A Route to Understanding the Ethane Adsorption Selectivity of the Zeolitic Imidazolate Framework-8 in Ethane–Ethylene Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZIF-8 in Methanol
2.2. Characterization
2.3. Adsorption Experiment and Analysis
2.4. Computational Methods
3. Results and Discussion
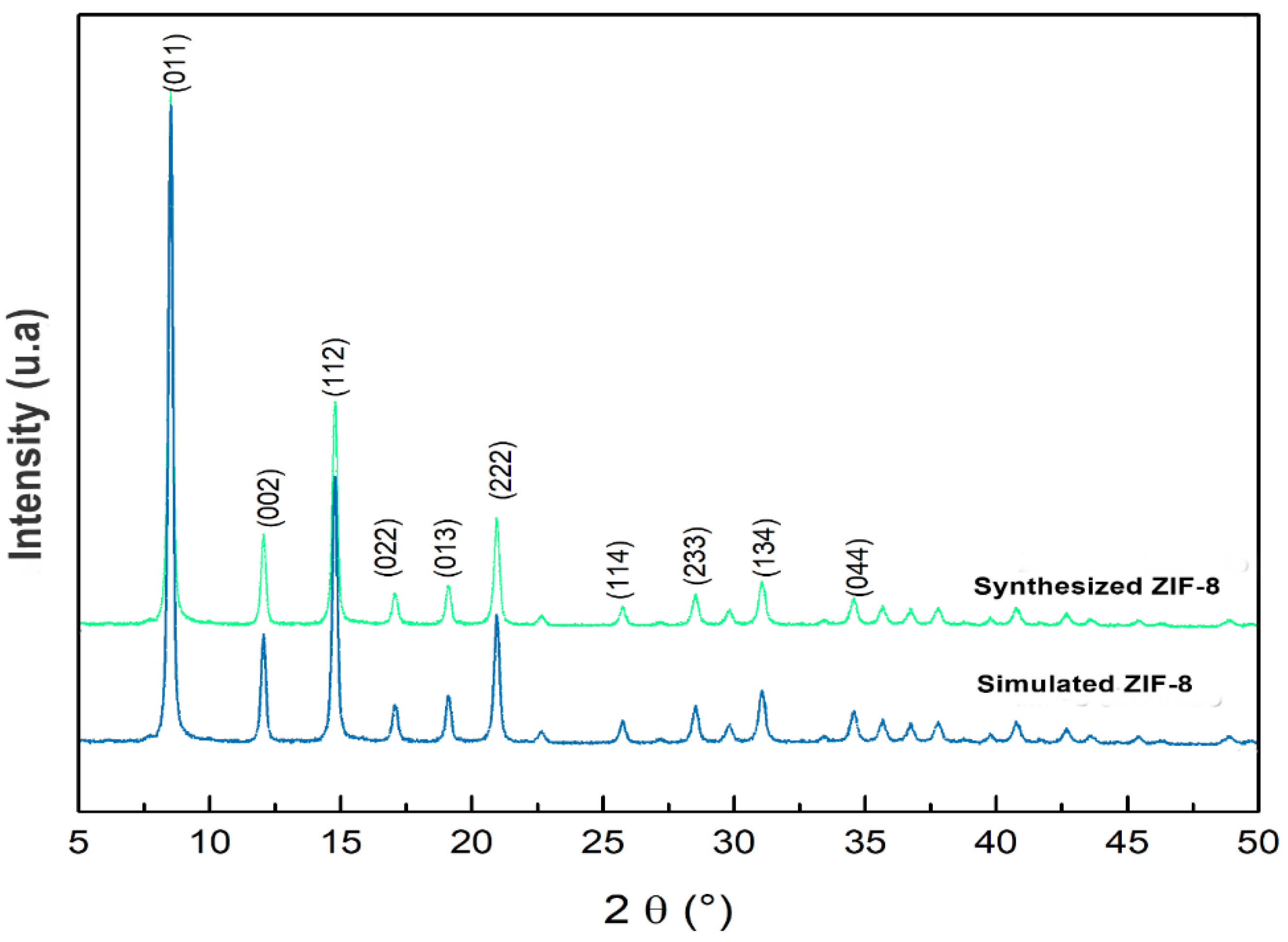
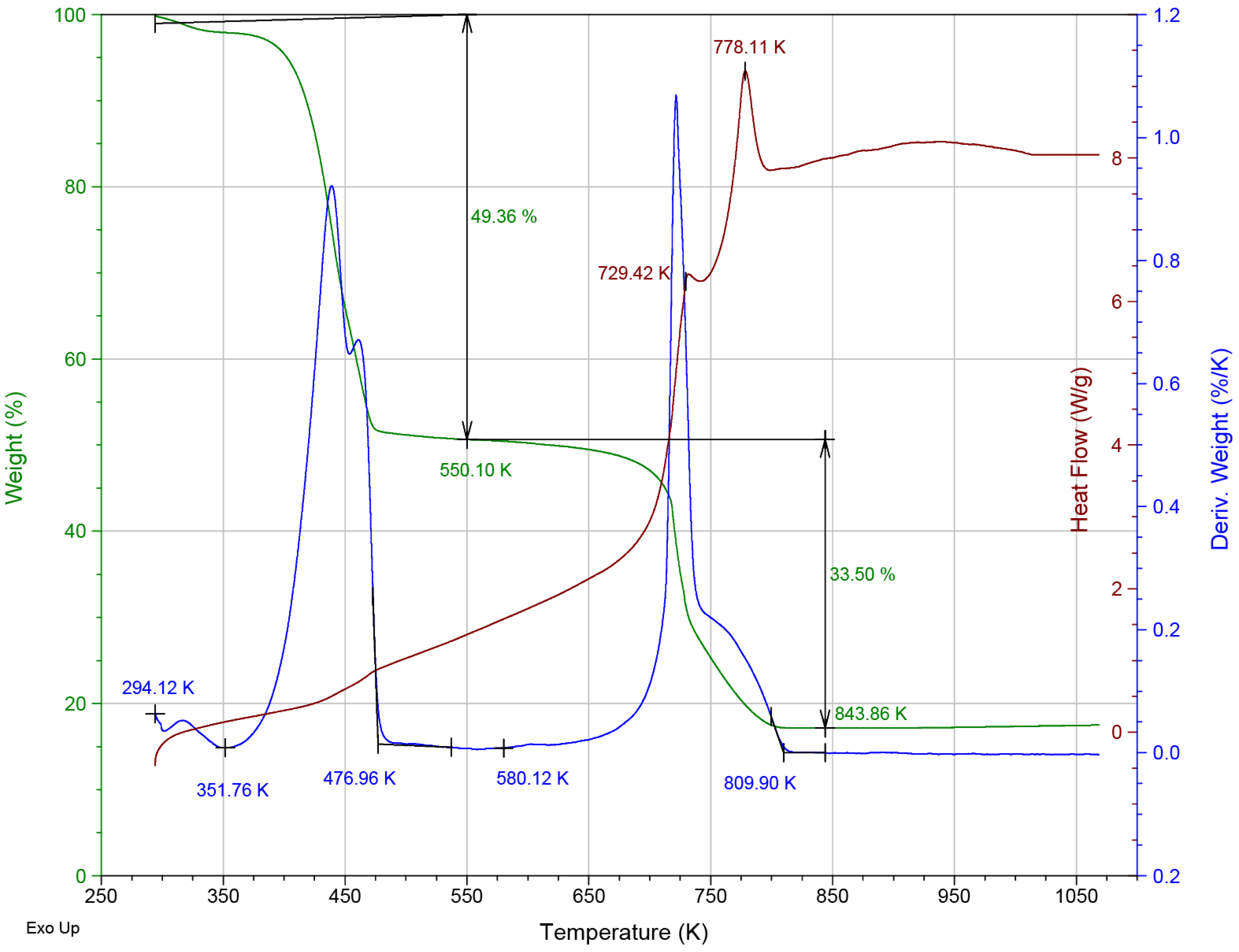
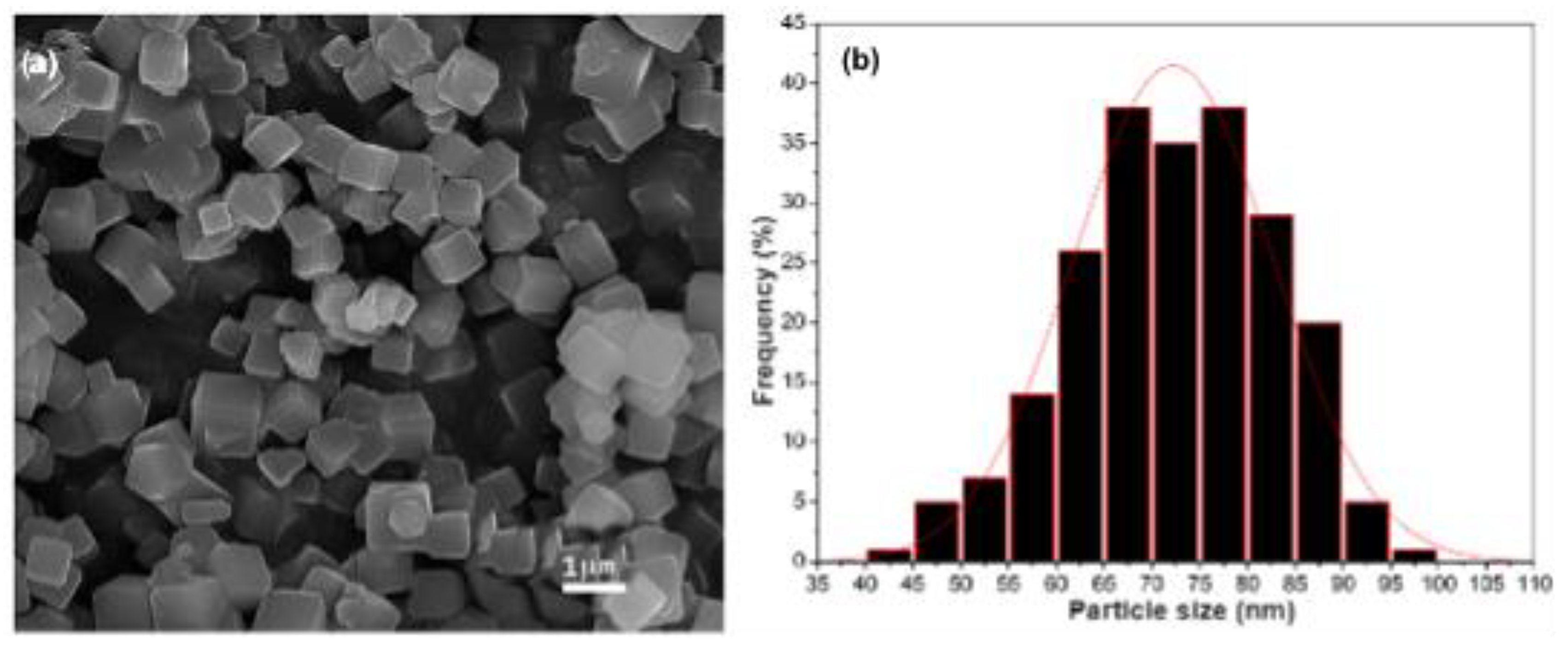
3.1. Material Characterization
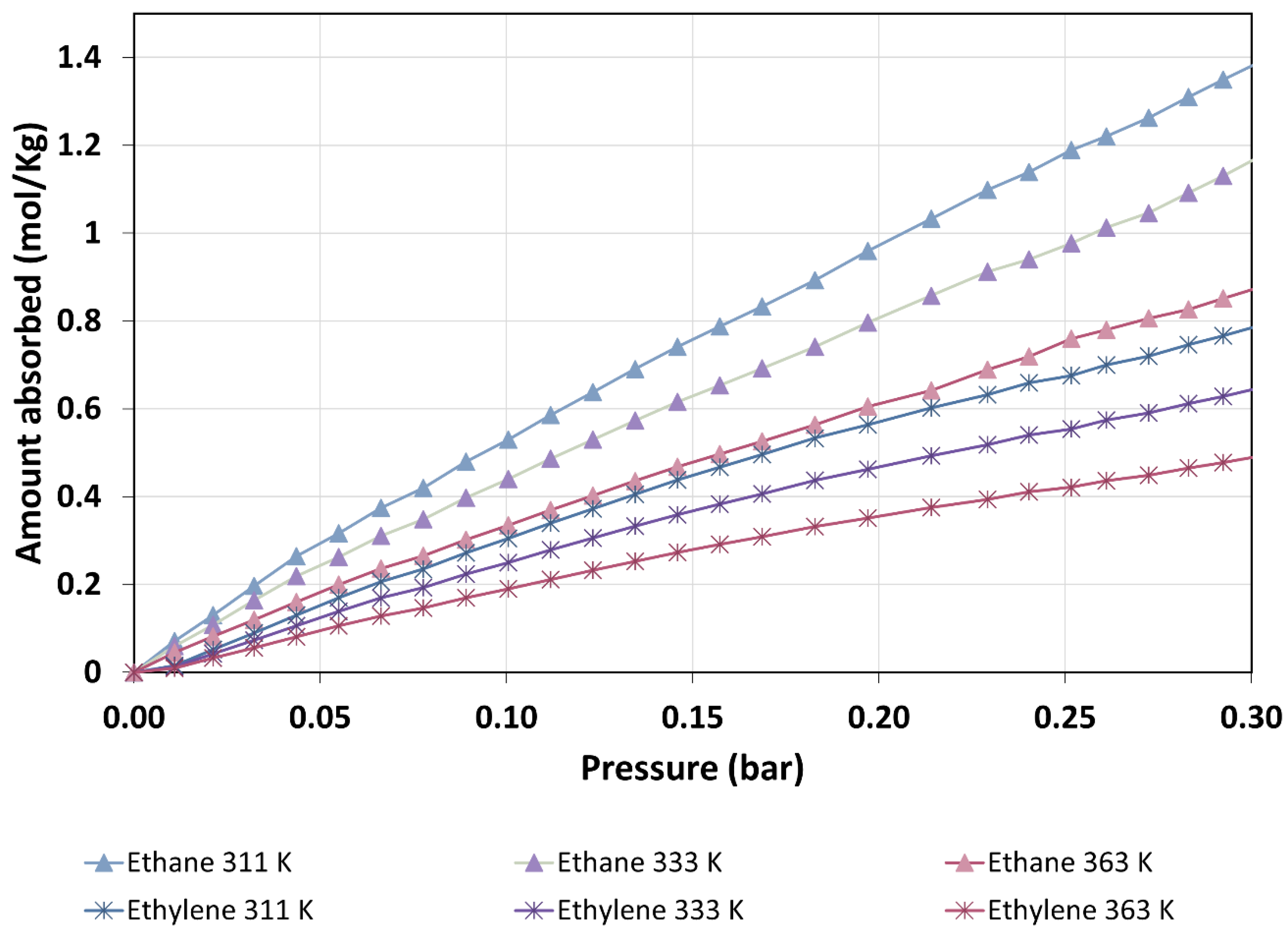
3.2. Ethane and Ethylene Adsorption
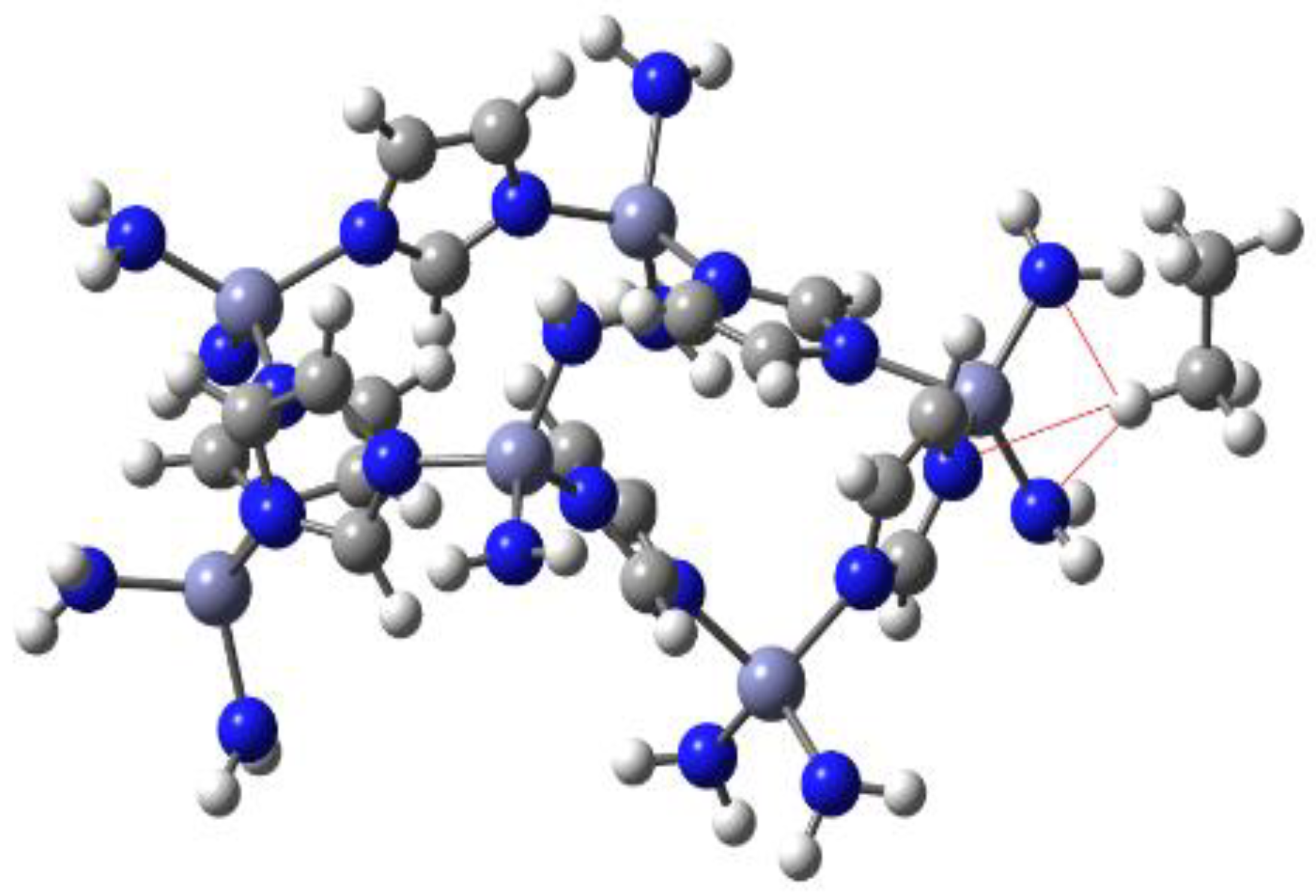
3.3. Theoretical Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Process: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Shao, L.; Liu, M.; Sang, Y.; Zhan, P.; Chen, J.; Huang, J. Nitrogen–Doped ultrahigh Microporous Carbons Derived from Two Nitrogen-Containing Post–Cross–Linked Polymers for Efficient CO2 Capture. J. Chem. Eng. 2020, 65, 2238–2250. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, X.; Chen, Y.; Huan, J.; Luo, H.; Deng, S. Adsoprtion of CO2, CH4, and N2 on Ordered Mesoporous Carbon: Approach for Greenhouse Gases Capture and Biogas Upgrading. Environ. Sci. Technol. 2013, 47, 5474–5480. [Google Scholar] [CrossRef]
- Pullumbi, P.; Brandani, F.; Brandani, S. Gas Separation by Adsorption: Technological Drivers and Opportunities for Improvement. Curr. Opin. Chem. Eng. 2019, 24, 131–142. [Google Scholar] [CrossRef]
- Amedi, H.R.; Aghajani, M. Economic Estimation of Various Membranes and Distillation for Propylene and Propane Separation. Ind. Eng. Chem. Res. 2018, 24, 4366. [Google Scholar] [CrossRef]
- Motelica, A.; Bruinsma, O.S.; Kreiter, R.; den Exter, M.; Vente, J.F. Membrane Retrofit Option for Paraffin/Olefin Separation-A Technoeconomic Evaluation. Ind. Eng. Chem. Res. 2012, 51, 6977–6986. [Google Scholar] [CrossRef]
- Bux, H.; Chmelik, C.; Krishna, R.; Caro, J. Ethane/ethane Separations by the MOF membrane ZIF-8: Molecular Correlation of Permation, Adsorption, Diffusion. J. Membr. Sci. 2010, 369, 284–289. [Google Scholar]
