Decreasing Hydrogen Content within Zirconium Using Au and Pd Nanoparticles as Sacrificial Agents under Pressurized Water at High Temperature
Abstract
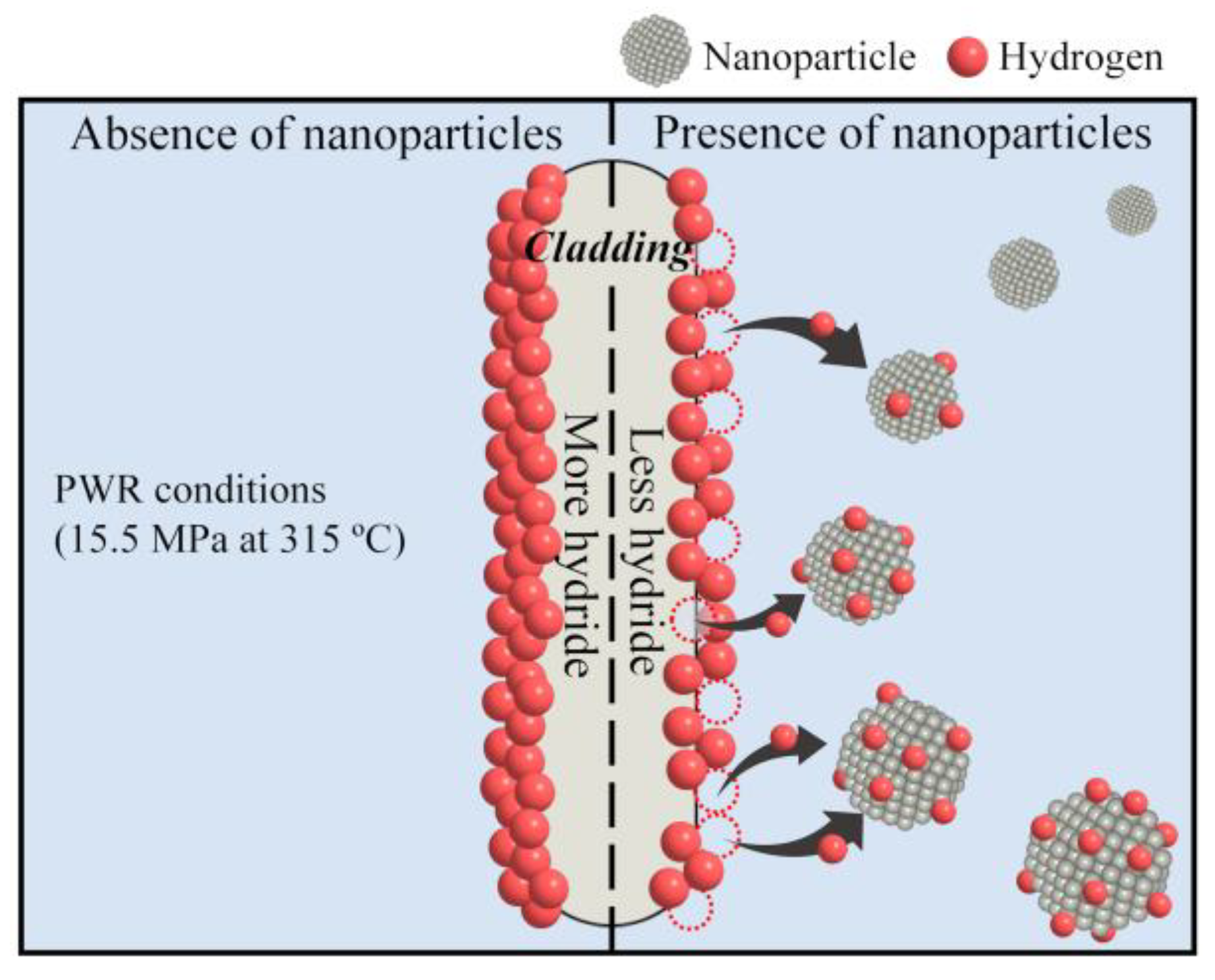
1. Introduction
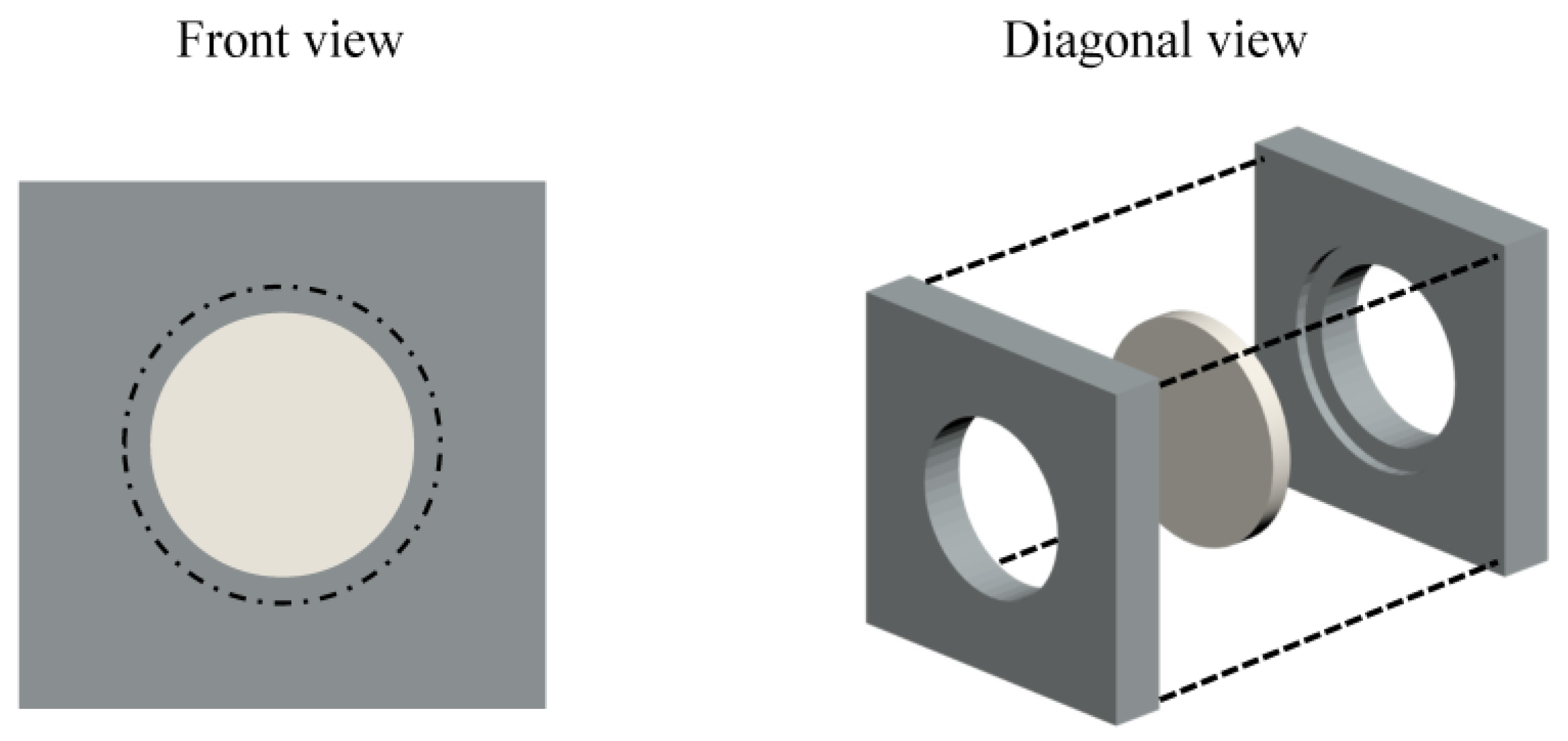
2. Materials and Methods
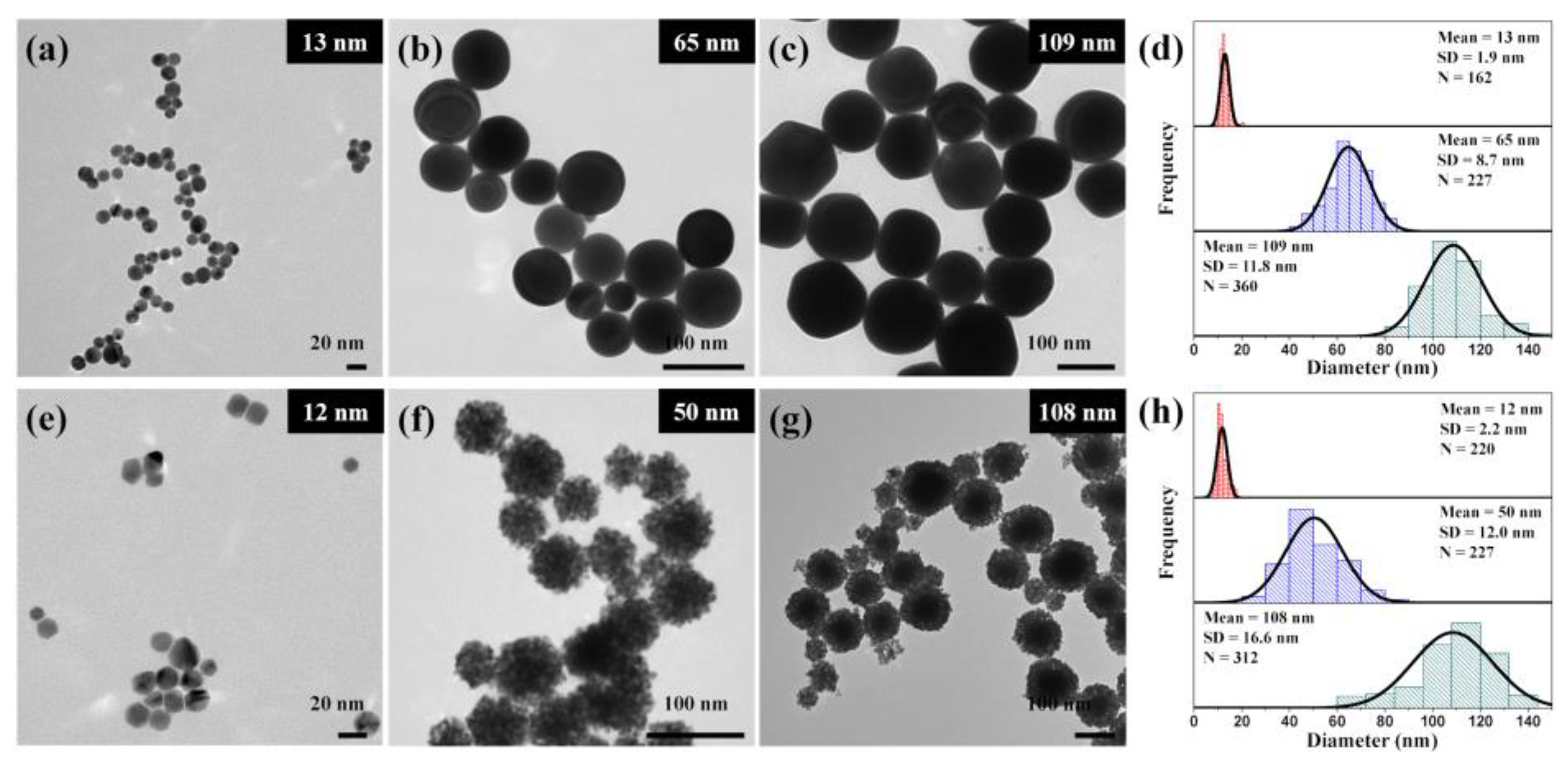
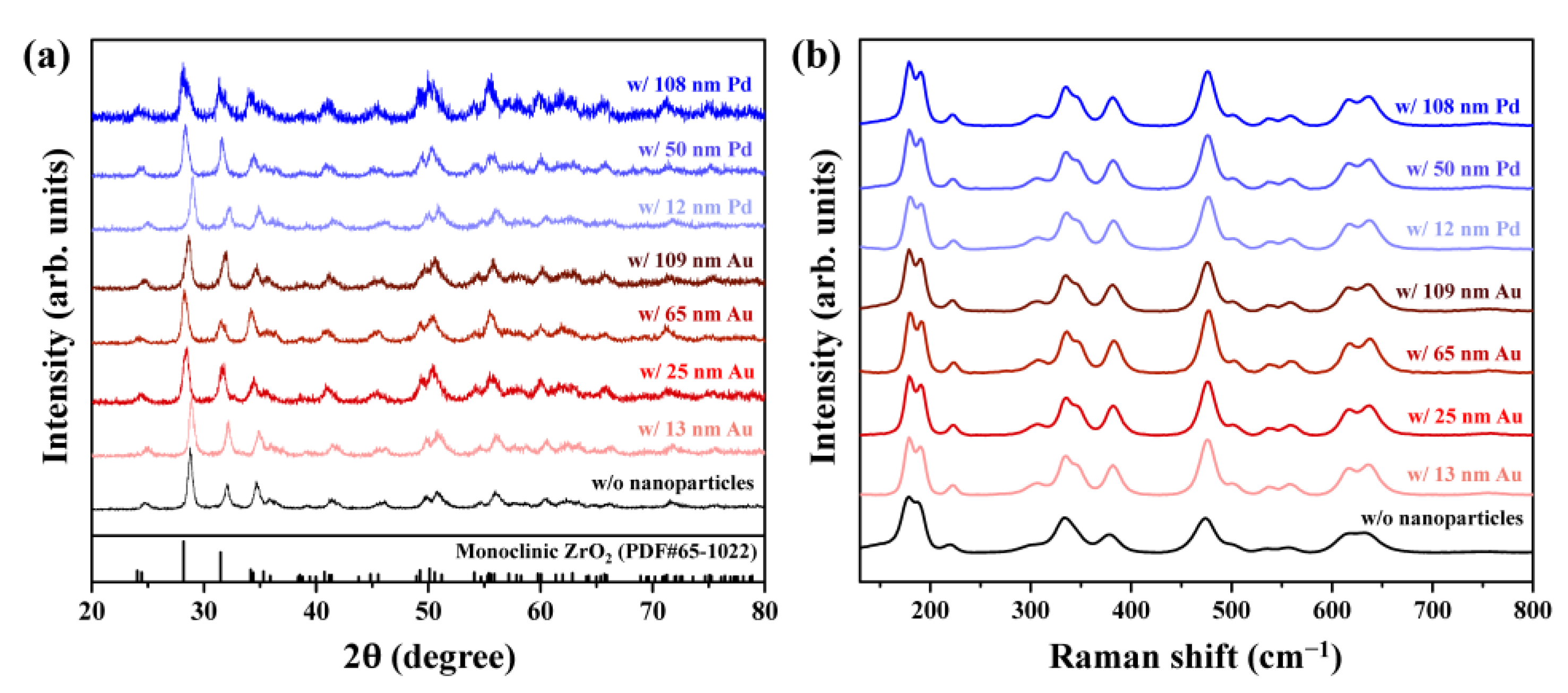
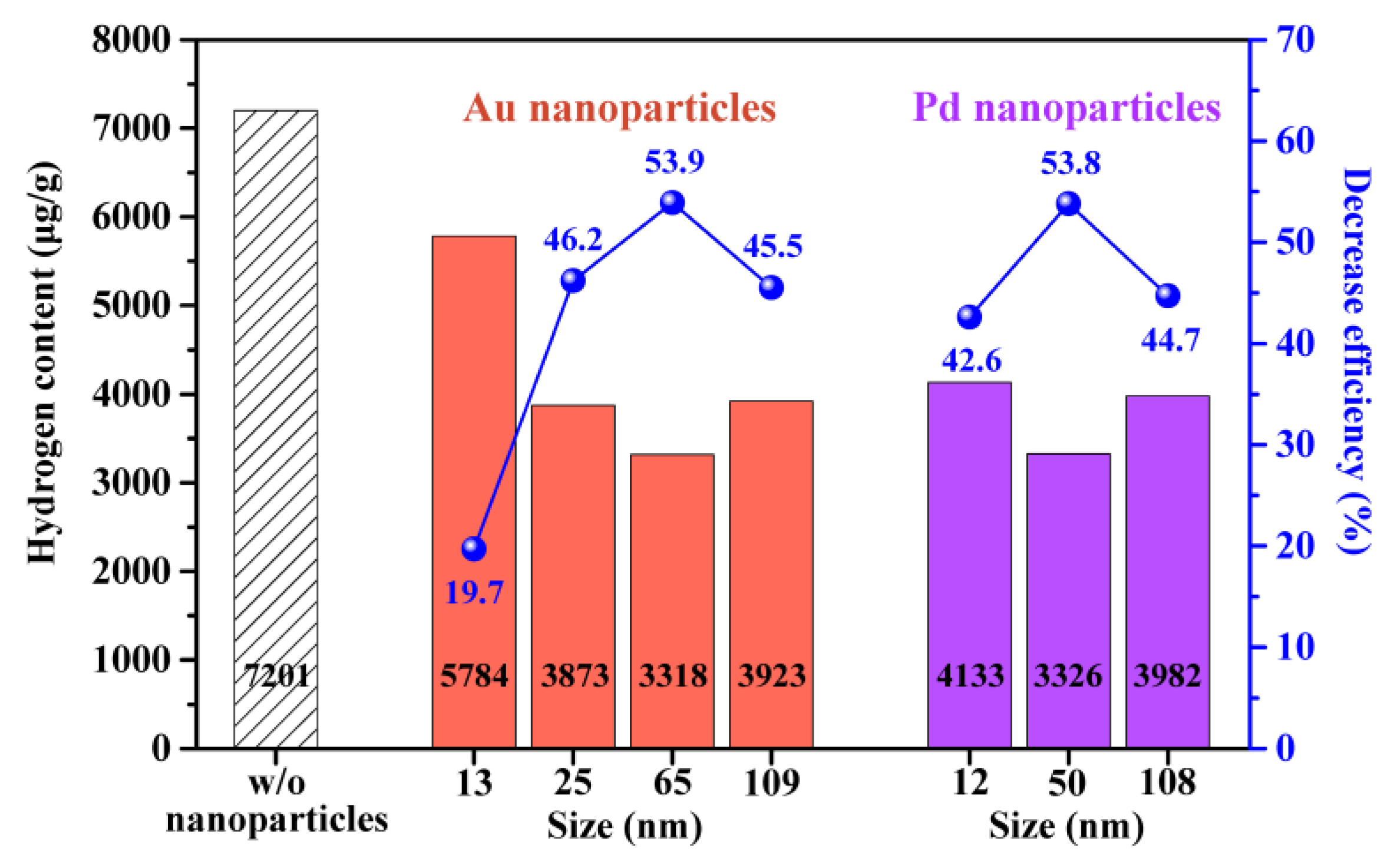
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Min, S.-J.; Won, J.-J.; Kim, K.-T. Terminal cool-down temperature-dependent hydride reorientations in Zr–Nb Alloy claddings under dry storage conditions. J. Nucl. Mater. 2014, 448, 172–183. [Google Scholar] [CrossRef]
- Youn, Y.-S.; Park, J.; Lim, S.H. Stable lattice thermal expansion of ZIRLO™: High-temperature X-ray diffraction results. J. Nucl. Mater. 2019, 523, 66–70. [Google Scholar] [CrossRef]
- Kim, S.-S.; Lim, S.; Ahn, D.-H.; Lee, G.-G.; Chang, K. Effect of Inhomogeneous Nucleation of Hydride at α/β Phase Boundary on Microstructure Evolution of Zr–2.5 wt% Nb Pressure Tube. Met. Mater. Int. 2019, 25, 838–845. [Google Scholar] [CrossRef]
- Park, S.; Kim, K.-j.; Lee, J.; Kim, J.-Y.; Lee, D.W.; Lim, S.H.; Youn, Y.-S. Synchrotron-based high-resolution photoemission spectroscopy study of ZIRLO cladding with H2O adsorption: Coverage and temperature dependence. Sci. Rep. 2020, 10, 6650. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, T.-H.; Kim, K.-m.; Kim, Y.-S. Terminal solid solubility of hydrogen of optimized-Zirlo and its effects on hydride reorientation mechanisms under dry storage conditions. Nucl. Eng. Technol. 2020, 52, 1742–1748. [Google Scholar] [CrossRef]
