Fabrication and Characterization of a Multifunctional Coating to Promote the Osteogenic Properties of Orthopedic Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bioglass, Chitosan and Heparin-Doped Hydroxyapatite (HAp, BG, Cs, Hep) Suspension
2.3. Preparation of Coatings
2.4. Characterization Methods
2.5. Cell Culture and Morphology
2.6. Potentiodynamic Polarization
3. Results and Discussion
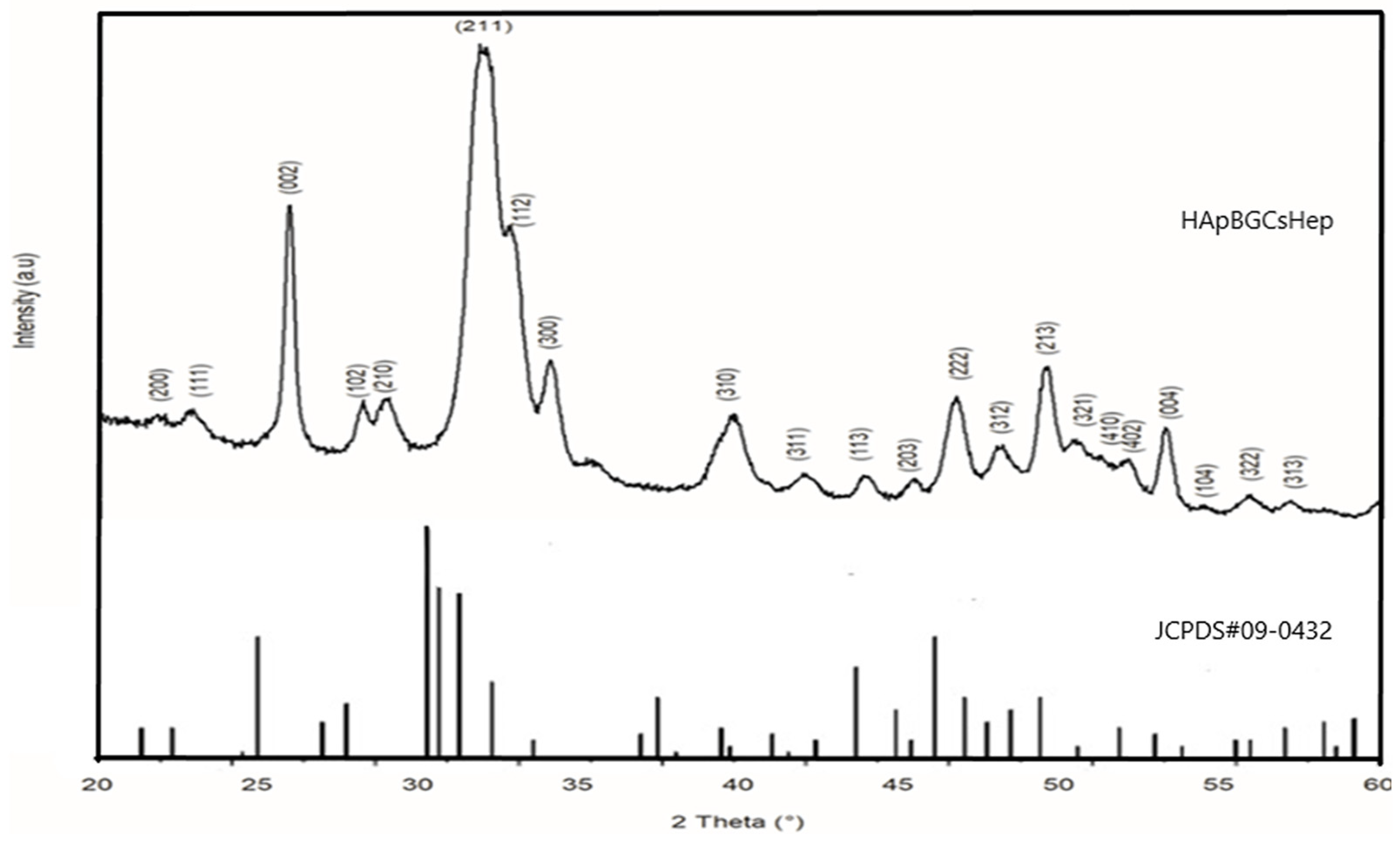
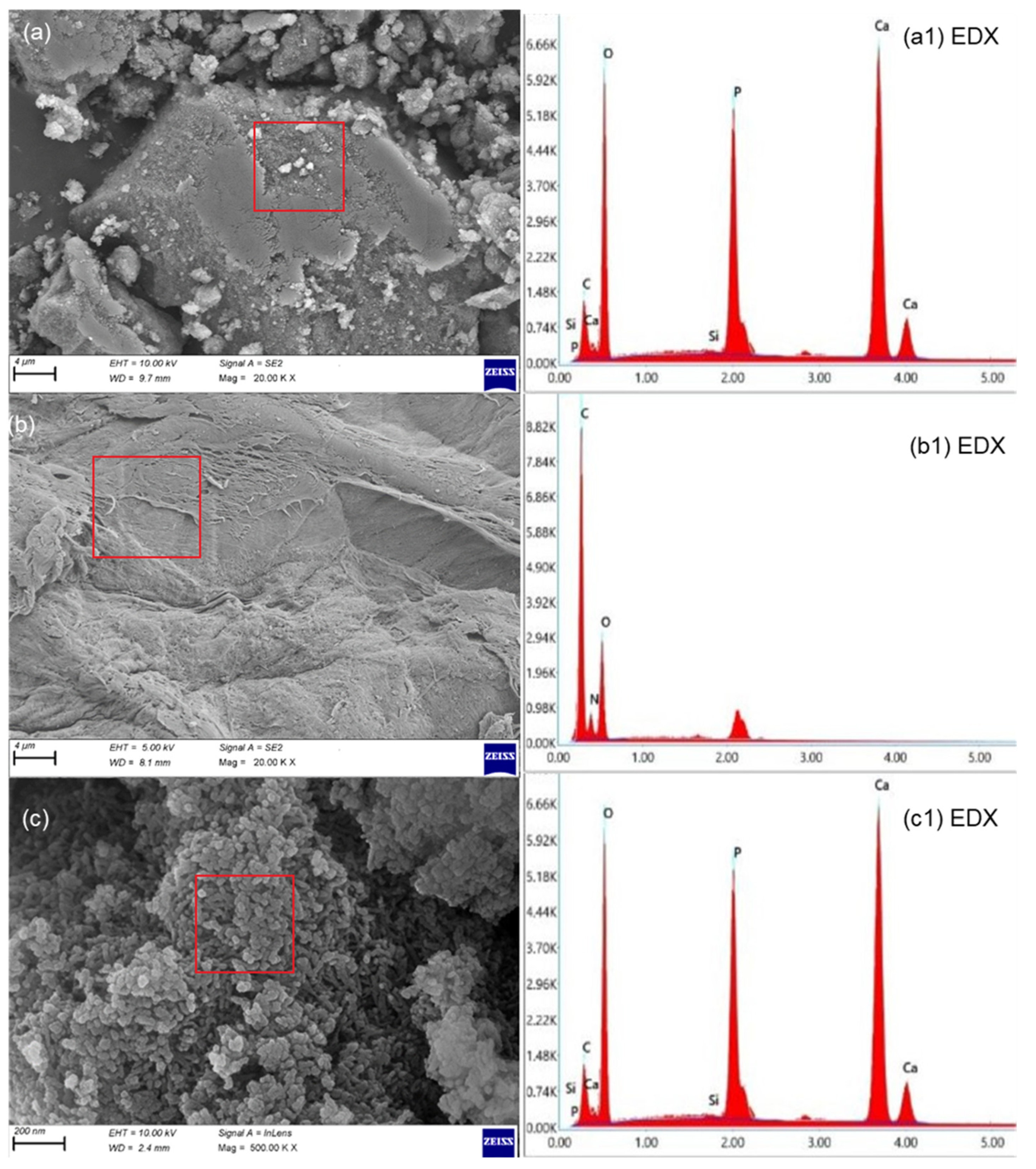
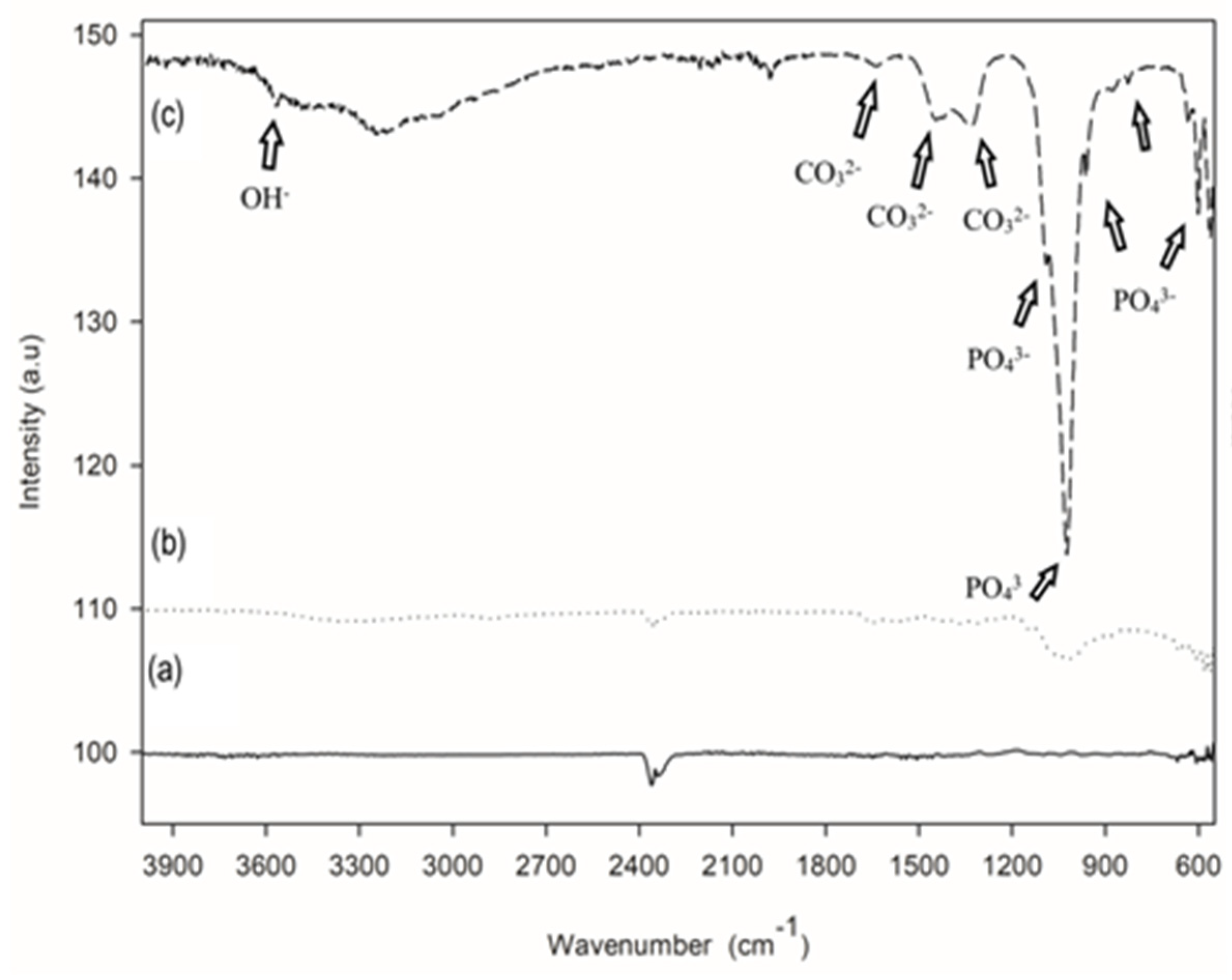
3.1. Coating (Physical) Characterization