- Wu, Y.; Chen, H.; Liu, D.; Qian, Y.; Xi, H. Adsorption and separation of ethane/ethylene on ZIFs with various topologies: Combining GCMC simulation with the ideal adsorbed solution theory (IAST). Chem. Eng. Sci. 2015, 124, 144–153. [Google Scholar] [CrossRef]
- Böhme, U.; Barth, B.; Paula, C.; Kuhnt, A.; Schwieger, W.; Mundstock, A.; Caro, J.; Hartmann, M. Ethene/ethane and propene/propane separation via the olefin and paraffin selective metal–organic framework adsorbents CPO-27 and ZIF-8. Langmuir 2013, 29, 8592–8600. [Google Scholar] [CrossRef]
- Lv, D.; Zhou, P.; Xu, J.; Tu, S.; Xu, F.; Yan, J.; Xi, H.; Yuan, W.; Fu, Q.; Chen, X.; et al. Recent advances in adsorptive separation of ethane and ethylene by C2H6-selective MOFs and other adsorbents. Chem. Eng. J. 2021, 431, 133208. [Google Scholar] [CrossRef]
- Brandani, S.; Mangano, E. The zero-length column technique to measure adsorption equilibrium and kinetics: Lessons learnt from 30 years of experience. Adsorption 2021, 27, 319–351. [Google Scholar] [CrossRef]
