Effect of the Equal Channel Angular Pressing on the Microstructure and Phase Composition of a 7xxx Series Al-Zn-Mg-Zr Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Mechanical and Microstructural Investigations
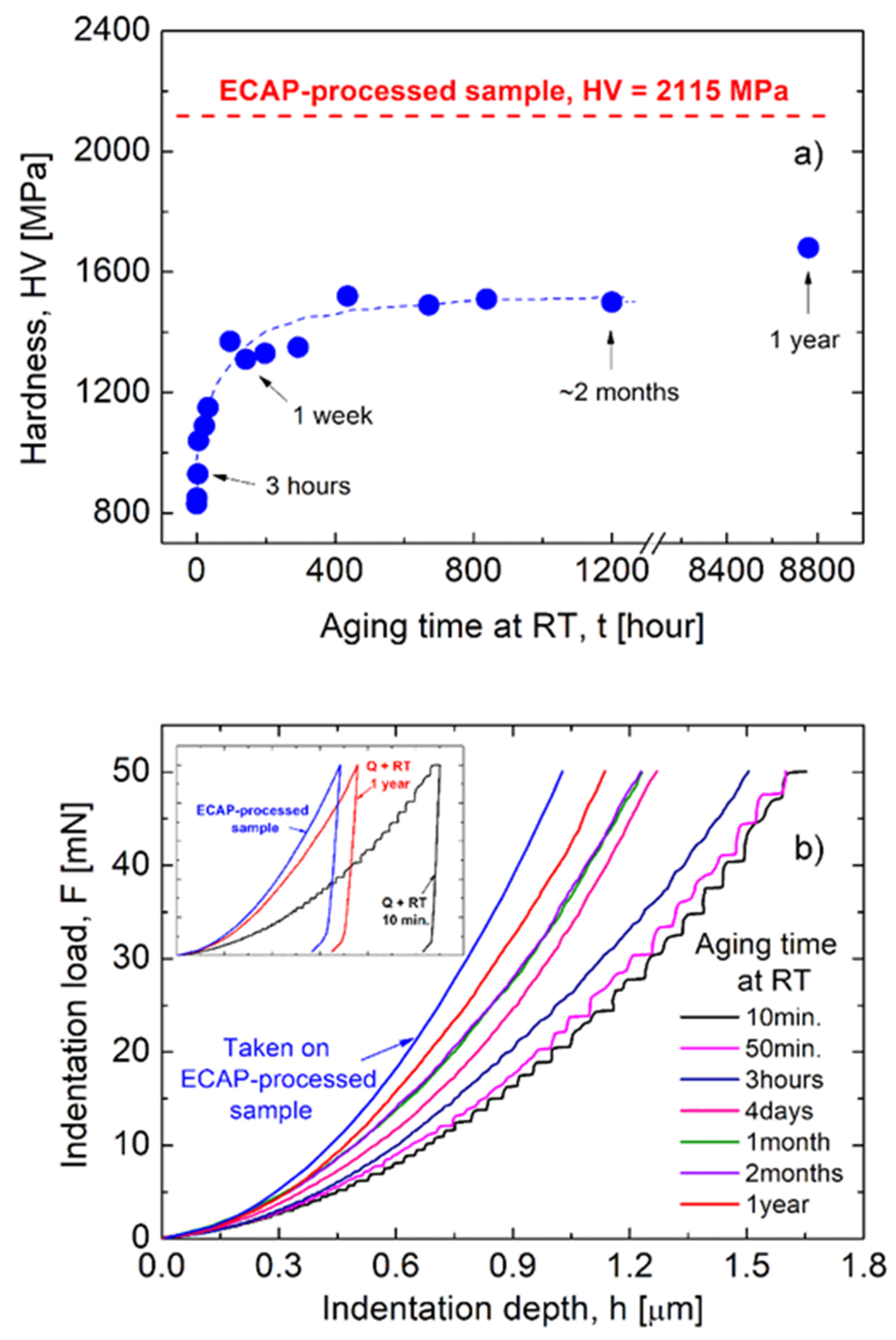
3.1.1. The Background: Hardness Behavior
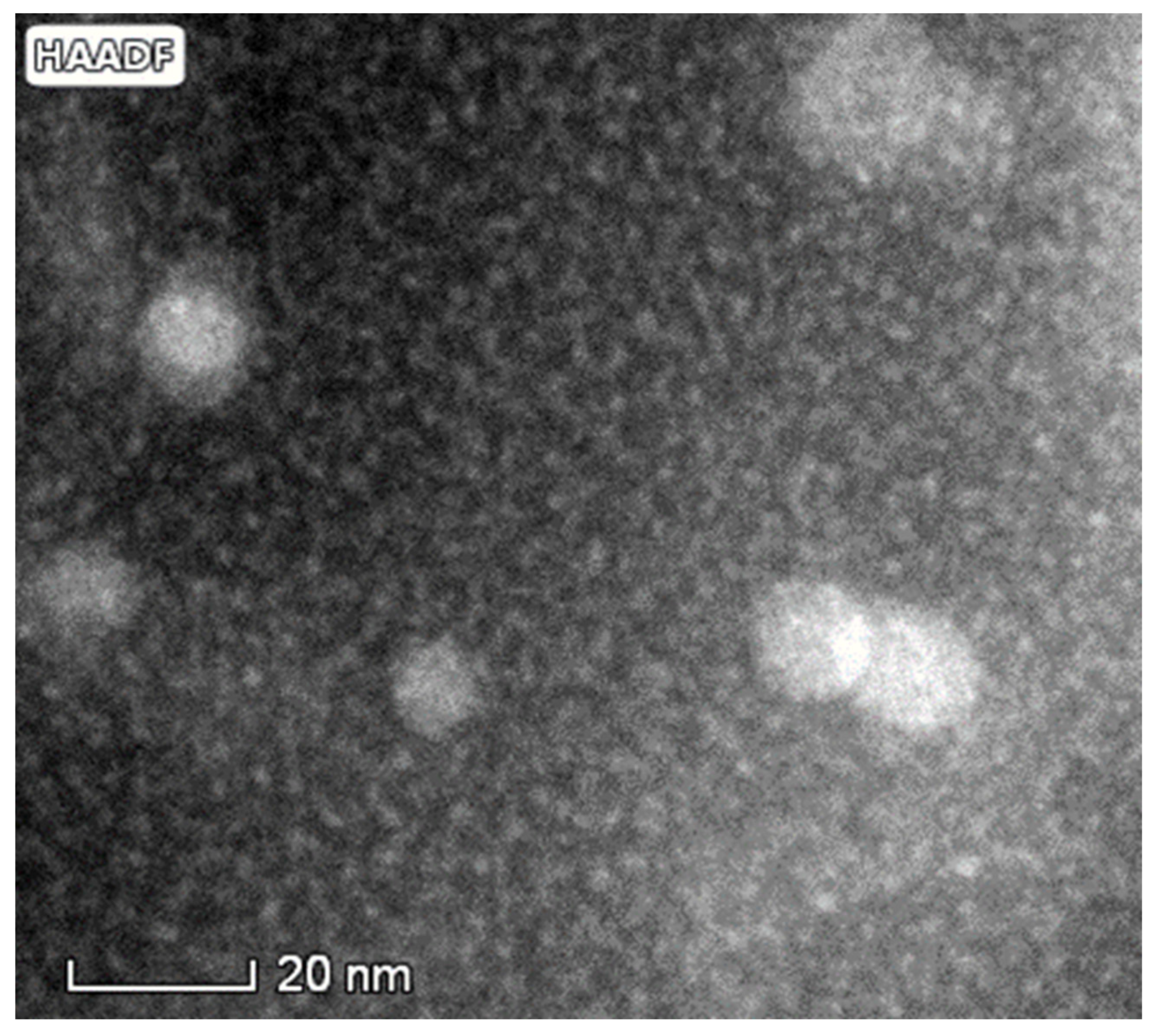
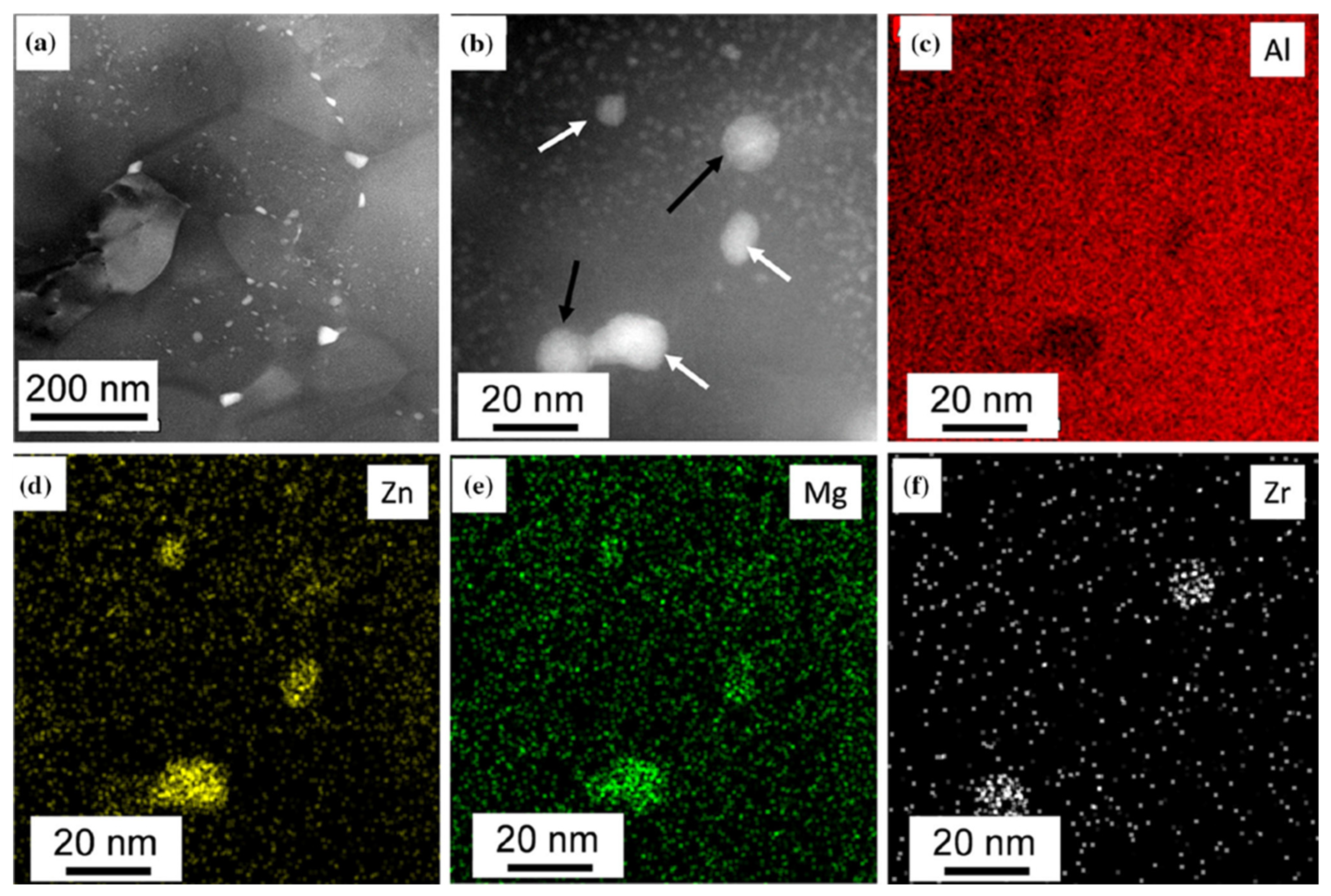
3.1.2. TEM Investigations
3.2. Characterization of Microstructure by DSC Investigations
3.2.1. Specific Enthalpies Characterizing the Dissolution and Precipitation Reactions
3.2.2. Kinetic Parameters for Dissolution and Precipitation Reactions
3.2.3. The Activation Energy of Dissolution and Precipitation Processes
4. Discussion
5. Conclusions
- It has been shown that the room-temperature severe plastic deformation exerted by ECAP resulted in not only an ultrafine-grained but also a strongly precipitated structure in the investigated AlZnMgZr alloy;
- As a collective consequence of grain-size (Hall–Petch) strengthening and precipitate-hardening, the ECAP process significantly—two and half times—increased the initial hardness (830 MPa) of the freshly quenched sample to 2115 MPa;
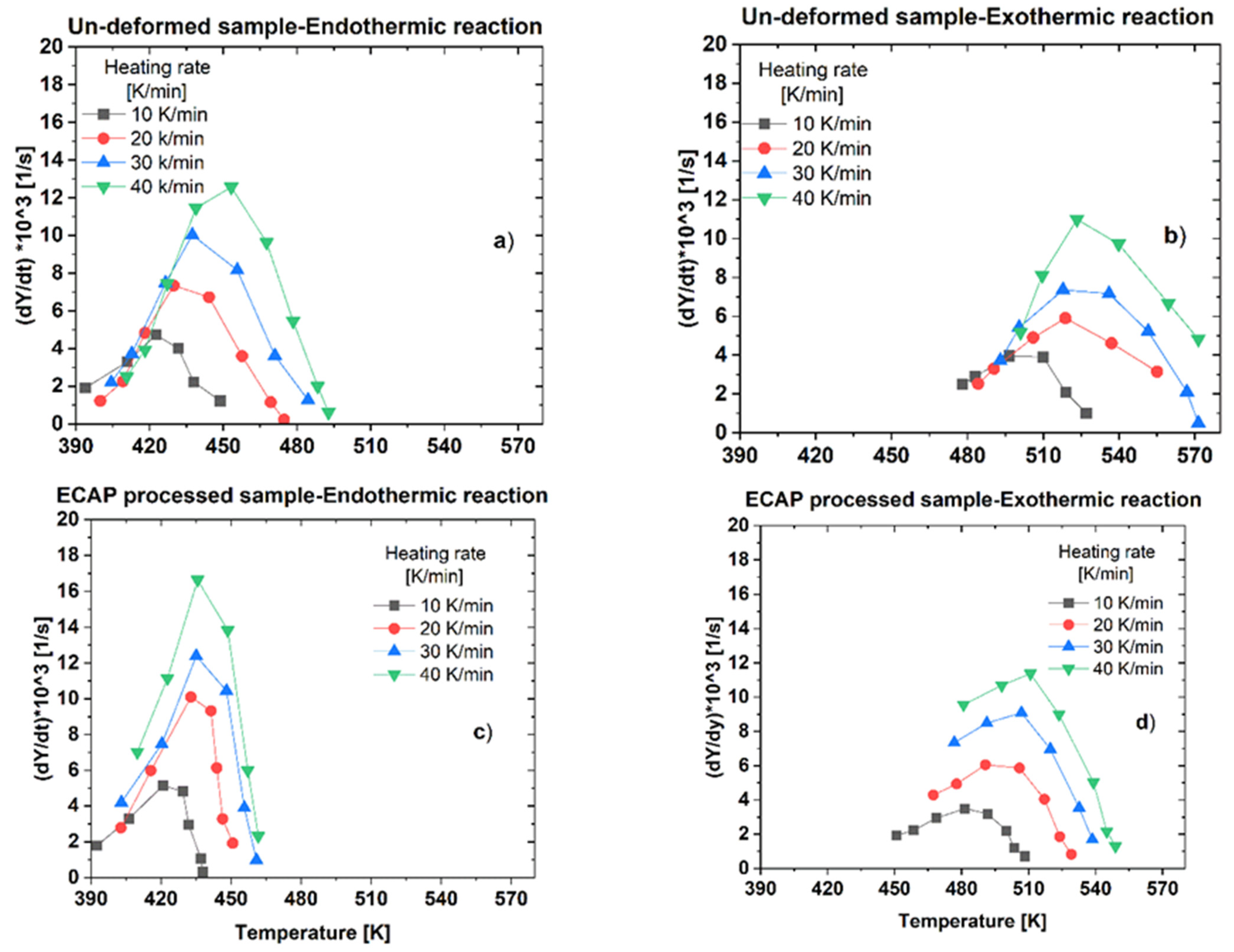
- It was demonstrated that the SPD via ECAP has a strong effect on the dissolution (endothermic) and precipitation (exothermic) processes during the DSC measurements because of the significant changes in the microstructure of the ECAP-processed samples;
- Results of the DSC tests have revealed that both the dissolution and precipitation processes are characterized by a specific enthalpy of dissolution, ΔH. As the effect of the ECAP process, while the specific enthalpy obtained for dissolution, ∆Hd decreased from a range of 5.0 to 6.2 to a range of 4.2 to 3.5 J/g, the value obtained for the precipitation, ∆Hp increased from a range of 5.9 to 7.0 to a range of 4.1 to 2.6 J/g;