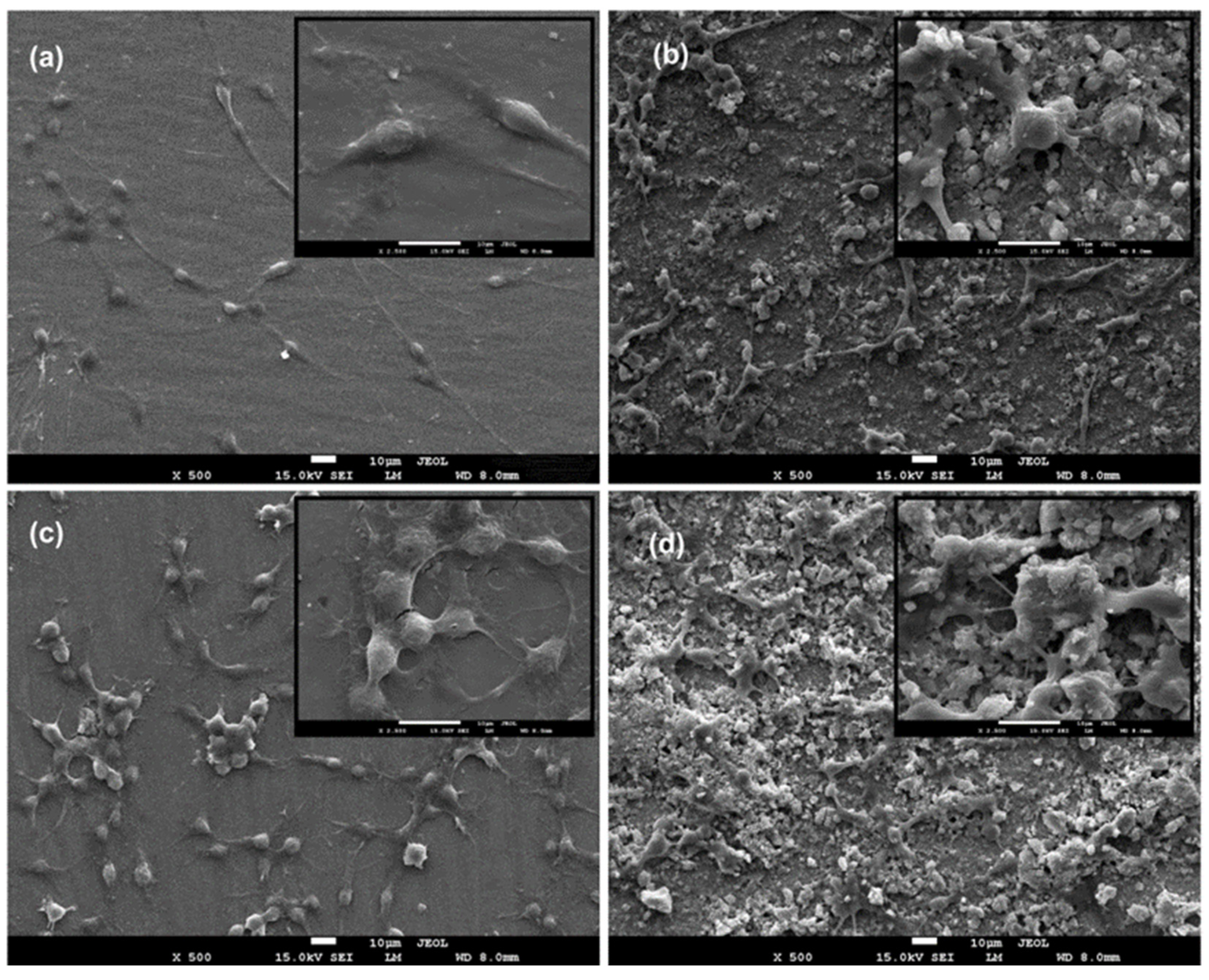
3.2. In Vitro Cell Behaviour (Scanning Electron Microscopy (Biocompatibility))
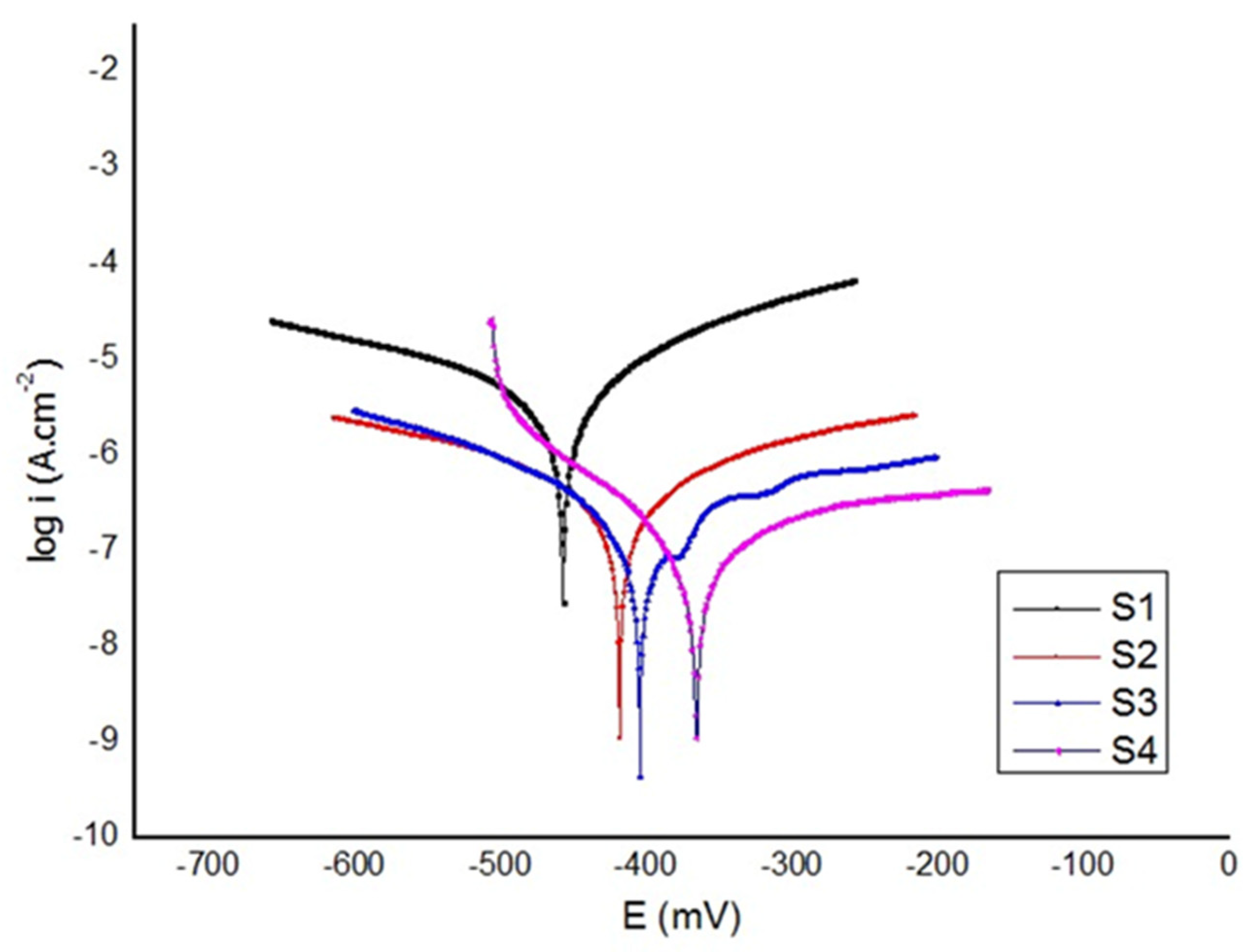
3.3. Electrochemical Test
4. Conclusions
- Doped HAp components remain similar in terms of chemical composition and crystallinity, regardless of the bioglass, chitosan, and heparin contents in the solution.