- Ha, J.M.; Park, S.; Kwon, E.; Lee, D.W.; Kwon, T.H.; Nam, J.-W.; Lee, J.; Kim, J.-Y.; Lee, H.; Lim, S.H.; et al. Effects of minor alloying elements added in simulated cladding on lattice thermal expansion. J. Nucl. Mater. 2021, 557, 153240. [Google Scholar] [CrossRef]
- Topping, M.; Long, F.; Cherubin, I.; Badr, N.N.; Cui, J.; Park, J.S.; Daymond, M.R. Investigating the stability of reoriented hydrides and their reprecipitation using in-situ heating experiments. J. Nucl. Mater. 2022, 564, 153670. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.-H.; Lee, Y. Suppressed hydride precipitation in the welding zone of a zirconium-based alloy cladding tube. J. Nucl. Mater. 2023, 580, 154406. [Google Scholar] [CrossRef]
- Stojilovic, N.; Ramsier, R.D. Oxidation of Zircaloy-4 by H2O followed by molecular desorption. Appl. Surf. Sci. 2006, 252, 5839–5845. [Google Scholar] [CrossRef]
- Hong, S.I.; Lee, K.W. Stress-induced reorientation of hydrides and mechanical properties of Zircaloy-4 cladding tubes. J. Nucl. Mater. 2005, 340, 203–208. [Google Scholar] [CrossRef]
- Colas, K.B.; Motta, A.T.; Daymond, M.R.; Almer, J.D. Effect of thermo-mechanical cycling on zirconium hydride reorientation studied in situ with synchrotron X-ray diffraction. J. Nucl. Mater. 2013, 440, 586–595. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K.-m.; Kim, J.-S.; Kim, Y.-S. Effects of hydride precipitation on the mechanical property of cold worked zirconium alloys in fully recrystallized condition. Nucl. Eng. Technol. 2020, 52, 352–359. [Google Scholar] [CrossRef]
- Barashev, A.V.; Zhao, Q.; Wang, Q.; Yan, Q.; Gao, F. Cluster dynamics simulation of Zr hydrides formation on grain boundaries in Zr. J. Nucl. Mater. 2022, 561, 153521. [Google Scholar] [CrossRef]