- Vargas-Bustamante, J.; Martínez-Ortiz, P.; Alvarado-Alvarado, D.; Torres-Herrera, U.; Balmaseda, J. Experimental Setup and Graphical User Interface for Zero-Length Column Chromatography. Appl. Sci. 2022, 12, 6694. [Google Scholar] [CrossRef]
- Liao, Y.T.; Dutta, S.; Chien, C.H.; Hu, C.C.; Shieh, F.K.; Lin, C.H.; Wu, K.C.W. Synthesis of Mixed-Ligand Zeolitic Imidazolate Framework (ZIF-8-90) for CO2 Adsorption. J. Inorg. Organomet. Polym. Mater. 2015, 25, 251–258. [Google Scholar] [CrossRef]
- Rasband, W.S.; ImageJ USA. National Institutes of Health, Bethesda, Maryland, USA. 1997. Available online: http://imagej.nih.gov/ij/ (accessed on 18 September 2021).
- Brandani, F.; Ruthven, D.; Coe, C.G. Measurement of Adsorption Equilibrium by the Zero Length Column (ZLC) Technique Part 1: Single-Component Systems. Ind. Eng. Chem. Res. 2003, 42, 1451–1461. [Google Scholar] [CrossRef]
- Iacomi, P.; Llewellyn, P.L. pyGAPS: A Python-based framework for adsorption isotherm processing and material characterisation. Adsorption 2019, 25, 1533–1542. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef]
- Raju, R.K.; Bengali, A.A.; Brothers, E.N. A unified set of experimental organometallic data used to evaluate modern theoretical methods. Dalton Trans. 2016, 45, 13766–13778. [Google Scholar] [CrossRef]
- He, M.; Yao, J.; Liu, Q.; Wang, K.; Chen, F.; Wang, H. Facile synthesis of zeolitic imidazolate framework-8 from a concentrated aqueous solution. Microporous Mesoporous Mater. 2014, 184, 55–60. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, M.; Yang, Y.; Lin, Y.S. Hydrolysis and condensation of ZIF-8 in water. Microporous Mesoporous Mater. 2019, 288, 109568. [Google Scholar] [CrossRef]
- Hachuła, B.; Nowak, M.; Kusz, J. Crystal and Molecular Structure Analysis of 2-Methylimidazole. J. Chem. Crystallogr. 2010, 40, 201–206. [Google Scholar] [CrossRef]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. Insitu high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef] [PubMed]
- Schejn, A.; Balan, L.; Falk, V.; Aranda, L.; Medjahdi, G.; Schneider, R. Controlling ZIF-8 nano-and microcrystal formation and reactivity through zinc salt variations. Cryst. Eng. Comm. 2014, 16, 4493–4500. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Lalonde, M.B.; Bury, W.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T. Opening ZIF-8: A Catalytically Active Zeolitic Imidazolate Framework of Sodalite Topology with Unsubstituted Linkers. J. Am. Chem. Soc. 2012, 134, 18790–18796. [Google Scholar] [CrossRef]
- Venna, S.R.; Jasinski, J.B.; Carreon, M.A. 2 imidazolate framework 8. J. Am. Chem. Soc. 2010, 132, 18030–18033. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Zhu, M.; Venna, S.R.; Jasinski, J.B.; Carreon, M.A. Room Temperature Synthesis of ZIF8 The Co-existence of ZnO Nano-needles. Chem. Mater. 2011, 23, 3590–3592. [Google Scholar] [CrossRef]
- Crystallography Open Data Base (COD). ZIF-8. Available online: http://www.crystallography.net/cod/ (accessed on 11 May 2021).