- With a proliferation rate of over 85% of the control, HApBGCSHep-coated discs proved to have greater biocompatibility than the other HAp+bioglass and chitosan+heparin coatings.
- The composite coatings had a smooth, crack-free surface with few micropores, according to the results of the SEM examination.
- Taken together, our data show that the HApBGCSHep-coated Ti6Al4V alloy is bioactive and predicts greater biocompatibility in vitro. Implantation of this composite material in animal models will be required next as proof of concept.
- The polarization tests revealed that the HApBGCsHep coating effectively enhanced the corrosion resistance of the coated samples.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706–2715. [Google Scholar] [CrossRef]
- Roche, B.; David, V.; Vanden-Bossche, A.; Peyrin, F.; Malaval, L.; Vico, K.; Lafage-Proust, M.-H. Structure and quantification of microvascularisation within mouse long bones: What and how should we measure. Bone 2012, 50, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Grüneboom, A.; Hawwari, İ.; Weicher, D.; Culeman, S.; Müller, S.; Henneberg, S.; Breizel, A.; Merz, S.; Borneman, S.; Zee, K. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat. Metab. 2019, 1, 236–250. [Google Scholar] [CrossRef]
- Dee, K.C.; PuleBizios, R. An Introduction to Tissue-Biomaterial Interactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Lee, Y.K.; Park, J.H.; Moon, H.T.; Lee, D.Y.; Yun, Y.H.; Byun, Y. The short-term effects on restenosis and thrombosis o echinomycin-eluting stents top coated with a hydrophobic heparin-containing polymer. Biomaterials 2007, 28, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, H.; Ghasemi, Z.; Kharaziha, M.; Karimzadeh, F.; Alihosseini, F. Chitosan-58S bioactive glass nanocomposite coatings on TiO2 nanotube: Structural and biological properties. Appl. Surf. Sci. 2018, 441, 138–149. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A Versatile Biopolymer for Orthopaedic Tissue-Engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitosan Composites with Inorganics, Morphogenetic Proteins and Stem Cells, for Bone Regeneration. Carbohydr. Polym. 2011, 83, 1433–1445. [Google Scholar] [CrossRef]
- Mohammadi, F.; Golafshan, N.; Kharaziha, M.; Ashrafi, A. Chitosan-heparin nanoparticle coating on anodized NiTi for improvement of blood compatibility and biocompatibility. Int. J. Biol. Macromol. 2009, 127, 159–168. [Google Scholar] [CrossRef]
- Ma, R.; Sask, K.N.; Shi, C.; Brash, J.L.; Zhitomirsky, I. Electrodeposition of polypyrrole-heparin and polypyrrole-hydroxyapatite films. Mater. Lett. 2011, 65, 681–684. [Google Scholar] [CrossRef]
- Muzamil, H.; Sami, U.; Muhammad, R.R.; Naseem, A.; Ahsan, A. Recent Developments in Zn-Based Biodegradable Materials for Biomedical Applications. J. Funct. Biomater. 2023, 14, 1. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Xiao, G.-Y.; Lu, Y.-P. Phosphate chemical conversion coatings on metallic substrates for biomedical application: A review. Mater. Sci. Eng. C 2015, 47, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bruni, S. Effects of surface treatment of Ti–6Al–4V titanium alloy on biocompatibility in cultured human umbilical vein endothelial cells. Acta Biomater. 2005, 1, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thovas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Oryhop. Relat. Res. 2010, 468, 45. [Google Scholar] [CrossRef]