- Results of the DSC tests have also revealed that both the dissolution and precipitation processes are characterized by an activation energy, Q. As the effect of the ECAP process, while the activation energy obtained for dissolution, Qd increased from 84.8 to 110.9 kJ/mole, the value obtained for the precipitation, Qp decreased from 147.4 to 117.7 kJ/mole.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altenbach, C.; Schnatterer, C.; Mercado, U.A.; Suuronen, J.P.; Zander, D.; Requena, G. Synchrotron-based holotomography and X-ray fluorescence study on the stress corrosion cracking behavior of the peak-aged 7075 aluminum alloy. J. Alloys Compd. 2020, 817, 152722. [Google Scholar] [CrossRef]
- Miller, W.; Zhuang, L.; Bottema, J.; Wittebrood, A.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W. Recent development in aluminium alloys for aerospace applications. Mater. Sci. Eng. A 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Hirsch, J. Aluminium in innovative light-weight car design. Mater. Trans. 2011, 52, 818–824. [Google Scholar] [CrossRef]
- Zheng, K.; Politis, D.J.; Wang, L.; Lin, J. A review on forming techniques for manufacturing lightweight complex-shaped aluminium panel components. Int. J. Lightweight Mater. Manuf. 2018, 1, 55–80. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Structure of the aluminium: Magnesium: Zinc alloys. Int. Metall. Rev. 1971, 153, 95–124. [Google Scholar] [CrossRef]
- Polmear, I.J. Light Alloys: Metallurgy of the Light Metals, 3rd ed.; Arnold: London, UK, 1995. [Google Scholar]
