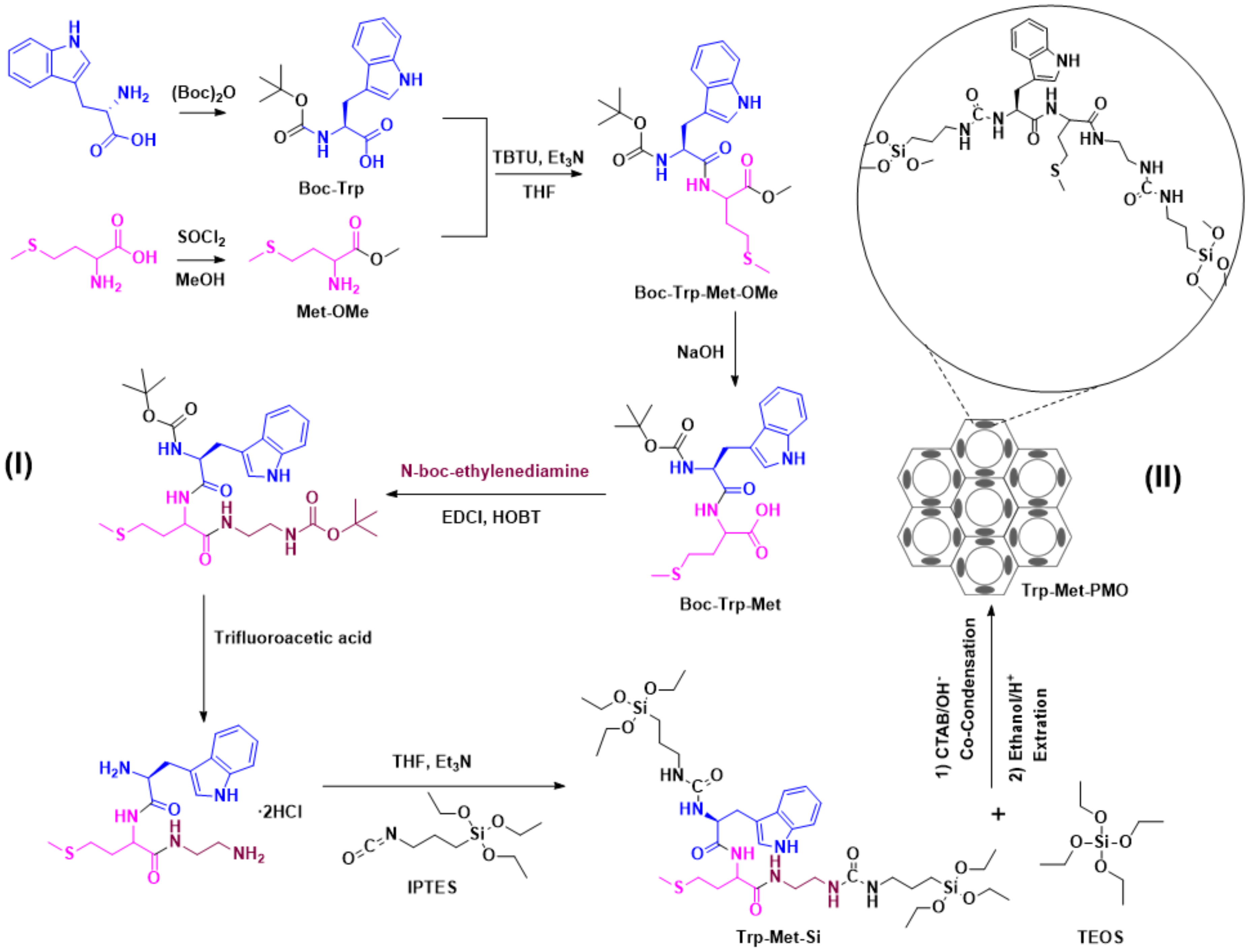
2.3. Synthesis of Dipeptide Organosilica Precursor (Trp-Met-Si)
2.3.1. Synthesis of Dipeptide BOC-Trp-Met-OMe
2.3.2. Synthesis of Dipeptide Organosilica Precursor Trp-Met-Si
2.4. Preparation of Mesoporous Organosilica (Trp-Met-PMO)
2.5. ABTS Radical-Scavenging Experiment
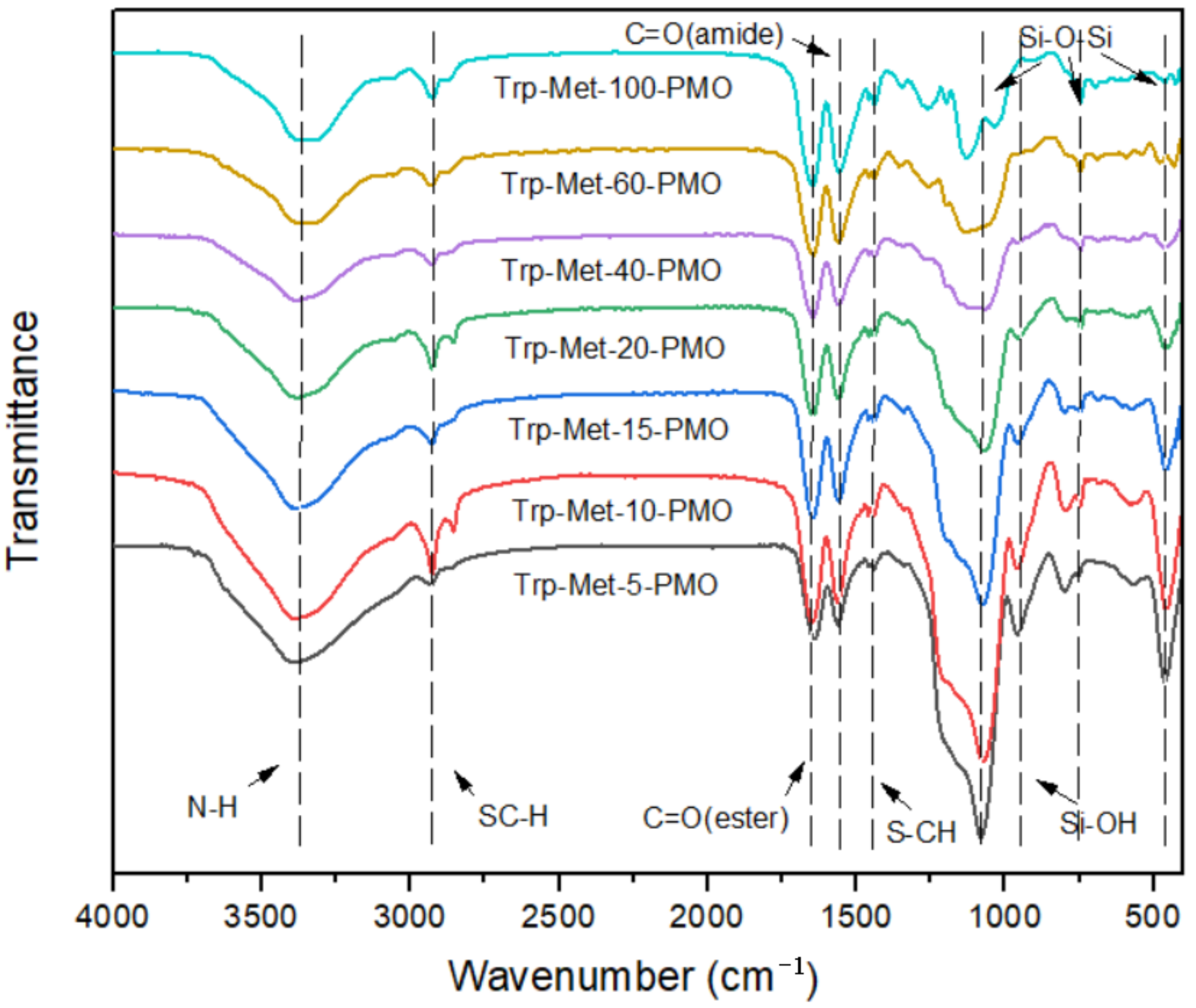
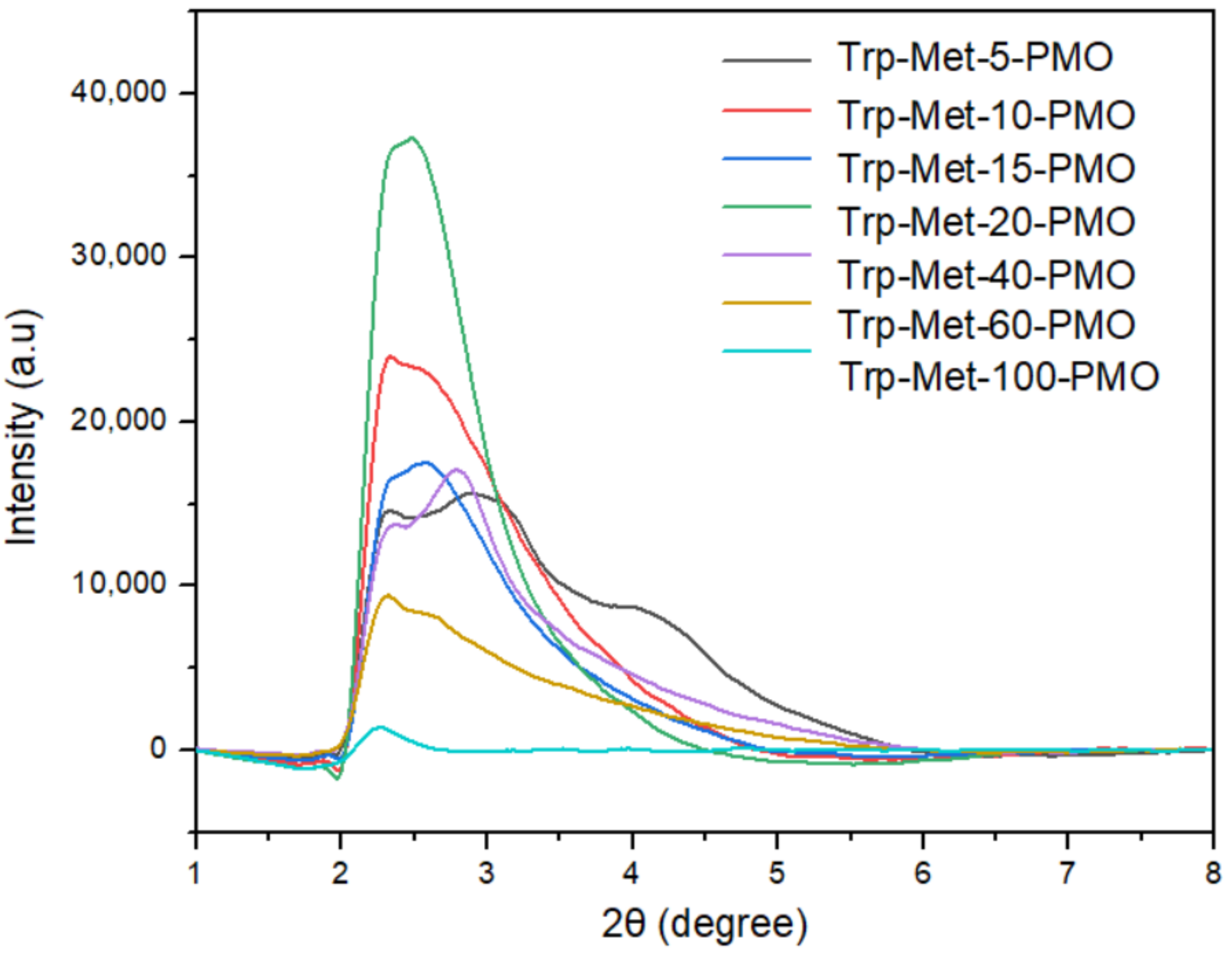
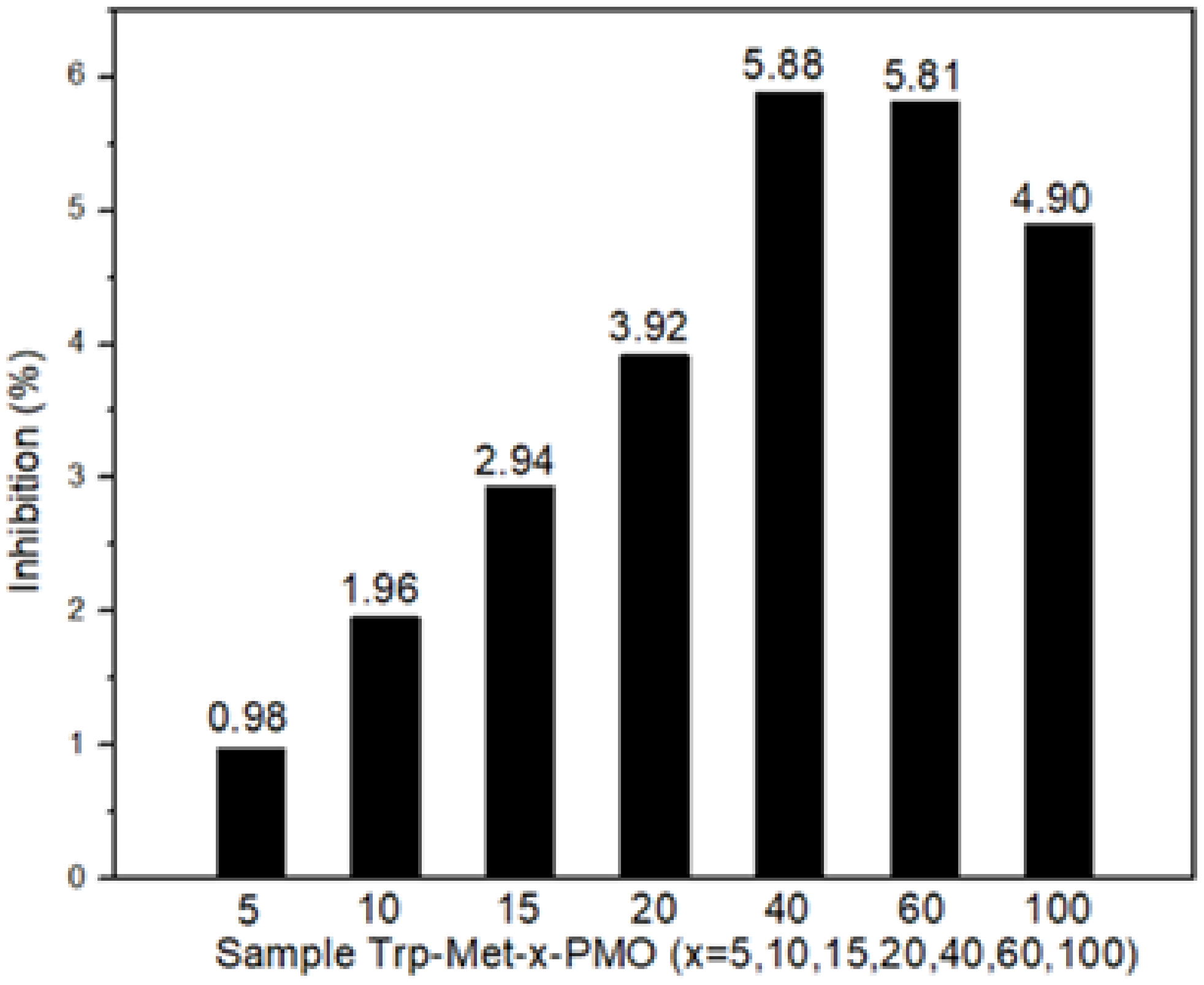
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, D.I.; Griendling, K.K. Regulation of Signal Transduction by Reactive Oxygen Species in the Cardiovascular System. Circ. Res. 2015, 116, 531–549. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef., I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals ((*)OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Nouman, W.; Basra, S.M.A.; Yasmeen, A.; Gull, T.; Hussain, S.B.; Zubair, M.; Gul, R. Seed