Influence of Low Temperature Plasma Oxidizing on the Bioactivity of NiTi Shape Memory Alloy for Medical Applications
Abstract
1. Introduction
2. Materials and Methods
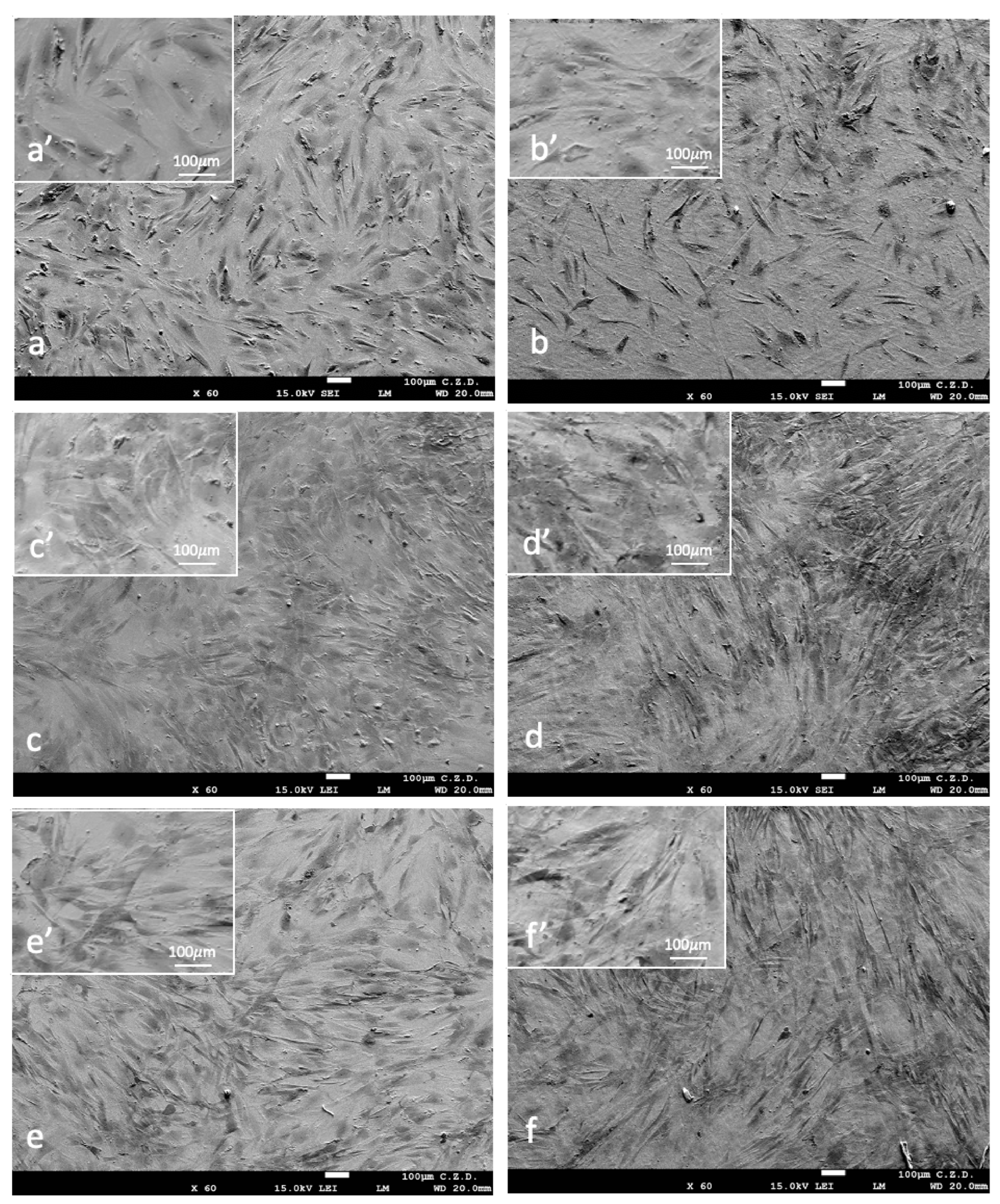
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biesiekierski, A.; Wang, J.; Abdel-Hady Gepreel, M.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Yoneyama, T.; Miyazaki, S. Shape Memory Alloys for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2008. [Google Scholar] [CrossRef]
- Duerig, T.; Pelton, A.; Stöckel, D. An overview of nitinol medical applications. Mater. Sci. Eng. A 1999, 273–275, 149–160. [Google Scholar] [CrossRef]
- Morgan, N. Medical shape memory alloy applications—The market and its products. Mater. Sci. Eng. A 2004, 378, 16–23. [Google Scholar] [CrossRef]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Rondelli, G.; Torricelli, P.; Fini, M.; Rimondini, L.; Giardino, R. In vitro corrosion study by EIS of an equiatomic NiTi alloy and an implant quality AISI 316 stainless steel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 79, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, Y.; Zhao, X.; Chen, H.; Zhang, T. Ni ion release, osteoblast-material interactions, and hemocompatibility of hafnium-implanted NiTi alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 646–659. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A.; Rondelli, G.C.; Undisz, A.L.; Anderegg, J.W.; Burleigh, T.D.; Rettenmayr, M.E. The electrochemical characteristics of native Nitinol surfaces. Biomaterials 2009, 30, 3662–3671. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Toker, S.; Canadinc, D.; Maier, H.; Birer, O. Evaluation of passive oxide layer formation–biocompatibility relationship in NiTi shape memory alloys: Geometry and body location dependency. Mater. Sci. Eng. C 2014, 36, 118–129. [Google Scholar] [CrossRef]
- Ryhänen, J. Biocompatibility of nitinol. Minim. Invasive Ther. Allied Technol. 2000, 9, 99–105. [Google Scholar] [CrossRef]
- Arndt, M.; Brück, A.; Scully, T.; Jäger, A.; Bourauel, C. Nickel ion release from orthodontic NiTi wires under simulation of realistic in-situ conditions. J. Mater. Sci. 2005, 40, 3659–3667. [Google Scholar] [CrossRef]
- Denkhaus, E.; Salnikow, K. Nickel essentiality, toxicity, and carcinogenicity. Crit. Rev. Oncol. 2002, 42, 35–56. [Google Scholar] [CrossRef]
- Chrzanowski, W.; Szade, J.; Hart, A.D.; Knowles, J.C.; Dalby, M.J. Biocompatible, Smooth, Plasma-Treated Nickel–Titanium Surface—An Adequate Platform for Cell Growth. J. Biomater. Appl. 2012, 26, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Plant, S.D.; Grant, D.M.; Leach, L. Behaviour of human endothelial cells on surface modified NiTi alloy. Biomaterials 2005, 26, 5359–5367. [Google Scholar] [CrossRef] [PubMed]
