Control of Fluoride Pollution in Cemented Phosphogypsum Backfill by Citric Acid Pretreatment
Abstract
:1. Introduction
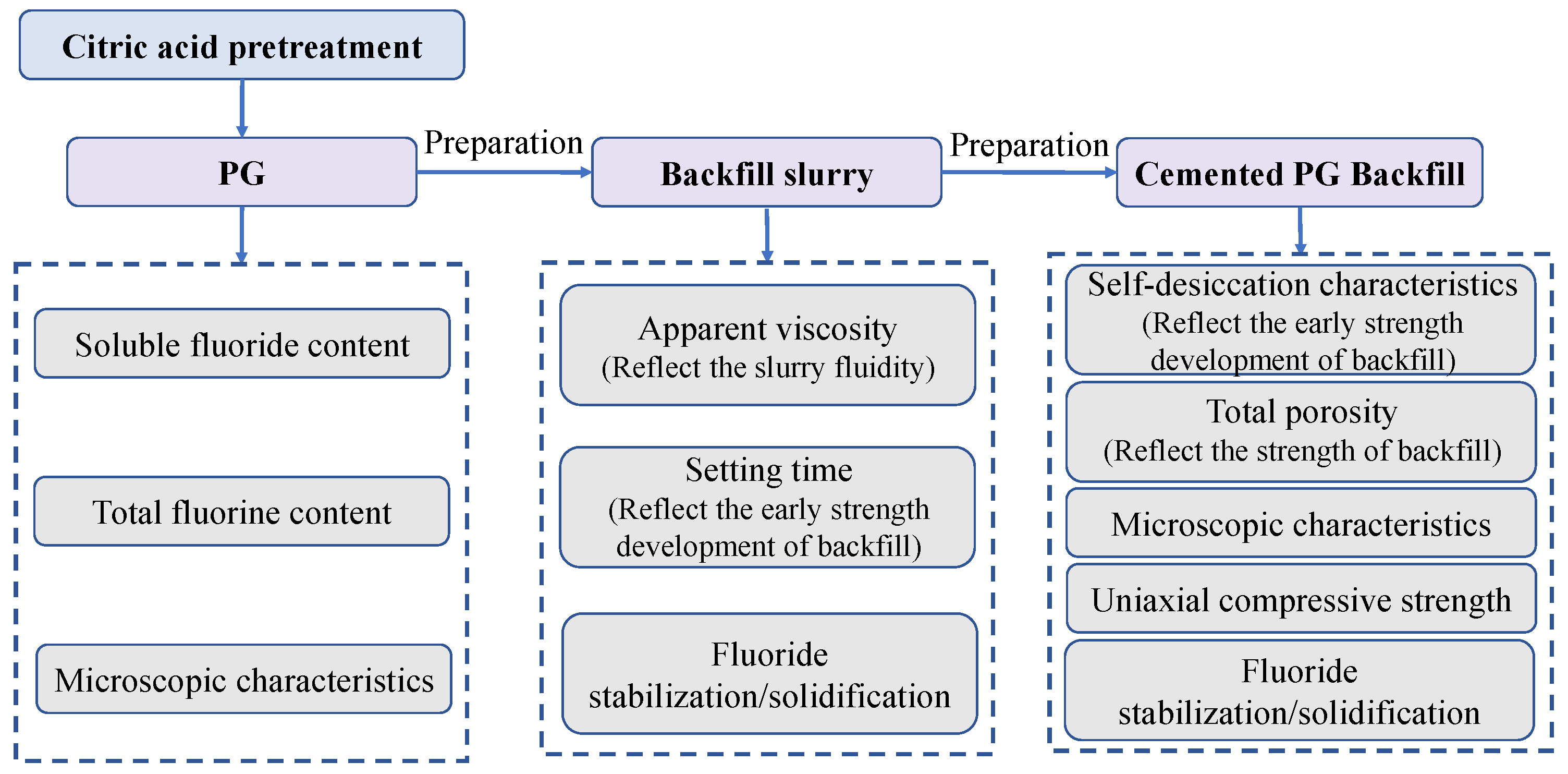
2. Materials and Methods
2.1. Raw Materials
2.2. Batch Purification of PG
2.3. Test Methodology
2.3.1. Preparation of Cemented Backfill Samples
2.3.2. Determination of Apparent Viscosity and Setting Time
2.3.3. Suction Monitoring
2.3.4. Porosity Measurement
2.3.5. Uniaxial Compressive Strength Test