- Aoba, T.; Kobayashi, M.; Miura, H. Effects of Aging on Mechanical Properties and Microstructure of Multi-Directionally Forged 7075 Aluminum Alloy. Mater. Sci. Eng. A 2017, 700, 220–225. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsuda, K.; Ikeno, S.; Yoshida, T.; Murakami, S. TEM observation of precipitate structures in Al–Zn–Mg alloys with addition of Cu/Ag. Metall. Mater. 2015, 60, 977–979. [Google Scholar] [CrossRef]
- Lee, S.; Tazoe, K.; Mohamed, I.F.; Horita, Z. Strengthening of AA7075 alloy by processing with high-pressure sliding (HPS) and subsequent aging. Mater. Sci. Eng. A 2015, 628, 56–61. [Google Scholar] [CrossRef]
- Zhao, T.; Luo, H.; Luo, J.; Wang, R. Enhanced intragranular dislocation capture ability in nano/ultrafine Al-Zn-Mg-Cu alloy by burnishing-assisted two-stage aging treatment-induced dynamic nucleation. J. Alloys Compd. 2023, 960, 170572. [Google Scholar] [CrossRef]
- Boldizsár, B.; Jenei, P.; Ahmed, A.Q.; Murashkin, M.Y.; Valiev, R.Z.; Chinh, N.Q. Low temperature super ductility and threshold stress of an ultrafine-grained Al–Zn–Mg–Zr alloy processed by equal-channel angular pressing. J. Mater. Sci. 2021, 56, 19244–19252. [Google Scholar] [CrossRef]
- Gubicza, J.; Labar, J.L.; Lendvai, J.; Chinh, N.Q. The influence of artificial aging on the microstructure and hardness of an Al–Zn–Mg–Zr alloy processed by equal channel angular pressing. J. Mater. Sci. 2019, 54, 10918–10928. [Google Scholar] [CrossRef]
- Xu, C.; Furukawa, M.; Horita, Z.; Langdon, T.G. Influence of ECAP on precipitate distributions in a spray-cast aluminum alloy. Acta Mater. 2005, 53, 749–758. [Google Scholar] [CrossRef]
- Samaee, M.; Najafi, S.; Eivani, A.R.; Jafarian, H.R.; Zhou, J. Simultaneous improvements of the strength and ductility of fine-grained AA6063 alloy with increasing number of ECAP passes. Mater. Sci. Eng. A 2016, 669, 350–357. [Google Scholar] [CrossRef]