- Plant, S.D.; Grant, D.M.; Leach, L. Surface modification of NiTi alloy and human platelet activation under static and flow conditions. Mater. Lett. 2007, 61, 2864–2867. [Google Scholar] [CrossRef]
- Barrett, R.D.; Bishara, S.E.; Quinn, J.K. Biodegradation of orthodontic appliances. Part I. Biodegradation of nickel and chromium in vitro. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, W. Engineering Surface Properties of Nickel-Titanium Alloy for Improved Osteointegration; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2012. [Google Scholar]
- Wataha, J.C.; Ratanasathien, S.; Hanks, C.T.; Sun, Z. In vitro IL-1β and TNF-α release from THP-1 monocytes in response to metal ions. Dent. Mater. 1996, 12, 322–327. [Google Scholar] [CrossRef]
- JWataha, C.; Lockwood, P.E.; Marek, M.; Ghazi, M. Ability of Ni-containing biomedical alloys to activate monocytes and en-dothelial cells in vitro. J. Biomed. Mater. Res. 1999, 45, 251–257. [Google Scholar] [CrossRef]
- Czarnowska, E.; Borowski, T.; Sowińska, A.; Lelątko, J.; Oleksiak, J.; Kamiński, J.; Tarnowski, M.; Wierzchoń, T. Structure and properties of nitrided surface layer produced on NiTi shape memory alloy by low temperature plasma nitriding. Appl. Surf. Sci. 2015, 334, 24–31. [Google Scholar] [CrossRef]
- Witkowska, J.; Sowińska, A.; Czarnowska, E.; Płociński, T.; Borowski, T.; Wierzchoń, T. NiTi shape-memory alloy oxidized in low-temperature plasma with carbon coating: Characteristic and a potential for cardiovascular applications. Appl. Surf. Sci. 2017, 421, 89–96. [Google Scholar] [CrossRef]
- Witkowska, J.; Sowińska, A.; Czarnowska, E.; Płociński, T.; Kamiński, J.; Wierzchoń, T. Hybrid a-CNH+TiO2+TiN-type surface layers produced on NiTi shape memory alloy for cardiovascular applications. Nanomedicine 2017, 12, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, J.; Kamiński, J.; Płociński, T.; Tarnowski, M.; Wierzchoń, T. Corrosion resistance of NiTi shape memory alloy after hybrid surface treatment using low-temperature plasma. Vacuum 2017, 137, 92–96. [Google Scholar] [CrossRef]
- Witkowska, J.; Sowińska, A.; Czarnowska, E.; Płociński, T.; Rajchel, B.; Tarnowski, M.; Wierzchoń, T. Structure and properties of composite surface layers produced on NiTi shape memory alloy by a hybrid method. J. Mater. Sci. Mater. Med. 2018, 29, 110. [Google Scholar] [CrossRef] [PubMed]