priming improves the emergence potential, growth and antioxidant system of Moringa oleifera under saline conditions. Plant Growth Regul. 2014, 73, 267–278. [Google Scholar] [CrossRef]
- Sui, L.; Wang, J.; Xiao, Z.; Yang, Y.; Yang, Z.; Ai, K. ROS-Scavenging Nanomaterials to Treat Periodontitis. Front. Chem. 2020, 8, 595530–595535. [Google Scholar] [CrossRef]
- Alvarez Echazu, M.I.; Olivetti, C.E.; Peralta, I.; Alonso, M.R.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. extract with antioxidant activity. Colloids Surf. B Biointerfaces 2018, 169, 82–91. [Google Scholar] [CrossRef]
- Saita, M.; Kaneko, J.; Sato, T.; Takahashi, S.S.; Wada-Takahashi, S.; Kawamata, R.; Sakurai, T.; Lee, M.C.; Hamada, N.; Kimoto, K.; et al. Novel antioxidative nanotherapeutics in a rat periodontitis model: Reactive oxygen species scavenging by redox injectable gel suppresses alveolar bone resorption. Biomaterials 2016, 76, 292–301. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Turan, O.; Bielecki, P.; Perera, V.; Lorkowski, M.; Covarrubias, G.; Tong, K.; Yun, A.; Rahmy, A.; Ouyang, T.; Raghunathan, S.; et al. Delivery of drugs into brain tumors using multicomponent silica nanoparticles. Nanoscale 2019, 11, 11910–11921. [Google Scholar] [CrossRef]
- Patino-Herrera, R.; Louvier-Hernandez, J.F.; Escamilla-Silva, E.M.; Chaumel, J.; Escobedo, A.G.P.; Perez, E. Prolonged release of metformin by SiO2 nanoparticles pellets for type II diabetes control. Eur. J. Pharm. Sci. 2019, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, L.; Gong, R.; Shi, J.; Zhong, L.; Duan, X.; Zhu, Y. A ROS-scavenging multifunctional nanoparticle for combinational therapy of diabetic nephropathy. Nanoscale 2020, 12, 23607–23619. [Google Scholar] [CrossRef] [PubMed]