- Liu, J.; Miao, X. Sol–gel derived bioglass as a coating material for porous alumina scaffolds. Ceram. Int. 2004, 30, 1781–1785. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Bioactive sol-gel foams for tissue repair. J. Biomed. Mater. Res. 2002, 59, 340–348. [Google Scholar] [CrossRef]
- Pishbin, F.; Simchi, A.; Ryan, M.P.; Boccaccini, A.R. A study of the electrophoretic deposition of bioglass suspension using the Taguchi experimental design approach. J. Eur. Ceram. Soc. 2010, 30, 2963–2970. [Google Scholar] [CrossRef]
- Dumont, V.C.; Mansur, A.A.P.; Carvalho, S.M.; Borsagli, F.G.L.M.; Pereira, M.M.; Mansur, H.S. Chitosan and carboxymethyl-chitosan capping ligands: Effect on the nucleation and growth of hydroxyapatite nanoparticles for producing biocomposite membranes. Mater. Sci. Eng. C 2016, 59, 265–277. [Google Scholar] [CrossRef]
- Farnoush, H.; Muhaffel, F.; Cimenoglu, H. Fabrication and characterization of nano-HA-45S5 bioglass composite coatings on calcium-phosphate containing micro-arc oxidized CP-Ti substrates. Appl. Surf. Sci. 2015, 324, 765–774. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A Review on Fundamentals and Applications of Electrophoretic Deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Hong, Z.; Liu, A.; Chen, L.; Chen, X.; Jing, X. Preparation of bioactive glass ceramic nanoparticles by combination of sol-gel and coprecipitation method. J. Non-Cryst. Solids 2009, 355, 368–372. [Google Scholar] [CrossRef]
- Fujii, S.; Okada, M.; Furuzono, T. Hydroxyapatite nanoparticles as stimulus-responsive particulate emulsifiers and building block for porous materials. J. Colloid Interface Sci. 2007, 315, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Alaei, M.; Atapour, M.; Labbaf, S. Electrophoretic deposition of chitosan-bioactive glass nanocomposite coatings on AZ91 Mg alloy for biomedical applications. Prog. Org. Coat. 2020, 147, 105803. [Google Scholar] [CrossRef]
- Dudek, K.; Goryczka, T.J.C.I. Electrophoretic deposition and characterization of thin hydroxyapatite coatings formed on the surface of NiTi shape memory alloy. Ceram. Int. 2016, 42, 19124–19132. [Google Scholar] [CrossRef]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F. Chacterization and biological properties of TiO2/PCL hybrid layers prepared via sol–gel dip coating for surface modification of titanium implants. J. Non-Cryst. Solids 2015, 415, 9–15. [Google Scholar] [CrossRef]
- Baboian, R. Corrosion Tests and Standards: Application and Interpretation, 2nd ed.; ASTM Manual Series; ASTM: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Ghomi, H.; Fathi, M.H.; Edris, H. Fabrication and characterization of bioactive glass/hydroxyapatite nanocomposite foam by gel casting method. Ceram. Int. 2011, 37, 1819–1824. [Google Scholar] [CrossRef]
- Cheng, X.M.; Li, Y.B.; Zuo, Y.; Zhang, L.; Li, J.D.; Wang, H.A. Properties and in vitro biological evaluation of nano-hydroxyapatite/chitosan membranes for bone guided regeneration. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2009, 29, 29–35. [Google Scholar]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Ou, K.L.; Weng, C.C.; Lin, Y.H.; Huang, M.S. A promising of alloying modified beta-type Titanium-Niobium implant for biomedical applications: Microstructural characteristics, in vitro biocompatibility and antibacterial performance. J. Alloys Compd. 2017, 697, 231–238. [Google Scholar] [CrossRef]
- Xu, J.; Bao, X.K.; Fu, T.; Lyu, Y.; Xie, Z.H. In vitro biocompatibility of a nanocrystalline β-Ta2O5 coating for orthopaedic implants. Ceram. Int. 2018, 44, 4660–4675. [Google Scholar] [CrossRef]