- Chlanda, A.; Witkowska, J.; Morgiel, J.; Nowińska, K.; Choińska, E.; Swieszkowski, W.; Wierzchoń, T. Multi-scale characterization and biological evaluation of composite surface layers produced under glow discharge conditions on NiTi shape memory alloy for potential cardiological application. Micron 2018, 114, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, J.; Svahn, F.; Jarmar, T.; Engqvist, H. Formation and adhesion of biomimetic hydroxyapatite deposited on titanium substrates. Acta Biomater. 2007, 3, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Jahan, A.; Ismail, M.; Sapuan, S.; Mustapha, F. Material screening and choosing methods—A review. Mater. Des. 2010, 31, 696–705. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Jahan, A. Material selection for femoral component of total knee replacement using comprehensive VIKOR. Mater. Des. 2011, 32, 4471–4477. [Google Scholar] [CrossRef]
- Chen, M.; Yang, X.; Hu, R.; Cui, Z.; Man, H. Bioactive NiTi shape memory alloy used as bone bonding implants. Mater. Sci. Eng. C 2004, 24, 497–502. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2005, 47, 49–121. [Google Scholar] [CrossRef]
- Lelątko, J.; Goryczka, T.; Wierzchoń, T.; Ossowski, M.; Łosiewicz, B.; Rówiński, E.; Morawiec, H. Structure of Low Temperature Nitrided/Oxidized Layer Formed on NiTi Shape Memory Alloy. Solid State Phenom. 2010, 163, 127–130. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.L.; Grasel, T.G.; Cooper, S.L.; Albrecht, R.M. Platelet shape change and cytoskeletal reorganization on polyurethaneureas. J. Biomed. Mater. Res. 1989, 23, 105–123. [Google Scholar] [CrossRef]
- Es-Souni, M.; Es-Souni, M.; Fischer-Brandies, H. Assessing the biocompatibility of NiTi shape memory alloys used for medical applications. Anal. Bioanal. Chem. 2005, 381, 557–567. [Google Scholar] [CrossRef]
- A Shabalovskaya, S. Surface, corrosion and biocompatibility aspects of Nitinol as an implant material. Bio-Med. Mater. Eng. 2002, 12, 69–109. [Google Scholar]
- Nagaraja, S.; Brown, R.; Saylor, D.; Undisz, A. Oxide Layer Formation, Corrosion, and Biocompatibility of Nitinol Cardiovascular Devices. Shape Mem. Superelasticity 2022, 8, 45–63. [Google Scholar] [CrossRef]
- Shabalovskaya, S.; Anderegg, J.; Van Humbeeck, J. Critical overview of Nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008, 4, 447–467. [Google Scholar] [CrossRef]
- Tan, L.; Crone, W. Surface characterization of NiTi modified by plasma source ion implantation. Acta Mater. 2002, 50, 4449–4460. [Google Scholar] [CrossRef]
- Shevchenko, N.; Pham, M.-T.; Maitz, M. Studies of surface modified NiTi alloy. Appl. Surf. Sci. 2004, 235, 126–131. [Google Scholar] [CrossRef]