2.3.6. Microstructure Test
2.3.7. Toxicity Leaching Test
2.3.8. Chemical Composition Measurement
3. Test Results and Discussion
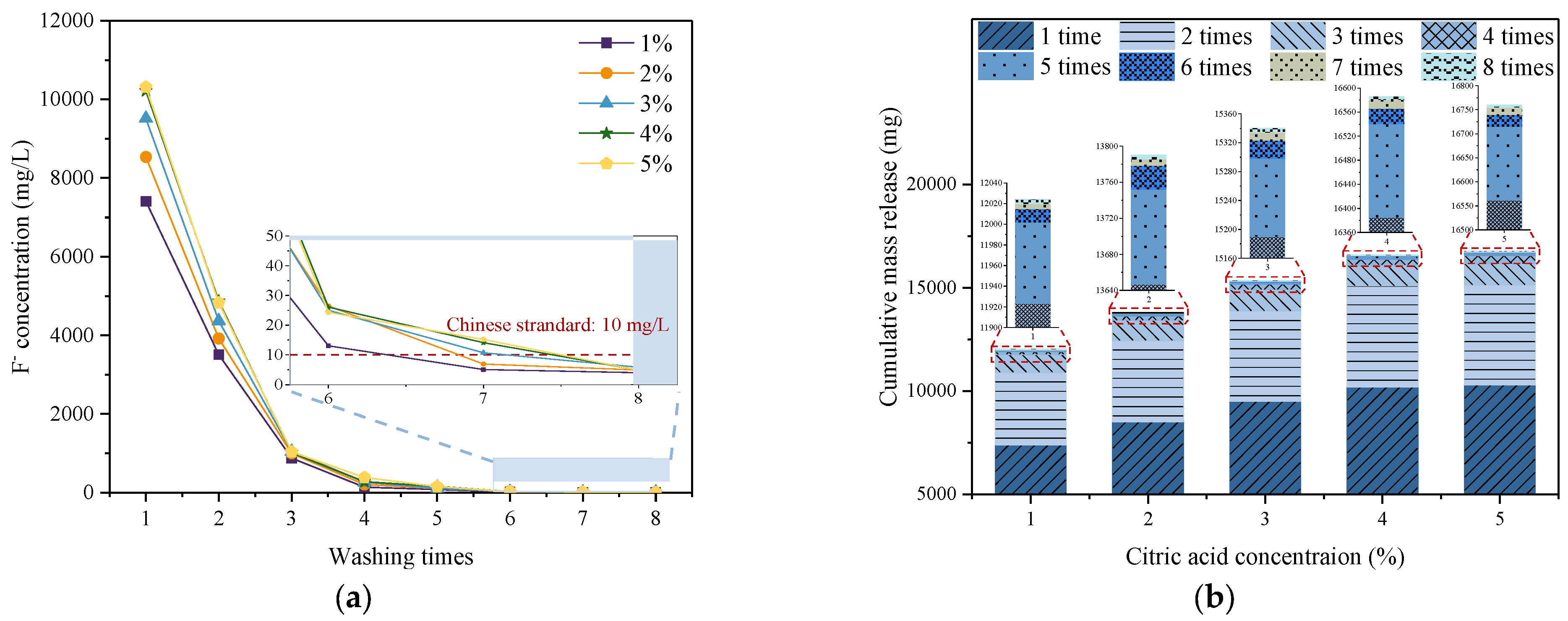
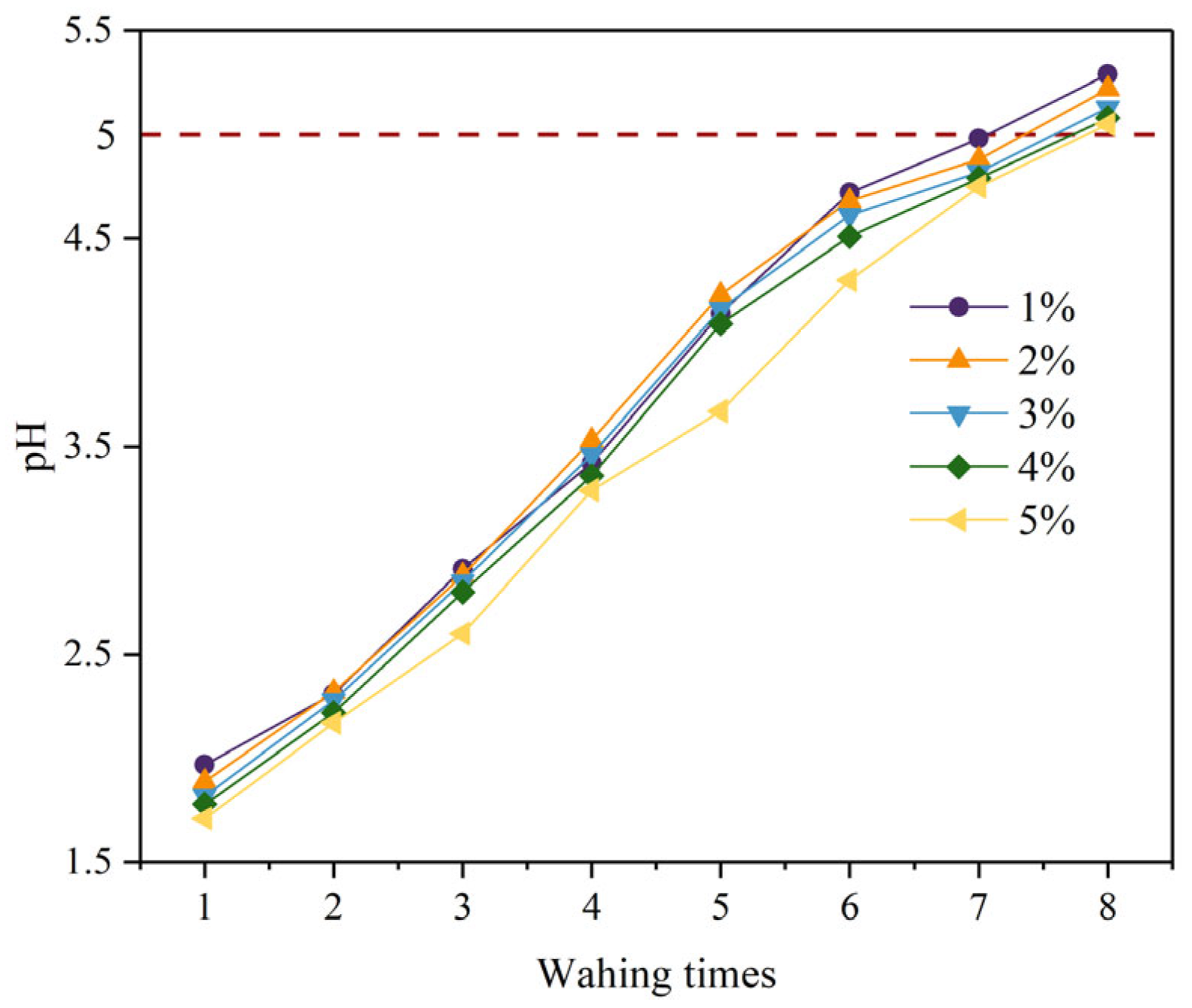
3.1. Removal of Fluoride from PG by Citric Acid Washing
3.1.1. Soluble Fluoride Content in PG
3.1.2. Total Fluorine Content in PG
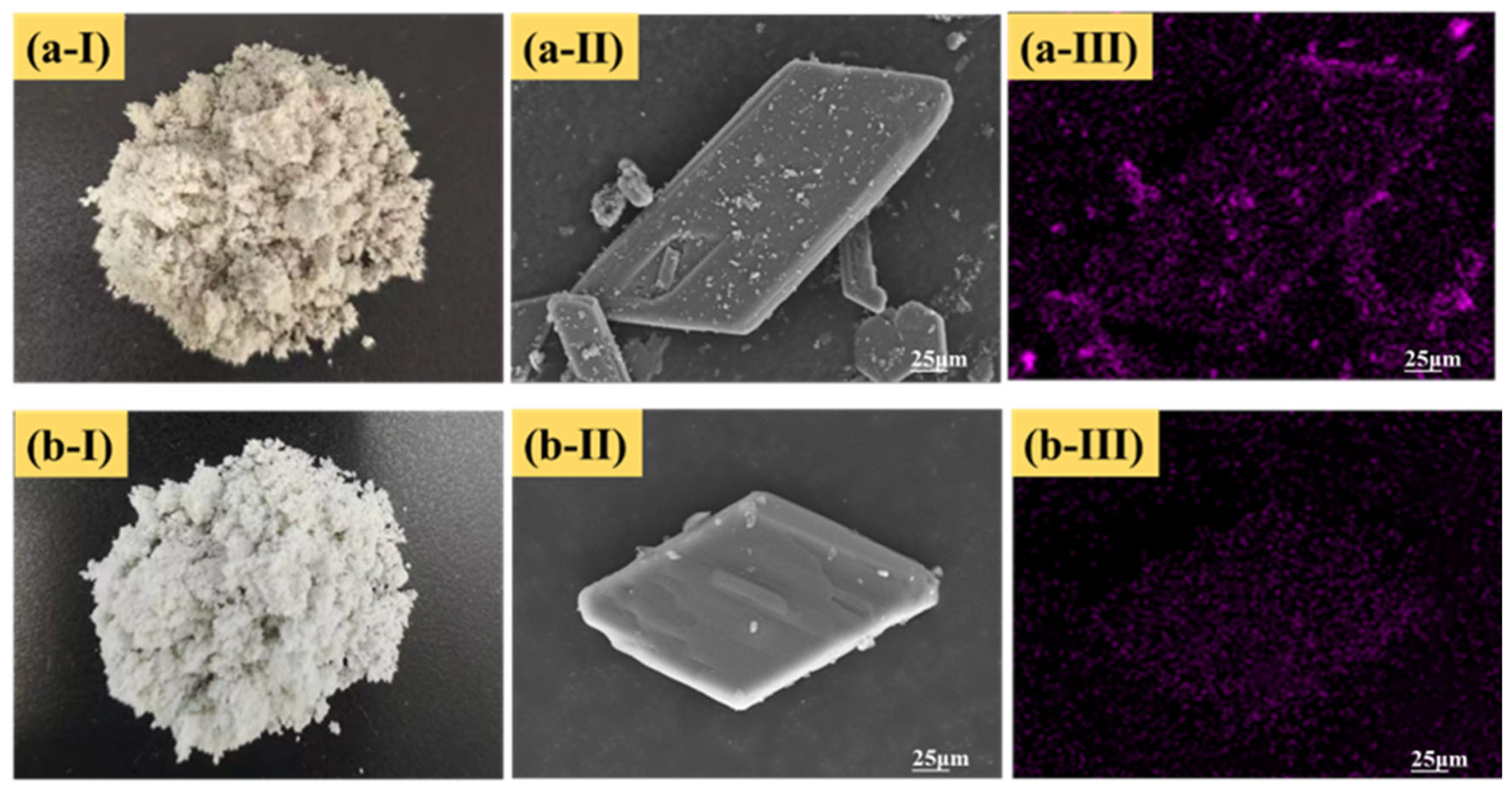
3.1.3. Microscopic Characteristics of PG