- Yao, J.; He, M.; Wang, K.; Chen, R.; Zhong, Z.; Wang, H. High-yield synthesis of zeolitic imidazolate frameworks from stoichiometric metal and ligand precursor aqueous solutions at room temperature. Cryst. Eng. Comm. 2013, 15, 3601–3606. [Google Scholar] [CrossRef]
- Tran, V.A.; Kadam, A.N.; Lee, S.-W. Adsorption-assisted photocatalytic degradation of methyl orange dye by zeolite-imidazole-framework-derived nanoparticles. J. Alloy. Compd. 2020, 835, 155414. [Google Scholar] [CrossRef]
- Wu, C.; Xie, D.; Mei, Y.; Xiu, Z.; Poduska, K.M.; Li, D.; Xu, B.; Sun, D. Unveiling the thermolysis natures of ZIF-8 and ZIF-67 by employing in situ structural characterization studies. Phys. Chem. Chem. Phys. 2019, 21, 17571–17577. [Google Scholar] [CrossRef] [PubMed]
- Brandani, S.; Ruthven, D.M. Analysis of ZLC desorption curves for gaseous systems. Adsorption 1996, 2, 133–143. [Google Scholar] [CrossRef]
- Brown, R.L.; Stein, S.E. Boiling Point Data. In NIST Chemistry WebBook; NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023. [Google Scholar] [CrossRef]
- Burgess, D.R., Jr. Thermochemical Data. In NIST Chemistry WebBook; NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023. [Google Scholar] [CrossRef]
- Majer, V.; Svoboda, V. Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation; Blackwell Scientific Publications: Oxford, UK, 1985; p. 300. [Google Scholar]
- National Institute of Standards and Technology. Thermophysical Properties of Fluid Systems: Ethane and Ethylene. 2022. Available online: https://webbook.nist.gov (accessed on 26 June 2023).
- Weitz, S.L.; Potoff, J.J. Effect of quadrupole moment on the phase behavior of binary mixtures containing ethene. Fluid Phase Equilibria 2005, 234, 144–150. [Google Scholar] [CrossRef]
- Van den Berghycol, J. Understanding the Anomalous Alkane Selectivity of ZIF-7 in the Separation of Light Alkane/Alkene Mixtures. Chem. A Eur. J. 2011, 17, 8832–8840. [Google Scholar] [CrossRef]
- Hughes, J.T.; Bennett, T.D.; Cheetham, A.K.; Navrotsky, A. Thermochemistry of Zeolitic Imidazolate Frameworks of Varying Porosity. J. Am. Chem. Soc. 2013, 135, 598–601. [Google Scholar] [CrossRef]
- Coudert, F.X.; Evans, J.D. Nanoscale metamaterials: Meta-MOFs and framework materials with anomalous behavior. Coord. Chem. Rev. 2019, 388, 48–62. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Bustamante, J.; Salcedo, R.; Balmaseda, J. A Route to Understanding the Ethane Adsorption Selectivity of the Zeolitic Imidazolate Framework-8 in Ethane–Ethylene Mixtures. Materials 2023, 16, 6587. https://doi.org/10.3390/ma16196587
Vargas-Bustamante J, Salcedo R, Balmaseda J. A Route to Understanding the Ethane Adsorption Selectivity of the Zeolitic Imidazolate Framework-8 in Ethane–Ethylene Mixtures. Materials. 2023; 16(19):6587. https://doi.org/10.3390/ma16196587
Chicago/Turabian StyleVargas-Bustamante, Jaquebet, Roberto Salcedo, and Jorge Balmaseda. 2023. "A Route to Understanding the Ethane Adsorption Selectivity of the Zeolitic Imidazolate Framework-8 in Ethane–Ethylene Mixtures" Materials 16, no. 19: 6587. https://doi.org/10.3390/ma16196587
APA StyleVargas-Bustamante, J., Salcedo, R., & Balmaseda, J. (2023). A Route to Understanding the Ethane Adsorption Selectivity of the Zeolitic Imidazolate Framework-8 in Ethane–Ethylene Mixtures. Materials, 16(19), 6587. https://doi.org/10.3390/ma16196587