- Firstov, G.; Vitchev, R.; Kumar, H.; Blanpain, B.; Van Humbeeck, J. Surface oxidation of NiTi shape memory alloy. Biomaterials 2002, 23, 4863–4871. [Google Scholar] [CrossRef]
- Michiardi, A.; Aparicio, C.; Planell, J.; Gil, F. New oxidation treatment of NiTi shape memory alloys to obtain Ni-free surfaces and to improve biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77B, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, W.; Neel, E.A.A.; Armitage, D.A.; Zhao, X.; Knowles, J.C.; Salih, V. In vitrostudies on the influence of surface modification of Ni–Ti alloy on human bone cells. J. Biomed. Mater. Res. Part A 2010, 93A, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Starosvetsky, D.; Gotman, I. TiN coating improves the corrosion behavior of superelastic NiTi surgical alloy. Surf. Coat. Technol. 2001, 148, 268–276. [Google Scholar] [CrossRef]
- Yokoyama, K.; Kanemura, T.; Sakai, J. Improvements in fracture properties of Ni–Ti superelastic alloy in physiological saline solution containing hydrogen peroxide by surface modification. Mater. Sci. Eng. A 2009, 513–514, 267–275. [Google Scholar] [CrossRef]
- Cheng, F.; Shi, P.; Man, H. Anatase coating on NiTi via a low-temperature sol–gel route for improving corrosion resistance. Scr. Mater. 2004, 51, 1041–1045. [Google Scholar] [CrossRef]
- Sui, J.; Cai, W. Formation of diamond-like carbon (DLC) film on the NiTi alloys via plasma immersion ion implantation and deposition (PIIID) for improving corrosion resistance. Appl. Surf. Sci. 2006, 253, 2050–2055. [Google Scholar] [CrossRef]
- Wong, M.; Cheng, F.; Man, H. Laser oxidation of NiTi for improving corrosion resistance in Hanks’ solution. Mater. Lett. 2007, 61, 3391–3394. [Google Scholar] [CrossRef]
- Man, H.C.; Cui, Z.D.; Yue, T.M. Surface characteristics and corrosion behavior of laser surface nitrided NiTi shape memory alloy for biomedical applications. J. Laser Appl. 2002, 14, 242–247. [Google Scholar] [CrossRef]
- Liu, J.-X.; Yang, D.-Z.; Shi, F.; Cai, Y.-J. Sol–gel deposited TiO2 film on NiTi surgical alloy for biocompatibility improvement. Thin Solid Films 2003, 429, 225–230. [Google Scholar] [CrossRef]
- Dudek, K.; Goryczka, T. Electrophoretic deposition and characterization of thin hydroxyapatite coatings formed on the surface of NiTi shape memory alloy. Ceram. Int. 2016, 42, 19124–19132. [Google Scholar] [CrossRef]
- Hu, T.; Chu, C.-L.; Yin, L.-H.; Pu, Y.-P.; Dong, Y.-S.; Guo, C.; Sheng, X.-B.; Chung, J.-C.; Chu, P.-K. In vitro biocompatibility of titanium-nickel alloy with titanium oxide film by H2O2 oxidation. Trans. Nonferrous Met. Soc. China 2007, 17, 553–557. [Google Scholar] [CrossRef]