- Purikova, O.; Tkachenko, I.; Šmíd, B.; Veltruská, K.; Dinhová, T.N.; Vorokhta, M.; Kopecký, V.; Hanyková, L.; Ju, X. Free-Blockage Mesoporous Silica Nanoparticles Loaded with Cerium Oxide as ROS-Responsive and ROS-Scavenging Nanomedicine. Adv. Funct. Mater. 2022, 32, 2208316–2208336. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, Y.P.; Liu, T.P.; Chien, F.C.; Chou, C.M.; Chen, C.T.; Mou, C.Y. Approach to Deliver Two Antioxidant Enzymes with Mesoporous Silica Nanoparticles into Cells. ACS Appl. Mater. Interfaces 2016, 8, 17944–17954. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Shiau, C.Y.; Chen, H.M.; Chiou, T.K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2020, 11, 148–153. [Google Scholar] [CrossRef]
- Xia, Y.; Bamdad, F.; Ganzle, M.; Chen, L. Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. 2012, 134, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Sun, J.; Liu, Y.; Zeng, H.; Su, Y.; Yang, Y. ACE inhibitory peptides and antioxidant peptides derived from in vitro digestion hydrolysate of hen egg white lysozyme. Food Chem. 2012, 135, 1245–1252. [Google Scholar] [CrossRef]
- Sadat, L.; Cakir-Kiefer, C.; N’Negue, M.-A.; Gaillard, J.-L.; Girardet, J.-M.; Miclo, L. Isolation and identification of antioxidative peptides from bovine α-lactalbumin. Int. Dairy J. 2011, 21, 214–221. [Google Scholar] [CrossRef]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef]
- Walcarius, A.; Sayen, S.; Gérardin, C.; Hamdoune, F.; Rodehüser, L. Dipeptide-functionalized mesoporous silica spheres. Colloids Surf. A 2004, 234, 145–151. [Google Scholar] [CrossRef]
- Ezzati, N.; Mahjoub, A.R.; Shahrnoy, A.A.; Syrgiannis, Z. Amino acid-functionalized hollow mesoporous silica nanospheres as efficient biocompatible drug carriers for anticancer applications. Int. J. Pharm. 2019, 572, 118709–118721. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.H.; Wang, J.Q.; Zhang, W.P.; Guo, C. Synthesis of phenylalanine and leucine dipeptide functionalized silica-based nanoporous material as a safe UV filter for sunscreen. J. Sol-Gel. Sci. Technol. 2021, 97, 466–478. [Google Scholar] [CrossRef]
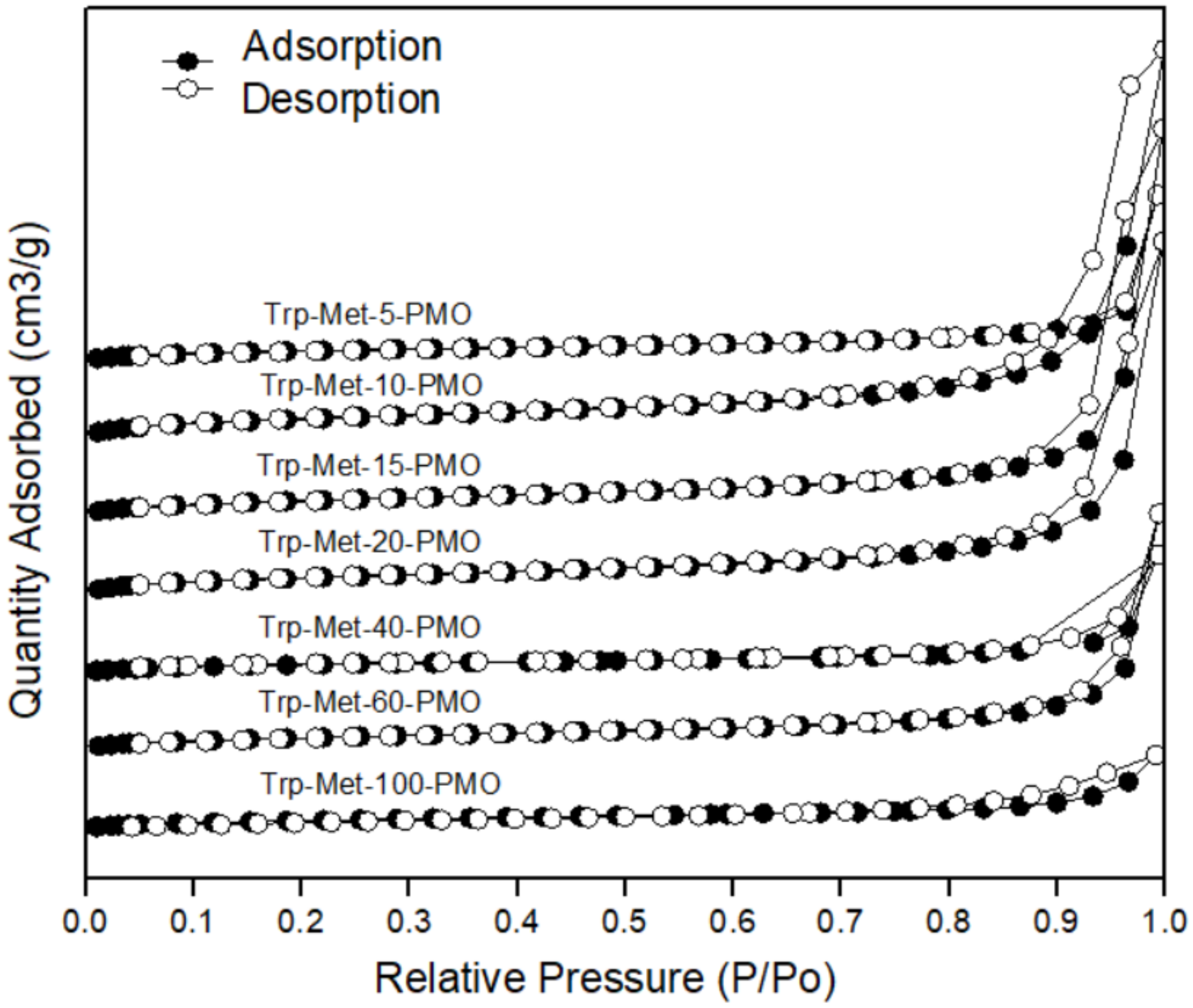
| Sample (Trp-Met-x-PMO) | d1001 (Å) | α0 2 (Å) | SBET (m2/g) | Vt (cm3/g) | Dp 3 (Å) | WT (Å) |
|---|---|---|---|---|---|---|
| x = 5 | 33.69 | 38.91 | 59 | 0.54 | 24.10 | 14.81 |
| x = 10 | 37.09 | 42.83 | 46 | 0.31 | 23.90 | 18.93 |
| x = 15 | 35.31 | 40.77 | 38 | 0.30 | 22.47 | 19.30 |
| x = 20 | 35.60 | 41.10 | 36 | 0.28 | 20.05 | 21.05 |
| x = 40 | 34.46 | 39.78 | 28 | 0.17 | 17.43 | 25.35 |
| x = 60 | 37.09 | 42.83 | 23 | 0.12 | 15.90 | 27.93 |