3.2. Optimization of the Properties of Cemented Backfill Slurry
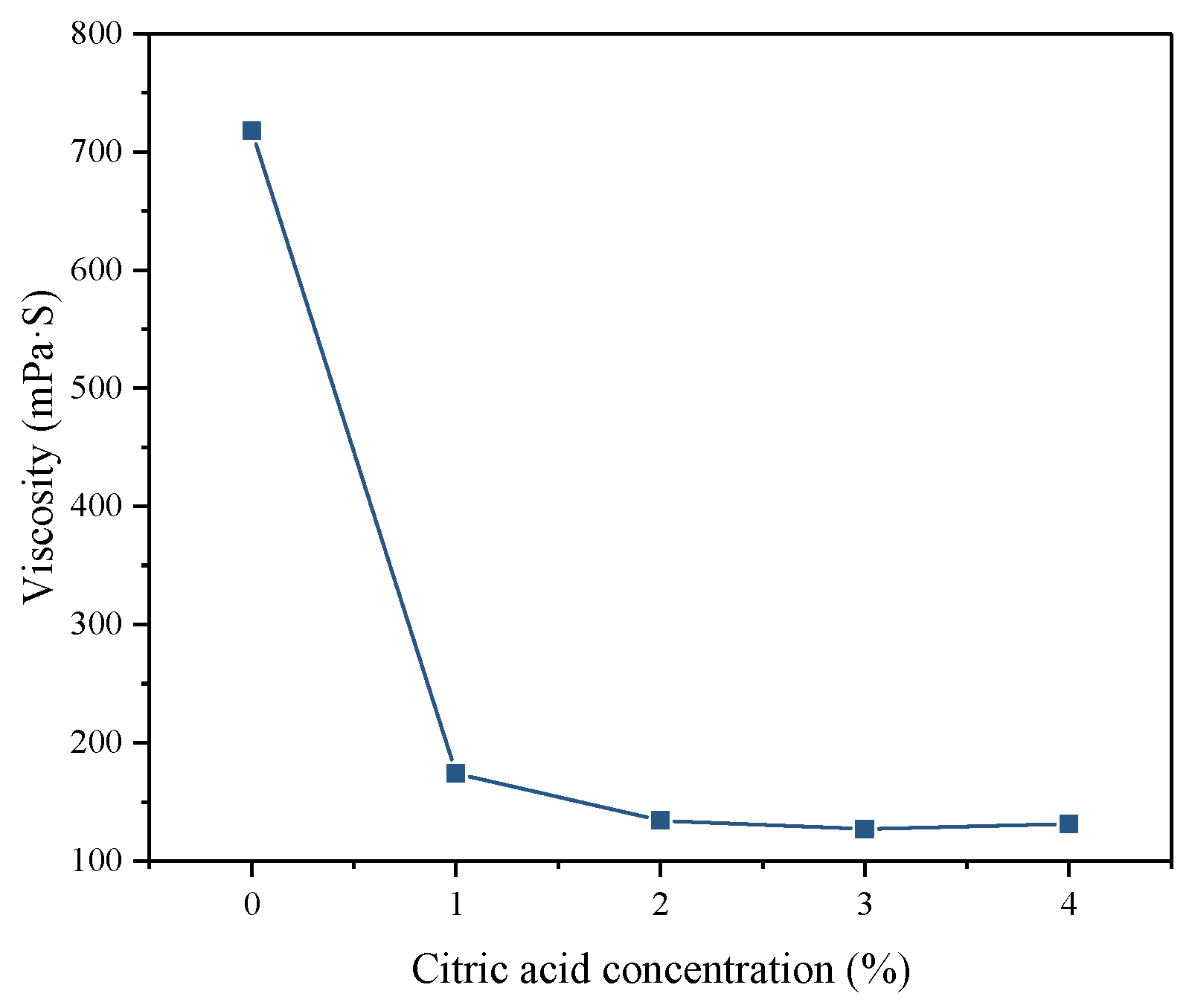
3.2.1. Apparent Viscosity
3.2.2. Setting Time
3.3. Optimization of the Mechanical Properties of the Cemented Backfill
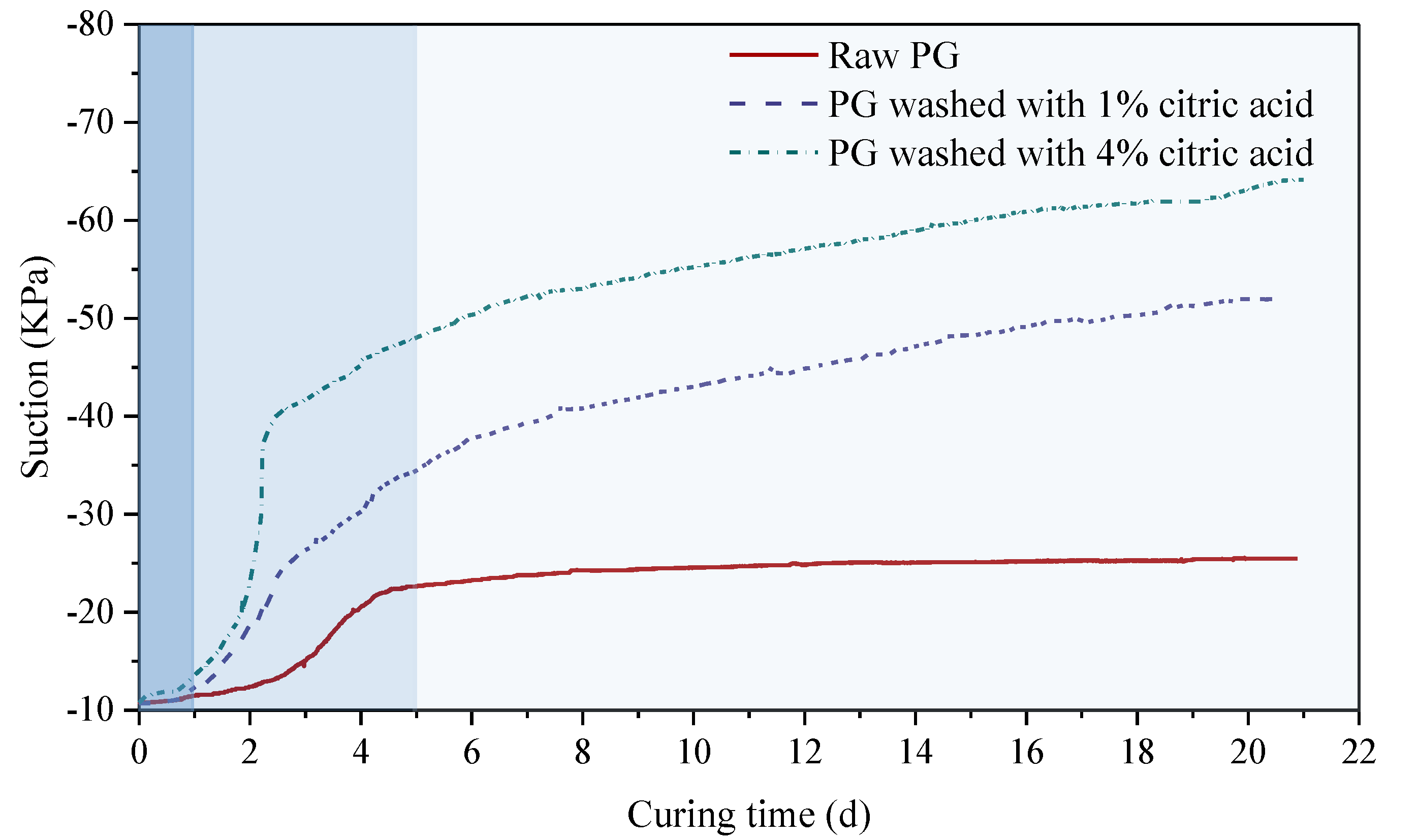
3.3.1. Self-Desiccation Characteristics
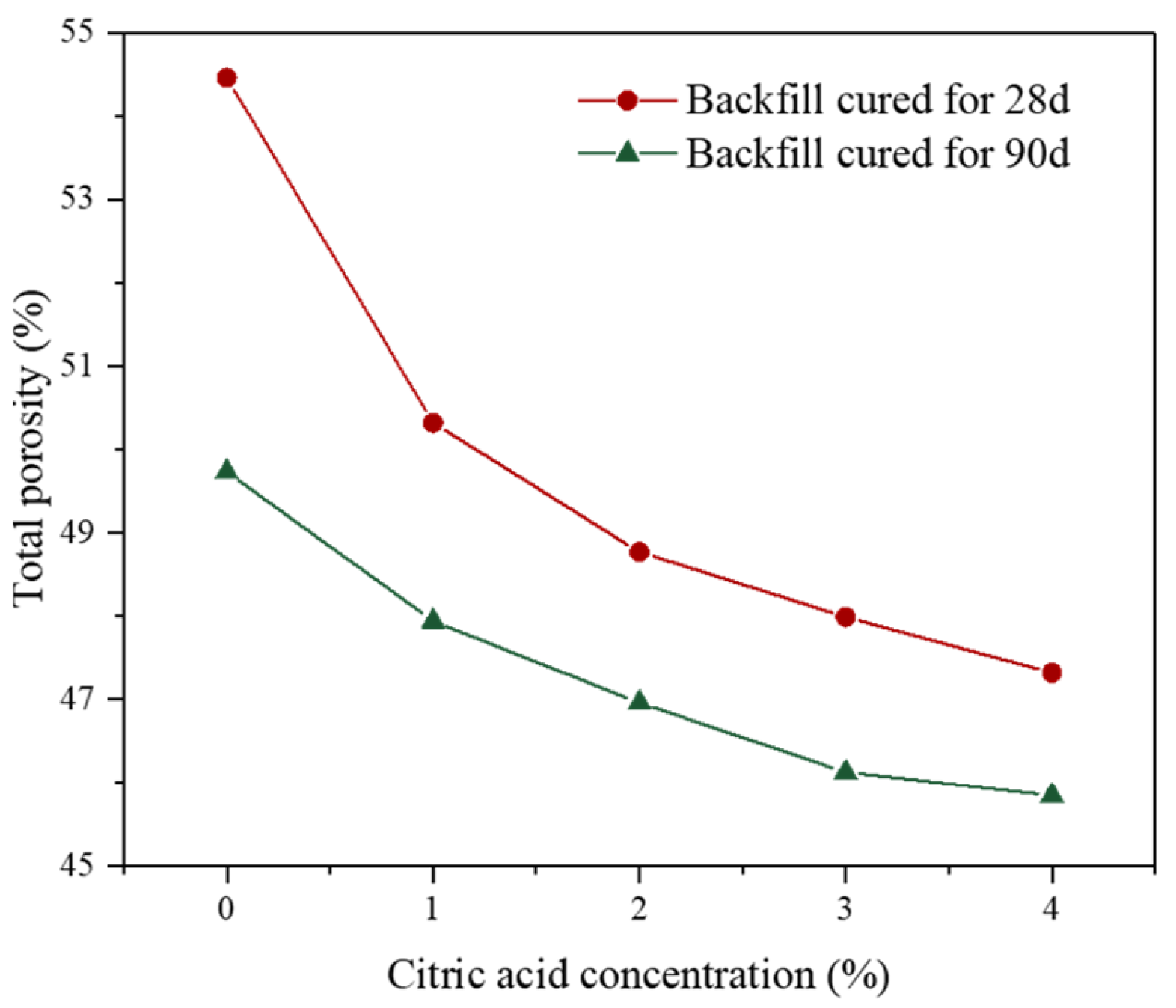
3.3.2. Total Porosity
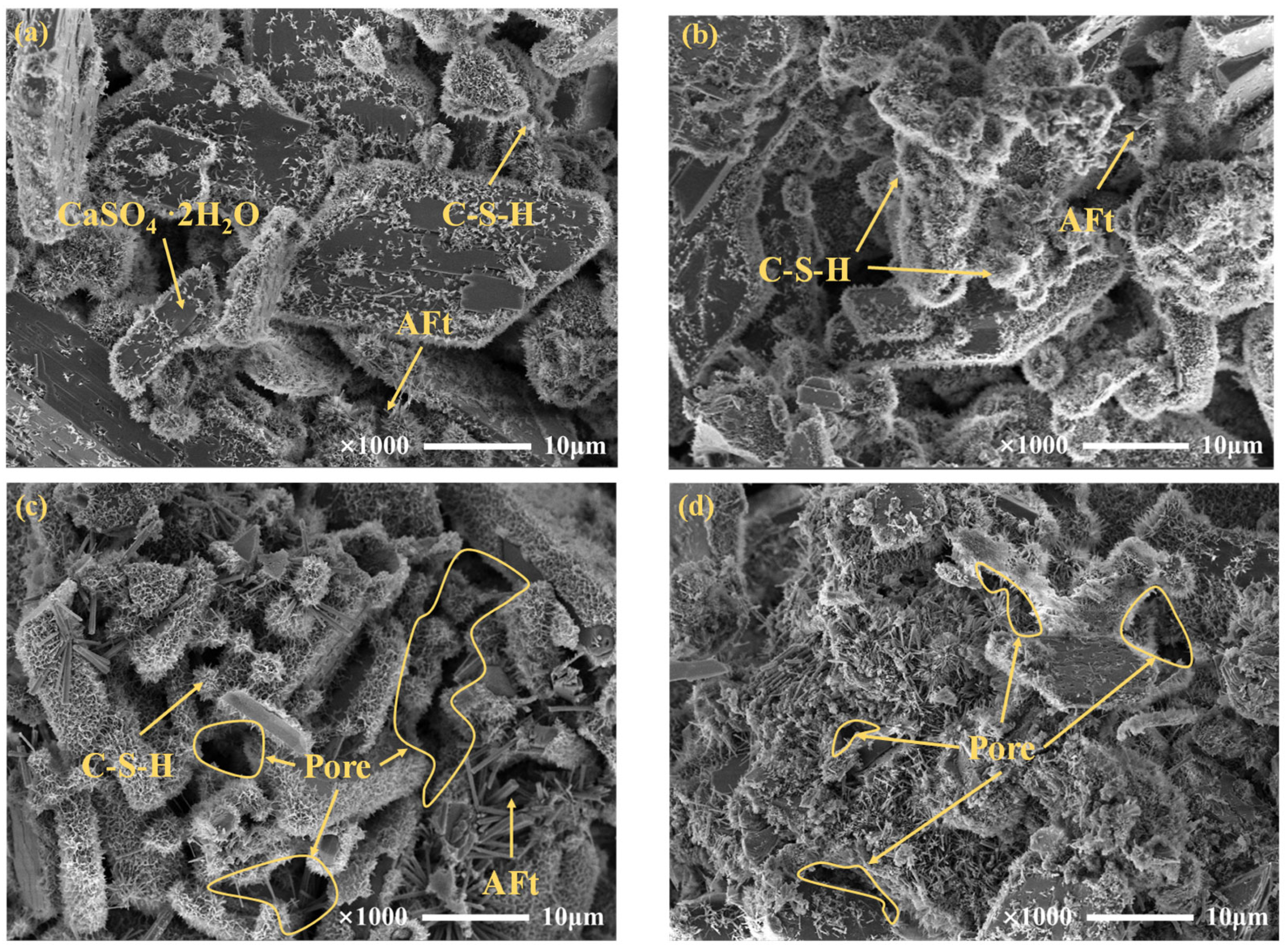
3.3.3. Microstructure of Cemented PG Backfill