- Poon, R.; Yeung, K.; Liu, X.; Chu, P.; Chung, C.; Lu, W.; Cheung, K.; Chan, D. Carbon plasma immersion ion implantation of nickel–titanium shape memory alloys. Biomaterials 2005, 26, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.; Chan, R.; Lam, K.; Wu, S.; Liu, X.; Chung, C.; Chu, P.K.; Lu, W.; Chan, D.; Luk, K.; et al. In vitro and in vivo characterization of novel plasma treated nickel titanium shape memory alloy for orthopedic implantation. Surf. Coat. Technol. 2007, 202, 1247–1251. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Khalil-Allafi, J.; Sadeghpour-Motlagh, M.; Shakeri, M.S.; Masoudfar, S.; Farrokhi, A.; Khosrowshahi, Y.B.; Nadernezhad, A.; Siadati, M.H.; Javidi, M.; et al. Effect of surface modification by nitrogen ion implantation on the electrochemical and cellular behaviors of super-elastic NiTi shape memory alloy. J. Mater. Sci. Mater. Med. 2014, 25, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Lelątko, J.; Freitag, M.; Rak, J.; Wierzchoń, T.; Goryczka, T. Structure of Nitride and Nitride/Oxide Layers Formed on NiTi Alloy. Solid State Phenom. 2012, 186, 259–262. [Google Scholar] [CrossRef]
- Lelatko, J.; Paczkowski, P.; Wierzchoń, T.; Morawiec, H. TEM studies of the nitrided Ni-Ti surface layer. J. Microsc. 2006, 223, 234–236. [Google Scholar] [CrossRef]
- Lelątko, J.; Lekston, Z.; Freitag, M.; Wierzchoń, T.; Goryczka, T. Influence of low temperature glow discharge nitriding and ox-ynitriding process on microstructural and shape memory effect in NiTi alloy. Mater. Eng. 2012, 4, 256–261. [Google Scholar]
- Loty, C.; Sautier, J.M.; Boulekbache, H.; Kokubo, T.; Kim, H.M.; Forest, N. In vitro bone formation on a bone-like apatite layer prepared by a biomimetic process on a bioactive glass-ceramic. J. Biomed. Mater. Res. 2000, 49, 423–434. [Google Scholar] [CrossRef]
- Koppala, S.; Swamiappan, S.; Gangarajula, Y.; Xu, L.; Sadasivuni, K.K.; Ponnamma, D.; Rajagopalan, V. Calcium deficiency in hydroxyapatite and its drug delivery applications. Micro Nano Lett. 2018, 13, 562–564. [Google Scholar] [CrossRef]
- Bourgeois, B.; Laboux, O.; Obadia, L.; Gauthier, O.; Betti, E.; Aguado, E.; Daculsi, G.; Bouler, J.-M. Calcium-deficient apatite: A firstin vivo study concerning bone ingrowth. J. Biomed. Mater. Res. 2003, 65, 402–408. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W. The fallacy of the calcium-phosphorus product. Kidney Int. 2007, 72, 792–796. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R. Formation and transformation of calcium phosphates: Relevance to vascular calcification. Z. Kardiol. 2001, 90 (Suppl. 3), 116–124. [Google Scholar] [CrossRef]