- Auzoux, Q.; Bouffioux, P.; Machiels, A.; Yagnik, S.; Bourdiliau, B.; Mallet, C.; Mozzani, N.; Colas, K. Hydride reorientation and its impact on mechanical properties of high burn-up and unirradiated cold-worked stress-relieved Zircaloy-4 and ZirloTM fuel cladding. J. Nucl. Mater. 2022, 568, 153893. [Google Scholar] [CrossRef]
- Kim, D.; Woo, D.; Lee, Y. Radial hydride fraction with various rod internal pressures and hydrogen contents for Zr-Nb alloy cladding tube. J. Nucl. Mater. 2022, 572, 154036. [Google Scholar] [CrossRef]
- Zanellato, O.; Preuss, M.; Buffiere, J.Y.; Ribeiro, F.; Steuwer, A.; Desquines, J.; Andrieux, J.; Krebs, B. Synchrotron diffraction study of dissolution and precipitation kinetics of hydrides in Zircaloy-4. J. Nucl. Mater. 2012, 420, 537–547. [Google Scholar] [CrossRef]
- Raynaud, P.A.; Koss, D.A.; Motta, A.T. Crack growth in the through-thickness direction of hydrided thin-wall Zircaloy sheet. J. Nucl. Mater. 2012, 420, 69–82. [Google Scholar] [CrossRef]
- Tupin, M.; Martin, F.; Bisor, C.; Verlet, R.; Bossis, P.; Chene, J.; Jomard, F.; Berger, P.; Pascal, S.; Nuns, N. Hydrogen diffusion process in the oxides formed on zirconium alloys during corrosion in pressurized water reactor conditions. Corros. Sci. 2017, 116, 1–13. [Google Scholar] [CrossRef]
- Qin, W.; Liang, J.L.; Cheng, Z.Q.; Shi, M.H.; Gu, D.; Li, T.L.; Zhu, W.L.; Szpunar, J.A. Threshold stress of hydride reorientation in zirconium alloy nuclear fuel cladding tubes: A theoretical determination. J. Nucl. Mater. 2022, 563, 153659. [Google Scholar] [CrossRef]