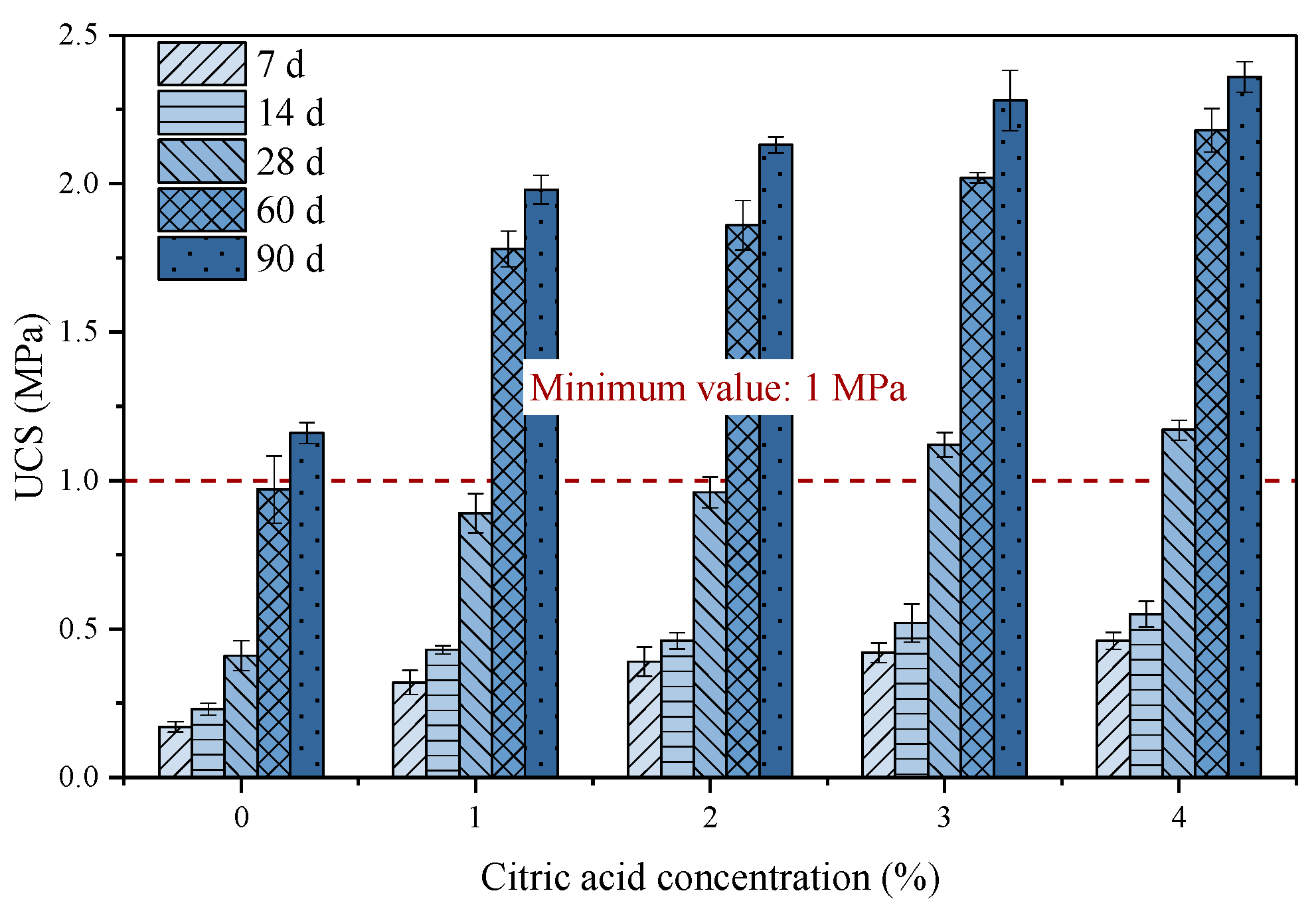
3.3.4. Strength Development
3.4. Optimization of Fluoride S/S during the Cemented Backfill Process
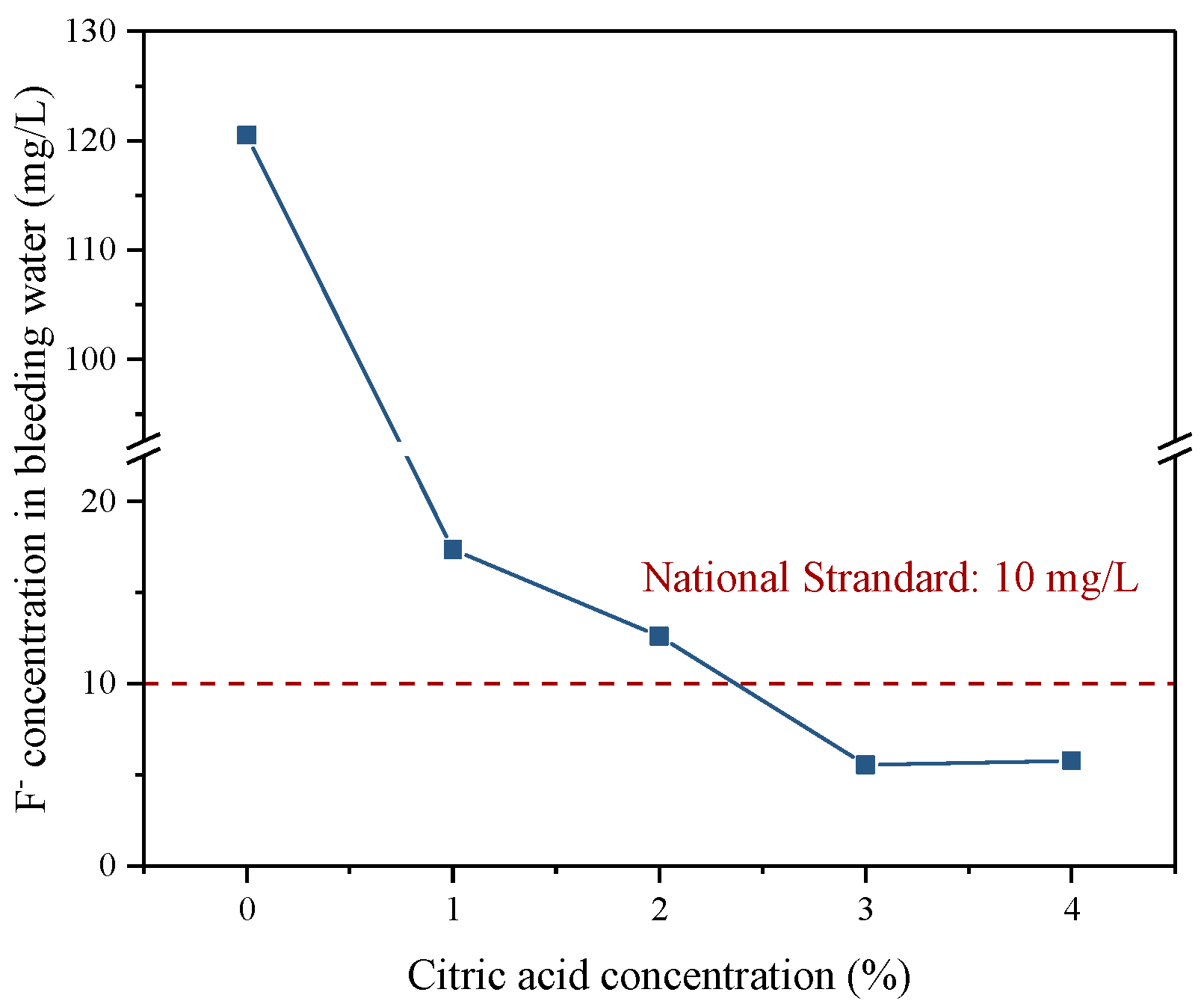
3.4.1. Fluoride S/S in Backfill Slurry
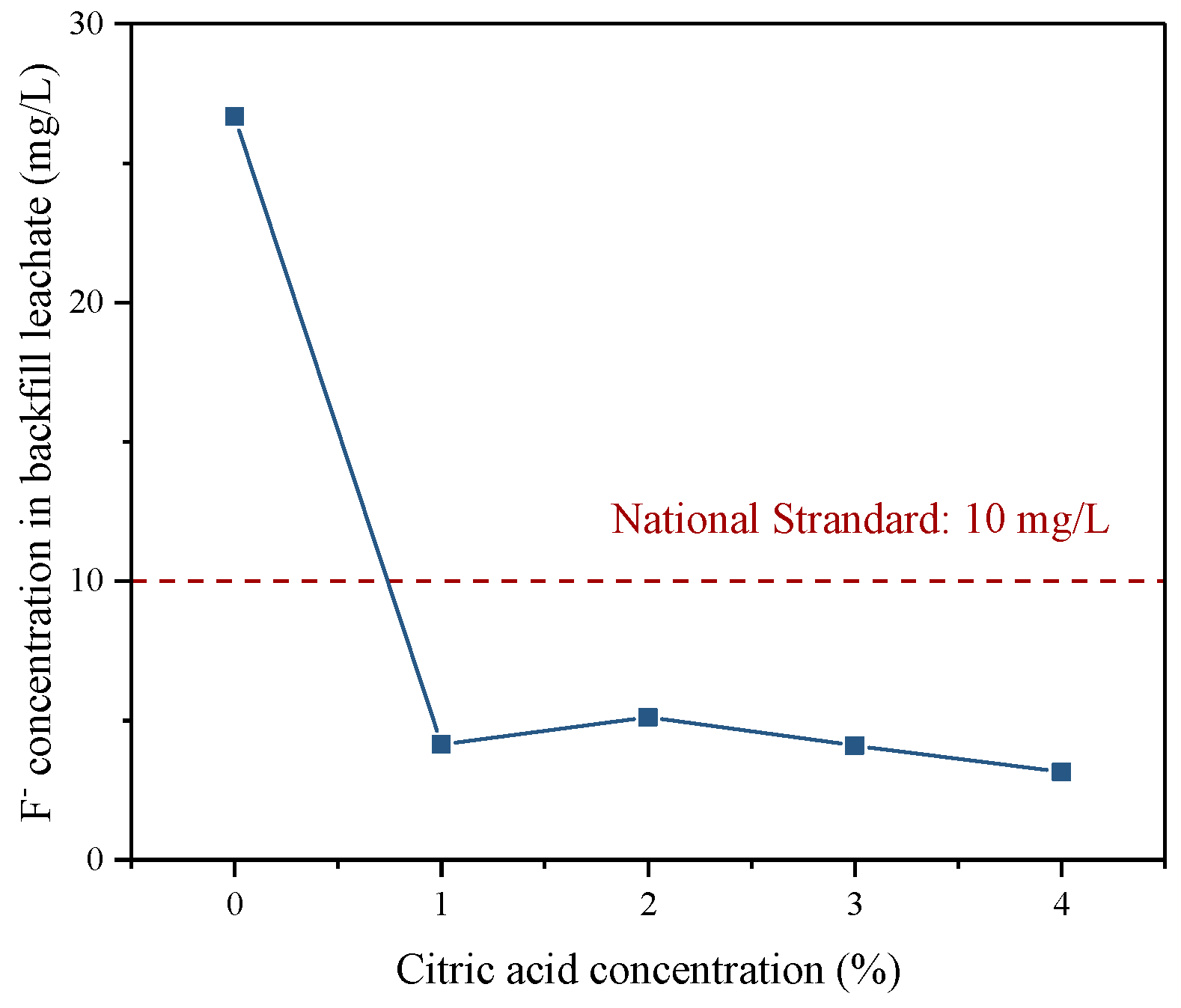
3.4.2. Fluoride S/S in Backfill
4. Conclusions
- (1)
- The content of fluoride washed out from PG increased with increasing citric acid concentrations and decreased with increasing washing times. Considering the efficiency of defluorination, the optimal citric acid concentration and washing times were determined to be 4% and 7–8 times, respectively.
- (2)
- After citric acid pretreatment, the viscosity and setting time of the backfill slurry were greatly reduced due to the reduction in fluoride in pretreated PG. Therefore, the citric acid washing pretreatment is beneficial to the pipeline transportation of backfill slurry and the early strength development of hardened backfill.
- (3)
- The citric acid pretreatment effectively improved the microstructure of cemented PG backfill by promoting the hydration reaction and reducing the backfill porosity, thus significantly enhancing the backfill strength. Furthermore, the use of pretreated PG as an aggregate could greatly reduce the usage and cost of the binder in actual production.