- Gasga, J.R.; Carbajal-De-La-Torre, G.; Bres, E.; Gil-Chavarria, I.M.; Rodríguez-Hernández, A.G.; Garcia-Garcia, R. STEM-HAADF electron microscopy analysis of the central dark line defect of human tooth enamel crystallites. J. Mater. Sci. Mater. Med. 2008, 19, 877–882. [Google Scholar] [CrossRef]
- Nancollas, G.H.; Wu, W. Biomineralization mechanisms: A kinetics and interfacial energy approach. J. Cryst. Growth 2000, 211, 137–142. [Google Scholar] [CrossRef]
- Wan, B.; Ruan, Y.; Shen, C.; Xu, G.; Forouzanfar, T.; Lin, H.; Wu, G. Biomimetically precipitated nanocrystalline hydroxyapatite. Nano TransMed 2022, 1, e9130008. [Google Scholar] [CrossRef]
- Griesiute, D.; Garskaite, E.; Antuzevics, A.; Klimavicius, V.; Balevicius, V.; Zarkov, A.; Katelnikovas, A.; Sandberg, D.; Kareiva, A. Synthesis, structural and luminescent properties of Mn-doped calcium pyrophosphate (Ca2P2O7) polymorphs. Sci. Rep. 2022, 12, 7116. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Anastasiou, A.; Nerantzaki, M.; Brown, A.; Jha, A.; Bikiaris, D. Drug loading capacity of microporous β-pyrophosphate crystals. Mater. Des. 2019, 168, 107661. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, L.; He, Z.; Jiang, Y.; Tan, J. Microstructure evolution, mechanical properties, and enhanced bioactivity of Ti-13Nb-13Zr based calcium pyrophosphate composites for biomedical applications. Mater. Sci. Eng. C 2019, 98, 279–287. [Google Scholar] [CrossRef]
- Lopez-Santos, C.; Colaux, J.L.; Laloy, J.; Fransolet, M.; Mullier, F.; Michiels, C.; Dogné, J.-M.; Lucas, S. Bioactivity and hemocompatibility study of amorphous hydrogenated carbon coatings produced by pulsed magnetron discharge. J. Biomed. Mater. Res. Part A 2013, 101A, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Logothetidis, S.; Karagkiozaki, V.; Karagiannidis, P.G.; Kalfagiannis, N.; Patsalas, P.; Georgiou, D.; Kavatzikidou, P. Novel nanostructured biomaterials: Implications for coronary stent thrombosis. Int. J. Nanomed. 2012, 7, 6063–6076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, F.; Huang, N.; Leng, Y.; Zhao, A.; Jing, F. Study on wettabilities and platelet adhesion behavior of C:H and C:N:H films prepared by DC-MFCVA. Appl. Surf. Sci. 2008, 255, 469–472. [Google Scholar] [CrossRef]
- Chun, Y.; Levi, D.S.; Mohanchandra, K.; Carman, G.P. Superhydrophilic surface treatment for thin film NiTi vascular applications. Mater. Sci. Eng. C 2009, 29, 2436–2441. [Google Scholar] [CrossRef]