- Courty, O.F.; Motta, A.T.; Piotrowski, C.J.; Almer, J.D. Hydride precipitation kinetics in Zircaloy-4 studied using synchrotron X-ray diffraction. J. Nucl. Mater. 2015, 461, 180–185. [Google Scholar] [CrossRef]
- Motta, A.T.; Capolungo, L.; Chen, L.-Q.; Cinbiz, M.N.; Daymond, M.R.; Koss, D.A.; Lacroix, E.; Pastore, G.; Simon, P.-C.A.; Tonks, M.R.; et al. Hydrogen in zirconium alloys: A review. J. Nucl. Mater. 2019, 518, 440–460. [Google Scholar] [CrossRef]
- Billone, M.C.; Burtseva, T.A.; Einziger, R.E. Ductile-to-brittle transition temperature for high-burnup cladding alloys exposed to simulated drying-storage conditions. J. Nucl. Mater. 2013, 433, 431–448. [Google Scholar] [CrossRef]
- Chu, H.C.; Wu, S.K.; Kuo, R.C. Hydride reorientation in Zircaloy-4 cladding. J. Nucl. Mater. 2008, 373, 319–327. [Google Scholar] [CrossRef]
- Won, J.-J.; Kim, M.-S.; Kim, K.-T. Heat-up and cool-down temperature-dependent hydride reorientation behaviors in zirconium alloy cladding tubes. Nucl. Eng. Technol. 2014, 46, 681–688. [Google Scholar] [CrossRef]
- Cha, H.-J.; Jang, K.-N.; An, J.-H.; Kim, K.-T. The effect of hydrogen and oxygen contents on hydride reorientations of zirconium alloy cladding tubes. Nucl. Eng. Technol. 2015, 47, 746–755. [Google Scholar] [CrossRef][Green Version]
- Lee, J.-M.; Kim, H.-A.; Kook, D.-H.; Kim, Y.-S. A study on the effects of hydrogen content and peak temperature on threshold stress for hydride reorientation in Zircaloy-4 cladding. J. Nucl. Mater. 2018, 509, 285–294. [Google Scholar] [CrossRef]
- Singh, R.N.; Kishore, R.; Singh, S.S.; Sinha, T.K.; Kashyap, B.P. Stress-reorientation of hydrides and hydride embrittlement of Zr–2.5 wt% Nb pressure tube alloy. J. Nucl. Mater. 2004, 325, 26–33. [Google Scholar] [CrossRef]