- Duan, Z.C.; Chinh, N.Q.; Xu, C.; Langdon, T.G. Developing processing routes for the equal-channel angular pressing of age-hardenable aluminum alloys. Metall. Mater. Trans. A 2010, 41, 802–809. [Google Scholar] [CrossRef]
- Wei, S.L.; Feng, Y.; Zhang, H.; Xu, C.T.; Wu, Y. Influence of aging on microstructure, mechanical properties and stress corrosion cracking of 7136 aluminum alloy. J. Cent. South Univ. 2021, 28, 2687–2700. [Google Scholar] [CrossRef]
- Singaravelu, A.S.S.; Williams, J.J.; Goyal, H.D.; Niverty, S.; Singh, S.S.; Stannard, T.J.; Chawla, N. 3D time-resolved observations of fatigue crack initiation and growth from corrosion pits in Al 7XXX alloys using in situ synchrotron X-ray tomography. Metall. Mater. Trans. A 2020, 51, 28–41. [Google Scholar] [CrossRef]
- Ma, K.; Hu, T.; Yang, H.; Topping, T.; Yousefiani, A.; Lavernia, E.J.; Schoenung, J.M. Coupling of dislocations and precipitates: Impact on the mechanical behavior of ultrafine grained Al–Zn–Mg alloys. Acta Mater. 2016, 103, 153–164. [Google Scholar] [CrossRef]
- Song, Z.; Niu, R.; Cui, X.; Bobruk, E.V.; Murashkin, M.Y.; Enikeev, N.A.; Ji, G.; Song, M.; Bhatia, V.; Ringer, S.P.; et al. Mechanism of room-temperature superplasticity in ultrafine-grained Al–Zn alloys. Acta Mater. 2023, 246, 118671. [Google Scholar] [CrossRef]
- Kumar, P.; Kawasaki, M.; Langdon, T.G. Review: Overcoming the paradox of strength and ductility in ultrafine–grained materials at low temperatures. J. Mater. Sci. 2016, 51, 7–18. [Google Scholar] [CrossRef]
- Li, G.; Xu, S.; Wan, T.; Liu, H.; Xie, L.; Zhang, M.; Li, J. Effect of intermediate-temperature severe plastic deformation on microstructure evolution, mechanical properties and corrosion behavior of an Al-Zn-Mg-Cu alloy. Mater. Charact. 2023, 205, 113248. [Google Scholar] [CrossRef]