- Popov, C.; Vasilchina, H.; Kulisch, W.; Danneil, F.; Stüber, M.; Ulrich, S.; Welle, A.; Reithmaier, J. Wettability and protein adsorption on ultrananocrystalline diamond/amorphous carbon composite films. Diam. Relat. Mater. 2009, 18, 895–898. [Google Scholar] [CrossRef]
- Tavakalyan, N.B.; Karapetyan, A.G.; Pogosyan, A.S.; Abrahamyan, A.K.; Corti, A.; Pompella, A.; Mihranyan, A. Influence of unsaturated carbonic acids on hemocompatibility and cytotoxicity of poly-vinylacetate based co-polymers. J. Mater. Sci. Mater. Med. 2010, 21, 1693–1702. [Google Scholar] [CrossRef]
- Dey, R.K.; Ray, A.R. Synthesis, characterization, and blood compatibility of polyamidoamines copolymers. Biomaterials 2003, 24, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.A.N.; Vieira, E.P.; Motschmann, H.; Möhwald, H.; Thünemann, A.F. Human Serum Albumin on Fluorinated Surfaces. Langmuir 2003, 19, 7544–7550. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]
- Xu, L.-C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef]
- Tang, C.-M.; Fan, F.-Y.; Lin, W.-T.; Wang, L.; Lin, W.-C. Effect of Bioactivity of Surface Topography and Coating Forming by Infrared Light-Induced on Titanium for Bone Repair. Appl. Sci. 2020, 10, 8158. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Hao, P. The effect of topography and wettability of biomaterials on platelet adhesion. J. Adhes. Sci. Technol. 2016, 30, 878–893. [Google Scholar] [CrossRef]
- Nagashima, S.; Hasebe, T.; Kamijo, A.; Yoshimoto, Y.; Hotta, A.; Morita, H.; Terada, H.; Tanaka, M.; Takahashi, K.; Suzuki, T. Effect of oxygen plasma treatment on non-thrombogenicity of diamond-like carbon films. Diam. Relat. Mater. 2010, 19, 861–865. [Google Scholar] [CrossRef]
- Wen, X.-W.; Pei, S.-P.; Li, H.; Ai, F.; Chen, H.; Li, K.-Y.; Wang, Q.; Zhang, Y.-M. Study on an antifouling and blood compatible poly(ethylene–vinyl acetate) material with fluorinated surface structure. J. Mater. Sci. 2010, 45, 2788–2797. [Google Scholar] [CrossRef]
- Skoog, S.A.; Lu, Q.; Malinauskas, R.A.; Sumant, A.V.; Zheng, J.; Goering, P.L.; Narayan, R.J.; Casey, B.J. Effects of nanotopography on the in vitro hemocompatibility of nanocrystalline diamond coatings. J. Biomed. Mater. Res. Part A 2017, 105, 253–264. [Google Scholar] [CrossRef]
- Shibata, H.; Yokoi, T.; Goto, T.; Kim, I.Y.; Kawashita, M.; Kikuta, K.; Ohtsuki, C. Behavior of hydroxyapatite crystals in a simulated body fluid: Effects of crystal face. J. Ceram. Soc. Jpn. 2013, 121, 807–812. [Google Scholar] [CrossRef]