- Daum, R.S.; Majumdar, S.; Liu, Y.; Billone, M.C. Radial-hydride Embrittlement of High-burnup Zircaloy-4 Fuel Cladding. J. Nucl. Sci. Technol. 2006, 43, 1054–1067. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, T.-H.; Kook, D.-H.; Kim, Y.-S. Effects of hydride morphology on the embrittlement of Zircaloy-4 cladding. J. Nucl. Mater. 2015, 456, 235–245. [Google Scholar] [CrossRef]
- Valance, S.; Bertsch, J. Hydrides reorientation investigation of high burn-up PWR fuel cladding. J. Nucl. Mater. 2015, 464, 371–381. [Google Scholar] [CrossRef]
- Woo, D.; Lee, Y. Understanding the mechanical integrity of Zircaloy cladding with various radial and circumferential hydride morphologies via image analysis. J. Nucl. Mater. 2023, 584, 154560. [Google Scholar] [CrossRef]
- Zlotea, C.; Oumellal, Y.; Provost, K.; Ghimbeu, C.M. Experimental Challenges in Studying Hydrogen Absorption in Ultrasmall Metal Nanoparticles. Front. Energy Res. 2016, 4, 24. [Google Scholar] [CrossRef]
- Narayan, T.C.; Hayee, F.; Baldi, A.; Leen Koh, A.; Sinclair, R.; Dionne, J.A. Direct visualization of hydrogen absorption dynamics in individual palladium nanoparticles. Nat. Commun. 2017, 8, 14020. [Google Scholar] [CrossRef]
- Namba, K.; Ogura, S.; Ohno, S.; Di, W.; Kato, K.; Wilde, M.; Pletikosić, I.; Pervan, P.; Milun, M.; Fukutani, K. Acceleration of hydrogen absorption by palladium through surface alloying with gold. Proc. Natl. Acad. Sci. USA 2018, 115, 7896–7900. [Google Scholar] [CrossRef] [PubMed]