- Lee, S.; Watanabe, K.; Matsuda, K.; Horita, Z. Low-temperature and high-strain-rate superplasticity of ultrafine-grained A7075 processed by high-pressure torsion. Mater. Trans. 2018, 59, 1341–1347. [Google Scholar] [CrossRef]
- Chinh, N.Q.; Murashkin, M.Y.; Bobruk, E.V.; Lábár, J.L.; Gubicza, J.; Kovács, Z.; Ahmed, A.Q.; Maier-Kiener, V.; Valiev, R.Z. Ultralow-temperature superplasticity and its novel mechanism in ultrafine-grained Al alloys. Mater. Res. Lett. 2021, 9, 475–482. [Google Scholar] [CrossRef]
- Wang, F.; Qui, D.; Liu, Z.L.; Taylor, J.A.; Easton, M.A.; Zhang, M.X. The grain refinement mechanism of cast aluminium by zirconium. Acta Mater. 2013, 61, 5636–5645. [Google Scholar] [CrossRef]
- Werenskiold, J.C. Equal Channel Angular Pressing (ECAP) of AA6082: Mechanical Properties, Texture and Microstructural Development. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2004. [Google Scholar]
- Langdon, T.G. Overview: Using Severe Plastic Deformation in the Processing of Superplastic Materials. Mater. Trans. 2023, 64, 1299–1305. [Google Scholar] [CrossRef]
- Jia, D.; He, T.; Song, M.; Huo, Y.; Hu, H. Effects of equal channel angular pressing and further cold upsetting process to the kinetics of precipitation during aging of 7050 aluminum alloy. J. Mater. Res. Technol. 2023, 26, 5126–5240. [Google Scholar] [CrossRef]
- Pharr, G.M.; Oliver, W.C.; Brotzen, F.R. Ont he generality of the relationship among contact stiffness, contact area, and elastic modulus during indentation. J. Mater. Res. 1992, 7, 613–617. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Chinh, N.Q.; Lendvai, J.; Ping, D.H.; Hono, K. The effect of Cu on mechanical and precipitation properties of Al–Zn–Mg alloys. J. Alloys. Compd. 2004, 378, 52–60. [Google Scholar] [CrossRef]