- Bodhak, S.; Bose, S.; Bandyopadhyay, A. Role of surface charge and wettability on early stage mineralization and bone cell–materials interactions of polarized hydroxyapatite. Acta Biomater. 2009, 5, 2178–2188. [Google Scholar] [CrossRef]
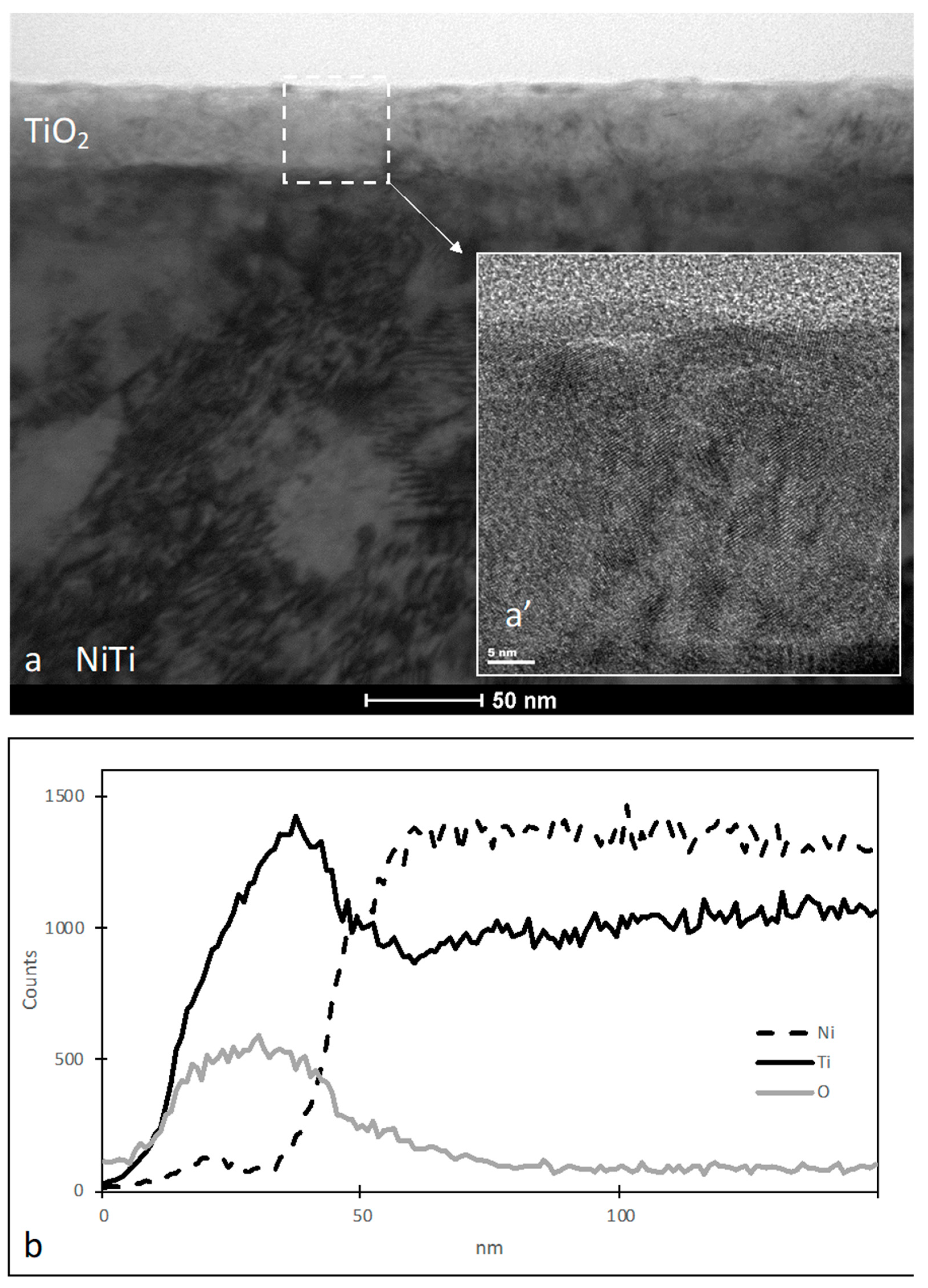

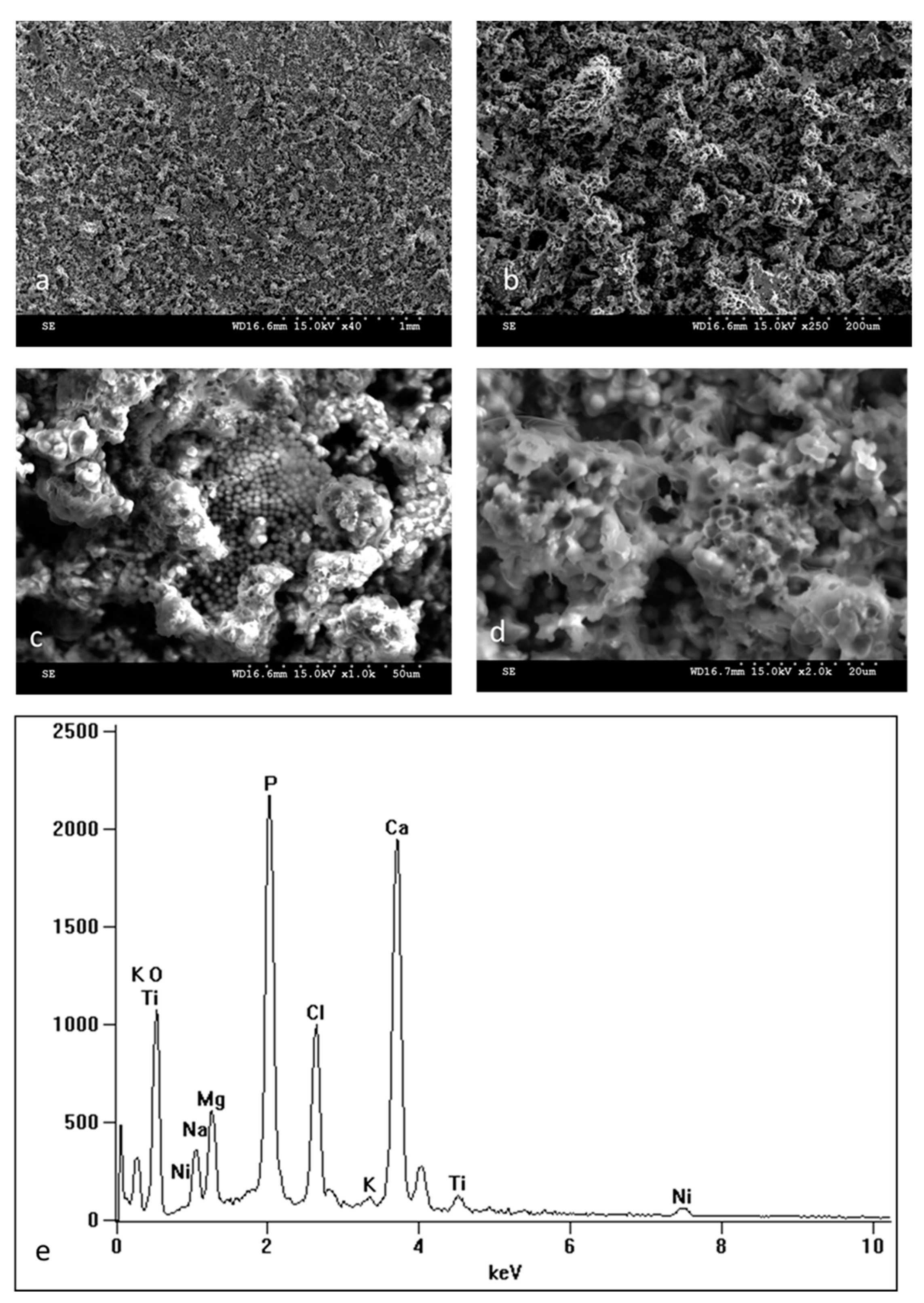
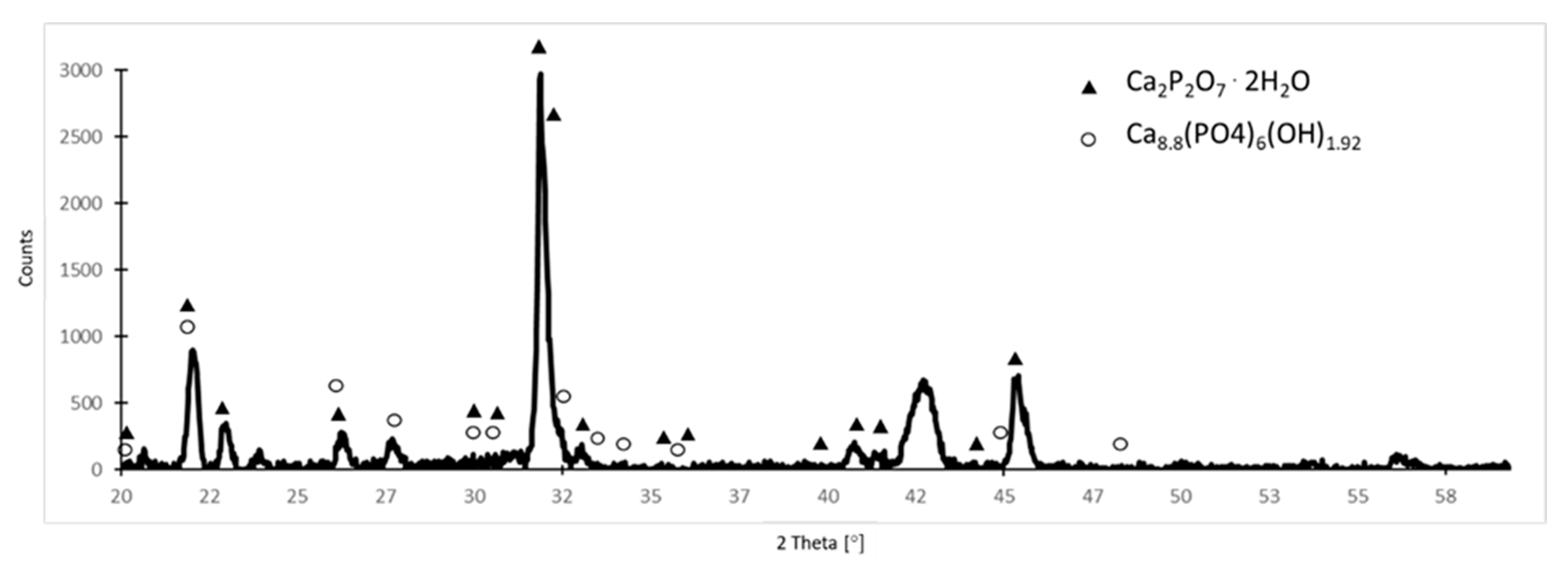
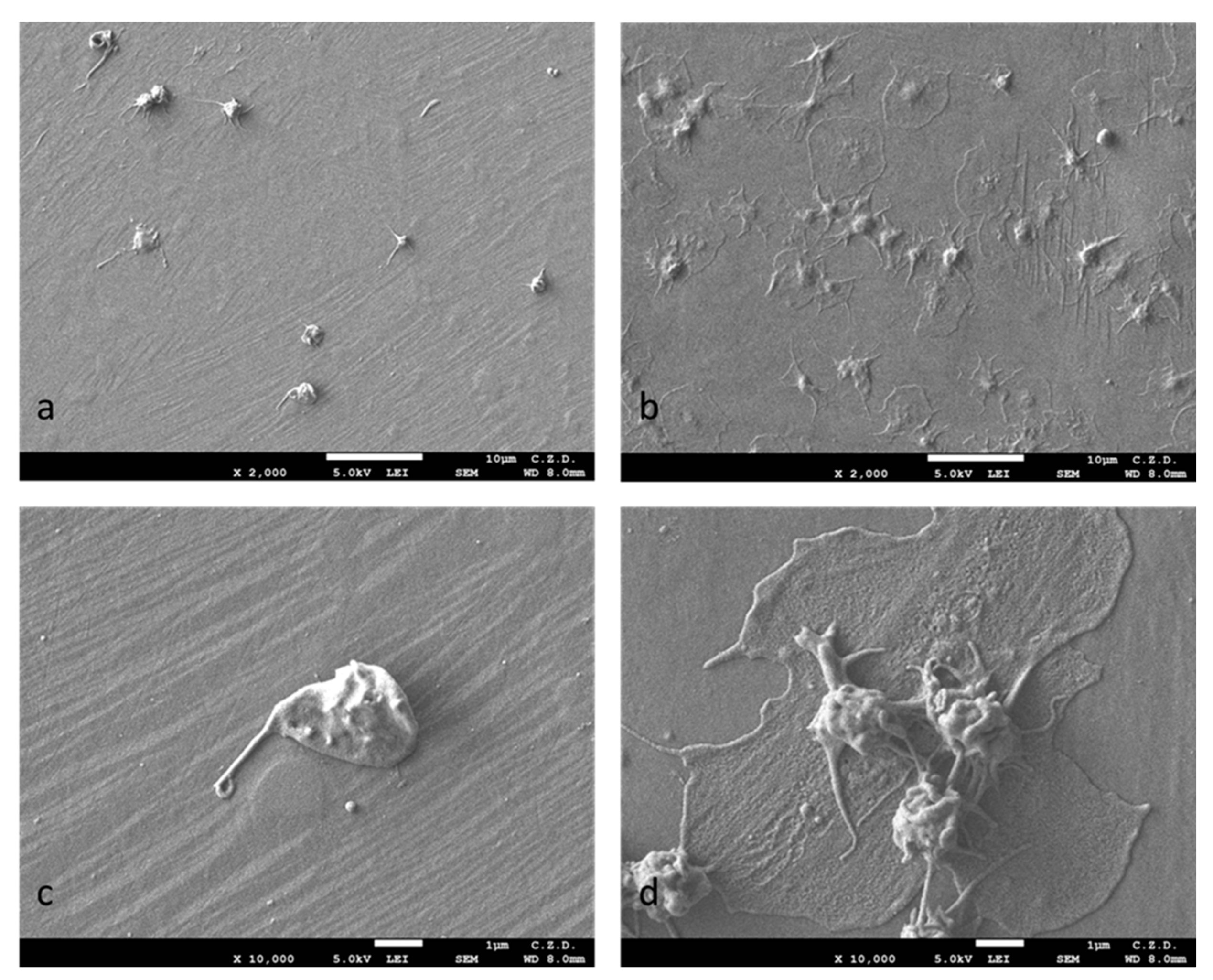


| Component | Concentration (g/L) |
|---|---|
| NaCl | 8.035 |
| NaHCO3 | 0.355 |
| KCl | 0.225 |
| K2HPO4·3H2O | 0.231 |
| MgCl2·6H2O | 0.311 |
| CaCl2 | 0.292 |
| Na2SO4 | 0.072 |
| (HOCH₂)₃CNH₂(Tris) | 6.118 |
| Ca (%at.) | P (%at.) | O (%at.) | Ca/P | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | Image Area | Point | Image Area | Point | Image Area | Point | Image Area | Point |
| NiTi (after 14 days in SBF) | 1.72 | 5.57 ± 0.13 | 1.32 | 3.78 ± 0.13 | 7.55 | 11.16 ± 0.35 | 1.30 | 1.47 |
| NiTi + TiO2 (after 14 days in SBF) | 19.77 | 21.70 ± 0.20 | 14.92 | 17.22 ± 0.11 | 48.00 | 55.34 ± 0.55 | 1.32 | 1.26 |
| NiTi + TiO2 (after 30 days in SBF) | 20.49 | 24.83 ± 0.18 | 14.72 | 16.19 ± 0.13 | 47.39 | 33.82 ± 0.73 | 1.39 | 1.53 |
| NiTi | NiTi + TiO2 | ||
|---|---|---|---|
| Contact Angle | distilled water | 84.5 ± 4.3 | 107.1 ± 0.7 |
| diiodomethane | 45.5 ± 1.3 | 66.5 ± 0.9 | |
| Surface Free Energy | γ (mN/m) | 36.37 | 25.78 |
| γd (mN/m) | 33.7 | 25.9 | |
| γp (mN/m) | 2.8 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowska, J.; Borowski, T.; Sowińska, A.; Choińska, E.; Moszczyńska, D.; Morgiel, J.; Sobiecki, J.; Wierzchoń, T. Influence of Low Temperature Plasma Oxidizing on the Bioactivity of NiTi Shape Memory Alloy for Medical Applications. Materials 2023, 16, 6086. https://doi.org/10.3390/ma16186086
Witkowska J, Borowski T, Sowińska A, Choińska E, Moszczyńska D, Morgiel J, Sobiecki J, Wierzchoń T. Influence of Low Temperature Plasma Oxidizing on the Bioactivity of NiTi Shape Memory Alloy for Medical Applications. Materials. 2023; 16(18):6086. https://doi.org/10.3390/ma16186086
Chicago/Turabian StyleWitkowska, Justyna, Tomasz Borowski, Agnieszka Sowińska, Emilia Choińska, Dorota Moszczyńska, Jerzy Morgiel, Jerzy Sobiecki, and Tadeusz Wierzchoń. 2023. "Influence of Low Temperature Plasma Oxidizing on the Bioactivity of NiTi Shape Memory Alloy for Medical Applications" Materials 16, no. 18: 6086. https://doi.org/10.3390/ma16186086
APA StyleWitkowska, J., Borowski, T., Sowińska, A., Choińska, E., Moszczyńska, D., Morgiel, J., Sobiecki, J., & Wierzchoń, T. (2023). Influence of Low Temperature Plasma Oxidizing on the Bioactivity of NiTi Shape Memory Alloy for Medical Applications. Materials, 16(18), 6086. https://doi.org/10.3390/ma16186086