- Gatin, A.; Grishin, M.; Dokhlikova, N.; Ozerin, S.; Sarvadii, S.; Kharitonov, V.; Shub, B. Effect of Size on Hydrogen Adsorption on the Surface of Deposited Gold Nanoparticles. Nanomaterials 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, M.; Chiorino, A.; Vindigni, F.; Boccuzzi, F. Hydrogen interaction with gold nanoparticles and clusters supported on different oxides: A FTIR study. Catal. Today 2012, 181, 62–67. [Google Scholar] [CrossRef]
- Watkins, W.L.; Borensztein, Y. Mechanism of hydrogen adsorption on gold nanoparticles and charge transfer probed by anisotropic surface plasmon resonance. Phys. Chem. Chem. Phys. 2017, 19, 27397–27405. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yamauchi, M.; Ikeda, R.; Yamamoto, T.; Matsumura, S.; Kitagawa, H. Double enhancement of hydrogen storage capacity of Pd nanoparticles by 20 at% replacement with Ir; systematic control of hydrogen storage in Pd–M nanoparticles (M = Ir, Pt, Au). Chem. Sci. 2018, 9, 5536–5540. [Google Scholar] [CrossRef]
- Dekura, S.; Kobayashi, H.; Kusada, K.; Kitagawa, H. Hydrogen in Palladium and Storage Properties of Related Nanomaterials: Size, Shape, Alloying, and Metal-Organic Framework Coating Effects. ChemPhysChem 2019, 20, 1158–1176. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Xiahou, Y.; Zhang, P.; Ding, W.; Wang, D. Revitalizing the Frens Method to Synthesize Uniform, Quasi-Spherical Gold Nanoparticles with Deliberately Regulated Sizes from 2 to 330 nm. Langmuir 2016, 32, 5870–5880. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhong, X.; Li, Z.; Xia, Y. Successive, Seed-Mediated Growth for the Synthesis of Single-Crystal Gold Nanospheres with Uniform Diameters Controlled in the Range of 5–150 nm. Part. Part. Syst. Charact. 2014, 31, 266–273. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Tumskiy, R.S.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Quantifying the Numbers of Gold Nanoparticles in the Test Zone of Lateral Flow Immunoassay Strips. ACS Appl. Nano Mater. 2019, 2, 5020–5028. [Google Scholar] [CrossRef]