- (4)
- During the cemented PG backfill process, the hydration reaction had a good S/S effect on the fluoride in the PG. Remarkably, when the PG was pretreated with a citric acid concentration of more than 3%, the concentration of fluoride in bleeding water and the backfill leachate was below the national standard limit, which is conducive to reducing the surrounding environmental pollution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Ren, Y.; Qu, G.; Liu, S.; Chen, B.; Liu, X.; Zhao, C.; Li, J. Utilization path of bulk industrial solid waste: A review on the multi-directional resource utilization path of phosphogypsum. J. Environ. Manag. 2022, 313, 114957. [Google Scholar] [CrossRef] [PubMed]
- Amrani, M.; Taha, Y.; Kchikach, A.; Benzaazoua, M.; Hakkou, R. Phosphogypsum recycling: New horizons for a more sustainable road material application. J. Build. Eng. 2020, 30, 101267. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Dalconi, M.C.; Molinari, S.; Valentini, L.; Wang, Y.; Sun, S.; Wang, P.; Artioli, G. Enhancing the sustainable immobilization of phosphogypsum by cemented paste backfill with the activation of γ-Al2O3. Constr. Build. Mater. 2022, 347, 128624. [Google Scholar] [CrossRef]
- Wang, J. Utilization effects and environmental risks of phosphogypsum in agriculture: A review. J. Clean. Prod. 2020, 276, 123337. [Google Scholar] [CrossRef]
- Tsioka, M.; Voudrias, E.A. Comparison of alternative management methods for phosphogypsum waste using life cycle analysis. J. Clean. Prod. 2020, 266, 121386. [Google Scholar] [CrossRef]
- Lütke, S.F.; Oliveira, M.L.S.; Silva, L.F.O.; Cadaval, T.R.S., Jr.; Dotto, G.L. Nanominerals assemblages and hazardous elements assessment in phosphogypsum from an abandoned phosphate fertilizer industry. Chemosphere 2020, 256, 127138. [Google Scholar] [CrossRef] [PubMed]
- Mashifana, T.; Ntuli, F.; Okonta, F. Leaching kinetics on the removal of phosphorus from waste phosphogypsum by application of shrinking core model. S. Afr. J. Chem. Eng. 2019, 27, 1–6. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Chen, X.; Ma, Y.; Zhou, M. Modification of phosphogypsum using circulating fluidized bed fly ash and carbide slag for use as cement retarder. Constr. Build. Mater. 2022, 338, 127630. [Google Scholar] [CrossRef]
- Sengupta, I.; Dhal, P.K. Impact of elevated phosphogypsum on soil fertility and its aerobic biotransformation through indigenous microorganisms (IMO’s) based technology. J. Environ. Manag. 2021, 297, 113195. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Ma, L.; Li, Y.; Deng, Y.; Chen, X.; Xu, Z. Adding an appropriate proportion of phosphogypsum ensured rice husk and urea composting to promote the compost as substrate utilization. Bioresour. Technol. 2022, 344, 126301. [Google Scholar] [CrossRef]
- Maierdan, Y.; Haque, M.A.; Chen, B.; Maimaitiyiming, M.; Ahmad, M.R. Recycling of waste river sludge into unfired green bricks stabilized by a combination of phosphogypsum, slag, and cement. Constr. Build. Mater. 2020, 260, 120666. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, H.; Chen, B.; Cui, Q. Development and optimization of phosphogypsum-based geopolymer cement. Constr. Build. Mater. 2023, 369, 130577. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Z.; Zhao, G.; Liu, Z. Utilization of phosphogypsum for backfilling, way to relieve its environmental impact. Gospod. Surowcami Miner. 2008, 24, 223–232. [Google Scholar]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Fuge, R. Fluorine in the environment, a review of its sources and geochemistry. Appl. Geochem. 2019, 100, 393–406. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Wang, Y.; Zhang, Q.; Liu, Y. Highly-efficient fluoride retention in on-site solidification/stabilization of phosphogypsum: Cemented paste backfill synergizes with poly-aluminum chloride activation. Chemosphere 2022, 309, 136652. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, S.; Liu, Y.; Qi, C.; Zhou, H.; Zhang, Q. Immobilization and leaching characteristics of fluoride from phosphogypsum-based cemented paste backfill. Int. J. Miner. Metall. Mater. 2021, 28, 1440–1542. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Kang, X.; Yu, J.; Fan, Y.; Dang, Y.; Zhang, W.; Wang, S. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Yang, J.; Ma, L.; Liu, H.; Guo, Z.; Dai, Q.; Zhang, W.; Bounkhong, K. Chemical behavior of fluorine and phosphorus in chemical looping gasification using phosphogypsum as an oxygen carrier. Chemosphere 2020, 248, 125979. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Shi, Y.; Zhu, Q. Fluoride immobilization and release in cemented PG backfill and its influence on the environment. Sci. Total Environ. 2023, 869, 161548. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L.; et al. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Juliastuti, S.R.; Hendrianie, N.; Pawitra, Y.D.; Putra, I.R. Reduction of P2O5 and F from Phosphogypsum by CaO addition. MATEC Web Conf. 2018, 156, 03021. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Dalconi, M.C.; Molinari, S.; Valentini, L.; Wang, Y.; Sun, S.; Wang, P.; Artioli, G. Retention of phosphorus and fluorine in phosphogypsum for cemented paste backfill: Experimental and numerical simulation studies. Environ. Res. 2022, 214(Pt. 1), 113775. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Q.; Chen, Q.; Qi, C.; Su, Z.; Huang, Z. Utilisation of water-washing pre-treated phosphogypsum for cemented paste backfill. Minerals 2019, 9, 175. [Google Scholar] [CrossRef]
- Tan, H.; Dong, F.; Bian, L.; He, X.; Liu, J. Preparation of Anhydrous Calcium Sulfate Whiskers from Phosphogypsum in H2O-H2SO4 Autoclave-Free Hydrothermal System. Mater. Trans. 2017, 58, 1111–1117. [Google Scholar] [CrossRef]
- Singh, M. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Mashifana, T.P. Chemical treatment of phosphogypsum and its potential application for building and construction. Procedia Manuf. 2019, 35, 641–648. [Google Scholar] [CrossRef]
- Mashifana, T.P.; Okonta, F.N.; Ntuli, F. Geotechnical Properties and Microstructure of Lime-Fly Ash-Phosphogypsum-Stabilized Soil. Adv. Civ. Eng. 2018, 2018, 3640868. [Google Scholar] [CrossRef]
- STM D2196-18; Standard test methods for rheological properties of non-newtonian materials by rotational viscountess. ASTM International: West Conshohocken, PA, USA, 2018.
- GB/T 1346-2011; Test methods for water requirement of normal consistency, setting time and soundness of the portland cement. Standards Press of China: Beijing, China, 2011.
- Wang, Y.; Yuan, Q.; Deng, D.; Ye, T.; Fang, L. Measuring the pore structure of cement asphalt mortar by nuclear magnetic resonance. Constr. Build. Mater. 2017, 137, 450–458. [Google Scholar] [CrossRef]
- Dong, X.; Meng, X.; Pu, R. Impacts of mineralogy and pore throat structure on the movable fluid of tight sandstone gas reservoirs in coal measure strata: A case study of the Shanxi formation along the southeastern margin of the Ordos Basin. J. Pet. Sci. Eng. 2023, 220, 111257. [Google Scholar] [CrossRef]
- JGJ/T70-2009; Standard for test method of performance on building mortar. China Ministry of Architecture and Building Press: Beijing, China, 2009.