- Portevin, A.; Le Chatelier, F. Sur un phénomène observé lors de l’essai de traction d’alliages en cours de transformation. Compt. Rend. Acad. Sci. Paris. 1923, 19, 176. [Google Scholar]
- Lu, J.; Song, Y.; Zhou, P.; Xu, H.; Liu, Y.; Hua, L. Effect of thermal strain on the microstructure evolution and post-aging mechanical properties of Al-Zn-Mg-Cu alloy in simulating hot stamping process. Mater. Sci. Eng. A 2023, 880, 145316. [Google Scholar] [CrossRef]
- Priya, P.; Krane, M.J.M.; Johnson, D.R. Precipitation of Al3Zr Dispersoids during Homogenization of Al–Zn–Cu–Mg–Zr Alloys. In Light Metals; Williams, E., Ed.; Springer: Cham, Switzerland, 2016; pp. 213–218. [Google Scholar]
- Jiang, X.J.; Tafto, J.; Noble, B.; Holme, B.; Waterloo, G. Differential scanning calorimetry and electron diffraction investigation on low-temperature aging in Al-Zn-Mg alloys. Metall. Mater. Trans. A 2000, 31, 339–348. [Google Scholar] [CrossRef]
- Löffler, H.; Kovács, I.; Lendvai, J. Decomposition processes in Al-Zn-Mg alloys. J. Mater. Sci. 1983, 18, 2215–2240. [Google Scholar] [CrossRef]
- Miesenberger, B.; Kozeschnik, E.; Milkereit, B.; Warczok, P.; Povoden-Karadeniz, E. Computational analysis of heterogeneous nucleation and precipitation in AA6005 Al-alloy during continuous cooling DSC experiments. Materialia 2022, 25, 101538. [Google Scholar] [CrossRef]
- Chemingui, M.; Ameur, R.; Optasanu, V.; Khitouni, M. DSC analysis of phase transformations during precipitation hardening in Al–Zn–Mg alloy (7020). J. Therm. Anal. Calorim. 2019, 136, 1887–1894. [Google Scholar] [CrossRef]
- Starink, M.J.; Gao, N.; Furukawa, M.; Horita, Z.; Xu, C.; Langdon, T.G. Microstructural developments in a spray-cast Al-7034 alloy processed by equal-channel angular pressing. Rev. Adv. Mater. Sci. 2004, 7, 1–12. [Google Scholar]