- Wang, Y.; Biby, A.; Xi, Z.; Liu, B.; Rao, Q.; Xia, X. One-Pot Synthesis of Single-Crystal Palladium Nanoparticles with Controllable Sizes for Applications in Catalysis and Biomedicine. ACS Appl. Nano Mater. 2019, 2, 4605–4612. [Google Scholar] [CrossRef]
- Wang, L.-m.; He, S.; Cui, Z.-m.; Guo, L. One-step synthesis of monodisperse palladium nanosphere and their catalytic activity for Suzuki coupling reactions. Inorg. Chem. Commun. 2011, 14, 1574–1578. [Google Scholar] [CrossRef]
- Baylon, R.A.L.; Sun, J.; Kovarik, L.; Engelhard, M.; Li, H.; Winkelman, A.D.; Wang, Y. Structural identification of ZnxZryOz catalysts for Cascade aldolization and self-deoxygenation reactions. Appl. Catal. B 2018, 234, 337–346. [Google Scholar] [CrossRef]
- Nelson, A.E.; Schulz, K.H. Surface chemistry and microstructural analysis of CexZr1−xO2−y model catalyst surfaces. Appl. Surf. Sci. 2003, 210, 206–221. [Google Scholar] [CrossRef]
- Lokesha, H.S.; Nagabhushana, K.R.; Singh, F.; Tatumi, S.H.; Prinsloo, A.R.E.; Sheppard, C.J. Unraveling the Charge State of Oxygen Vacancies in Monoclinic ZrO2 and Spectroscopic Properties of ZrO2:Sm3+ Phosphor. J. Phys. Chem. C 2021, 125, 27106–27117. [Google Scholar] [CrossRef]
- Yan, Y.; Qian, S.; Garrison, B.; Smith, T.; Kim, P. Nondestructive hydrogen analysis of steam-oxidized Zircaloy-4 by wide-angle neutron scattering. J. Nucl. Mater. 2018, 502, 191–200. [Google Scholar] [CrossRef]