- Hussain, M.; Askari Rizvi, S.H.; Abbas, N.; Sajjad, U.; Shad, M.R.; Badshah, M.A.; Malik, A.I. Recent developments in coatings for orthopedic metallic implants. Coatings 2021, 11, 791. [Google Scholar] [CrossRef]
- Pan, C.J.; Pang, L.Q.; Gao, F.; Wang, Y.N.; Liu, T.; Ye, W.; Hou, Y.H. Anticoagulation and endothelial cell behaviors of heparin-loaded graphene oxide coating on titanium surface. Mater. Sci. Eng. C 2016, 63, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Ji, J.; Yuan, W.; Shen, J. Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomaterials 2005, 26, 6684–6692. [Google Scholar] [CrossRef]
- Hasan, A.; Pandey, L.M. Polymers, surface-modified polymers, and self-assembled monolayers as surface-modifying agents for biomaterials. Polym. Plast. Technol. Eng. 2015, 54, 1358–1378. [Google Scholar] [CrossRef]
- Follmann, H.D.; Naves, A.F.; Martins, A.F.; Félix, O.; Decher, G.; Muniz, E.C.; Silva, R. Advanced fibroblast proliferation inhibition for biocompatible coating by electrostatic layer-by-layer assemblies of heparin and chitosan derivatives. J. Colloid Interface Sci. 2016, 474, 9–17. [Google Scholar] [CrossRef]
- Chupa, J.M.; Foster, A.M.; Sumner, S.R.; Madihally, S.V.; Matthew, H.W. Vascular cell responses to polysaccharide materials: In vitro and in vivo evaluations. Biomaterials 2000, 21, 2315–2322. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, S.M.; Al-Khaled, K.; Ayadi, M.; Abbas, N.; Chammam, W. Performance analysis of biodegradable materials for orthopedic applications. Mater. Today Commun. 2022, 31, 103167. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Kamali, H.A. Microstructural characterization and corrosion resistance evaluation of nanostructured Al and Al/AlCr coated Mg–Zn–Ce–La alloy. J. Alloys Compd. 2014, 615, 657–671. [Google Scholar] [CrossRef]


| Substance | Composition (gL−1) |
|---|---|
| NaCl | 8 |
| KCl | 0.4 |
| NaH2PO4.2H2O | 0.25 |
| NaHCO3 | 0.35 |
| Na2HPO4.2H2O | 0.06 |
| CaCl2.2H2O | 0.19 |
| MgCl2.6H2O | 0.4 |
| MgSO4.7H2O | 0.06 |
| Glucose | 1 |
| Material No | Material | Ecorr (V) | icorr (µA.cm−2) | Corrosion Rate (µm/yr) |
|---|---|---|---|---|
| S1 | Bare Ti6Al4V | −0.457 | 2.51 | 24.18 |
| S2 | HA+Bioglass (HApBG) | −0.415 | 0.63 | 6.11 |
| S3 | Chitosan+Heparin (CsHep) | −0.400 | 0.32 | 3.1 |
| S4 | HA+Bioglass+Chitosan+Heparin (HApBGCsHep) | −0.365 | 0.06 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koc, S.; Baygar, T.; Özarslan, S.; Sarac, N.; Ugur, A. Fabrication and Characterization of a Multifunctional Coating to Promote the Osteogenic Properties of Orthopedic Implants. Materials 2023, 16, 6608. https://doi.org/10.3390/ma16196608
Koc S, Baygar T, Özarslan S, Sarac N, Ugur A. Fabrication and Characterization of a Multifunctional Coating to Promote the Osteogenic Properties of Orthopedic Implants. Materials. 2023; 16(19):6608. https://doi.org/10.3390/ma16196608
Chicago/Turabian StyleKoc, Serap (Gungor), Tuba Baygar, Selma Özarslan, Nurdan Sarac, and Aysel Ugur. 2023. "Fabrication and Characterization of a Multifunctional Coating to Promote the Osteogenic Properties of Orthopedic Implants" Materials 16, no. 19: 6608. https://doi.org/10.3390/ma16196608
APA StyleKoc, S., Baygar, T., Özarslan, S., Sarac, N., & Ugur, A. (2023). Fabrication and Characterization of a Multifunctional Coating to Promote the Osteogenic Properties of Orthopedic Implants. Materials, 16(19), 6608. https://doi.org/10.3390/ma16196608