- Ahmed, A.Q.; Ugi, D.; Lendvai, J.; Murashkin, M.Y.; Bobruk, E.V.; Valiev, R.Z.; Chinh, N.Q. Effect of Zn content on microstructure evolution in Al–Zn alloys processed by high-pressure torsion. J. Mater. Res. 2023, 38, 3602–3612. [Google Scholar] [CrossRef]
- Hutchinson, C.R. Modeling the Kinetics of Precipitation in Aluminium Alloys. In Fundamentals of Aluminium Metallurgy; Woodhead Publishing: Sawston, UK, 2011; pp. 422–467. [Google Scholar]
- Hansen, N. Hall–Petch relation and boundary strengthening. Scripta Mater. 2004, 51, 801–806. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, Y.; Marceau, R.; Wang, L.; Zhang, Q.; Gao, X.; Hutchinson, C. Precipitation strengthening of aluminum alloys by room-temperature cyclic plasticity. Science 2019, 363, 972–975. [Google Scholar] [CrossRef]
- Birol, Y. DSC analysis of the precipitation reaction in AA6005 alloy. J. Therm. Anal. Calorim. 2008, 93, 977–981. [Google Scholar] [CrossRef]
- Osten, J.; Milkereit, B.; Schick, C.; Kessler, O. Dissolution and precipitation behaviour during continuous heating of Al–Mg–Si alloys in a wide range of heating rates. Materials 2015, 8, 2830–2848. [Google Scholar] [CrossRef]
- Tschopp, M.A.; McDowell, D.L. Asymmetric tilt grain boundary structure and energy in copper and aluminium. Philos. Mag. 2007, 87, 3871–3892. [Google Scholar] [CrossRef]
- Stiller, K.; Warren, P.J.; Hansen, V.; Angenete, J.; Gjønnes, J. Investigation of precipitation in an Al–Zn–Mg alloy after two-step ageing treatment at 100° and 150 °C. Mater. Sci. Eng. A 1999, 270, 55–63. [Google Scholar] [CrossRef]
- Deschamps, A.; Livet, F.; Bréchet, Y. Influence of predeformation on ageing in an Al–Zn–Mg alloy—I. Microstructure evolution and mechanical properties. Acta Mater. 1998, 47, 281–292. [Google Scholar] [CrossRef]