- HJ 557-2010; Solid waste-extraction procedure for leaching toxicity-horizontal vibration method. China Ministry of Ecology and Environment Press: Beijing, China, 2009.
- JCT 2073-2011; Determination of phosphorus and fluorine content in phosphogypsum. China Ministry of Industry and Information Technology Press: Beijing, China, 2011.
- Li, Y.; Yue, W.; Pan, C.; Zhang, S. Study on Influence of Pretreatment Process on Phosphogypsum and Slag Cement. Non-Met. Mines 2018, 41, 15–17. [Google Scholar]
- Li, X.; Liu, B.; Yao, J.; Shi, Y.; Li, D.; Du, S.; He, Z.; Gao, L.; Wang, X.; Zhao, G.; et al. Theory and practice of green mine backfill with whole phosphate waste. Chin. J. Nonferrous Met. 2018, 9, 1845–1865. [Google Scholar]
- Chen, S.; Chen, J.; He, X.; Su, Y.; Jin, Z.; Wang, B. Micromicelle-mechanical coupling method for high-efficiency phosphorus removal and whiteness improvement of phosphogypsum. Constr. Build. Mater. 2022, 354, 129220. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Zhu, Q.; Zhou, S.; Min, C.; Shi, Y. Slurry preparation effects on the cemented phosphogypsum backfill through an orthogonal experiment. Minerals 2019, 9, 31. [Google Scholar] [CrossRef]
- Wang, H.; Mao, Q.J.; Gao, Y.; Wang, Z.M.; Cui, S.P. Effect and Mechanism of Sodium Fluosilicate on Setting Time of Cement. Mater. Sci. Forum 2017, 898, 1978–1983. [Google Scholar] [CrossRef]
- Patel, D.; Ein-Mozaffari, F.; Mehrvar, M. Characterization of the continuous-flow mixing of non-Newtonian fluids using the ratio of residence time to batch mixing time. Chem. Eng. Res. Des. 2013, 91, 1223–1234. [Google Scholar] [CrossRef]
- Min, C.; Li, X.; He, S.; Zhou, S.; Zhou, Y.; Yang, S.; Shi, Y. Effect of mixing time on the properties of phosphogypsum-based cemented backfill. Constr. Build. Mater. 2019, 210, 564–573. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Zhou, Y.; Min, C.; Shi, Y. Effect of phosphorus on the properties of phosphogypsum-based cemented backfill. J. Hazard. Mater. 2020, 399, 122993. [Google Scholar] [CrossRef]
- Shi, Y.; Song, Y.; Min, C.; Du, J.; Wang, X.; Min, J.; Wang, S. Phosphogypsum pretreatment with CaCO3 to improve the backfill performance. Case Stud. Constr. Mater. 2023, 18, e01954. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, S.; Wang, Y.; Zhang, Q.; Zhu, L.; Liu, Y. In-situ remediation of phosphogypsum in a cement-free pathway: Utilization of ground granulated blast furnace slag and NaOH pretreatment. Chemosphere 2023, 313, 137412. [Google Scholar] [CrossRef]
- Costa, R.P.; de Medeiros, M.H.G.; Martinez, E.D.R.; Quarcioni, V.A.; Suzuki, S.; Kirchheim, A.P. Effect of soluble phosphate, fluoride, and pH in Brazilian phosphogypsum used as setting retarder on Portland cement hydration. Case Stud. Constr. Mater. 2022, 17, e01413. [Google Scholar] [CrossRef]
- Holanda, F.D.C.; Schmidt, H.; Quarcioni, V.A. Influence of phosphorus from phosphogypsum on the initial hydration of Portland cement in the presence of superplasticizers. Cem. Concr. Compos. 2017, 83, 384–393. [Google Scholar] [CrossRef]
- Cavusoglu, I.; Yilmaz, E.; Yilmaz, A.O. Sodium silicate effect on setting properties, strength behavior and microstructure of cemented coal fly ash backfill. Powder Technol. 2021, 384, 17–28. [Google Scholar] [CrossRef]
- Li, W.; Fall, M. Strength and self-desiccation of slag-cemented paste backfill at early ages: Link to initial sulphate concentration. Cem. Concr. Compos. 2018, 89, 160–168. [Google Scholar] [CrossRef]
- Kermani, M.; Hassani, F.P.; Aflaki, E.; Benzaazoua, M.; Nokken, M. Evaluation of the effect of sodium silicate addition to mine backfill, Gelfill—Part 2: Effects of mixing time and curing temperature. J. Rock Mech. Geotech. Eng. 2015, 7, 668–673. [Google Scholar] [CrossRef]
- Li, C.; Jiang, L.; Xu, N.; Jiang, S. Pore structure and permeability of concrete with high volume of limestone powder addition. Powder Technol. 2018, 338, 416–424. [Google Scholar] [CrossRef]
- Wang, H.; Ju, C.; Zhou, M.; Chen, J.; Dong, Y.; Hou, H. Sustainable and efficient stabilization/solidification of Pb, Cr, and Cd in lead-zinc tailings by using highly reactive pozzolanic solid waste. J. Environ. Manag. 2022, 306, 114473. [Google Scholar] [CrossRef]
- Stegemann, J.A.; Zhou, Q. Screening tests for assessing treatability of inorganic industrial wastes by stabilization/solidification with cement. J. Hazard. Mater. 2009, 161, 300–306. [Google Scholar] [CrossRef]
- Guan, W.; Zhao, X. Fluoride recovery using porous calcium silicate hydrates via spontaneous Ca2+ and OH− release. Sep. Purif. Technol. 2016, 165, 71–77. [Google Scholar] [CrossRef]


| Chemical Composition | PG (wt%) | Binder (wt%) |
|---|---|---|
| SO3 | 54.93 | 5.72 |
| CaO | 36.99 | 47.94 |
| P2O5 | 2.03 | 1.56 |
| SiO2 | 1.90 | 26.01 |
| Al2O3 | 0.56 | 8.25 |
| Fe2O3 | 0.44 | 4.14 |
| K2O | 0.14 | 1.04 |
| MgO | 0.11 | 1.41 |
| Na2O | 0.07 | 0.54 |
| TiO2 | 0.05 | 0.63 |
| F | 1.38 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Shi, Y.; Zhu, Q. Control of Fluoride Pollution in Cemented Phosphogypsum Backfill by Citric Acid Pretreatment. Materials 2023, 16, 6493. https://doi.org/10.3390/ma16196493
Zhou Y, Shi Y, Zhu Q. Control of Fluoride Pollution in Cemented Phosphogypsum Backfill by Citric Acid Pretreatment. Materials. 2023; 16(19):6493. https://doi.org/10.3390/ma16196493
Chicago/Turabian StyleZhou, Yanan, Ying Shi, and Quanqi Zhu. 2023. "Control of Fluoride Pollution in Cemented Phosphogypsum Backfill by Citric Acid Pretreatment" Materials 16, no. 19: 6493. https://doi.org/10.3390/ma16196493
APA StyleZhou, Y., Shi, Y., & Zhu, Q. (2023). Control of Fluoride Pollution in Cemented Phosphogypsum Backfill by Citric Acid Pretreatment. Materials, 16(19), 6493. https://doi.org/10.3390/ma16196493