- Yamauchi, M.; Ikeda, R.; Kitagawa, H.; Takata, M. Nanosize Effects on Hydrogen Storage in Palladium. J. Phys. Chem. C 2008, 112, 3294–3299. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamauchi, M.; Ikeda, R.; Kitagawa, H. Atomic-level Pd–Au alloying and controllable hydrogen-absorption properties in size-controlled nanoparticles synthesized by hydrogenreduction. Chem. Commun. 2009, 32, 4806–4808. [Google Scholar] [CrossRef][Green Version]
- Bond, G.C. Hydrogenation by gold catalysts: An unexpected discovery and a current assessment. Gold Bull. 2016, 49, 53–61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.J.; Ha, J.; Choi, S.J.; Kim, H.I.; Ryu, S.; Kim, Y.; Youn, Y.-S. Decreasing Hydrogen Content within Zirconium Using Au and Pd Nanoparticles as Sacrificial Agents under Pressurized Water at High Temperature. Materials 2023, 16, 6164. https://doi.org/10.3390/ma16186164
Lee YJ, Ha J, Choi SJ, Kim HI, Ryu S, Kim Y, Youn Y-S. Decreasing Hydrogen Content within Zirconium Using Au and Pd Nanoparticles as Sacrificial Agents under Pressurized Water at High Temperature. Materials. 2023; 16(18):6164. https://doi.org/10.3390/ma16186164
Chicago/Turabian StyleLee, Yeon Ju, Juhee Ha, Su Ji Choi, Hyeok Il Kim, Sumin Ryu, Youngsoo Kim, and Young-Sang Youn. 2023. "Decreasing Hydrogen Content within Zirconium Using Au and Pd Nanoparticles as Sacrificial Agents under Pressurized Water at High Temperature" Materials 16, no. 18: 6164. https://doi.org/10.3390/ma16186164
APA StyleLee, Y. J., Ha, J., Choi, S. J., Kim, H. I., Ryu, S., Kim, Y., & Youn, Y.-S. (2023). Decreasing Hydrogen Content within Zirconium Using Au and Pd Nanoparticles as Sacrificial Agents under Pressurized Water at High Temperature. Materials, 16(18), 6164. https://doi.org/10.3390/ma16186164