- Ghosh, K.S.; Gao, N. Determination of kinetic parameters from calorimetric study of solid state reactions in 7150 Al-Zn-Mg alloy. Trans. Nonferrous Met. Soc. China 2011, 21, 1199–1209. [Google Scholar] [CrossRef]



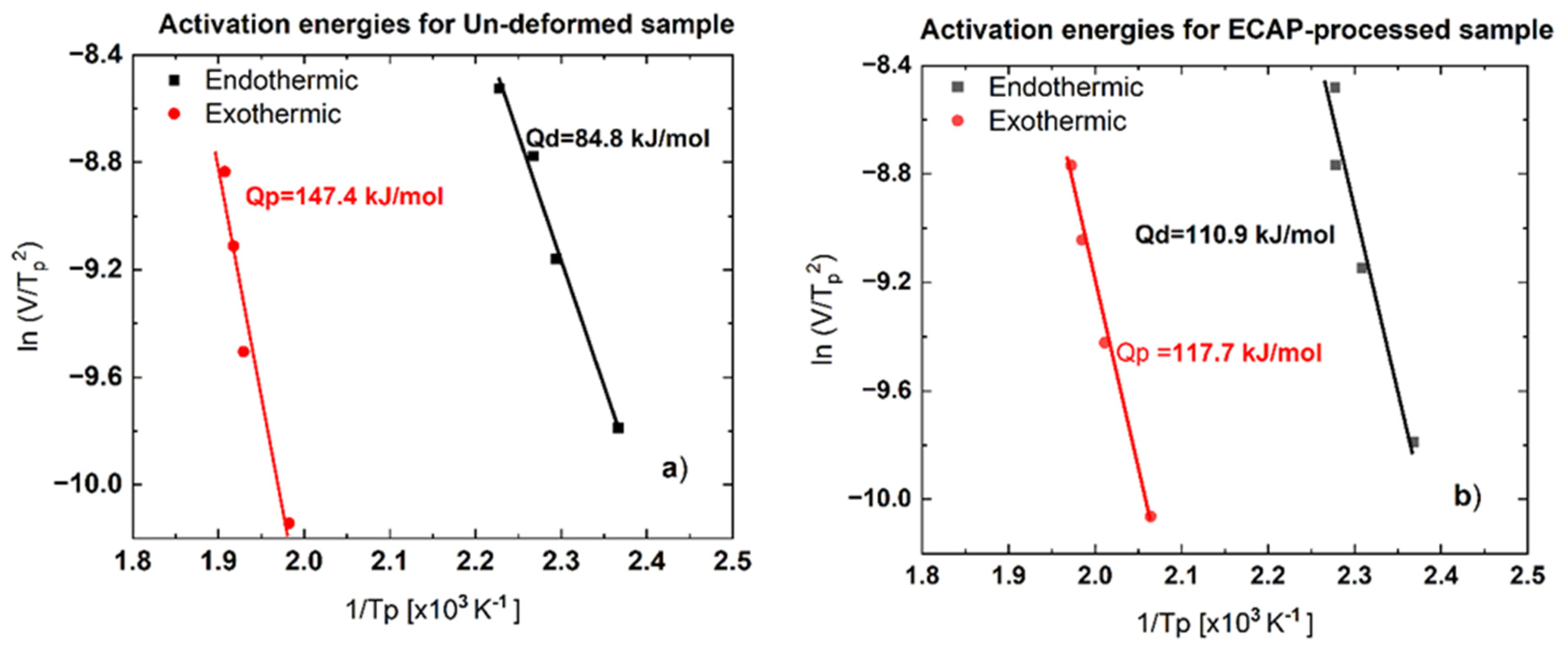
| Reaction Type | Un-Deformed Sample | ECAP-Processed Sample |
|---|---|---|
| Dissolution (endothermic) reaction | 5.0 6.2 J/g | 4.2 3.5 J/g |
| Precipitation (exothermic) reaction | 5.9 7.0 J/g | 4.1 2.6 J/g |
| Dissolution reaction, Qd | 84.8 kJ/mol | 110.9 kJ/mol |
| Precipitation reaction, Qp | 147.4 kJ/mol | 117.7 kJ/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.Q.; Olasz, D.; Bobruk, E.V.; Valiev, R.Z.; Chinh, N.Q. Effect of the Equal Channel Angular Pressing on the Microstructure and Phase Composition of a 7xxx Series Al-Zn-Mg-Zr Alloy. Materials 2023, 16, 6593. https://doi.org/10.3390/ma16196593
Ahmed AQ, Olasz D, Bobruk EV, Valiev RZ, Chinh NQ. Effect of the Equal Channel Angular Pressing on the Microstructure and Phase Composition of a 7xxx Series Al-Zn-Mg-Zr Alloy. Materials. 2023; 16(19):6593. https://doi.org/10.3390/ma16196593
Chicago/Turabian StyleAhmed, Anwar Qasim, Dániel Olasz, Elena V. Bobruk, Ruslan Z. Valiev, and Nguyen Q. Chinh. 2023. "Effect of the Equal Channel Angular Pressing on the Microstructure and Phase Composition of a 7xxx Series Al-Zn-Mg-Zr Alloy" Materials 16, no. 19: 6593. https://doi.org/10.3390/ma16196593
APA StyleAhmed, A. Q., Olasz, D., Bobruk, E. V., Valiev, R. Z., & Chinh, N. Q. (2023). Effect of the Equal Channel Angular Pressing on the Microstructure and Phase Composition of a 7xxx Series Al-Zn-Mg-Zr Alloy. Materials, 16(19), 6593. https://doi.org/10.3390/ma16196593