| x = 100 | 39.06 | 45.10 | 19 | 0.06 | 12.19 | 32.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Ma, H.; Dai, Y.; Du, Y.; Guo, C.; Wang, J. Architecture of Nanoantioxidant Based on Mesoporous Organosilica Trp-Met-PMO with Dipeptide Skeleton. Materials 2023, 16, 638. https://doi.org/10.3390/ma16020638
Zhou W, Ma H, Dai Y, Du Y, Guo C, Wang J. Architecture of Nanoantioxidant Based on Mesoporous Organosilica Trp-Met-PMO with Dipeptide Skeleton. Materials. 2023; 16(2):638. https://doi.org/10.3390/ma16020638
Chicago/Turabian StyleZhou, Wanli, Haohua Ma, Yunqiao Dai, Yijing Du, Cheng Guo, and Jianqiang Wang. 2023. "Architecture of Nanoantioxidant Based on Mesoporous Organosilica Trp-Met-PMO with Dipeptide Skeleton" Materials 16, no. 2: 638. https://doi.org/10.3390/ma16020638
APA StyleZhou, W., Ma, H., Dai, Y., Du, Y., Guo, C., & Wang, J. (2023). Architecture of Nanoantioxidant Based on Mesoporous Organosilica Trp-Met-PMO with Dipeptide Skeleton. Materials, 16(2), 638. https://doi.org/10.3390/ma16020638






